Abstract
The circumventricular organs (CVOs) are specialised neuroepithelial structures found in the midline of the brain, grouped around the third and fourth ventricles. They mediate the communication between the brain and the periphery by performing sensory and secretory roles, facilitated by increased vascularisation and the absence of a blood‐brain barrier. Surprisingly little is known about the origins of the CVOs (both developmental and evolutionary), but their functional and organisational similarities raise the question of the extent of their relationship. Here, I review our current knowledge of the embryonic development of the seven major CVOs (area postrema, median eminence, neurohypophysis, organum vasculosum of the lamina terminalis, pineal organ, subcommissural organ, subfornical organ) in embryos of different vertebrate species. Although there are conspicuous similarities between subsets of CVOs, no unifying feature characteristic of their development has been identified. Cross‐species comparisons suggest that CVOs also display a high degree of evolutionary flexibility. Thus, the term ‘CVO’ is merely a functional definition, and features shared by multiple CVOs may be the result of homoplasy rather than ontogenetic or phylogenetic relationships.
Keywords: adenohypophysis, bone morphogenetic proteins, cellular specification, fate maps, fibroblast growth factors, homeobox genes, infundibulum, pituitary gland, Rathke's pouch, sonic hedgehog, tanycytes, transcription factors, Wnts
Introduction
Our brain is continuously communicating with the rest of our body and hormonal signals play an important role in this communication, in particular in homeostatic and adaptive‐regulatory processes. However, the shuttling of larger molecules such as hormones between the brain and the periphery is tightly controlled by the blood–brain barrier (BBB; Abbott & Friedman, 2012). Specialised neuroepithelial structures called circumventricular organs (CVOs), which are grouped around the third and fourth ventricle along the midline of the brain, allow for the regulated exchange of hormones and other molecules between the bloodstream and the brain (Fig. 1A).
Figure 1.
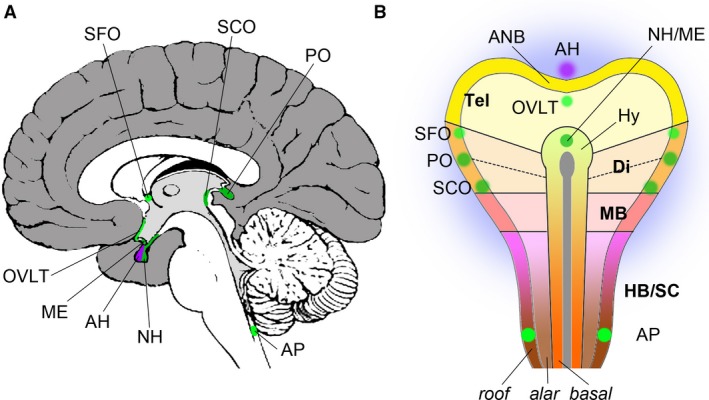
Topographic anatomy and embryonic origins of the CVOs. (A) Mid‐sagittal section of a human brain; view of medial surface, anterior points to the left, superior to the top. The cerebral cortex is coloured in dark grey, the corpus callosum, septum pellucidum, fornix, dorsal and ventral diencephalon, brain stem and cerebellum are shown in white, and the walls of the ventricles in light grey. Sensory CVOs are highlighted in light green, secretory CVOs in dark green. The adenohypophysis (AH) is shown in purple. (B) Schematic depiction of the neural plate of a vertebrate embryo with the approximate positions of the progenitor domains of the CVOs indicated; anterior points to the top. The telencephalon (Tel) is yellow, the diencephalon (Di) orange, the midbrain (MB) red and the hindbrain/spinal cord (HB/SC) purple/brown. The notochord is shown as a grey line along the midline that ends in an oval, the prechordal plate. The basal, alar and roof plates are outlined. The presumptive location of the zona limitans intrathalamica is indicated with a dotted line. The surrounding non‐neural ectoderm is shaded in blue. ANB, anterior neural border; AP, area postrema; Hy, hypothalamus; ME, median eminence; OVLT, organum vasculosum of the lamina terminalis; PO, pinal organ; SCO, subcommissural organ; SFO, subfornical organ.
CVOs can be secretory, such as the neurohypophysis/median eminence, the pineal gland and the subcommissural organ (SCO), or sensory, such as the area postrema, the organum vasculosum of the lamina terminalis (OVLT) and the subfornical organ (SFO). Although CVOs are functionally and morphologically diverse, they also share a range of common features: as their capillaries are fenestrated, they lack the BBB that is characteristic of the rest of the central nervous system, they tend to comprise specialised neuroglial cells (pituicytes, tanycytes) and they provide an interface not only between the bloodstream and the brain, but also between brain tissue and the cerebrospinal fluid in the ventricular system of the central nervous system (Joly et al. 2007; Guerra et al. 2015; Kaur & Ling, 2017).
This raises the question whether the term ‘CVO’ is purely a functional classification, or whether there are underlying genetic relationships between different CVOs. In this essay I will review our current knowledge of (i) the embryonic origins of the CVOs and their molecular specification and (ii) their conservation between different species. The neurohypophysis has received considerable attention as a component of the endocrinologically important pituitary gland and the pineal organ as a model for brain asymmetry in zebrafish; however, far less is known about the formation of the area postrema, OVLT, SCO and SFO.
The neurohypophysis/median eminence
The neurohypophysis forms the posterior lobe of the pituitary gland (hypophysis cerebri), a pea‐sized structure that protrudes ventrally from the hypothalamus via the stalk‐like infundibular stem. The anterior lobe of the pituitary gland, the adenohypophysis, originates from the roof of the stomodeum (oral ectoderm) and is therefore non‐neural. The median eminence, a small swelling that is often regarded as a CVO in its own right, is found at the interface between the ventral hypothalamus and the infundibular recess (Figs 1A and 2C). The mature neurohypophysis receives axons from magnocellular neurons in the paraventricular and supraoptic nuclei of the hypothalamus, and it releases the hormones oxytocin and vasopressin. By contrast, the adenohypophysis is controlled by peptide hormones that are released from hypothalamic neurons close to the median eminence and then transported to the anterior lobe of the pituitary gland via a system of vessels called the pituitary portal system. The pituitary gland is a major regulator of growth, homeostasis, metabolism and reproduction, and its functions have been studied and reviewed in countless publications. For the purpose of this review, I am going to focus on key steps in pituitary development. I will consider the neurohypophysis and the median eminence together because they emerge in direct proximity and are thus likely to share a large number of developmental mechanisms, and because they are connected to form a functional unit (Szarek et al. 2010; Davis et al. 2013; Clarke, 2015).
Figure 2.
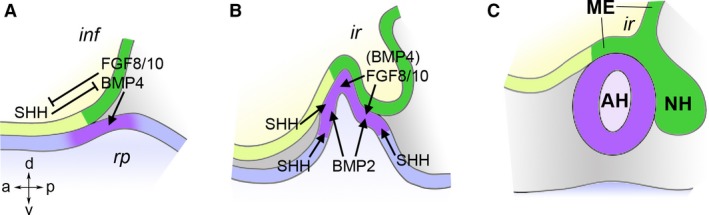
Embryonic development of the pituitary gland. Schematic diagrams representing the interactions between, and morphogenesis of, involved tissues (based on Treier et al. 1998). Neural tissues are shown in yellow and green, non‐neural ectoderm in blue and purple, the embryonic axes are indicated (A/P/D/V). (A) At embryonic day (E) 9.0 in the mouse, the ventroposterior‐medial hypothalamus (infundibulum, inf, dark green) expresses BMP4, FGF8 and FGF10, which induce Rathke's pouch (rp, adenohypophyseal placode, purple) in the underlying oral ectoderm. The infundibular signals are antagonised by SHH from the anterior hypothalamus. (B) At E10.5, Rathke's pouch has started to evaginate dorsally and the infundibulum ventrally. At this stage, cell fates are patterned in the pouch by opposing gradients of FGFs from the infundibulum and BMP2 from underlying mesoderm. SHH from surrounding tissues promotes proliferation of the pouch. (C) At E12.5, the pouch has budded off from the stomodeal roof, forming the progenitor of the adenohypophysis (AH). The neurohypophysis (NH) is connected to the hypothalamus via the infundibular stalk. The median eminence (ME) forms the area around the infundibular recess (ir).
During embryonic development, the neurohypophysis appears as a localised outpocketing of the floor of the hypothalamus. Fate mapping studies in chick, frog, mouse and zebrafish identified progenitors of the hypothalamus in the centre of the anterior neural plate (Fig. 1B; Couly & le Douarin, 1985; Eagleson & Harris, 1990; Woo & Fraser, 1995; Cobos et al. 2001; Staudt & Houart, 2007). The prechordal plate, a mesodermal midline structure at the anterior end of the notochord, lies just beneath this region, and ablation and transplantation studies in chick embryos demonstrated that the prechordal plate is a signalling center that is both necessary and sufficient for the induction of the hypothalamus. The signalling factors responsible for the inductive activity of the prechordal plate are Sonic Hedgehog (SHH) and Bone Morphogenetic Protein 7 (BMP7) (Dale et al. 1997; Pera & Kessel, 1997). For example, Shh mutant mice lack all ventral brain structures including the hypothalamus and pituitary gland (Chiang et al. 1996). The homeobox gene Nkx2.1 is a target of SHH signalling in the ventral forebrain and an early marker of hypothalamic differentiation, and genetic ablation of this gene in mice also results in absence of the pituitary gland (Takuma et al. 1998).
After the hypothalamus has been induced, it is patterned by overlapping signals: the ventroposterior region (infundibulum) produces BMP4 and the Fibroblast Growth Factors 8 and 10 (FGF8/10). These signals induce Rathke's pouch, the precursor of the adenohypophysis, in the underlying oral ectoderm (Fig. 2A; Ericson et al. 1998; Takuma et al. 1998; Treier et al. 1998). Because of this inductive role, the infundibular region has been suggested to function as an ‘organiser’ of pituitary development (Davis et al. 2013). Whereas BMP4 appears to be the inducer of Rathke's pouch proper, FGF8 and FGF10 seem to be more important for its survival and expansion (Takuma et al. 1998; McCabe et al. 2011). A hypothalamic domain of SHH expression is found anterior to the infundibular BMP/FGF domain, and these two groups of signals mutually restrict their expression (Fig. 2A; Treier et al. 1998). Conditional ablation of Shh function from the hypothalamic neuroepithelium leads to anterior expansion of the BMP/FGF domain and a concomitant anterior displacement of the neurohypophysis, whereas posterior expansion of Shh expression results in pituitary hypoplasia (Zhao et al. 2012; Trowe et al. 2013).
