ABSTRACT
An increase in the prevalence of commensal Escherichia coli carrying blaCTX-M genes among dairy cattle was observed between 2008 and 2012 in Washington State. To study the molecular epidemiology of this change, we selected 126 blaCTX-M-positive and 126 blaCTX-M-negative isolates for determinations of the multilocus sequence types (MLSTs) and antibiotic resistance phenotypes from E. coli obtained during a previous study. For 99 isolates, we also determined the blaCTX-M alleles using PCR and sequencing and identified the replicon types of blaCTX-M-carrying plasmids. The blaCTX-M-negative E. coli isolates comprised 76 sequence types (STs) compared with 32 STs in blaCTX-M-positive E. coli isolates. The blaCTX-M-positive E. coli isolates formed three MLST clonal complexes, accounting for 83% of these isolates; 52% of blaCTX-M-negative E. coli isolates clustered into 10 clonal complexes, and the remainder were singletons. Overall, blaCTX-M-negative E. coli isolates had more diverse genotypes that were distinct to farms, whereas blaCTX-M-positive E. coli isolates had a clonal population structure and were widely disseminated on farms in both regions included in the study. Plasmid replicon types included IncI1 which predominated, followed by IncFIB and IncFIA/FIB. blaCTX-M-15 was the predominant CTX-M gene allele, followed by blaCTX-M-27 and blaCTX-M-14. There was no significant association between plasmid replicon types and bacterial STs, and neither clonal complexes nor major plasmid groups were associated with two discrete dairy-farming regions of Washington State.
IMPORTANCE Infections caused by extended-spectrum β-lactamase (ESBL)-producing Escherichia coli occur globally and present treatment challenges because of their resistance to multiple antimicrobial drugs. Cattle are potential reservoirs of ESBL-producing Enterobacteriaceae, and so understanding the causes of successful dissemination of blaCTX-M genes in commensal bacteria will inform future approaches for the prevention of antibiotic-resistant pathogen emergence.
KEYWORDS: Escherichia coli, extended-spectrum β-lactamases, dairy cattle, molecular epidemiology, plasmids, sequence types
INTRODUCTION
Infections caused by Enterobacteriaceae such as Escherichia coli or Klebsiella pneumoniae that are resistant to third-generation cephalosporins are a public health concern because they are challenging to treat, and their prevalence has increased in the United States (1) and worldwide (2) in recent decades. Enterobacteriaceae exhibit resistance to expanded-spectrum cephalosporins mainly by producing extended-spectrum β-lactamases (ESBLs), which are enzymes that hydrolyze β-lactam antibiotics. The ESBL families include TEM, SHV, OXA, CTX-M, and other variants. The CTX-M β-lactamases are of particular interest because they have become the predominant ESBL worldwide (3–5), and more than 120 CTX-M enzymes have been described (6, 7). The success of CTX-M β-lactamase-producing lineages has been attributed to several factors, such as an efficient mobilization and dissemination of CTX-M genes by mobile genetic elements (insertion sequences, transposons, and plasmids), the association of plasmids that harbor CTX-M genes with successful bacterial clones that are widely disseminated, the low fitness cost of CTX-M β-lactamase production, and antibiotic selection pressure (7).
The global problem of E. coli isolates that are resistant to critically important antibiotics requires investigations of how and where antibiotic selection is occurring, where reservoirs of resistant bacterial strains exist, and how these strains disseminate. We recently reported that E. coli isolated from dairy cattle in Washington State in 2008 did not carry CTX-M genes, while isolates collected in 2011 did carry CTX-M genes (8), confirming the recent emergence of these strains in the cattle population in that region. To investigate whether this relatively sudden widespread prevalence of blaCTX-M-carrying E. coli isolates was due to the dissemination of one or a few clones, we applied multilocus sequence typing to determine the genotypes among blaCTX-M-carrying and non-blaCTX-M-carrying E. coli isolates and examined their distributions across farms and regions. To assess the possibility of plasmid horizontal transfer rather than clonal expansion, we also characterized CTX-M genes and plasmids that encode CTX-M enzymes and determined the associations between blaCTX-M alleles, plasmid replicon types, and genotypes. We also compared blaCTX-M alleles, plasmid replicon types, and genotypes from this study with those from other studies, particularly studies of blaCTX-M-carrying E. coli isolates that are known to cause clinical infections in humans.
