Abstract
Its high metabolic rate and high polyunsaturated fatty acid content make the brain very sensitive to oxidative damage. In the brain, neuronal metabolism occurs at a very high rate and generates considerable amounts of reactive oxygen species and free radicals, which accumulate inside neurons, leading to altered cellular homeostasis and integrity and eventually irreversible damage and cell death. A misbalance in redox metabolism and the subsequent neurodegeneration increase throughout the course of normal aging, leading to several age‐related changes in learning and memory as well as motor functions. The neuroprotective function of antioxidants is crucial to maintain good brain homeostasis and adequate neuronal functions. Vitamins E and C are two important antioxidants that are taken up by brain cells via the specific carriers αTTP and SVCT2, respectively. The aim of this study was to use immunohistochemistry to determine the distribution pattern of these vitamin transporters in the brain in a mouse model that shows fewer signs of brain aging and a higher resistance to oxidative damage. Both carriers were distributed widely throughout the entire brain in a pattern that remained similar in 4‐, 12‐, 18‐ and 24‐month‐old mice. In general, αTTP and SVCT2 were located in the same regions, but they seemed to have complementary distribution patterns. Double‐labeled cell bodies were detected only in the inferior colliculus, entorhinal cortex, dorsal subiculum, and several cortical areas. In addition, the presence of αTTP and SVCT2 in neurons was analyzed using double immunohistochemistry for NeuN and the results showed that αTTP but not SVCT2 was present in Bergmann's glia. The presence of these transporters in brain regions implicated in learning, memory and motor control provides an anatomical basis that may explain the higher resistance of this animal model to brain oxidative stress, which is associated with better motor performance and learning abilities in old age.
Keywords: aging, antioxidants, oxidative damage, transporters, vitamin C, vitamin E
Introduction
Ageing is a biologically complex process that involves many changes in live organisms. One feature that is phylogenetically conserved in aging is the induction of stress response pathways (Haigis & Yankner, 2010). Some of the most important alterations occur in learning, memory and motor performance (Yeoman et al. 2012). The phenotypic changes that occur during aging reflect morphological and physiological changes in the brain. For example, aging has been associated with an increase in neuroinflammatory processes and the generation of reactive oxygen species (ROS) (Harman, 1972; Bishop et al. 2010; Haigis & Yankner, 2010; Venkateshappa et al. 2012; Yeoman et al. 2012). The natural activity of the neuroprotective system that combats the effects of ROS declines during aging, leading to an imbalance between damage and repair. Consequently, the levels of oxidative stress increase, and the excessive accumulation of ROS in combination with a reduction in mitochondrial function and respiratory metabolism result in neuronal damage. In addition, changes in synaptic physiology may contribute to alterations in neuronal connectivity and higher order integration (Bishop et al. 2010).
The preservation of good antioxidant mechanisms during aging is crucial to avoid aged‐related oxidation. Two compounds with excellent antioxidant properties are vitamin E (VitE) and vitamin C (VitC). VitE is the most important lipid‐soluble antioxidant (for review, see Brigelius‐Flohe, 2006; Mocchegiani et al. 2014). VitE, and especially its α‐tocopherol isoform, is a ROS scavenger that functions in membranes (Gohil et al. 2004; Ulatowski et al. 2014), where it also exhibits many non‐antioxidant properties, including the inhibition of inflammatory signaling and the regulation of normal glial cell functions, adult hippocampal neurogenesis (Cecchini et al. 2003) and genetic functions (Brigelius‐Flohe, 2006; Mocchegiani et al. 2014; Selvaraju et al. 2014). Of all of the VitE isomers, organisms preferentially retain RRR‐α‐tocopherol (Brigelius‐Flohe, 2006) because it is selectively recognized by the α‐tocopherol transfer protein (αTTP). VitE is taken up from food and stored in the liver, where αTTP selectively binds the RRR‐α isoform (probably internalizing it via an endocytic process, Qian et al. 2004) and subsequently incorporates it into plasma lipoproteins and distributes it to other tissues, including