Rathke's pouch is induced in the oral ectoderm that lies beneath the infundibular organiser. Its progenitors can be traced back to the non‐neural ectoderm just anterior to the early neural plate (Fig. 1B; Couly & le Douarin, 1985; Sánchez‐Arrones et al. 2015). These progenitors are found in a horseshoe‐shaped domain of ectoderm that surrounds the anterior neural plate and that is known as the pre‐placodal region which gives rise to a number of placodes, ectodermal thickenings that contribute to many of the sensory organs of the head region (Baker & Bronner‐Fraser, 2001; Schlosser, 2006; Streit, 2007). Indeed, the ectoderm that forms Rathke's pouch is also called the (adeno)hypophyseal placode. Thus, the mature pituitary gland has both a neural and a non‐neural, placodal contribution.
Various homeobox genes are essential for pituitary gland development. Hesx1 is initially expressed broadly throughout the forebrain anlage, but becomes restricted to Rathke's pouch later on. Mice lacking Hesx1 function display a range of forebrain defects – consistent with Hesx1's early expression – including pituitary dysplasia. Strikingly similar defects are observed in human patients with septo‐optic dysplasia (SOD), and loss‐of‐function mutations in the human HESX1 gene have been identified in a subset of SOD patients (Dattani et al. 1998). The homeobox gene Six3 and the high mobility group gene Sox2 are also required for normal pouch formation (Gaston‐Massuet et al. 2008; Jayakody et al. 2012).
The homeobox genes Otx1 and Otx2 are essential for the development of various structures of the vertebrate head (Acampora et al. 2000). Otx1 is postnatally expressed in the pituitary gland, and mice that lack Otx1 gene function display transient dwarfism and hypogonadism characteristic of pituitary malfunction (Acampora et al. 1998). Thus, Otx1 appears to play a later role in cell type specification in this region (like a number of other factors such as Pitx1, Pitx2 and Pit1) (Tremblay et al. 1998). Otx2 −/− mice completely lack anterior head structures (Acampora et al. 1995; Matsuo et al. 1995; Ang et al. 1996); however, specific defects of the pituitary are observed in patients with hypomorphic OTX2 mutations (Beby & Lamonerie, 2013; Schoenmakers et al. 2015). A recent study investigating the effects of selectively eliminating Otx2 function from either the oral ectoderm or the infundibular neuroectoderm demonstrated a requirement for this gene in the latter: FGF signalling is absent from this region in such mice, resulting in severe hypoplasia of the pituitary stalk and posterior lobe and, secondarily, of the (Otx2‐positive) anterior lobe, consistent with the proposed role of the infundibulum as an organiser of pituitary development (Mortensen et al. 2015).
Cell type patterning within the developing pituitary gland is regulated by opposing FGF and BMP gradients from the infundibular organiser and ventral mesoderm, respectively (Fig. 2B; Ericson et al. 1998; Treier et al. 1998). Shh is not expressed within Rathke's pouch itself; however, cellular proliferation within the pouch depends on SHH signalling, presumably from surrounding tissues (Treier et al. 2001; Wang et al. 2010). Furthermore, multiple Wnt genes are expressed in this region, but their respective roles have not been conclusively dissected (Treier et al. 1998; Cha et al. 2004; Potok et al. 2008). A number of transcription factors have been implicated in the specification of cell lineages and growth of the pituitary: the LIM homeodomain factors LHX3 and LHX4, the POU transcription factor POU1F1/PIT1, and the paired‐like homeodomain factor PROP1 (Li et al. 1990; Sheng et al. 1997, 1996; Wu et al. 1998). Their individual roles have been reviewed elsewhere (Prince et al. 2011; de Moraes et al. 2012; Schoenmakers et al. 2015).
Taken together, pituitary gland formation involves an interaction between the neuroepithelium and placodal ectoderm. Signals from the neurohypophysis are essential for the positioning and proliferation of the adenohypophysis, but there is no evidence of signals acting in the opposite direction, from Rathke's pouch to the infundibulum. The finding that some patients with mutations in HESX1 who display severe anterior pituitary aplasia retain a fairly normal neurohypophysis provides genetic evidence that signals from Rathke's pouch are not required for posterior lobe formation (Sobrier et al. 2006).
The median eminence is a prominence in the midline of the ventral hypothalamus immediately adjacent to the infundibular stalk (Figs 1A and 2C). Functionally, it provides a link between the hypothalamus and the pituitary gland: it mediates the secretion of the hypothalamic releasing factors into the pituitary portal system and it contains axons from several hypothalamic nuclei on their way into the neurohypophysis. The pars tuberalis, a thin layer of cells wrapped around the pituitary stalk that produce pituitary tropic hormones, is also often counted as part of the median eminence. The pars tuberalis expresses melatonin receptors and has been implicated in regulating seasonal reproductive behaviour (Lewis & Ebling, 2017).
How separable are the neurohypophysis and the median eminence molecularly? There is evidence that the expression of thyrotropin‐releasing hormone is higher in the median eminence than in other divisions of the ventral hypothalamus (Geris et al. 1999); however, genetic mechanisms that regulate a cell fate switch between the neurohypophysis and median eminence remain to be discovered.
The vascular organ of the lamina terminalis (OVLT)
The OVLT is a sensory CVO involved in osmoregulation that responds to factors such as angiotensin II and that regulates thirst via its connections to the median preoptic nucleus (Bourque, 2008; Cancelliere et al. 2015; McKinley et al. 2015). OVLT neurons are found within the lamina terminalis, a thin layer of neuroepithelium that forms part of the anterior wall of the third ventricle (Fig. 1A). In early embryos, lamina terminalis progenitors can be found medially in the preoptic area of the anterior neural plate, just in front of the anlage of the hypothalamus and posterior to the anterior neural border (ANB; Fig. 1B; Cobos et al. 2001). Upon neural tube closure, the ANB folds up into the commissural plate, a template for the formation of all telencephalic commissures (Suarez et al. 2014). The ANB/commissural plate also functions as a signalling centre that secretes several FGFs and that is involved in the patterning of the cerebral cortex (Shimogori et al. 2004). Thus, the lamina terminalis emerges between the FGF‐secreting ANB and the SHH‐producing prechordal plate/hypothalamus, and there is substantial cross‐regulation between these two signalling centres (Ohkubo et al. 2002; Storm et al. 2006). At the same time, BMPs and WNTs from the dorsal midline of the telencephalon antagonise the ventroanterior domains of FGF and SHH signalling (Ohkubo et al. 2002; Gunhaga et al. 2003; Shimogori et al. 2004; Storm et al. 2006).
Homeobox genes important for the formation of the lamina terminalis are Six3, which appears to integrate FGF and SHH signalling (Lagutin et al. 2003; Storm et al. 2006; Geng et al. 2008; Jeong et al. 2008b), and the SHH target gene Nkx2.1, which is essential for hypothalamus and ventral telencephalon formation (Dale et al. 1997; Pera & Kessel, 1997; Corbin et al. 2003). Taken together, the lamina terminalis is formed under the control of those signals that pattern the emerging telencephalon more generally, but how exactly the OVLT is established within the preoptic region remains unknown. In a recent re‐evaluation of hypothalamic regionalisation, Puelles and Rubenstein (2015) introduced an ‘acroterminal hypothalamic domain’ (ATD), a medial strip of neuroepithelium that connects the anterior ends of the floor and roof plates. The OVLT, median eminence and neurohypophysis originate within this ATD. However, as several other structures such as the optic stalk and chiasm and the postoptic commissure also form within the ATD, it is not just a progenitor domain of the anteroventral CVOs.
The subfornical organ (SFO)
Like the OVLT, the SFO is a sensory CVO involved in osmoregulation (Bourque, 2008), but it has also been implicated in energy homeostasis (Medeiros et al. 2012; Hindmarch & Ferguson, 2016; Matsuda et al. 2017). The SFO is located in the dorsoanterior corner of the third ventricle in the roof plate, beneath the fornix and close to the interventricular foramen of Monro (Fig. 1A). The SFO receives neuronal projections from the hindbrain, and it is also interconnected with multiple regions in the forebrain including the lamina terminalis and various hypothalamic nuclei (e.g. Tanaka et al. 2002a,b).
None of the available fate maps of the early neural plate/tube includes the SFO, but based on its location in the adult brain, SFO progenitors would be expected in the roof plate adjacent to the eminentia thalami, a dorsal subdivision of the neural tube that is located between the mid‐diencephalic zona limitans intrathalamica and the border between the telencephalon and the diencephalon (Fig. 1B). Consistent with this, the paired box/homeobox gene Pax2, a marker of the eminentia thalami, is later on also expressed in the SFO (Fotaki et al. 2008). Pax2‐deficient mice display various neurological deficits but the development of the SFO has not specifically been investigated in such mutants (Torres et al. 1996). It is likely that the SFO has failed to attract much attention from embryologists because it differentiates comparatively late in embryonic development (Castaneyra‐Perdomo et al. 1992).
The pineal organ
The pineal organ is a secretory CVO found in the posterior roof of the diencephalon (Fig. 1A). Historically, it piqued the interest of both anatomists and philosophers due both to its central location in the human brain and to the fact that it appeared to be the only azygous (unpaired) structure there. For example, René Descartes speculated in his Treatise of Man and his The Passions of the Soul that the pineal organ constitutes the ‘principal seat of the soul’.
The pineal organ begins to form as an outpocketing of the dorsal midline of the diencephalon shortly after neural tube closure (Oksche, 1965). As the pineal organ grows, it elongates into a thin stalk that carries a pine cone‐shaped body at its distal end, not dissimilar from the stalk‐like attachment of the pituitary gland on the opposite side of the third ventricle (Fig. 3). The pineal evagination is first visible as a little knob midway along the anteroposterior axis of the roof of the diencephalon, but differential growth or tissue movements lead to an apparent posterior shift such that, in the mature brain, the pineal stalk appears to be connected to the third ventricle close to the border between the diencephalon and midbrain.
Figure 3.