RESULTS
Comparison of MLST diversity and distribution between blaCTX-M-positive and -negative E. coli isolates.
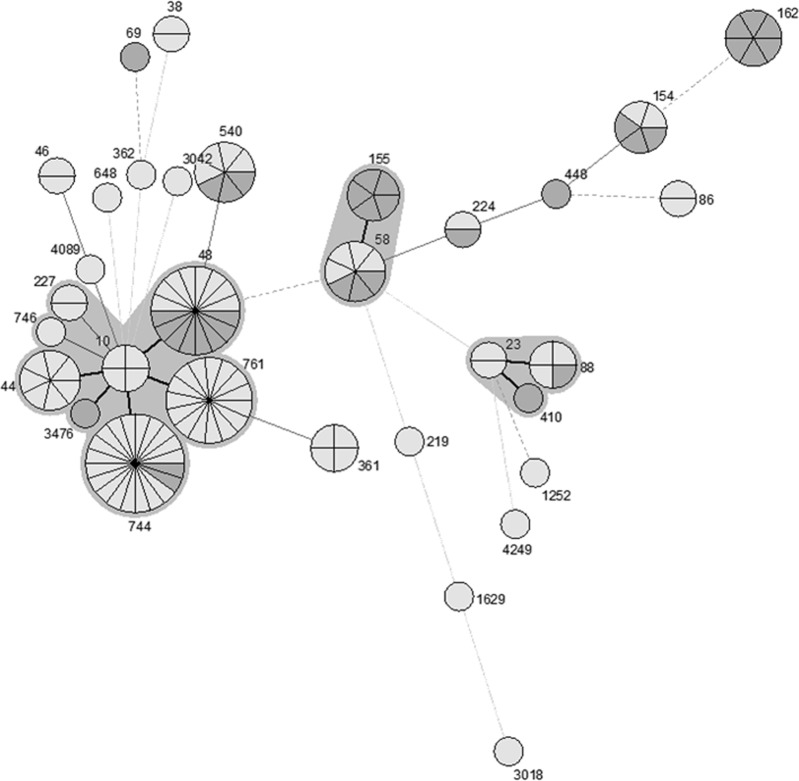
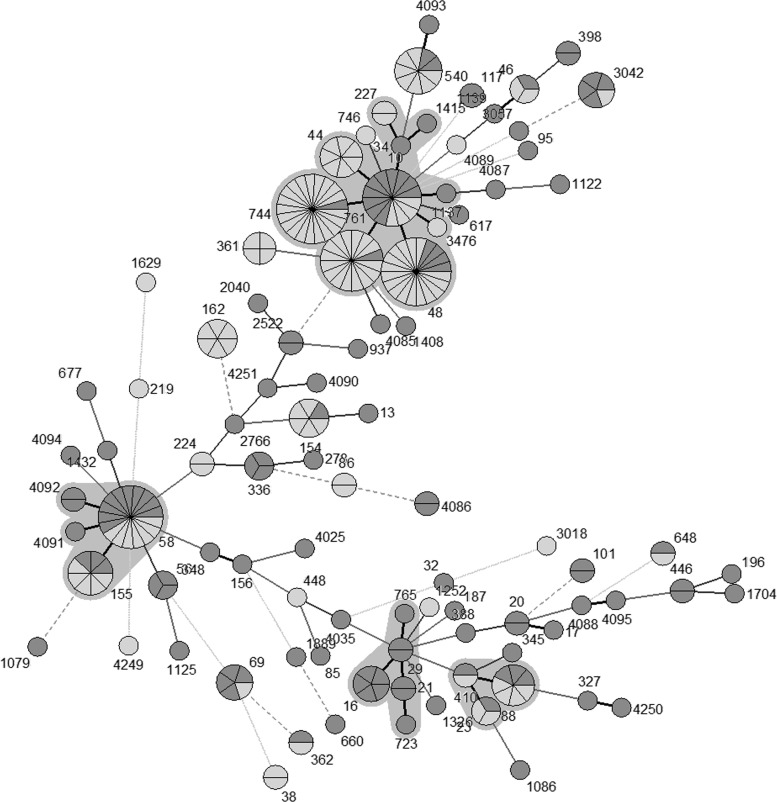
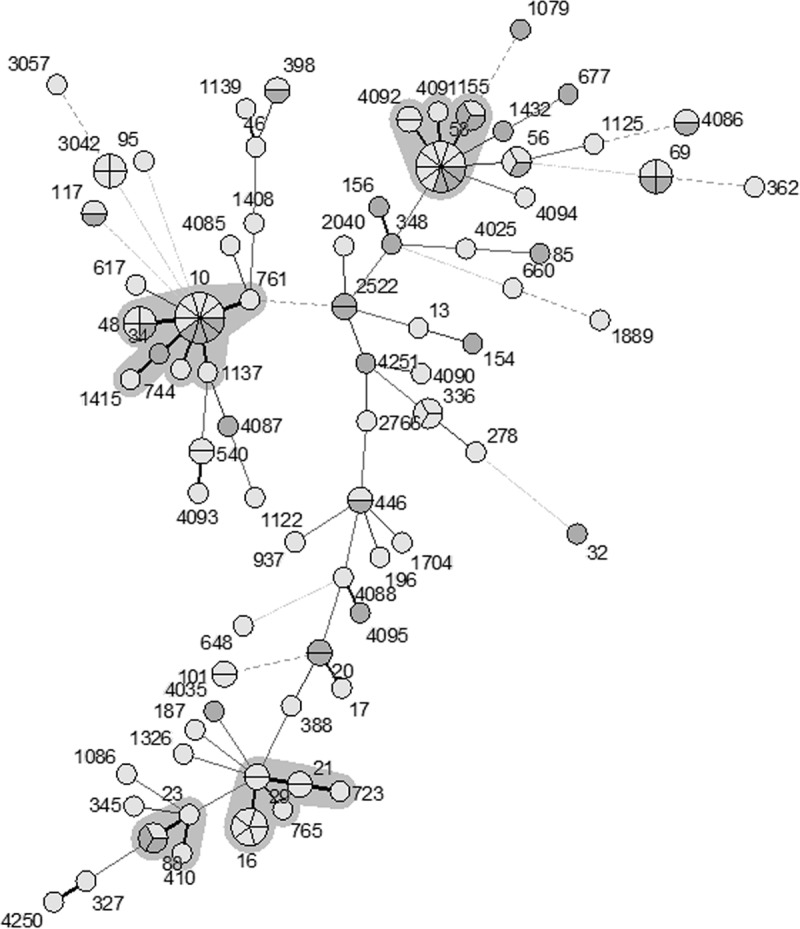
The sequence type (ST) distribution was less diverse in E. coli isolates carrying blaCTX-M (Simpson's index of diversity [SID] = 0.98; 95% confidence interval [CI], 0.97 to 0.99) than in E. coli isolates lacking blaCTX-M (SID = 0.93; 95% CI, 0.91 to 0.95). We found 76 different STs among the 126 blaCTX-M-negative E. coli isolates and 32 STs among the 126 blaCTX-M-positive E. coli isolates. An eBURST analysis of the blaCTX-M-positive E. coli isolates based on multilocus sequence type (MLST) genotypes identified three clonal complexes (CC10, CC58, and CC88), which accounted for 105 isolates (83.3% of the blaCTX-M-positive E. coli isolates) and 21 singletons (see Table S3 in the supplemental material). CC10 was the predominant clonal complex, which included 6 STs and accounted for 50% of the blaCTX-M-positive isolates, and was found on 16 of 18 farms sampled. Among the 63 blaCTX-M-positive isolates within the CC10 complex, ST744 was most frequent (n = 20), followed by ST48 (n = 16) and ST761 (n = 15). CC58 was the second largest clonal complex, accounting for 9.5% of blaCTX-M-positive E. coli isolates from 5 different farms. The third largest clonal complex (CC88) was also found on 5 separate farms. Overall, 65.1% of blaCTX-M-positive E. coli isolates were associated with these three clonal complexes (Fig. 1; Table 1). As was true for blaCTX-M-positive E. coli isolates, CC10 and CC58 were the most common clonal complexes among the blaCTX-M-negative E. coli isolates but represented smaller proportions of the total (15.1% and 12.7%, respectively). The blaCTX-M-negative E. coli isolates included a significantly higher proportion of singleton STs than the blaCTX-M-positive E. coli isolates (P < 0.05) (Fig. 2; Table 1). When all of the isolates were analyzed together, the blaCTX-M-negative and -positive E. coli isolates were often associated with the same or closely linked STs, with the exception of a few STs; for example, ST44, ST162, and ST361 had 4 to 6 isolates each and were exclusively blaCTX-M positive. The association between ST744, ST761, and ST48 and blaCTX-M carriage is noticeable in this analysis (Fig. 3).
FIG 1.
Minimum spanning tree analysis of 126 blaCTX-M-positive Escherichia coli isolates based on MLST genotypes. Each circle represents a genotype, and the partitions within a circle are individual isolates. There are three main clonal complexes, and each is surrounded by a gray zone. Thick lines connect genotypes that differ at one locus, and thin lines connect genotypes that differ by two or more loci. Shading indicates the region from which isolates were obtained: pale gray indicates eastern Washington (n = 78) and dark gray indicates northwestern Washington (n = 48).
TABLE 1.
Multilocus sequence types and clonal complexes observed in 126 blaCTX-M-positive and 126 blaCTX-M-negative E. coli isolates from cattle on 18 dairy farms in Washington State
| Clonal complexa |
blaCTX-M-positive E. coli |
blaCTX-M-negative E. coli |
||
|---|---|---|---|---|
| ST | No. of isolates (%) | ST | No. of isolates (%) | |
| 10 | 10, 44, 48, 744, 761, 3476 | 63 (50) | 10, 34, 48, 744, 761, 1137, 1415 | 19 (15.1) |
| 58 | 58, 155 | 12 (9.5) | 58, 155, 4091, 4092 | 16 (12.7) |
| 88 | 23, 88, 90 | 7 (5.5) | 23, 88, 90 | 5 (3.97) |
| 29 | 21, 29, 16, 723, 765 | 11 (8.7) | ||
| No founder | 14 (11.1) | |||
| Singletons | 44 (34.9) | 61 (48.4) | ||
| Total | 126 (100) | 126 (100) | ||
Clonal complexes were defined using the eBURST algorithm from blaCTX-M-negative E. coli and blaCTX-M-positive E. coli MLST data. The clonal complexes correspond to the predicted founder. “No founder” refers to the inability of the algorithm to predict the founder. The likelihood ratio chi-square P value was ≤0.001 for a 2 × k test for differences in the percentages of each clonal complex within blaCTX-M-negative and blaCTX-M-positive E. coli isolates.
FIG 2.
Minimum spanning tree analysis of 126 blaCTX-M-negative Escherichia coli isolates based on MLST genotypes. Each circle or node represents a genotype, and the partitions within a node are individual isolates. Each clonal complex is surrounded by a gray zone. Thick lines connect genotypes that differ by one locus, and thin lines connect genotypes that differ by two or more loci. Shading indicates the region from which isolates were obtained: pale gray indicates eastern Washington (n = 78) and dark gray indicates northwestern Washington (n = 48).