the brain (Spector & Johanson, 2007; Mocchegiani et al. 2014). VitE transfer to neurons by simple diffusion occurs at a relatively low rate (Marin et al. 2014) that does not allow enough nutrients to be delivered to brain cells (Spector & Johanson, 2007). The amount of VitE in the brain (and specifically α‐tocopherol) is highly regulated by an efficient homeostatic system that ensures that the correct levels of this neuroprotective nutrient are supplied to the brain by a carrier system (Martin et al. 1999; Manor & Morley, 2007; Spector & Johanson, 2007). The results of some studies have shown that the ability of the brain to uptake α‐tocopherol is limited (Martin et al. 1999; Baxter et al. 2012) because of the regulatory effects exerted by αTTP. Alterations in αTTP have been shown to cause locomotion disorders as well as anxiety and loss of memory functions with age (Gohil et al. 2004). In humans, a deficiency or mutation in this transporter can lead to a pathology known as AVED (ataxia associated with vitamin E deficiency, see (Copp et al. 1999; Gohil et al. 2004; Marin et al. 2014; Mocchegiani et al. 2014; Spector & Johanson, 2007)). All disorders associated with alterations in αTTP are ameliorated by treatment with high doses of VitE (Copp et al. 1999; Gohil et al. 2004; Brigelius‐Flohe, 2006; Spector & Johanson, 2007; Mocchegiani et al. 2014; Ulatowski et al. 2014). Surprisingly, despite the fact that a large number of studies have indicated that αTTP is important for the proper internalization of VitE into the central nervous system and the fact that this antioxidant is not homogeneously distributed in cerebral tissues (Martin et al. 1999), no previous studies have provided a detailed description of the distribution of αTTP in the mammalian brain.
VitC is a water‐soluble antioxidant, physiologically present as an ascorbate anion (Rice, 2000; Hansen et al. 2014; Harrison et al. 2014; Covenas et al. 2015). VitC is involved in numerous functions, including its role as a co‐enzyme in noradrenalin synthesis and an agent in glutamate‐ and dopamine‐mediated transmission (Rebec & Pierce, 1994; Coveñas et al. 2011; Covenas et al. 2015), liberation of gonadotropins (Figueroa‐Mendez & Rivas‐Arancibia, 2015) or neuronal maturation and myelin formation (Hansen et al. 2014). However, its best known function is its neuroprotective effect against the excessive accumulation of ROS (Rice, 2000; Harrison & May, 2009; Harrison et al. 2010, 2014). Because it operates as an electron donor, ascorbate can decrease excessive and neurotoxic levels of glutamate via a heteroexchange mechanism (Rice, 2000; Qiu et al. 2007). Similar to VitE, brain levels of VitC are homeostatically regulated and preferentially retained in the brain at the expense of its presence in other organs (Rice, 2000; Harrison & May, 2009; Harrison et al. 2010, 2014). The homeostatic regulation of VitC in the brain is considered a highly efficient process because it is very difficult to induce VitC deficiency in adult mammals even under conditions of dietary deficiency (for review see Spector & Johanson, 2007). The access of VitC to the brain parenchyma involves a two‐step mechanism. First, it travels from the blood to the cerebrospinal fluid (CSF), then it travels from the CSF into brain cells (Harrison & May, 2009). Ascorbate can enter into cells in one of two forms. In its oxidized form, dehydroascorbic acid, VitC can use the glucose transporters present on cell membranes, and this process does not consume energy. Alternatively, the reduced form of VitC uses the saturable and sodium‐dependent VitC transporter 2 (SVCT2), which requires a concentration gradient and energy consumption (Mun et al. 2006). This latter transporter is present in the choroid plexus epithelium and specialized tanycytes (Harrison & May, 2009). In the brain, SVCT2 is present on neurons but not on glial cells (Rice, 2000; Castro et al. 2001; Garcia Mde et al. 2005; Mun et al. 2006; Harrison & May, 2009; Harrison et al. 2010, 2014; Hansen et al. 2014), and neurons can therefore preferentially uptake ascorbate via active transport. As in the case for αTTP, SVCT2 is saturable by extracellular ascorbate, and this mode of entry of VitC into neurons is therefore limited (Qiu et al. 2007; Harrison & May, 2009). VitC can act synergistically with VitE because ascorbate can recycle the oxidated form of α‐tocopherol (α‐tocopheroxyl radical) back to α‐tocopherol (Rice, 2000; Harrison & May, 2009; Harrison et al. 2010).