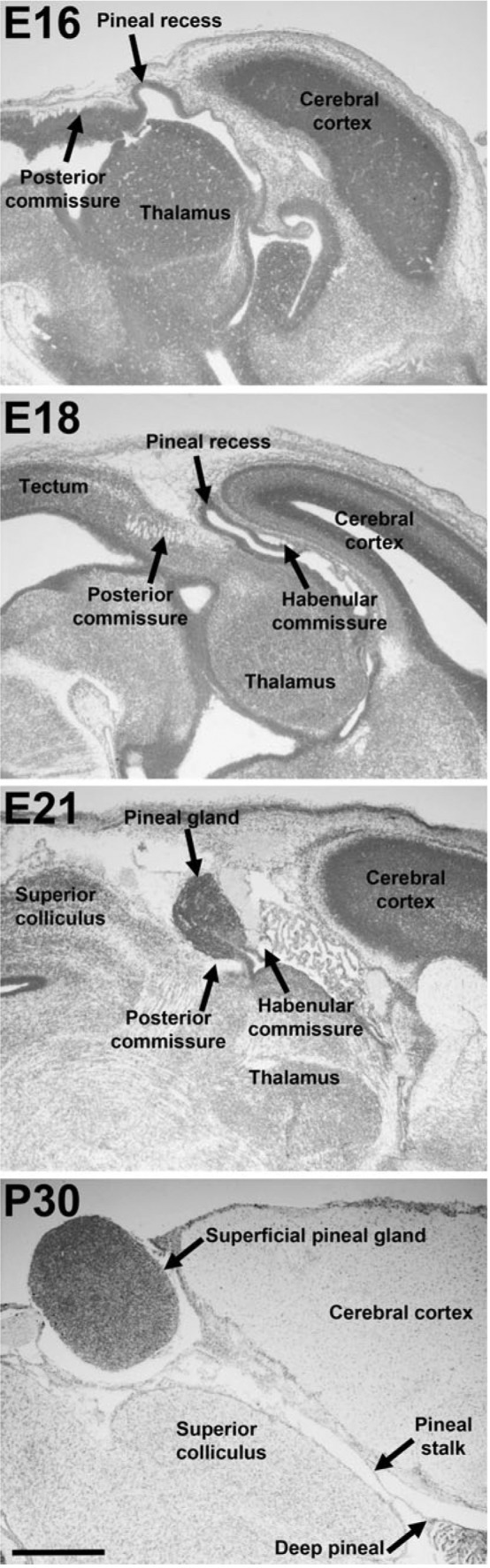
Development of the pineal organ of the rat. Nissl‐stained sagittal sections through rat brains at embryonic days (E) 16, 18, 21 and postnatal day (P) 30; anterior points to the right. Scale bar: 1 mm. Reproduced from Rath et al. (2013) with the kind permission of Springer Publishing.
In mammals, the pineal organ serves as a neurosecretory organ that releases the hormone melatonin, thereby modulating sleep, mood, food intake, breeding and sexual maturation in the context of diurnal and seasonal rhythms (Macchi & Bruce, 2004; Arendt & Skene, 2005). It receives afferent sympathetic and parasympathetic innervation from the superior cervical, sphenopalatine and otic ganglia. Furthermore, the retina sends projections indirectly to the pineal gland via the hypothalamus, thereby relaying information about ambient light (Møller & Baeres, 2002). In many non‐mammalian vertebrates the pineal organ functions as a photosensor itself, and the pinealocytes (the major cell type of the pineal organ) of these species share a striking resemblance with retinal photoreceptors; however, mammalian pinealocytes have lost this photoreceptive function (Ekström & Meissl, 2003). Like retinal photoreceptors, pinealocytes also express opsins (Peirson et al. 2009).
In zebrafish, the development of the pineal complex has been studied as a model for asymmetric neurogenesis (Bianco & Wilson, 2009). However, relatively little is known about how pineal progenitors become initially specified early in development. Fate mapping studies in chick and frog embryos located pineal progenitors in the anterolateral neural plate (prospective roof plate), indicating that the pineal organ is not really azygous, as has been suggested by Descartes and others (Fig. 1B; Couly & le Douarin, 1987; Eagleson & Harris, 1990). The anlage of the pineal organ expands anteriorly in zebrafish embryos carrying a mutation in masterblind, encoding an intracellular antagonist of WNT signalling, suggesting that its anterior limit is established through WNT inhibition (Masai et al. 1997; Heisenberg et al. 2001). Thus, the induction of pineal progenitors is governed by the same signals that are known to pattern the early neural plate in a more global fashion (Wilson & Houart, 2004; Kiecker & Lumsden, 2012).
Several homeodomain transcription factors are required for pineal development and most of those are also involved in retinal development, such as PAX6, OTX2, RAX, CRX, PAX4 and TBX2B (Estivill‐Torrus et al. 2001; Nishida et al. 2003; Foucher et al. 2006; Snelson et al. 2008; Rath et al. 2013; Chatterjee et al. 2014). For example, PAX6 mutations in humans result in pineal aplasia (Mitchell et al. 2003; Abouzeid et al. 2009). The homeodomain transcription factor NOT1 (encoded by the gene floating head in zebrafish), one of the earliest markers of pineal specification and a factor essential for pinealogenesis, provides an exception to this, as it is not expressed in the developing retina (Masai et al. 1997).
The subcommissural organ (SCO)
The SCO is found posterior to the evagination of the pineal stalk, anterior to the entrance to the cerebral aquaeduct and inferior to the posterior commissure (Fig. 1A). The SCO secretes a number of factors such as SCO‐spondin, the thyroid hormone transporter transthyretrin, basic FGF and various high molecular weight glycoproteins, some of which are insoluble and form Reissner's fibre, a long fibrous aggregate that reaches from the SCO all the way through the central canal of the spinal cord (Lehmann et al. 2001; Meiniel et al. 2008; Guerra et al. 2015). The relevance of the SCO is somewhat mysterious but it has been speculated that both SCO and Reissner's fibre are involved in water homeostasis and the regulation of cerebrospinal fluid composition. Consistently, abnormal SCO development has been implicated as a potential cause of congenital hydrocephalus (Baas et al. 2006; Meiniel, 2007; Huh et al. 2009; Lee et al. 2012; Ortloff et al. 2013). A role in regulating neurogenesis has been suggested for the soluble factors secreted by the SCO (Guerra et al. 2015).
Classic neural plate fate maps do not include the SCO progenitor region, but based on its location in the adult brain, one can assume that they are derived from the lateral neural plate (prospective roof plate) between the pineal progenitor region and the boundary between the forebrain and the midbrain at the level of the posterior commissure (Fig. 1B). Unlike the SFO, the SCO develops comparably early in development – in fact it has been reported as one of the first structures of the brain to differentiate (Guerra et al. 2015). Its formation is closely linked to that of the posterior commissure, and there is evidence that the SCO is required for proper pathfinding of commissural axons (Grondona et al. 2012). Genes that specifically affect the SCO have not yet been discovered, but both SCO and pineal development are affected in Pax6 mutants (see above) (Estivill‐Torrus et al. 2001).
The area postrema
The area postrema is the only CVO associated with the fourth rather than the third ventricle. It is a sensory CVO found at the posterior end of the floor of the fourth ventricle and is involved in the detection of toxins in the bloodstream, motion sickness, food aversion, nausea and vomiting as well as cardiorespiratory homeostasis (Fig. 1A; Price et al. 2008). Similar to several of the other CVOs, the area postrema has not been mapped in classic lineage tracing studies, but its location in the mature brain suggests that it originates from the caudal myelencephalon, possibly from the non‐segmented part of the caudal hindbrain (Fig. 1B; Cambronero & Puelles, 2000).
Very little is known about the ontogenetic mechanisms that regulate area postrema formation. Hindbrain development is governed by retinoic acid (RA) signalling, and the formation of the noradrenergic neurons of the area postrema also depends on this signal. Work in zebrafish has indicated that the effects of RA in the area postrema are mediated via induction of tfap2a, encoding a transcriptional activator (Holzschuh et al. 2003). Lmx1b is a member of the LIM homeobox gene family and is also required for noradrenergic differentiation in the area postrema (Filippi et al. 2007). Finally, neuronal area postrema defects are found in mice mutant for the homeobox gene Gsx2 (Szucsik et al. 1997).
Transcription factors in CVO development
Neural development is organised by secreted factors that are released from signalling centres such as the prechordal plate and by transcription factors that represent a readout of these signals, endow cells with a pre‐pattern, and determine how cells respond to the next signalling event (Kiecker & Lumsden, 2012; Kiecker et al. 2016). Transcription factors encoded by genes of the homeobox superfamily are key regulators of neural development that function in a broadly conserved manner across the animal kingdom (Hirth, 2010; Arendt et al. 2016). As reviewed above, a plethora of homeobox genes are expressed in developing CVOs and many of those are required for proper cell fate acquisition there.
Otx gene function is strictly required for the development of the pituitary and pineal organ, but Otx genes are expressed broadly throughout the developing forebrain, suggesting that at least some of the defects in pituitary and pineal development observed in Otx loss‐of‐function scenarios are likely to be due to broader effects of regional misspecification (Acampora et al. 1995; Matsuo et al. 1995; Ang et al. 1996). Nonetheless, pituitary‐specific defects are found in some OTX2 hypomorphic patients (Beby & Lamonerie, 2013; Schoenmakers et al. 2015), and Otx1 plays a later role in pituitary maturation in the mouse (Acampora et al. 1998). Furthermore, other genes that belong to the same subfamily of homeobox genes (the bicoid subfamily) also play important roles in cell fate specification in the pituitary gland: Pit1, Pitx1 and Pitx2 (Gage & Camper, 1997; Tremblay et al. 1998). Similarly, there is an ongoing requirement for Otx2 function in pinealogenesis (Nishida et al. 2003), and other Otx‐related genes such as Crx and otx5 (in frog and zebrafish) are necessary for pinealocyte formation (Vignali et al. 2000; Gamse et al. 2002; Rath et al. 2013). Furthermore, sustained expression of both OTX1 and OTX2 is found in the SCO of human embryos at later stages of neural development (Larsen et al. 2010). Collectively, these findings could indicate a dedicated role for the Otx gene family in CVO formation; however, Otx genes are not expressed in the developing hindbrain and are therefore likely to be dispensable for area postrema formation.
Similar to the Otx genes, Pax genes play multiple roles in neurodevelopment, and Pax6 in particular is broadly expressed throughout the developing forebrain (Georgala et al. 2011). Pituitary cell type specification is affected in Pax6 mutant mice (Bentley et al. 1999; Kioussi et al. 1999); however, the expression of Pax6 seems to be stronger in the adenohypophyseal placode than in the neurohypophysis, suggesting that it may be the non‐neural part of the pituitary gland that is affected in such mutants (Sjödal & Gunhaga, 2008). Pax6 plays a key role in the earliest steps of pineal organ induction: Pax6 mutant mice and zebrafish fail to form a pineal evagination, and the pineal gland is absent in human patients with PAX6 mutations (Estivill‐Torrus et al. 2001; Mitchell et al. 2003; Chatterjee et al. 2014). The central importance of this gene's function in pinealogenesis has been interpreted as strong evidence for the similarity between pinealocyte and retinal photoreceptor specification, and indeed the sequence of expression of transcriptional regulators in pinealocytes and in the retina is virtually exchangeable (Rath et al. 2013). The Pax6‐related gene Pax4 is also found in the developing pineal organ, although at later stages (Rath et al. 2009) and Pax6 is required for SCO formation (Estivill‐Torrus et al. 2001). As mentioned above, expression of Pax2 is found in the SFO, but its specific role in this CVO has not yet been addressed (Fotaki et al. 2008). No Pax gene expression has been described in the area postrema.