FIG 3.
Minimum spanning tree analysis of 252 Escherichia coli isolates based on MLST genotypes. Each circle represents a sequence type, and the partitions within each circle represent individual isolates. Clonal complexes are surrounded by gray zones. Within each circle, dark gray represents blaCTX-M-positive Escherichia coli isolates (n = 126) and light gray represents blaCTX-M-negative Escherichia coli isolates (n = 126).
Human disease-associated sequence types.
ST69 (n = 4) and ST95 (n = 1) were detected among the blaCTX-M-negative E. coli isolates, and one ST69 isolate was blaCTX-M positive. These sequence types are considered pandemic human disease-associated STs (Table 2; Fig. 1 and 2) (9).
TABLE 2.
Distribution of CTX-M genes and plasmid replicon types among 99 blaCTX-M-positive E. coli isolates from dairy cattle in Washington State
| No. of isolates |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CCa | STb | CTX-M group 1 |
CTX-M group 9 |
Plasmid replicon types |
|||||||||||||
| CTX-M-15 | CTX-M-55 | CTX-M-14 | CTX-M-24 | CTX-M-27 | CTX-M-65 | I1 | FIB | NT | FIA/FIB | N | FIA | F | FIA/I1 | B/0 | A/C | ||
| CC10 | ST761 | 4 | 1 | 8 | 3 | 5 | 1 | 1 | 2 | ||||||||
| ST48 | 1 | 1 | 5 | 2 | 2 | 1 | 3 | ||||||||||
| ST744 | 10 | 2 | 4 | 7 | 3 | 2 | 1 | 2 | 1 | ||||||||
| ST10 | 2 | 1 | 1 | ||||||||||||||
| ST4096 | 1 | 1 | |||||||||||||||
| ST44 | 5 | 1 | 4 | ||||||||||||||
| ST685 | 1 | 1 | |||||||||||||||
| CC58 | ST58 | 4 | 2 | 4 | 2 | ||||||||||||
| ST155 | 5 | 4 | 1 | ||||||||||||||
| CC88 | ST23 | 1 | 1 | ||||||||||||||
| ST88 | 9 | 3 | 2 | 3 | 1 | ||||||||||||
| ST90 | 1 | 1 | |||||||||||||||
| Singletons or no founders | ST162 | 3 | 2 | 1 | |||||||||||||
| ST86 | 2 | 1 | 3 | ||||||||||||||
| ST361 | 4 | 1 | 1 | 1 | 1 | ||||||||||||
| ST540 | 2 | 3 | 1 | 1 | 2 | 1 | |||||||||||
| ST1163 | 1 | 1 | 1 | 1 | |||||||||||||
| ST154 | 1 | 1 | |||||||||||||||
| ST345 | 1 | 1 | |||||||||||||||
| ST448 | 1 | 1 | |||||||||||||||
| ST219 | 1 | 1 | |||||||||||||||
| ST1629 | 1 | 1 | |||||||||||||||
| ST3018 | 1 | 1 | |||||||||||||||
| ST32 | 1 | 1 | |||||||||||||||
| ST4249 | 1 | 1 | |||||||||||||||
| ST227 | 1 | 1 | |||||||||||||||
| ST3042 | 1 | 1 | |||||||||||||||
| ST163 | 1 | ||||||||||||||||
| ST362 | 1 | 1 | |||||||||||||||
| ST69 | 1 | 1 | |||||||||||||||
| ST746 | 1 | 1 | |||||||||||||||
| ST38 | 1 | ||||||||||||||||
| ST349 | 1 | 1 | |||||||||||||||
| Total | 50 | 1 | 16 | 1 | 26 | 5 | 31 | 27 | 10 | 9 | 8 | 7 | 4 | 1 | 1 | 1 | |
CC, clonal complex.
ST, sequence type.
Plasmid replicon types and CTX-M alleles among blaCTX-M-positive E. coli isolates.
Plasmid replicon typing was performed on 99 blaCTX-M-positive E. coli isolates from which we were able to isolate the blaCTX-M-carrying plasmids. Nine replicon types were identified among 89 isolates; an additional 10 isolates could not be typed using the multiplex PCR method (10, 11). IncI1 plasmids were most frequent (n = 31), followed by IncFIB (n = 27), IncFIA/FIB, IncN, IncFIA, and others (Table 3).
TABLE 3.
Relationship of plasmid replicon types and CTX-M genes among 99 blaCTX-M-positive E. coli isolates from cattle on dairy farms in Washington State
| Plasmid replicon type | No. of isolates |
||||||
|---|---|---|---|---|---|---|---|
| CTX-M group 1 |
CTX-M group 9 |
Subtotal | |||||
| CTX-M-15 | CTX-M-55 | CTX-M-14 | CTX-M-24 | CTX-M-27 | CTX-M-65 | ||
| IncI1 | 24 | 2 | 4 | 1 | 31 | ||
| IncFIB | 8 | 7 | 1 | 10 | 1 | 27 | |
| IncFIA/FIB | 7 | 1 | 1 | 9 | |||
| IncFIA | 1 | 1 | 2 | 3 | 7 | ||
| IncF | 2 | 2 | 4 | ||||
| IncFIA/I1 | 1 | 1 | |||||
| IncA/C | 1 | 1 | |||||
| IncB/O | 1 | 1 | |||||
| IncN | 1 | 4 | 3 | 8 | |||
| NTa | 6 | 2 | 2 | 10 | |||
| Total | 50 | 1 | 16 | 1 | 26 | 5 | 99 |
NT, nontypeable: no PCR product was obtained using the standard set of primers for replicon typing (10).
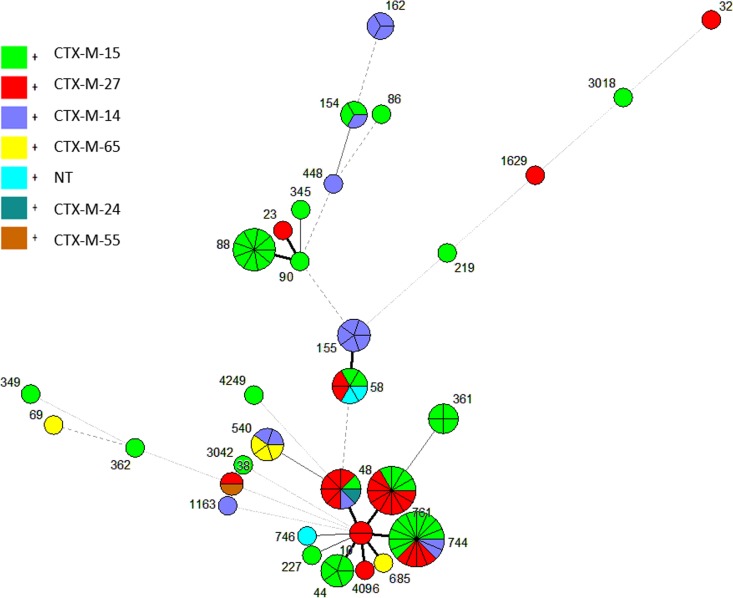
We detected 6 different blaCTX-M alleles, of which the most frequent was blaCTX-M-15 (n = 50), followed by blaCTX-M-27 (n = 26) and blaCTX-M-14 (n = 16), while blaCTX-M-65, blaCTX-M-24, and blaCTX-M-55 were uncommon (Table 3). Overall, blaCTX-M-15 and blaCTX-M-27 were widely distributed among the different STs. There was an association between MLST clonal complexes and CTX-M alleles (P = 0.0008); the isolates in CC10 tended to carry blaCTX-M-15 or blaCTX-M-27 alleles; those in CC88 mostly carried blaCTX-M-15 alleles (Fig. 4).
FIG 4.
Distributions of blaCTX-M genes among the different MLST genotypes in blaCTX-M-positive E. coli isolates (n = 99) from dairy cattle in Washington State. NT, nontypeable.
There was an association between replicon types and blaCTX-M alleles (P = 0.007). For instance, IncI1 and IncFIA/FIB predominantly carried blaCTX-M-15 alleles, while IncFIB carried blaCTX-M-27, blaCTX-M-15, and blaCTX-M-14 alleles in almost equal proportions. There was no clear association between clonal complex and plasmid replicon type, including the 10 plasmids that were nontypeable (P = 0.178) (Table 2).
Geographic distribution of MLSTs.
The four major clonal complexes did not differ significantly in the proportions that were found in the two regions of Washington State (Table S3).
Antibiotic resistance among blaCTX-M-positive and blaCTX-M-negative E. coli isolates.
The proportions of blaCTX-M-positive E. coli isolates with resistance to ampicillin, ceftiofur, cephalothin, chloramphenicol, nalidixic acid, streptomycin, sulfisoxazole, tetracycline, and trimethoprim-sulfamethoxazole were higher than those of blaCTX-M-negative isolates (Table 4).
TABLE 4.
Percentages of blaCTX-M-positive and blaCTX-M-negative E. coli isolates resistant to a panel of 12 antibiotics
| Antibiotic | Point estimate (% [95% CI]) |
|
|---|---|---|
| CTX-M-positive E. coli | CTX-M-negative E. coli | |
| Amikacin | 4.0 (1.7–9.0) | 4.7 (2.2–10.0) |
| Amoxicillin-clavulanic acid | 15.1 (9.9–22.3) | 22.2 (15.8–30.2) |
| Ampicillin | 99.2 (95.6–100)a | 38.9 (30.8–47.6)a |
| Ceftiofur | 99.2 (95.6–100)a | 13.4 (8.6–20.5)a |
| Cephalothin | 99.2 (95.6–100)a | 27.0 (20.0–35.3)a |
| Chloramphenicol | 52.3 (43.7–60.9)a | 30.2 (22.8–38.7)a |
| Gentamicin | 7.9 (4.4–14.0) | 9.5 (5.5–15.9) |
| Nalidixic acid | 35.7 (27.9–44.4)a | 7.9 (4.4–14.0)a |
| Streptomycin | 77.8 (69.8–84.2)a | 46.0 (37.6–54.7)a |
| Sulfisoxazole | 73.0 (64.7–80.0)a | 45.2 (36.8–53.9)a |
| Tetracycline | 86.5 (79.5–91.4)a | 51.6 (43.0–60.1)a |
| Trimethoprim-sulfamethoxazole | 66.7 (58.1–74.3)a | 27.8 (20.7–36.2)a |
Higher percentage of blaCTX-M-positive isolates are resistant than blaCTX-M-negative isolates.
DISCUSSION
We recently reported an increase in commensal E. coli isolates carrying blaCTX-M resistance genes in dairy cattle, and to assess the possibility that this represented the clonal dissemination of one or a few clones of E. coli, we genotyped blaCTX-M-positive and blaCTX-M-negative commensal E. coli isolates from dairy cattle across Washington State. We found that a large proportion of blaCTX-M-positive E. coli isolates belonged to a few MLST clonal complexes, while blaCTX-M-negative E. coli isolates were significantly more diverse with regard to sequence types. The predominance of a few clones among blaCTX-M-positive E. coli isolates is consistent with other studies that report few genotypes accounting for a large proportion of multidrug-resistant strains (9).
The primary clonal complexes of commensal blaCTX-M-positive E. coli isolates were a subset of those found in blaCTX-M-negative E. coli isolates, suggesting a scenario in which the acquisition of blaCTX-M plasmids by E. coli genotypes that were already fit and established in the dairy animal niche resulted in an increase in their fitness in the presence of antimicrobial selection pressure. The wide distribution of blaCTX-M-positive E. coli clones across a broad geography could be the result of clonal dissemination via movement of cattle, humans, contaminated vehicles, feed, and possibly birds and insects (8, 12, 13), followed by a diversification through normal microevolutionary mechanisms, such as mutation and horizontal gene transfer mediated by plasmids and bacteriophages (14, 15). An expectation of this scenario would be that a single ST of E. coli could be associated with a diversity of plasmid types, which is consistent with our observations. For example, in our data, ST744 isolates (n = 16) were associated with six different plasmid types associated with three different blaCTX-M alleles (Table 2).
We detected small numbers of ST69 and ST95, which, like ST131 and ST393, have been referred to as pandemic clonal lineages of human-associated extraintestinal pathogenic E. coli (ExPEC). The ST69 isolates in this study were primarily blaCTX-M negative, which is consistent with reports that ST69 strains rarely produce ESBL. To our knowledge, these were the only human disease-associated pandemic STs detected in our study (9). It has been suggested that food-producing animals are reservoirs of resistant bacteria that cause clinical infections in humans (16–18); however, compelling quantitative evidence is lacking. For example, a systematic review of 34 original studies found that six studies provided evidence for whole bacterium transmission, and 13 studies provided evidence for the transmission of resistance via mobile genetic elements from food animals to humans; most of these were of poultry or retail meats. Conversely, findings from 17 studies did not support whole bacterium transmission, and two studies did not provide evidence for the transfer of resistance via mobile genetic elements from food animal sources to humans (19).
Among the STs within the ST10 clonal complex, ST48, ST744, and ST761 were the most frequent and had the strongest association with blaCTX-M (Fig. 1 through 3). Although ST761 was not detected in the northwestern region of Washington State, the other two in that complex (ST48 and ST744) were detected in both regions, suggesting that they represent widely disseminated types (Fig. 2). ST744 has been frequently reported from widely dispersed locations around the world, including from food animals in Australia (20), broiler chickens in Algeria (21), bovine mastitis in Germany (22), cattle in France (23), and hospitalized patients in Hong Kong (24), many with ESBL and/or carbapenem resistance characteristics. ST48 has also frequently been reported from diverse sources and locations and is reportedly associated with resistance (25–30).
The IncI1 and IncF plasmid families were the most frequent replicon types detected in this study, which is consistent with reports that IncI1 and IncF were the most frequent replicon types among blaCTX-M-15-carrying plasmids in Enterobacteriaceae from human, livestock, and environmental sources in European studies (31–33). IncFIB has been reported to be the most common replicon type in commensal and pathogenic E. coli isolates originating from avian, human, and poultry meat (34), and it has been associated with virulence (35). Both IncI and IncF plasmid groups are considered to be easily transmissible narrow-host-range plasmids and clustered with each other in an analysis of their gene content in comparison with that of IncN, IncP, and IncW plasmids (36, 37). Unlike IncI1 and IncFIA/FIB that were mainly associated with blaCTX-M-15, IncFIB, the second most common replicon type in this study, was associated with blaCTX-M-15, blaCTX-M-14, and blaCTX-M-27 genes (Table 3).
blaCTX-M-15 was the most frequent blaCTX-M allele in this study, followed by blaCTX-M-27 and blaCTX-M-14 (Table 3). Consistent with our findings, blaCTX-M-15 was the dominant allele among cattle-derived ESBL-producing E. coli isolates in the United Kingdom and Lebanon (38, 39). In contrast with our results, the prevalence of blaCTX-M-15-producing E. coli was reported to be low among ESBL-producing E. coli isolates from nonhuman sources (33, 40). A review of ESBL genes in E. coli isolates from human and nonhuman sources from Europe and Asia found that overall, blaCTX-M-1 was the most frequent allele in cattle/pig-derived isolates (72%), followed by blaCTX-M-14 (14%) and blaCTX-M-15 (8%); however, regional differences in prevalence were observed (40). Other studies indicate that blaCTX-M-14 is prevalent among cattle-derived ESBL-producing E. coli isolates (41, 42), while blaCTX-M-27 is uncommon in ESBL-producing E. coli isolates from cattle/pigs (40). Whereas the real explanation for the observed differences in the distribution of blaCTX-M alleles is unknown, plausible explanations include environmental or geographical factors, differences in β-lactamase activity against specific antimicrobial substrates, regional founder effects, the mobility of plasmids that mediate these genes, and other resistance and virulence determinants (43, 44).