Three of the CVOs develop from the roof of the diencephalon: the pineal organ, SCO and SFO. BMPs are key signals in this area, and their effects are relayed by homedomain transcription factors of the Msx family. Disruption of Msx1 in mouse embryos leads to a general loss of dorsal midline strcutures and, subsequently, of the pineal organ and SCO (Bach et al. 2003; Fernandez‐Llebrez et al. 2004). The other tissue originating within this region is the choroid plexus epithelium of the third ventricle. Due to its selective barrier properties, the choroid plexus is sometimes regarded as a CVO in its own right (see next section). This could indicate that the entire roof of the third ventricle is a common CVO progenitor domain.
Finally, neuronal subtype specification in many subdivisions of the neural tube is known to be regulated by a ‘code’ of LIM homeobox gene expression, for example in the spinal cord, where different combinations of LIM factors specify different subtypes of motor neurons (Shirasaki & Pfaff, 2002). LIM genes also play a role in the developing pituitary gland: Lhx3 and Lhx4 cooperate in Rathke's pouch formation (Sheng et al. 1997) and Lhx2 is required for the formation of the neurohypophysis (Zhao et al. 2010). The neurohypophysis defects in Lhx2 mutants lead to a disorganised anterior lobe, consistent with the suggested role of the neurohypophysis as an organiser of pituitary morphogenesis. Lhx3 is also expressed in the pineal organ of zebrafish and chick embryos and Lhx4 has been detected in the pineal organ of the rat, but their respective roles in pinealogenesis have not yet been determined (Glasgow et al. 1997; Zhang et al. 2006; Bailey et al. 2009). However, the pineal organ is severely hypoplastic in mice lacking Lhx9, a paralog of Lhx2 that is highly expressed in pineal progenitors (Yamazaki et al. 2015). Lhx gene expression has not systematically been analysed in any of the other CVOs; however, the observation that Lhx9 mutant mice have a high incidence of hydrocephalus could suggest that the SCO is also affected in such mice (Yamazaki et al. 2015).
Taken together, there is considerable overlap between sets of transcriptional regulators that are expressed in developing CVOs; however, most of these factors are also expressed in many other subdivisions of the neural tube, and no transcriptional regulator that is expressed in all CVOs – a ‘master regulator’ of CVO formation – has been identified. Although a comprehensive atlas of gene expression in the CVOs remains to be compiled, the current literature does not suggest a common ‘transcriptional signature’ that is a unifying characteristic of all CVOs.
Later specialisations of CVOs
Despite divergent embryonic origins and transcriptional signatures, all CVOs develop specific common characteristics at later stages of embryogenesis. For example, their capillaries are more permeable due to fenestrations and discontinuous tight junctions (Wilhelm et al. 2016). The glucose transporter GLUT1, the BBB markers Cadherin‐10 and EBA (endothelial barrier antigen), the transferrin receptor as well as tight junction proteins such as Claudin‐5 and Zona Occludens‐1 are specifically downregulated in microvessels that infiltrate CVOs (Zeller et al. 1996; Williams et al. 2005; Jeong et al. 2008a; Norsted et al. 2008; Maolood & Meister, 2009; Morita et al. 2016). At the same time, several aquaporins (AQPs) are upregulated in CVOs (Nico et al. 2001; Goren et al. 2006; Wilson et al. 2010), and the pathological expression of AQP4 autoantibodies has been identified as a cause of neuromyelitis optica, an inflammatory autoimmune disease that also affects CVOs, resulting in intractable nausea and vomiting (Lennon et al. 2005; Matiello et al. 2007).
CVOs tend to be highly vascularised by local plexi, presumably to facilitate the exchange of hormones and other substances between the bloodstream and neural tissue. In the case of the neurohypophysis, FGF3 and FGF10 have been demonstrated to promote vascularisation (Liu et al. 2013). Whether this role of FGFs is conserved in other CVOs remains to be determined. Various developmental signalling pathways are involved in vasculogenesis, and a systematic analysis of whether and how these have been modified in developing CVOs could help establish the mechanisms that distinguish CVOs from other parts of the brain (Roca & Adams, 2007; Mancuso et al. 2008; Adams & Eichmann, 2010; Liebner & Plate, 2010). Recently vascularisation in the pars tuberalis of the median eminence was shown to be modulated in a circannual fashion through melatonin‐regulated differential splicing of vascular endothelial growth factor (Castle‐Miller et al. 2017). This fascinating study suggests that CVOs remain dynamic structures throughout life.
Sometimes considered a CVO in its own right (Joly et al. 2007), the choroid plexus (the tissue that produces the cerebrospinal fluid) shares some of the features of the CVOs discussed here: it is highly vascularised, its vessels are leaky and lack a BBB, its epithelial cells create a barrier between blood and cerebrospinal fluid, and dedicated water channels such as AQP1 are upregulated there (Wilson et al. 2010; Wolburg & Paulus, 2010).
Most CVOs – with the exception of the evaginating neurohypophysis and pineal organ – are lined with specialised ependymal cells called tanycytes that may serve as a selective barrier between the blood circulation and the cerebrospinal fluid and that contact both neurons and blood vessels via long, slender processes (Langlet et al. 2013). Very little is known about the earliest steps in the specification of tanycytes, but the finding that they form a resident stem cell population in the adult brain is likely to ensure continued interest in their cell biology (Xu et al. 2005; Robins et al. 2013; Rizzoti & Lovell‐Badge, 2017). Other ependymal cells in CVOs are often flattened with a squamous appearance, some of them lack cilia, and many display microvilli (e.g. Mark & Farmer, 1984).
CVOs in evolution
So far, I have reviewed our knowledge of the genetic mechanisms that regulate the formation of CVOs during embryogenesis (ontogenetically). I will conclude this review with a brief survey of research on the evolutionary (phylogenetic) origins of the CVOs.
CVOs can be incredibly diverse even between closely related phyla. In a true tour de force, Tsuneki (1986) compared 17 potential CVOs between 31 vertebrate species. The neurohypophysis, median eminence and SCO were found in all species examined, and a pineal organ was present in most species, with the exception of hagfish and caimans, leading to the conclusion that these four are likely to be the phylogenetically oldest CVOs. An SCO equivalent, producing a Reissner's fibre, has even been identified in cephalochordates (Guerra et al. 2015). This apparent evolutionary conservation is somewhat surprising, given how little we understand of the biological role of the SCO. Notably, a SCO‐spondin orthologue has been found in echinoderms, one of the deuterostome clades that is most distantly related to the vertebrates. This could indicate that the SCO has an even deeper origin somewhere close to the base of the deuterostome superphylum, or that the SCO‐spondin gene preceded the emergence of a chordate‐like proper SCO gland (Meiniel et al. 2008). Interestingly, the SCO is also frequently described as one of the first structures in the brain to differentiate (Schoebitz et al. 1986).
The co‐conservation of neurohypophysis and median eminence supports the view that they form a functional unit. It has been suggested that the arcuate nucleus of the hypothalamus and the median eminence also form a functional complex (Yi et al. 2006), and the paraventricular hypothalamic nucleus which is tightly connected with the neurohypophysis displays CVO‐like characteristics (Joly et al. 2007). In view of the interconnectedness of these structures as well as their common developmental origin from one progenitor domain, it may make sense to regard this entire region as one complex CVO, rather than a mosaic of multiple smaller CVOs and nuclei.
In amphioxus, a lobe extends asymmetrically from the ventral brain to contact Hatschek's pit (the ‘pre‐oral organ’), the putative homologue of the vertebrate adenohypophysis (Gorbman et al. 1999), and the amphioxus orthologue of the pituitary marker gene Pit1 is expressed there (Candiani & Pestarino, 1998). Although there is no evidence for a morphologically distinct pituitary gland in echinoderms, the GnRH (gonadotropin‐releasing hormone) signalling system is present in both sea urchins and starfish (Zandawala et al. 2017), and GnRH induces spawning in hemichordates and even in molluscs, indicating that the evolution of at least some pituitary hormones preceded the emergence of the actual pituitary gland (Gorbman et al. 2003). Cells with both sensory and neurosecretory properties that express orthologues of markers of the ventral vertebrate forebrain were found in the brain of the annelid Platynereis dumerilii, a marine worm (Tessmar‐Raible et al. 2007). It is conceivable that the emergence of these cell types provided the basis for the formation of neurosecretory signalling centres such as the neurohypophysis/median eminence in the vertebrate brain.
The pineal organ is particularly remarkable from an evolutionary perspective. In most vertebrates it is a photosensory organ, but this function has been lost in mammals, where the pineal organ is purely secretory. In anamniotes the pineal organ is often associated with accessory organs such as the parapineal organ of lampreys and fish, the frontal organ of some amphibians and the parietal eye of lizards and tuataras (Ekström & Meissl, 2003; Mano & Fukada, 2007). The skulls of many of these species display foramina that allow their pineal/parapineal organs to directly receive ambient light. The evolutionary transformation of the pineal organ from a photosensor to a secretory gland is mirrored by a regression of its principal cell type, the pinealocyte, from a typical retina‐like photoreceptor with a primary cilium that has been modified into an outer segment to a non‐photoreceptive pinealocyte with an ordinary, small primary cilium (Ekström & Meissl, 2003; Mano & Fukada, 2007).
The presumed homologue of the pineal organ in amphioxus is called the lamellar organ. Like the vertebrate pineal organ, the lamellar body expresses the amphioxus orthologue of pax6 and the LIM homeobox gene islet1 (Glardon et al. 1998; Jackman et al. 2000). The pineal organ seems to have been lost in hagfish, crocodilians and in some tropical mammals (but these clades still have melatonin‐producing pinealocyte‐like cells in other regions of their brains), whereas some polar mammals have disproportionaly large pineal glands (Ralph, 1975; Vollrath, 1979; Ekström & Meissl, 2003). Taken together these observations indicate that the pineal organ is highly variable, suggesting the absence of a strong selective pressure favouring its maintenance and morphological conservation.