Conclusion.
We observed low MLST diversity among commensal E. coli isolates carrying blaCTX-M in dairy cattle across Washington State and a lack of association between geographic region and MLST clonal complexes, suggesting that clonal bacterial spread played a role in the rapid increase of blaCTX-M prevalence that was observed between 2008 and 2012 (8). However, we did not detect any association between the blaCTX-M plasmid replicon types and the ST or clonal complexes of their host bacteria. In addition, the blaCTX-M-negative E. coli isolates, although more diverse, included the same clonal complexes that were prominent among the blaCTX-M-positive E. coli isolates. Certain E. coli STs are apparently host associated, while blaCTX-M plasmid replicon types are found in multiple vertebrate hosts and have been referred to as “epidemic plasmids,” suggesting an important role of horizontal gene transfer in the epidemiology of blaCTX-M-associated E. coli (45).
To elucidate all of the causal factors driving a population change in resistant bacteria, we would need a complex model of multiple interacting forces, including animal movements, environmental distribution systems such as water flow, local antimicrobial use, and selective pressure, and the fitness of bacteria due to plasmid and other genetic acquisitions. Ideally, such a model could simulate changes in any of these factors to predict changes in commensal as well as clinical bacterial populations that may impact public health.
MATERIALS AND METHODS
Sample collection.
Samples for this study were collected during the summer and fall of 2012 as described in a previous study (8). The 30 dairy farms in that study were chosen based on their willingness to collaborate and their geographic dispersion across the state. For 21 of the 30 farms sampled in that study, susceptible E. coli isolates with no blaCTX-M were available because nonselective as well as selective media were used, but three of those 21 farms had no E. coli isolates carrying blaCTX-M. To be able to compare the genotypes of E. coli isolates with no blaCTX-M to genotypes of E. coli isolates carrying blaCTX-M, we used isolates from the remaining 18 dairy farms. They were located in northwestern (region 1) and eastern (region 2) Washington State. Fecal samples were collected from the rectums of individual calves or pooled from fresh fecal pats from adult lactating cow pens, were placed on ice, and were transported to the laboratory for processing (8).
Bacterial culture, isolation, and confirmation.
Escherichia coli isolates were cultured from fecal samples as previously described (46). Briefly, each sample was directly plated onto unsupplemented MacConkey agar and incubated overnight, and three presumptive E. coli colonies were inoculated in a 96-well plate containing brain heart infusion (BHI) broth and incubated. Buffered glycerol was added to each well, and the plates were stored at −80°C. In addition, to select for ESBL strains, each fecal sample (5 g) was placed in nutrient broth supplemented with cefotaxime (2 μg/ml) (Research Products International Corp., Mount Prospect, IL, USA) and incubated overnight. Then, 100 μl nutrient broth was plated onto MacConkey agar plates supplemented with cefepime (4 μg/ml) (Apotex Corp., Weston, FL, USA). Similarly, three single colonies were picked from each plate, inoculated in 96-well plates, and stored. The isolates were confirmed as E. coli by PCR amplification of the uidA gene (47).
Detection of the blaCTX-M gene and isolate selection.
The presence of blaCTX-M was determined by PCR (48). We grouped preweaned calves into age groups of 7 days each (1 to 7 days, 8 to 14 days, 14 to 21 days, 22 to 28 days, 35 to 42 days, 43 to 49 days, 50 to 56 days, and 57 to 63 days) and adults into one age group. From each group, one to four samples were chosen by generating a random number list using the Rand function in Excel version 3 (Microsoft, Redmond, WA), sorting by random number within the group, and choosing the first isolate in the list. Because we selected similar numbers of blaCTX-M-positive and -negative isolates from each age group, we could not make inferences about the distribution of CTX-M by animal age. From each of the 18 farms, an average of 14 isolates (standard deviation [SD], 5.5), including one to four from adult cows and 1 to 4 from each age group of preweaned calves, were chosen for a final isolate set of 126 blaCTX-M-negative and 126 blaCTX-M-positive isolates (total, 252 isolates). Ninety-six isolates were from farms in northwestern Washington and 156 isolates were from farms in eastern Washington (see Table S1 in the supplemental material). To add to the sample size of isolates for the purpose of studying the distribution of plasmid types and blaCTX-M alleles, an additional 23 isolates from farms not included in the current study, but from which plasmids could be isolated, were used. These additional isolates were obtained from nine different farms in eastern Washington (region 2) during the previous study (8), one to three isolates per farm. They were obtained during the same time period as the 252 isolates that were chosen for the MLST comparison between blaCTX-M-carrying and noncarrying E. coli isolates. Although northwestern Washington (region 1) was not represented in this additional set, the aim was to get as complete a picture as possible of replicon types, and so we used these for that purpose only (8, 49) (see Table S2).
Nucleic acid extraction.
Each isolate was streaked on a blood agar plate, and a single colony was inoculated into 3 ml BHI broth and incubated overnight at 37°C. Genomic DNA from each isolate was extracted using a Qiagen DNeasy blood and tissue kit according to the manufacturer's instructions (Qiagen Sciences, Inc., Germantown, MD) and stored at 4°C.
MLST determination.
All isolates were genotyped according to the Achtman seven-locus MLST scheme. Briefly, internal fragments of seven housekeeping genes (adk, fumC, gyrB, icd, mdh, purA, and recA) were amplified using published primers and protocols (http://mlst.warwick.ac.uk/mlst/dbs/Ecoli/documents/primersColi_html). The amplified products were sequenced bidirectionally, the raw trace files were read, and the sequences were assembled into single contigs using BioNumerics version 6.6 (Applied Maths, Sint-Martens-Latem, Belgium). Each unique allele was assigned a number, the allelic profile for each isolate was determined, and sequence types (STs) were assigned using BioNumerics (http://mlst.warwick.ac.uk/mlst/dbs/Ecoli). The sequences of novel alleles were submitted to the curator for new allele and ST assignment. The genotypic relationships among isolates were determined by generating minimum spanning trees using BioNumerics. Within a clonal complex, each ST has to match with at least one other ST at 6 loci. The eBURST algorithm was used to determine the founder ST of a clonal complex (http://eburst.mlst.net/).
Antibiotic resistance testing.
The 252 E. coli isolates were tested for resistance to 12 antibiotics (amikacin, 30 μg; amoxicillin-clavulanic acid, 20/10 μg; ampicillin, 10 μg; ceftiofur, 30 μg; cephalothin, 30 μg; chloramphenicol, 30 μg; gentamicin, 10 μg; nalidixic acid, 30 μg; streptomycin, 10 μg; sulfisoxazole, 0.25 μg; tetracycline, 30 μg; and trimethoprim-sulfamethoxazole, 1.25/23.75 μg) using the disc diffusion assay according to the Clinical and Laboratory Standards Institute guidelines (50).
CTX-M groups and types.
To determine blaCTX-M groups, we first screened for blaCTX-M group 1 using previously described protocols and primers, followed by PCR to detect blaCTX-M group 9 (51). To determine blaCTX-M types, we performed PCR using sequencing primers as previously described (46, 52). The primers used to determine blaCTX-M groups and types are shown in Table 5. The PCR products were submitted to Functional Biosciences (Madison, WI, USA) for sequencing, and the specific blaCTX-M alleles were identified using the NCBI Basic Local Alignment Search Tool (53).
TABLE 5.
PCR Primers used in this study
| Target | Primer name | Sequence (5′→3′) | Reference |
|---|---|---|---|
| blaCTX-M | Pan CTX-M forward | TTT GCG ATG TGC AGT ACC AGT AA | 48 |
| Pan CTX-M reverse | CGA TAT CGT TGG TGG TGC CAT A | ||
| blaCTX-M (group I) | CTXM1-F3 forward | GAC GAT GTC ACT GGC TGA GC | 59 |
| CTXM1-R2 reverse | AGC CGC CGA CGC TAA TAC A | ||
| blaCTX-M (group II) | TOHO1-2F forward | GCG ACC TGG TTA ACT ACA ATC C | 59 |
| TOHO1-1R reverse | CGG TAG TAT TGC CCT TAA GCC | ||
| blaCTX-M (group III) | CTXM8.WSAgroupIII.F forward | AGA CCT GAT TAA CTA CAA TCC CAT TA | 51 |
| CTXM8.WSAgroupIII.R reverse | ACT TTC TGC CTT CTG CTC TGG C | ||
| blaCTX-M (group IV) | CTXM914F forward | GCT GGA GAA AAG CAG CGG AG | 59 |
| CTXM914R reverse | GTA AGC TGA CGC AAC GTC TG | ||
| blaCTX-M sequencing primers | CTXM-1 upstream forward | ATG TTG TTG TTA ATT CGT CTC | 46 |
| CTXM-1 upstream reverse | CGT TAT CGC TGT ACT GTA G | ||
| CTXM-2 downstream forward | TTA ACT ATA ATC CGA TTG CG | 46 | |
| CTXM-2 downstream reverse | TTT CTG CCT TAG GTT GAG | ||
| blaCTX-M (CTX-M-9 group) | M9 upper forward | ATG GTG ACA AAG AGA GTG CA | 60 |
| M9 lower reverse | CCC TTC GGC GAT GAT TCT C | ||
| uidA | UAL-754 forward | AAA ACG GCA AGA AAA AGC AG | 47 |
| UAR-900 Reverse | ACG CGT GGT TAC AGT CTT GCG |
Plasmid isolation, characterization, and replicon typing.
To isolate and characterize blaCTX-M-associated plasmids, two procedures were used. Conjugation experiments were performed by growing blaCTX-M-positive E. coli and GeneHog recipient cells (Invitrogen) separately in LB broth at 37°C for 6 h. Then, blaCTX-M-positive E. coli and GeneHog recipient cells were plated together on LB medium supplemented with rifampin (100 μg/ml) and cefepime (16 μg/ml) to select for recipient cells carrying the blaCTX-M plasmid. Conjugants were screened by denaturing chromosomal DNA using alkaline sodium dodecyl sulfate, removing protein and cell debris by phenol-chloroform extraction (54), and confirming the presence of blaCTX-M using PCR (48). If conjugation was not successful, plasmid DNA was extracted using a GeneJET plasmid Midiprep kit (Thermo Fisher Scientific, Waltham, MA, USA) or by using a published protocol for larger plasmids (55). The plasmid DNA was then electroporated into competent E. coli DH10B or GeneHog cells (Invitrogen) in 2-mm-path-length cuvettes using a GenePulser (Bio-Rad). Transformants were immediately placed into SOC medium (MP Biomedicals, Solon, OH, USA) (500 ml) and incubated for 1 h at 37°C with agitation (300 rpm). Transformants were then plated on LB agar plates supplemented with cefepime (16 μg/ml). The isolates were confirmed to have a single plasmid by preparing plasmid DNA as above (54), and the blaCTX-M gene was confirmed using PCR (48).
Plasmid replicon typing was performed as described by Johnson and Nolan (10), using a modification of the PCR method described by Carattoli et al. (11). The procedure involves three multiplex PCR panels using previously described primer pairs, targets, control strains, and run conditions, and the approach can detect 18 replicon types.
Statistical analyses.
The proportions of blaCTX-M-carrying and non-blaCTX-M-carrying E. coli isolates that were resistant to the antibiotics tested were compared using R package Hmisc. The associations between genotype and blaCTX-M allele and between plasmid replicon type and blaCTX-M allele were determined using Pearson's chi-square test of independence in R software. The comparisons between proportions were tested for significance using the Pearson's chi-square test in the software WinPepi (56). The Simpson's index of diversity was calculated according to the formula developed by Hunter and Gaston (57), and 95% confidence intervals surrounding the indices of diversity were calculated using the formula of Grundmann et al. (58).
Supplementary Material
ACKNOWLEDGMENT
This project was supported by funding from National Integrated Food Safety Initiative (NIFSI; grant no. 2010-51110-21131) from the USDA National Institute of Food and Agriculture.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.02430-17.
REFERENCES
- 1.McDanel J, Schweizer M, Crabb V, Nelson R, Samore M, Khader K, Blevins AE, Diekema D, Chiang HY, Nair R, Perencevich E. 2017. Incidence of extended-spectrum β-lactamase (ESBL)-producing Escherichia coli and Klebsiella infections in the United States: a systematic literature review. Infect Control Hosp Epidemiol 38:1209–1215. doi: 10.1017/ice.2017.156. [DOI] [PubMed] [Google Scholar]
- 2.Lynch JP III, Clark NM, Zhanel GG. 2013. Evolution of antimicrobial resistance among Enterobacteriaceae (focus on extended spectrum β-lactamases and carbapenemases). Expert Opin Pharmacother 14:199–210. doi: 10.1517/14656566.2013.763030. [DOI] [PubMed] [Google Scholar]
- 3.Weissman SJ, Adler A, Qin X, Zerr DM. 2013. Emergence of extended-spectrum β-lactam resistance among Escherichia coli at a U.S. academic children's hospital is clonal at the sequence type level for CTX-M-15, but not for CMY-2. Int J Antimicrob Agents 41:414–420. doi: 10.1016/j.ijantimicag.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bush K. 2013. Proliferation and significance of clinically relevant β-lactamases. Ann N Y Acad Sci 1277:84–90. doi: 10.1111/nyas.12023. [DOI] [PubMed] [Google Scholar]
- 5.Naseer U, Sundsfjord A. 2011. The CTX-M conundrum: dissemination of plasmids and Escherichia coli clones. Microb Drug Resist 17:83–97. doi: 10.1089/mdr.2010.0132. [DOI] [PubMed] [Google Scholar]
- 6.Gutkind GO, Di Conza J, Power P, Radice M. 2013. β-Lactamase-mediated resistance: a biochemical, epidemiological and genetic overview. Curr Pharm Des 19:164–208. doi: 10.2174/138161213804070320. [DOI] [PubMed] [Google Scholar]
- 7.D'Andrea MM, Arena F, Pallecchi L, Rossolini GM. 2013. CTX-M-type β-lactamases: a successful story of antibiotic resistance. Int J Med Microbiol 303:305–317. doi: 10.1016/j.ijmm.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 8.Davis MA, Sischo WM, Jones LP, Moore DA, Ahmed S, Short DM, Besser TE. 2015. Recent emergence of Escherichia coli with cephalosporin resistance conferred by blaCTX-M on Washington State dairy farms. Appl Environ Microbiol 81:4403–4410. doi: 10.1128/AEM.00463-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Riley LW. 2014. Pandemic lineages of extraintestinal pathogenic Escherichia coli. Clin Microbiol Infect 20:380–390. doi: 10.1111/1469-0691.12646. [DOI] [PubMed] [Google Scholar]
- 10.Johnson TJ, Nolan LK. 2009. Plasmid replicon typing. Methods Mol Biol 551:27–35. doi: 10.1007/978-1-60327-999-4_3. [DOI] [PubMed] [Google Scholar]