The key function of the pineal organ is the secretion of melatonin. However, melatonin is an ancient molecule that is found even in photosynthetically active cyanobacteria, where its most likely role is to serve as a free radical scavenger and antioxidant (Manchester et al. 2015). The key enzyme for melatonin biosynthesis is arylalkylamine N‐acetyltransferase (AANAT), of which there are two classes: vertebrate AANATs and non‐vertebrate AANATs that are found in fungi and protists (Vetting et al. 2005; Coon & Klein, 2006). Interestingly, all seven aanat genes in the amphioxus genome have non‐vertebrate characteristics and urochordates also lack vertebrate‐like aanats (Pavlicek et al. 2010). By contrast, agnathans possess vertebrate‐type aanat genes, suggesting that a major transition about 500 mio years ago, possibly based on a duplication of the ancient aanat gene, led to a cooption of the melatonin system for the regulation of diurnal rhythmicity (Falcon et al. 2014). However, a recent study revealed that in the protostome Platynereis, melatonin is produced in opsin‐containing photoreceptor cells in the brain and that it regulates diurnally cyclical swimming behaviour (Tosches et al. 2014). This spectacular finding suggests that the role of melatonin in governing circadian rhythms is more ancient than previously assumed.
The SFO and area postrema seem less conserved than the SCO, neurohypophyseal system and pineal organ, and a number of non‐mammalian CVOs have been found in other species such as the lateral septal organ in birds and the saccus vasculosus (SV) in fish (Kuenzel & van Tienhoven, 1982; Tsuneki, 1986; Bardet et al. 2006; Sueiro et al. 2007). Thus, it appears that CVOs have emerged multiple times independently and many of them have undergone significant modifications throughout evolution. The SV has recently been identified as the functional equivalent of the amniote pituitary gland pars tuberalis in fish, as it regulates seasonal changes in reproductive behaviour (Nakane et al. 2013). Surprisingly little overlap in the expression of developmental regulator genes was found between the anlagen of the SV and pituitary gland of trout embryos, underlining the distinct origins of these two structures. However, various pituitary marker genes such as Lhx3 and Pit‐1 become expressed in the adult SV, suggesting that they may be required for functional differentiation rather than embryonic specification (Maeda et al. 2015).
Conclusions
This review of the mechanisms that underlie the development of the CVOs and their evolution demonstrates that CVOs are a highly diverse group of neuroepithelial specialisations that are characterised by similarities in function rather than common genetic programmes. Progenitors of CVOs are induced by signals that regulate global patterning in the developing neural plate and neural tube, according to their relative position in a quasi‐Cartesian coordinate system that is established by local signalling centres (Kiecker & Lumsden, 2012; Puelles & Rubenstein, 2015). For example, the neurohypophysis/median eminence depends on SHH signalling from the prechordal plate, and progenitors of the pineal organ are positioned along the neural plate border by a specific level of WNT, a factor that is also involved in patterning the rest of the forebrain.
Despite considerable overlap in the expression of groups of transcription factors between different CVOs, no single ‘master regulator’ or unique ‘transcriptional signature’ of CVO development has been identified. In the case of the pineal organ, the temporal sequence of homeobox gene expression is more similar to that of the developing retina than that of other CVOs. Of course, we cannot rule out that a common transcriptional profile of CVO development remains to be identified, and a systematic comparison of their transcriptomes is pending, but the currently available data suggest a lack of a ‘common genetic denominator’.
Nevertheless, it is obvious that there are striking similarities between CVOs with regard to their cell biology. Thus, a search for common regulators of the differentiation of these later features – such as the lack of a BBB, the emergence of tanycytes and/or the high degree of vascularisation – might prove more successful in identifying overlapping genetic mechanisms in the future. Another underlying similarity is that CVOs develop in places where neural and non‐neural ectoderm interact: area postrema, OVLT, pineal organ, SCO and SFO progenitors are induced along the border of the neural plate and the neurohypophyseal complex interacts with the non‐neural ectoderm of the adenohypophyseal placode (Figs 1B and 2). Of course, this may simply be a consequence of their role as interfaces between the brain and the periphery, but a detailed analysis of the cell lineages that contribute to the CVOs will be an important future avenue of research in this area.
Thus, although embryologists and developmental neurobiologists have started to piece together the puzzle of CVO development, many questions remain unanswered: the embryonic origins of the area postrema, OVLT, SCO and SFO are fairly enigmatic and the genetic networks regulating their development remain uncharted territory. Undoubtedly, more research is required to further our understanding of the formation of these important structures that link the brain with the blood circulation and the cerebrospinal fluid, thereby integrating homeostatic and adaptive mechanisms. During this enterprise, it will be important to keep in mind that, at least for the time being, ‘CVO’ is a purely functional definition that describes a heterogeneous group of neuroepithelial specialisations. Different CVOs have most probably emerged independently in evolution, and their similarities are a likely result of homoplasy rather than genetic relatedness.
Author contributions
C. Kiecker conceived and wrote the manuscript.
Acknowledgements
I would like to thank Malcolm Logan and Richard Wingate for helpful comments on the manuscript.
References
- Abbott NJ, Friedman A (2012) Overview and introduction: the blood‐brain barrier in health and disease. Epilepsia 53(Suppl 6), 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abouzeid H, Youssef MA, Elshakankiri N, et al. (2009) PAX6 aniridia and interhemispheric brain anomalies. Mol Vis 15, 2074–2083. [PMC free article] [PubMed] [Google Scholar]
- Acampora D, Mazan S, Lallemand Y, et al. (1995) Forebrain and midbrain regions are deleted in Otx2 −/− mutants due to a defective anterior neuroectoderm specification during gastrulation. Development 121, 3279–3290. [DOI] [PubMed] [Google Scholar]
- Acampora D, Mazan S, Tuorto F, et al. (1998) Transient dwarfism and hypogonadism in mice lacking Otx1 reveal prepubescent stage‐specific control of pituitary levels of GH, FSH and LH. Development 125, 1229–1239. [DOI] [PubMed] [Google Scholar]
- Acampora D, Postiglione MP, Avantaggiato V, et al. (2000) The role of Otx and Otp genes in brain development. Int J Dev Biol 44, 669–677. [PubMed] [Google Scholar]
- Adams RH, Eichmann A (2010) Axon guidance molecules in vascular patterning. Cold Spring Harb Perspect Biol 2, a001875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang SL, Jin O, Rhinn M, et al. (1996) A targeted mouse Otx2 mutation leads to severe defects in gastrulation and formation of axial mesoderm and to deletion of rostral brain. Development 122, 243–252. [DOI] [PubMed] [Google Scholar]
- Arendt J, Skene DJ (2005) Melatonin as a chronobiotic. Sleep Med Rev 9, 25–39. [DOI] [PubMed] [Google Scholar]
- Arendt D, Tosches MA, Marlow H (2016) From nerve net to nerve ring, nerve cord and brain – evolution of the nervous system. Nat Rev Neurosci 17, 61–72. [DOI] [PubMed] [Google Scholar]
- Baas D, Meiniel A, Benadiba C, et al. (2006) A deficiency in RFX3 causes hydrocephalus associated with abnormal differentiation of ependymal cells. Eur J Neurosci 24, 1020–1030. [DOI] [PubMed] [Google Scholar]
- Bach A, Lallemand Y, Nicola MA, et al. (2003) Msx1 is required for dorsal diencephalon patterning. Development 130, 4025–4036. [DOI] [PubMed] [Google Scholar]
- Bailey MJ, Coon SL, Carter DA, et al. (2009) Night/day changes in pineal expression of >600 genes: central role of adrenergic/cAMP signaling. J Biol Chem 284, 7606–7622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker CV, Bronner‐Fraser M (2001) Vertebrate cranial placodes I. Embryonic induction. Dev Biol 232, 1–61. [DOI] [PubMed] [Google Scholar]
- Bardet SM, Cobos I, Puelles E, et al. (2006) Chicken lateral septal organ and other circumventricular organs form in a striatal subdomain abutting the molecular striatopallidal border. J Comp Neurol 499, 745–767. [DOI] [PubMed] [Google Scholar]
- Beby F, Lamonerie T (2013) The homeobox gene Otx2 in development and disease. Exp Eye Res 111, 9–16. [DOI] [PubMed] [Google Scholar]
- Bentley CA, Zidehsarai MP, Grindley JC, et al. (1999) Pax6 is implicated in murine pituitary endocrine function. Endocrine 10, 171–177. [DOI] [PubMed] [Google Scholar]
- Bianco IH, Wilson SW (2009) The habenular nuclei: a conserved asymmetric relay station in the vertebrate brain. Philos Trans R Soc Lond B Biol Sci 364, 1005–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourque CW (2008) Central mechanisms of osmosensation and systemic osmoregulation. Nat Rev Neurosci 9, 519–531. [DOI] [PubMed] [Google Scholar]
- Cambronero F, Puelles L (2000) Rostrocaudal nuclear relationships in the avian medulla oblongata: a fate map with quail chick chimeras. J Comp Neurol 427, 522–545. [DOI] [PubMed] [Google Scholar]
- Cancelliere NM, Black EA, Ferguson AV (2015) Neurohumoral integration of cardiovascular function by the lamina terminalis. Curr Hypertens Rep 17, 93. [DOI] [PubMed] [Google Scholar]
- Candiani S, Pestarino M (1998) Evidence for the presence of the tissue‐specific transcription factor Pit‐1 in lancelet larvae. J Comp Neurol 400, 310–316. [DOI] [PubMed] [Google Scholar]