- 11.Carattoli A, Bertini A, Villa L, Falbo V, Hopkins KL, Threlfall EJ. 2005. Identification of plasmids by PCR-based replicon typing. J Microbiol Methods 63:219–228. doi: 10.1016/j.mimet.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 12.Solà-Ginés M, Gonzalez-Lopez JJ, Cameron-Veas K, Piedra-Carrasco N, Cerda-Cuellar M, Migura-Garcia L. 2015. Houseflies (Musca domestica) as vectors for extended-spectrum β-lactamase-producing Escherichia coli on Spanish broiler farms. Appl Environ Microbiol 81:3604–3611. doi: 10.1128/AEM.04252-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Medhanie GA, Pearl DL, McEwen SA, Guerin MT, Jardine CM, Schrock J, LeJeune JT. 2016. On-farm starling populations and other environmental and management factors associated with the presence of cefotaxime and ciprofloxacin resistant E. coli among dairy cattle in Ohio. Prev Vet Med 134:122–127. doi: 10.1016/j.prevetmed.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 14.Feil EJ, Spratt BG. 2001. Recombination and the population structures of bacterial pathogens. Annu Rev Microbiol 55:561–590. doi: 10.1146/annurev.micro.55.1.561. [DOI] [PubMed] [Google Scholar]
- 15.Brabban AD, Hite E, Callaway TR. 2005. Evolution of foodborne pathogens via temperate bacteriophage-mediated gene transfer. Foodborne Pathog Dis 2:287–303. doi: 10.1089/fpd.2005.2.287. [DOI] [PubMed] [Google Scholar]
- 16.Vieira AR, Collignon P, Aarestrup FM, McEwen SA, Hendriksen RS, Hald T, Wegener HC. 2011. Association between antimicrobial resistance in Escherichia coli isolates from food animals and blood stream isolates from humans in Europe: an ecological study. Foodborne Pathog Dis 8:1295–1301. doi: 10.1089/fpd.2011.0950. [DOI] [PubMed] [Google Scholar]
- 17.Kennedy D. 2013. Time to deal with antibiotics. Science 342:777. doi: 10.1126/science.1248056. [DOI] [PubMed] [Google Scholar]
- 18.Manges AR. 2016. Escherichia coli and urinary tract infections: the role of poultry-meat. Clin Microbiol Infect 22:122–129. doi: 10.1016/j.cmi.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 19.Lazarus B, Paterson DL, Mollinger JL, Rogers BA. 2015. Do human extraintestinal Escherichia coli infections resistant to expanded-spectrum cephalosporins originate from food-producing animals? A systematic review. Clin Infect Dis 60:439–452. doi: 10.1093/cid/ciu785. [DOI] [PubMed] [Google Scholar]
- 20.Abraham S, Jordan D, Wong HS, Johnson JR, Toleman MA, Wakeham DL, Gordon DM, Turnidge JD, Mollinger JL, Gibson JS, Trott DJ. 2015. First detection of extended-spectrum cephalosporin- and fluoroquinolone-resistant Escherichia coli in Australian food-producing animals. J Glob Antimicrob Resist 3:273–277. doi: 10.1016/j.jgar.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 21.Belmahdi M, Bakour S, Al Bayssari C, Touati A, Rolain JM. 2016. Molecular characterisation of extended-spectrum β-lactamase- and plasmid AmpC-producing Escherichia coli strains isolated from broilers in Bejaia, Algeria. J Glob Antimicrob Resist 6:108–112. doi: 10.1016/j.jgar.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 22.Eisenberger D, Carl A, Balsliemke J, Kampf P, Nickel S, Schulze G, Valenza G. 27 September 2017. Molecular characterization of extended-spectrum β-lactamase-producing Escherichia coli isolates from milk samples of dairy cows with mastitis in Bavaria, Germany. Microb Drug Resist doi: 10.1089/mdr.2017.018. [DOI] [PubMed] [Google Scholar]
- 23.Haenni M, Beyrouthy R, Lupo A, Chatre P, Madec JY, Bonnet R. 22 November 2017. Epidemic spread of Escherichia coli ST744 isolates carrying mcr-3 and blaCTX-M-55 in cattle in France. J Antimicrob Chemother doi: 10.1093/jac/dkx418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ho PL, Cheung YY, Wang Y, Lo WU, Lai EL, Chow KH, Cheng VC. 2016. Characterization of carbapenem-resistant Escherichia coli and Klebsiella pneumoniae from a healthcare region in Hong Kong. Eur J Clin Microbiol Infect Dis 35:379–385. doi: 10.1007/s10096-015-2550-3. [DOI] [PubMed] [Google Scholar]
- 25.Agabou A, Lezzar N, Ouchenane Z, Khemissi S, Satta D, Sotto A, Lavigne JP, Pantel A. 2016. Clonal relationship between human and avian ciprofloxacin-resistant Escherichia coli isolates in north-eastern Algeria. Eur J Clin Microbiol Infect Dis 35:227–234. doi: 10.1007/s10096-015-2534-3. [DOI] [PubMed] [Google Scholar]
- 26.Ben Said L, Jouini A, Alonso CA, Klibi N, Dziri R, Boudabous A, Ben Slama K, Torres C. 2016. Characteristics of extended-spectrum β-lactamase (ESBL)- and pAmpC beta-lactamase-producing Enterobacteriaceae of water samples in Tunisia. Sci Total Environ 550:1103–1109. doi: 10.1016/j.scitotenv.2016.01.042. [DOI] [PubMed] [Google Scholar]
- 27.Dissanayake DR, Octavia S, Lan R. 2014. Population structure and virulence content of avian pathogenic Escherichia coli isolated from outbreaks in Sri Lanka. Vet Microbiol 168:403–412. doi: 10.1016/j.vetmic.2013.11.028. [DOI] [PubMed] [Google Scholar]
- 28.Hasan B, Melhus A, Sandegren L, Alam M, Olsen B. 2014. The gull (Chroicocephalus brunnicephalus) as an environmental bioindicator and reservoir for antibiotic resistance on the coastlines of the Bay of Bengal. Microb Drug Resist 20:466–471. doi: 10.1089/mdr.2013.0233. [DOI] [PubMed] [Google Scholar]
- 29.Roderova M, Halova D, Papousek I, Dolejska M, Masarikova M, Hanulik V, Pudova V, Broz P, Htoutou-Sedlakova M, Sauer P, Bardon J, Cizek A, Kolar M, Literak I. 2016. Characteristics of quinolone resistance in Escherichia coli isolates from humans, animals, and the environment in the Czech Republic. Front Microbiol 7:2147. doi: 10.3389/fmicb.2016.02147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soufi L, Saenz Y, Vinue L, Abbassi MS, Hammami S, Torres C. 2013. Characterization of Pc promoter variants of class 1 integrons in Escherichia coli isolates from poultry meat. Foodborne Pathog Dis 10:1075–1077. doi: 10.1089/fpd.2013.1542. [DOI] [PubMed] [Google Scholar]
- 31.Hopkins KL, Liebana E, Villa L, Batchelor M, Threlfall EJ, Carattoli A. 2006. Replicon typing of plasmids carrying CTX-M or CMY beta-lactamases circulating among Salmonella and Escherichia coli isolates. Antimicrob Agents Chemother 50:3203–3206. doi: 10.1128/AAC.00149-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zurfluh K, Glier M, Hachler H, Stephan R. 2015. Replicon typing of plasmids carrying blaCTX-M-15 among Enterobacteriaceae isolated at the environment, livestock and human interface. Sci Total Environ 521–522:75–78. doi: 10.1016/j.scitotenv.2015.03.079. [DOI] [PubMed] [Google Scholar]
- 33.Fischer J, Rodriguez I, Baumann B, Guiral E, Beutin L, Schroeter A, Kaesbohrer A, Pfeifer Y, Helmuth R, Guerra B. 2014. blaCTX-M-15-carrying Escherichia coli and Salmonella isolates from livestock and food in Germany. J Antimicrob Chemother 69:2951–2958. doi: 10.1093/jac/dku270. [DOI] [PubMed] [Google Scholar]