- Castaneyra‐Perdomo A, Meyer G, Heylings DJ (1992) Early development of the human area postrema and subfornical organ. Anat Rec 232, 612–619. [DOI] [PubMed] [Google Scholar]
- Castle‐Miller J, Bates DO, Tortonese DJ (2017) Mechanisms regulating angiogenesis underlie seasonal control of pituitary function. Proc Natl Acad Sci U S A 114, E2514–E2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha KB, Douglas KR, Potok MA, et al. (2004) WNT5A signaling affects pituitary gland shape. Mech Dev 121, 183–194. [DOI] [PubMed] [Google Scholar]
- Chatterjee M, Guo Q, Weber S, et al. (2014) Pax6 regulates the formation of the habenular nuclei by controlling the temporospatial expression of Shh in the diencephalon in vertebrates. BMC Biol 12, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang C, Litingtung Y, Lee E, et al. (1996) Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature 383, 407–413. [DOI] [PubMed] [Google Scholar]
- Clarke IJ (2015) Hypothalamus as an endocrine organ. Compr Physiol 5, 217–253. [DOI] [PubMed] [Google Scholar]
- Cobos I, Shimamura K, Rubenstein JL, et al. (2001) Fate map of the avian anterior forebrain at the four‐somite stage, based on the analysis of quail‐chick chimeras. Dev Biol 239, 46–67. [DOI] [PubMed] [Google Scholar]
- Coon SL, Klein DC (2006) Evolution of arylalkylamine N‐acetyltransferase: emergence and divergence. Mol Cell Endocrinol 252, 2–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin JG, Rutlin M, Gaiano N, et al. (2003) Combinatorial function of the homeodomain proteins Nkx2.1 and Gsh2 in ventral telencephalic patterning. Development 130, 4895–4906. [DOI] [PubMed] [Google Scholar]
- Couly GF, le Douarin NM (1985) Mapping of the early neural primordium in quail‐chick chimeras. I. Developmental relationships between placodes, facial ectoderm, and prosencephalon. Dev Biol 110, 422–439. [DOI] [PubMed] [Google Scholar]
- Couly GF, le Douarin NM (1987) Mapping of the early neural primordium in quail‐chick chimeras. II. The prosencephalic neural plate and neural folds: implications for the genesis of cephalic human congenital abnormalities. Dev Biol 120, 198–214. [DOI] [PubMed] [Google Scholar]
- Dale JK, Vesque C, Lints TJ, et al. (1997) Cooperation of BMP7 and SHH in the induction of forebrain ventral midline cells by prechordal mesoderm. Cell 90, 257–269. [DOI] [PubMed] [Google Scholar]
- Dattani MT, Martinez‐Barbera JP, Thomas PQ, et al. (1998) Mutations in the homeobox gene HESX1/Hesx1 associated with septo‐optic dysplasia in human and mouse. Nat Genet 19, 125–133. [DOI] [PubMed] [Google Scholar]
- Davis SW, Ellsworth BS, Perez Millan MI, et al. (2013) Pituitary gland development and disease: from stem cell to hormone production. Curr Top Dev Biol 106, 1–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagleson GW, Harris WA (1990) Mapping of the presumptive brain regions in the neural plate of Xenopus laevis . J Neurobiol 21, 427–440. [DOI] [PubMed] [Google Scholar]
- Ekström P, Meissl H (2003) Evolution of photosensory pineal organs in new light: the fate of neuroendocrine photoreceptors. Philos Trans R Soc Lond B Biol Sci 358, 1679–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson J, Norlin S, Jessell TM, et al. (1998) Integrated FGF and BMP signaling controls the progression of progenitor cell differentiation and the emergence of pattern in the embryonic anterior pituitary. Development 125, 1005–1015. [DOI] [PubMed] [Google Scholar]
- Estivill‐Torrus G, Vitalis T, Fernandez‐Llebrez P, et al. (2001) The transcription factor Pax6 is required for development of the diencephalic dorsal midline secretory radial glia that form the subcommissural organ. Mech Dev 109, 215–224. [DOI] [PubMed] [Google Scholar]
- Falcon J, Coon SL, Besseau L, et al. (2014) Drastic neofunctionalization associated with evolution of the timezyme AANAT 500 Mya. Proc Natl Acad Sci U S A 111, 314–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez‐Llebrez P, Grondona JM, Perez J, et al. (2004) Msx1‐deficient mice fail to form prosomere 1 derivatives, subcommissural organ, and posterior commissure and develop hydrocephalus. J Neuropathol Exp Neurol 63, 574–586. [DOI] [PubMed] [Google Scholar]
- Filippi A, Durr K, Ryu S, et al. (2007) Expression and function of nr4a2, lmx1b, and pitx3 in zebrafish dopaminergic and noradrenergic neuronal development. BMC Dev Biol 7, 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotaki V, Price DJ, Mason JO (2008) Newly identified patterns of Pax2 expression in the developing mouse forebrain. BMC Dev Biol 8, 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foucher I, Mione M, Simeone A, et al. (2006) Differentiation of cerebellar cell identities in absence of Fgf signalling in zebrafish Otx morphants. Development 133, 1891–1900. [DOI] [PubMed] [Google Scholar]
- Gage PJ, Camper SA (1997) Pituitary homeobox 2, a novel member of the bicoid‐related family of homeobox genes, is a potential regulator of anterior structure formation. Hum Mol Genet 6, 457–464. [DOI] [PubMed] [Google Scholar]
- Gamse JT, Shen YC, Thisse C, et al. (2002) Otx5 regulates genes that show circadian expression in the zebrafish pineal complex. Nat Genet 30, 117–121. [DOI] [PubMed] [Google Scholar]
- Gaston‐Massuet C, Andoniadou CL, Signore M, et al. (2008) Genetic interaction between the homeobox transcription factors HESX1 and SIX3 is required for normal pituitary development. Dev Biol 324, 322–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng X, Speirs C, Lagutin O, et al. (2008) Haploinsufficiency of Six3 fails to activate Sonic hedgehog expression in the ventral forebrain and causes holoprosencephaly. Dev Cell 15, 236–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgala PA, Carr CB, Price DJ (2011) The role of Pax6 in forebrain development. Dev Neurobiol 71, 690–709. [DOI] [PubMed] [Google Scholar]
- Geris KL, D'Hondt E, Kuhn ER, et al. (1999) Thyrotropin‐releasing hormone concentrations in different regions of the chicken brain and pituitary: an ontogenetic study. Brain Res 818, 260–266. [DOI] [PubMed] [Google Scholar]
- Glardon S, Holland LZ, Gehring WJ, et al. (1998) Isolation and developmental expression of the amphioxus Pax‐6 gene (AmphiPax‐6): insights into eye and photoreceptor evolution. Development 125, 2701–2710. [DOI] [PubMed] [Google Scholar]
- Glasgow E, Karavanov AA, Dawid IB (1997) Neuronal and neuroendocrine expression of lim3, a LIM class homeobox gene, is altered in mutant zebrafish with axial signaling defects. Dev Biol 192, 405–419. [DOI] [PubMed] [Google Scholar]
- Gorbman A, Nozaki M, Kubokawa K (1999) A brain‐Hatschek's pit connection in amphioxus. Gen Comp Endocrinol 113, 251–254. [DOI] [PubMed] [Google Scholar]
- Gorbman A, Whiteley A, Kavanaugh S (2003) Pheromonal stimulation of spawning release of gametes by gonadotropin releasing hormone in the chiton, Mopalia sp. Gen Comp Endocrinol 131, 62–65. [DOI] [PubMed] [Google Scholar]
- Goren O, Adorjan I, Kalman M (2006) Heterogeneous occurrence of aquaporin‐4 in the ependyma and in the circumventricular organs in rat and chicken. Anat Embryol (Berl) 211, 155–172. [DOI] [PubMed] [Google Scholar]
- Grondona JM, Hoyo‐Becerra C, Visser R, et al. (2012) The subcommissural organ and the development of the posterior commissure. Int Rev Cell Mol Biol 296, 63–137. [DOI] [PubMed] [Google Scholar]
- Guerra MM, Gonzalez C, Caprile T, et al. (2015) Understanding how the subcommissural organ and other periventricular secretory structures contribute via the cerebrospinal fluid to neurogenesis. Front Cell Neurosci 9, 480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunhaga L, Marklund M, Sjödal M, et al. (2003) Specification of dorsal telencephalic character by sequential Wnt and FGF signaling. Nat Neurosci 6, 701–707. [DOI] [PubMed] [Google Scholar]
- Heisenberg CP, Houart C, Take‐Uchi M, et al. (2001) A mutation in the Gsk3‐binding domain of zebrafish Masterblind/Axin1 leads to a fate transformation of telencephalon and eyes to diencephalon. Genes Dev 15, 1427–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindmarch CC, Ferguson AV (2016) Physiological roles for the subfornical organ: a dynamic transcriptome shaped by autonomic state. J Physiol 594, 1581–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirth F (2010) On the origin and evolution of the tripartite brain. Brain Behav Evol 76, 3–10. [DOI] [PubMed] [Google Scholar]
- Holzschuh J, Barrallo‐Gimeno A, Ettl AK, et al. (2003) Noradrenergic neurons in the zebrafish hindbrain are induced by retinoic acid and require tfap2a for expression of the neurotransmitter phenotype. Development 130, 5741–5754. [DOI] [PubMed] [Google Scholar]
- Huh MS, Todd MA, Picketts DJ (2009) SCO‐ping out the mechanisms underlying the etiology of hydrocephalus. Physiology (Bethesda) 24, 117–126. [DOI] [PubMed] [Google Scholar]
- Jackman WR, Langeland JA, Kimmel CB (2000) islet reveals segmentation in the Amphioxus hindbrain homolog. Dev Biol 220, 16–26. [DOI] [PubMed] [Google Scholar]
- Jayakody SA, Andoniadou CL, Gaston‐Massuet C, et al. (2012) SOX2 regulates the hypothalamic‐pituitary axis at multiple levels. J Clin Invest 122, 3635–3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong JY, Kwon HB, Ahn JC, et al. (2008a) Functional and developmental analysis of the blood‐brain barrier in zebrafish. Brain Res Bull 75, 619–628. [DOI] [PubMed] [Google Scholar]
- Jeong Y, Leskow FC, El‐Jaick K, et al. (2008b) Regulation of a remote Shh forebrain enhancer by the Six3 homeoprotein. Nat Genet 40, 1348–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joly JS, Osorio J, Alunni A, et al. (2007) Windows of the brain: towards a developmental biology of circumventricular and other neurohemal organs. Semin Cell Dev Biol 18, 512–524. [DOI] [PubMed] [Google Scholar]
- Kaur C, Ling EA (2017) The circumventricular organs. Histol Histopathol 32, 879–892. [DOI] [PubMed] [Google Scholar]
- Kiecker C, Lumsden A (2012) The role of organizers in patterning the nervous system. Annu Rev Neurosci 35, 347–367. [DOI] [PubMed] [Google Scholar]