- 34.Johnson TJ, Wannemuehler YM, Johnson SJ, Logue CM, White DG, Doetkott C, Nolan LK. 2007. Plasmid replicon typing of commensal and pathogenic Escherichia coli isolates. Appl Environ Microbiol 73:1976–1983. doi: 10.1128/AEM.02171-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnson TJ, Siek KE, Johnson SJ, Nolan LK. 2006. DNA sequence of a ColV plasmid and prevalence of selected plasmid-encoded virulence genes among avian Escherichia coli strains. J Bacteriol 188:745–758. doi: 10.1128/JB.188.2.745-758.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suzuki H, Yano H, Brown CJ, Top EM. 2010. Predicting plasmid promiscuity based on genomic signature. J Bacteriol 192:6045–6055. doi: 10.1128/JB.00277-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Villa L, Garcia-Fernandez A, Fortini D, Carattoli A. 2010. Replicon sequence typing of IncF plasmids carrying virulence and resistance determinants. J Antimicrob Chemother 65:2518–2529. doi: 10.1093/jac/dkq347. [DOI] [PubMed] [Google Scholar]
- 38.Day MJ, Rodriguez I, van Essen-Zandbergen A, Dierikx C, Kadlec K, Schink AK, Wu G, Chattaway MA, DoNascimento V, Wain J, Helmuth R, Guerra B, Schwarz S, Threlfall J, Woodward MJ, Coldham N, Mevius D, Woodford N. 2016. Diversity of STs, plasmids and ESBL genes among Escherichia coli from humans, animals and food in Germany, the Netherlands and the UK. J Antimicrob Chemother 71:1178–1182. doi: 10.1093/jac/dkv485. [DOI] [PubMed] [Google Scholar]
- 39.Diab M, Hamze M, Madec JY, Haenni M. 2017. High prevalence of non-ST131 CTX-M-15-producing Escherichia coli in healthy cattle in Lebanon. Microb Drug Resist 23:261–266. doi: 10.1089/mdr.2016.0019. [DOI] [PubMed] [Google Scholar]
- 40.Ewers C, Bethe A, Semmler T, Guenther S, Wieler LH. 2012. Extended-spectrum β-lactamase-producing and AmpC-producing Escherichia coli from livestock and companion animals, and their putative impact on public health: a global perspective. Clin Microbiol Infect 18:646–655. doi: 10.1111/j.1469-0691.2012.03850.x. [DOI] [PubMed] [Google Scholar]
- 41.Liebana E, Batchelor M, Hopkins KL, Clifton-Hadley FA, Teale CJ, Foster A, Barker L, Threlfall EJ, Davies RH. 2006. Longitudinal farm study of extended-spectrum beta-lactamase-mediated resistance. J Clin Microbiol 44:1630–1634. doi: 10.1128/JCM.44.5.1630-1634.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Horton RA, Randall LP, Snary EL, Cockrem H, Lotz S, Wearing H, Duncan D, Rabie A, McLaren I, Watson E, La Ragione RM, Coldham NG. 2011. Fecal carriage and shedding density of CTX-M extended-spectrum β-lactamase-producing Escherichia coli in cattle, chickens, and pigs: implications for environmental contamination and food production. Appl Environ Microbiol 77:3715–3719. doi: 10.1128/AEM.02831-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tenaillon O, Skurnik D, Picard B, Denamur E. 2010. The population genetics of commensal Escherichia coli. Nat Rev Microbiol 8:207–217. doi: 10.1038/nrmicro2298. [DOI] [PubMed] [Google Scholar]
- 44.Smet A, Martel A, Persoons D, Dewulf J, Heyndrickx M, Herman L, Haesebrouck F, Butaye P. 2010. Broad-spectrum β-lactamases among Enterobacteriaceae of animal origin: molecular aspects, mobility and impact on public health. FEMS Microbiol Rev 34:295–316. doi: 10.1111/j.1574-6976.2009.00198.x. [DOI] [PubMed] [Google Scholar]
- 45.Bevan ER, Jones AM, Hawkey PM. 2017. Global epidemiology of CTX-M β-lactamases: temporal and geographical shifts in genotype. J Antimicrob Chemother 72:2145–2155. doi: 10.1093/jac/dkx146. [DOI] [PubMed] [Google Scholar]
- 46.Wittum TE, Mollenkopf DF, Daniels JB, Parkinson AE, Mathews JL, Fry PR, Abley MJ, Gebreyes WA. 2010. CTX-M-type extended-spectrum β-lactamases present in Escherichia coli from the feces of cattle in Ohio, United States. Foodborne Pathog Dis 7:1575–1579. doi: 10.1089/fpd.2010.0615. [DOI] [PubMed] [Google Scholar]
- 47.Bej AK, DiCesare JL, Haff L, Atlas RM. 1991. Detection of Escherichia coli and Shigella spp. in water by using the polymerase chain reaction and gene probes for uid. Appl Environ Microbiol 57:1013–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Edelstein M, Pimkin M, Palagin I, Edelstein I, Stratchounski L. 2003. Prevalence and molecular epidemiology of CTX-M extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae in Russian hospitals. Antimicrob Agents Chemother 47:3724–3732. doi: 10.1128/AAC.47.12.3724-3732.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ahmed S, Besser TE, Call DR, Weissman SJ, Jones LP, Davis MA. 2016. Evaluation of two multi-locus sequence typing schemes for commensal Escherichia coli from dairy cattle in Washington State. J Microbiol Methods 124:57–61. doi: 10.1016/j.mimet.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 50.Clinical and Laboratory Standards Institute. 2013. Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals; approved standards— 4th ed VET01-A4. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 51.Lewis JS II, Herrera M, Wickes B, Patterson JE, Jorgensen JH. 2007. First report of the emergence of CTX-M-type extended-spectrum beta-lactamases (ESBLs) as the predominant ESBL isolated in a U.S. health care system. Antimicrob Agents Chemother 51:4015–4021. doi: 10.1128/AAC.00576-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ruppé E, Hem S, Lath S, Gautier V, Ariey F, Sarthou JL, Monchy D, Arlet G. 2009. CTX-M beta-lactamases in Escherichia coli from community-acquired urinary tract infections, Cambodia. Emerg Infect Dis 15:741–748. doi: 10.3201/eid1505.071299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. J Mol Biol 215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 54.Kado CI, Liu ST. 1981. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol 145:1365–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sambrook J, Fritsch EF, Maniatis T. 1989. Molecular cloning: a laboratory manual, 2nd ed Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 56.Abramson JH. 2011. WINPEPI updated: computer programs for epidemiologists, and their teaching potential. Epidemiol Perspect Innov 8:1. doi: 10.1186/1742-5573-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hunter PR, Gaston MA. 1988. Numerical index of the discriminatory ability of typing systems: an application of Simpson's index of diversity. J Clin Microbiol 26:2465–2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grundmann H, Hori S, Tanner G. 2001. Determining confidence intervals when measuring genetic diversity and the discriminatory abilities of typing methods for microorganisms. J Clin Microbiol 39:4190–4192. doi: 10.1128/JCM.39.11.4190-4192.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pitout JD, Hossain A, Hanson ND. 2004. Phenotypic and molecular detection of CTX-M-beta-lactamases produced by Escherichia coli and Klebsiella spp. J Clin Microbiol 42:5715–5721. doi: 10.1128/JCM.42.12.5715-5721.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Eckert C, Gautier V, Arlet G. 2006. DNA sequence analysis of the genetic environment of various blaCTX-M genes. J Antimicrob Chemother 57:14–23. doi: 10.1093/jac/dki398. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.