- Kiecker C, Graham A, Logan M (2016) Differential cellular responses to hedgehog signalling in vertebrates‐what is the role of competence? J Dev Biol 4, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kioussi C, O'Connell S, St‐Onge L, et al. (1999) Pax6 is essential for establishing ventral‐dorsal cell boundaries in pituitary gland development. Proc Natl Acad Sci U S A 96, 14378–14382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuenzel WJ, van Tienhoven A (1982) Nomenclature and location of avian hypothalamic nuclei and associated circumventricular organs. J Comp Neurol 206, 293–313. [DOI] [PubMed] [Google Scholar]
- Lagutin OV, Zhu CC, Kobayashi D, et al. (2003) Six3 repression of Wnt signaling in the anterior neuroectoderm is essential for vertebrate forebrain development. Genes Dev 17, 368–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlet F, Mullier A, Bouret SG, et al. (2013) Tanycyte‐like cells form a blood‐cerebrospinal fluid barrier in the circumventricular organs of the mouse brain. J Comp Neurol 521, 3389–3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen KB, Lutterodt MC, Møllgård K, et al. (2010) Expression of the homeobox genes OTX2 and OTX1 in the early developing human brain. J Histochem Cytochem 58, 669–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Tan J, Morris MB, et al. (2012) Congenital hydrocephalus and abnormal subcommissural organ development in Sox3 transgenic mice. PLoS One 7, e29041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann W, Wagner U, Naumann WW (2001) Multiple forms of glycoproteins in the secretory product of the bovine subcommissural organ – an ancient glial structure. Acta Histochem 103, 99–112. [DOI] [PubMed] [Google Scholar]
- Lennon VA, Kryzer TJ, Pittock SJ, et al. (2005) IgG marker of optic‐spinal multiple sclerosis binds to the aquaporin‐4 water channel. J Exp Med 202, 473–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JE, Ebling FJ (2017) Tanycytes as regulators of seasonal cycles in neuroendocrine function. Front Neurol 8, 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Crenshaw EB 3rd, Rawson EJ, et al. (1990) Dwarf locus mutants lacking three pituitary cell types result from mutations in the POU‐domain gene pit‐1 . Nature 347, 528–533. [DOI] [PubMed] [Google Scholar]
- Liebner S, Plate KH (2010) Differentiation of the brain vasculature: the answer came blowing by the Wnt. J Angiogenes Res 2, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Pogoda HM, Pearson CA, et al. (2013) Direct and indirect roles of Fgf3 and Fgf10 in innervation and vascularisation of the vertebrate hypothalamic neurohypophysis. Development 140, 1111–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macchi MM, Bruce JN (2004) Human pineal physiology and functional significance of melatonin. Front Neuroendocrinol 25, 177–195. [DOI] [PubMed] [Google Scholar]
- Maeda R, Shimo T, Nakane Y, et al. (2015) Ontogeny of the saccus vasculosus, a seasonal sensor in fish. Endocrinology 156, 4238–4243. [DOI] [PubMed] [Google Scholar]
- Manchester LC, Coto‐Montes A, Boga JA, et al. (2015) Melatonin: an ancient molecule that makes oxygen metabolically tolerable. J Pineal Res 59, 403–419. [DOI] [PubMed] [Google Scholar]
- Mancuso MR, Kuhnert F, Kuo CJ (2008) Developmental angiogenesis of the central nervous system. Lymphat Res Biol 6, 173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mano H, Fukada Y (2007) A median third eye: pineal gland retraces evolution of vertebrate photoreceptive organs. Photochem Photobiol 83, 11–18. [DOI] [PubMed] [Google Scholar]
- Maolood N, Meister B (2009) Protein components of the blood‐brain barrier (BBB) in the brainstem area postrema‐nucleus tractus solitarius region. J Chem Neuroanat 37, 182–195. [DOI] [PubMed] [Google Scholar]
- Mark MH, Farmer PM (1984) The human subfornical organ: an anatomic and ultrastructural study. Ann Clin Lab Sci 14, 427–442. [PubMed] [Google Scholar]
- Masai I, Heisenberg CP, Barth KA, et al. (1997) floating head and masterblind regulate neuronal patterning in the roof of the forebrain. Neuron 18, 43–57. [DOI] [PubMed] [Google Scholar]
- Matiello M, Jacob A, Wingerchuk DM, et al. (2007) Neuromyelitis optica. Curr Opin Neurol 20, 255–260. [DOI] [PubMed] [Google Scholar]
- Matsuda T, Hiyama TY, Niimura F, et al. (2017) Distinct neural mechanisms for the control of thirst and salt appetite in the subfornical organ. Nat Neurosci 20, 230–241. [DOI] [PubMed] [Google Scholar]
- Matsuo I, Kuratani S, Kimura C, et al. (1995) Mouse Otx2 functions in the formation and patterning of rostral head. Genes Dev 9, 2646–2658. [DOI] [PubMed] [Google Scholar]
- McCabe MJ, Gaston‐Massuet C, Tziaferi V, et al. (2011) Novel FGF8 mutations associated with recessive holoprosencephaly, craniofacial defects, and hypothalamo‐pituitary dysfunction. J Clin Endocrinol Metab 96, E1709–E1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinley MJ, Yao ST, Uschakov A, et al. (2015) The median preoptic nucleus: front and centre for the regulation of body fluid, sodium, temperature, sleep and cardiovascular homeostasis. Acta Physiol (Oxf) 214, 8–32. [DOI] [PubMed] [Google Scholar]
- Medeiros N, Dai L, Ferguson AV (2012) Glucose‐responsive neurons in the subfornical organ of the rat – a novel site for direct CNS monitoring of circulating glucose. Neuroscience 201, 157–165. [DOI] [PubMed] [Google Scholar]
- Meiniel A (2007) The secretory ependymal cells of the subcommissural organ: which role in hydrocephalus? Int J Biochem Cell Biol 39, 463–468. [DOI] [PubMed] [Google Scholar]
- Meiniel O, Meiniel R, Lalloue F, et al. (2008) The lengthening of a giant protein: when, how, and why? J Mol Evol 66, 1–10. [DOI] [PubMed] [Google Scholar]
- Mitchell TN, Free SL, Williamson KA, et al. (2003) Polymicrogyria and absence of pineal gland due to PAX6 mutation. Ann Neurol 53, 658–663. [DOI] [PubMed] [Google Scholar]
- Møller M, Baeres FM (2002) The anatomy and innervation of the mammalian pineal gland. Cell Tissue Res 309, 139–150. [DOI] [PubMed] [Google Scholar]
- de Moraes DC, Vaisman M, Conceicao FL, et al. (2012) Pituitary development: a complex, temporal regulated process dependent on specific transcriptional factors. J Endocrinol 215, 239–245. [DOI] [PubMed] [Google Scholar]
- Morita S, Furube E, Mannari T, et al. (2016) Heterogeneous vascular permeability and alternative diffusion barrier in sensory circumventricular organs of adult mouse brain. Cell Tissue Res 363, 497–511. [DOI] [PubMed] [Google Scholar]
- Mortensen AH, Schade V, Lamonerie T, et al. (2015) Deletion of OTX2 in neural ectoderm delays anterior pituitary development. Hum Mol Genet 24, 939–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakane Y, Ikegami K, Iigo M, et al. (2013) The saccus vasculosus of fish is a sensor of seasonal changes in day length. Nat Commun 4, 2108. [DOI] [PubMed] [Google Scholar]
- Nico B, Frigeri A, Nicchia GP, et al. (2001) Role of aquaporin‐4 water channel in the development and integrity of the blood‐brain barrier. J Cell Sci 114, 1297–1307. [DOI] [PubMed] [Google Scholar]
- Nishida A, Furukawa A, Koike C, et al. (2003) Otx2 homeobox gene controls retinal photoreceptor cell fate and pineal gland development. Nat Neurosci 6, 1255–1263. [DOI] [PubMed] [Google Scholar]
- Norsted E, Gomuc B, Meister B (2008) Protein components of the blood‐brain barrier (BBB) in the mediobasal hypothalamus. J Chem Neuroanat 36, 107–121. [DOI] [PubMed] [Google Scholar]
- Ohkubo Y, Chiang C, Rubenstein JL (2002) Coordinate regulation and synergistic actions of BMP4, SHH and FGF8 in the rostral prosencephalon regulate morphogenesis of the telencephalic and optic vesicles. Neuroscience 111, 1–17. [DOI] [PubMed] [Google Scholar]
- Oksche A (1965) Survey of the development and comparative morphology of the pineal organ. Prog Brain Res 10, 3–29. [DOI] [PubMed] [Google Scholar]
- Ortloff AR, Vio K, Guerra M, et al. (2013) Role of the subcommissural organ in the pathogenesis of congenital hydrocephalus in the HTx rat. Cell Tissue Res 352, 707–725. [DOI] [PubMed] [Google Scholar]
- Pavlicek J, Sauzet S, Besseau L, et al. (2010) Evolution of AANAT: expansion of the gene family in the cephalochordate amphioxus. BMC Evol Biol 10, 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirson SN, Halford S, Foster RG (2009) The evolution of irradiance detection: melanopsin and the non‐visual opsins. Philos Trans R Soc Lond B Biol Sci 364, 2849–2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pera EM, Kessel M (1997) Patterning of the chick forebrain anlage by the prechordal plate. Development 124, 4153–4162. [DOI] [PubMed] [Google Scholar]
- Potok MA, Cha KB, Hunt A, et al. (2008) WNT signaling affects gene expression in the ventral diencephalon and pituitary gland growth. Dev Dyn 237, 1006–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, Hoyda TD, Ferguson AV (2008) The area postrema: a brain monitor and integrator of systemic autonomic state. Neuroscientist 14, 182–194. [DOI] [PubMed] [Google Scholar]
- Prince KL, Walvoord EC, Rhodes SJ (2011) The role of homeodomain transcription factors in heritable pituitary disease. Nat Rev Endocrinol 7, 727–737. [DOI] [PubMed] [Google Scholar]
- Puelles L, Rubenstein JL (2015) A new scenario of hypothalamic organization: rationale of new hypotheses introduced in the updated prosomeric model. Front Neuroanat 9, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph CL (1975) The pineal gland and geographical distribution of animals. Int J Biometeorol 19, 289–303. [DOI] [PubMed] [Google Scholar]
- Rath MF, Bailey MJ, Kim JS, et al. (2009) Developmental and diurnal dynamics of Pax4 expression in the mammalian pineal gland: nocturnal down‐regulation is mediated by adrenergic‐cyclic adenosine 3′,5′‐monophosphate signaling. Endocrinology 150, 803–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rath MF, Rohde K, Klein DC, et al. (2013) Homeobox genes in the rodent pineal gland: roles in development and phenotype maintenance. Neurochem Res 38, 1100–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzoti K, Lovell‐Badge R (2017) Pivotal role of median eminence tanycytes for hypothalamic function and neurogenesis. Mol Cell Endocrinol 445, 7–13. [DOI] [PubMed] [Google Scholar]
- Robins SC, Stewart I, McNay DE, et al. (2013) α‐Tanycytes of the adult hypothalamic third ventricle include distinct populations of FGF‐responsive neural progenitors. Nat Commun 4, 2049. [DOI] [PubMed] [Google Scholar]
- Roca C, Adams RH (2007) Regulation of vascular morphogenesis by Notch signaling. Genes Dev 21, 2511–2524. [DOI] [PubMed] [Google Scholar]
- Sánchez‐Arrones L, Ferrán JL, Hidalgo‐Sanchez M, et al. (2015) Origin and early development of the chicken adenohypophysis. Front Neuroanat 9, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlosser G (2006) Induction and specification of cranial placodes. Dev Biol 294, 303–351. [DOI] [PubMed] [Google Scholar]
- Schoebitz K, Garrido O, Heinrichs M, et al. (1986) Ontogenetical development of the chick and duck subcommissural organ. An immunocytochemical study. Histochemistry 84, 31–40. [DOI] [PubMed] [Google Scholar]
- Schoenmakers N, Alatzoglou KS, Chatterjee VK, et al. (2015) Recent advances in central congenital hypothyroidism. J Endocrinol 227, R51–R71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng HZ, Zhadanov AB, Mosinger B Jr, et al. (1996) Specification of pituitary cell lineages by the LIM homeobox gene Lhx3 . Science 272, 1004–1007. [DOI] [PubMed] [Google Scholar]
- Sheng HZ, Moriyama K, Yamashita T, et al. (1997) Multistep control of pituitary organogenesis. Science 278, 1809–1812. [DOI] [PubMed] [Google Scholar]
- Shimogori T, Banuchi V, Ng HY, et al. (2004) Embryonic signaling centers expressing BMP, WNT and FGF proteins interact to pattern the cerebral cortex. Development 131, 5639–5647. [DOI] [PubMed] [Google Scholar]
- Shirasaki R, Pfaff SL (2002) Transcriptional codes and the control of neuronal identity. Annu Rev Neurosci 25, 251–281. [DOI] [PubMed] [Google Scholar]
- Sjödal M, Gunhaga L (2008) Expression patterns of Shh, Ptc2, Raldh3, Pitx2, Isl1, Lim3 and Pax6 in the developing chick hypophyseal placode and Rathke's pouch. Gene Expr Patterns 8, 481–485. [DOI] [PubMed] [Google Scholar]
- Snelson CD, Santhakumar K, Halpern ME, et al. (2008) Tbx2b is required for the development of the parapineal organ. Development 135, 1693–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobrier ML, Maghnie M, Vie‐Luton MP, et al. (2006) Novel HESX1 mutations associated with a life‐threatening neonatal phenotype, pituitary aplasia, but normally located posterior pituitary and no optic nerve abnormalities. J Clin Endocrinol Metab 91, 4528–4536. [DOI] [PubMed] [Google Scholar]
- Staudt N, Houart C (2007) The prethalamus is established during gastrulation and influences diencephalic regionalization. PLoS Biol 5, e69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm EE, Garel S, Borello U, et al. (2006) Dose‐dependent functions of Fgf8 in regulating telencephalic patterning centers. Development 133, 1831–1844. [DOI] [PubMed] [Google Scholar]
- Streit A (2007) The preplacodal region: an ectodermal domain with multipotential progenitors that contribute to sense organs and cranial sensory ganglia. Int J Dev Biol 51, 447–461. [DOI] [PubMed] [Google Scholar]
- Suarez R, Gobius I, Richards LJ (2014) Evolution and development of interhemispheric connections in the vertebrate forebrain. Front Hum Neurosci 8, 497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sueiro C, Carrera I, Ferreiro S, et al. (2007) New insights on Saccus vasculosus evolution: a developmental and immunohistochemical study in elasmobranchs. Brain Behav Evol 70, 187–204. [DOI] [PubMed] [Google Scholar]
- Szarek E, Cheah PS, Schwartz J, et al. (2010) Molecular genetics of the developing neuroendocrine hypothalamus. Mol Cell Endocrinol 323, 115–123. [DOI] [PubMed] [Google Scholar]
- Szucsik JC, Witte DP, Li H, et al. (1997) Altered forebrain and hindbrain development in mice mutant for the Gsh‐2 homeobox gene. Dev Biol 191, 230–242. [DOI] [PubMed] [Google Scholar]
- Takuma N, Sheng HZ, Furuta Y, et al. (1998) Formation of Rathke's pouch requires dual induction from the diencephalon. Development 125, 4835–4840. [DOI] [PubMed] [Google Scholar]
- Tanaka J, Kariya K, Miyakubo H, et al. (2002a) Attenuated drinking response induced by angiotensinergic activation of subfornical organ projections to the paraventricular nucleus in estrogen‐treated rats. Neurosci Lett 324, 242–246. [DOI] [PubMed] [Google Scholar]
- Tanaka J, Mashiko N, Kawakami A, et al. (2002b) GABAergic systems in the nucleus tractus solitarius regulate noradrenaline release in the subfornical organ area in the rat. Auton Neurosci 100, 58–65. [DOI] [PubMed] [Google Scholar]
- Tessmar‐Raible K, Raible F, Christodoulou F, et al. (2007) Conserved sensory‐neurosecretory cell types in annelid and fish forebrain: insights into hypothalamus evolution. Cell 129, 1389–1400. [DOI] [PubMed] [Google Scholar]
- Torres M, Gomez‐Pardo E, Gruss P (1996) Pax2 contributes to inner ear patterning and optic nerve trajectory. Development 122, 3381–3391. [DOI] [PubMed] [Google Scholar]
- Tosches MA, Bucher D, Vopalensky P, et al. (2014) Melatonin signaling controls circadian swimming behavior in marine zooplankton. Cell 159, 46–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treier M, Gleiberman AS, O'Connell SM, et al. (1998) Multistep signaling requirements for pituitary organogenesis in vivo. Genes Dev 12, 1691–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treier M, O'Connell S, Gleiberman A, et al. (2001) Hedgehog signaling is required for pituitary gland development. Development 128, 377–386. [DOI] [PubMed] [Google Scholar]
- Tremblay JJ, Lanctot C, Drouin J (1998) The pan‐pituitary activator of transcription, Ptx1 (pituitary homeobox 1), acts in synergy with SF‐1 and Pit1 and is an upstream regulator of the Lim‐homeodomain gene Lim3/Lhx3 . Mol Endocrinol 12, 428–441. [DOI] [PubMed] [Google Scholar]
- Trowe MO, Zhao L, Weiss AC, et al. (2013) Inhibition of Sox2‐dependent activation of Shh in the ventral diencephalon by Tbx3 is required for formation of the neurohypophysis. Development 140, 2299–2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuneki K (1986) A survey of occurrence of about seventeen circumventricular organs in brains of various vertebrates with special reference to lower groups. J Hirnforsch 27, 441–470. [PubMed] [Google Scholar]
- Vetting MW, Lp SDC, Yu M, et al. (2005) Structure and functions of the GNAT superfamily of acetyltransferases. Arch Biochem Biophys 433, 212–226. [DOI] [PubMed] [Google Scholar]
- Vignali R, Colombetti S, Lupo G, et al. (2000) Xotx5b, a new member of the Otx gene family, may be involved in anterior and eye development in Xenopus laevis . Mech Dev 96, 3–13. [DOI] [PubMed] [Google Scholar]
- Vollrath L (1979) Comparative morphology of the vertebrate pineal complex. Prog Brain Res 52, 25–38. [DOI] [PubMed] [Google Scholar]
- Wang Y, Martin JF, Bai CB (2010) Direct and indirect requirements of Shh/Gli signaling in early pituitary development. Dev Biol 348, 199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm I, Nyul‐Toth A, Suciu M, et al. (2016) Heterogeneity of the blood‐brain barrier. Tissue Barriers 4, e1143544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MJ, Lowrie MB, Bennett JP, et al. (2005) Cadherin‐10 is a novel blood‐brain barrier adhesion molecule in human and mouse. Brain Res 1058, 62–72. [DOI] [PubMed] [Google Scholar]
- Wilson SW, Houart C (2004) Early steps in the development of the forebrain. Dev Cell 6, 167–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson AJ, Carati CJ, Gannon BJ, et al. (2010) Aquaporin‐1 in blood vessels of rat circumventricular organs. Cell Tissue Res 340, 159–168. [DOI] [PubMed] [Google Scholar]
- Wolburg H, Paulus W (2010) Choroid plexus: biology and pathology. Acta Neuropathol 119, 75–88. [DOI] [PubMed] [Google Scholar]
- Woo K, Fraser SE (1995) Order and coherence in the fate map of the zebrafish nervous system. Development 121, 2595–2609. [DOI] [PubMed] [Google Scholar]
- Wu W, Cogan JD, Pfaffle RW, et al. (1998) Mutations in PROP1 cause familial combined pituitary hormone deficiency. Nat Genet 18, 147–149. [DOI] [PubMed] [Google Scholar]
- Xu Y, Tamamaki N, Noda T, et al. (2005) Neurogenesis in the ependymal layer of the adult rat 3rd ventricle. Exp Neurol 192, 251–264. [DOI] [PubMed] [Google Scholar]
- Yamazaki F, Møller M, Fu C, et al. (2015) The Lhx9 homeobox gene controls pineal gland development and prevents postnatal hydrocephalus. Brain Struct Funct 220, 1497–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi CX, van der Vliet J, Dai J, et al. (2006) Ventromedial arcuate nucleus communicates peripheral metabolic information to the suprachiasmatic nucleus. Endocrinology 147, 283–294. [DOI] [PubMed] [Google Scholar]
- Zandawala M, Tian S, Elphick MR (2017) The evolution and nomenclature of GnRH‐type and corazonin‐type neuropeptide signaling systems. Gen Comp Endocrinol doi: 10.1016/j.ygcen.2017.06.007 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Zeller K, Vogel J, Kuschinsky W (1996) Postnatal distribution of Glut1 glucose transporter and relative capillary density in blood‐brain barrier structures and circumventricular organs during development. Brain Res Dev Brain Res 91, 200–208. [DOI] [PubMed] [Google Scholar]
- Zhang JH, Liu JL, Wu YJ, et al. (2006) LIM homeodomain proteins Islet‐1 and Lim‐3 expressions in the developing pineal gland of chick embryo by immunohistochemistry. J Pineal Res 41, 247–254. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Mailloux CM, Hermesz E, et al. (2010) A role of the LIM‐homeobox gene Lhx2 in the regulation of pituitary development. Dev Biol 337, 313–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Zevallos SE, Rizzoti K, et al. (2012) Disruption of SoxB1‐dependent Sonic hedgehog expression in the hypothalamus causes septo‐optic dysplasia. Dev Cell 22, 585–596. [DOI] [PMC free article] [PubMed] [Google Scholar]