The effect of VitC on brain functions has been studied using a variety of approaches. The distribution patterns of VitC and SVCT2 in the brain have been reported in several mammalian species (Rice, 2000; Castro et al. 2001; Garcia Mde et al. 2005; Mun et al. 2006; Harrison & May, 2009; Mangas et al. 2009; Harrison et al. 2010, 2014; Coveñas et al. 2011; Hansen et al. 2014; Covenas et al. 2015). Moreover, several animal models have been developed to study the effects of VitC deprivation or SVCT2 mutation (for review, see Harrison et al. 2014), and in some of these models, the combined effects of VitC and VitE deprivation have been explored. Studies performed in guinea pigs with moderate VitE deficiency showed that these animals were healthy despite the fact that α‐tocopherol levels were decreased in the brain and plasma. Interestingly, when VitC was removed from the diet of these animals, most of them developed progressive paralysis within 5–6 days and subsequently died within 24 h. Finally, administering ascorbate and VitE to aged mice improved their performance in memory tasks (Harrison & May, 2009).
VitE and VitC are transported into cells by separate carriers present throughout the BBB or choroid plexus, and the two vitamins are accumulated in brain cells via separate and specialized systems (Spector & Johanson, 2007). These two antioxidants perform many beneficial functions in the brain, and it is widely believed that they act synergistically. However, the antioxidant properties of these vitamins can change with aging, resulting in an imbalance in the brain redox state. Several studies have reported that deficient antioxidant activity leads to an increase in oxidative stress and cognitive decline (Hansen et al. 2014; Harrison et al. 2014; Mocchegiani et al. 2014). Other authors have proposed that the age‐dependent changes in vitamin levels reflects maturation rather than aging (Lykkesfeldt & Moos, 2005). Moreover, although the neuroprotective properties of VitE family members and VitC are clear, the numerous relevant studies available in the literature, most of which have focused on aging in humans, describe contradictory results (Hansen et al. 2014; Harrison et al. 2014; Mocchegiani et al. 2014). It has been suggested that the inconclusive nature of these studies results from difficulties in selecting proper controls, partially because of a number of allelic differences that can affect vitamin metabolism (Mocchegiani et al. 2014). Hence, for this study, we chose a genetically stable mouse strain in which we age‐matched the knockout mice.
In this study, we compared the distribution patterns of the vitamin transporters αTTP and SVCT2 in the brains of mice lacking DNA polymerase μ. This animal model is characterized by delayed brain aging, increased liver regenerative capacity, increased resistance to apoptosis, preserved motor function (Escudero et al. 2014) and improved learning capabilities and long‐term potentiation during aging (Lucas et al. 2009).
The goals of this study were the following: (i) to use immunohistochemistry to map in detail the distribution patterns of αTTP and SVCT2 during aging in the brains of these animals; (ii) to use double‐labeling methods to analyze the anatomical relationships between the two carriers; and (iii) to use double immunohistochemistry with neuronal nuclear antigen (NeuN), a marker of neurons, and glial fibrillary acid protein (GFAP), a marker of astrocytes, to determine whether each transporter is present in neurons or glial cells.
Methods
Sixty male mice were used in this study. They were separated into 12 groups (n = 5 in each group) by age and genotype, which was confirmed in each mouse using PCR. The following ages were used: 4, 12, 18 and 24 months old. The following genotypes were used: wild type (wt); polymerase μ (Pol μ−/−) knock‐out (KO), which resulted in animals that were highly resistant to aging processes, and heterozygote (+/−). The process used to generate Pol μ−/− mice and their wt counterpart has been previously described. The genetic background included mixed 129/SVxBALB/c and wt littermates as well as C57BL/6J and FVB/N mice (Lucas et al. 2009).
All animals were housed under standard conditions for humidity, light and temperature, and provided free access to food and tap water. The animals were handled according to Spanish laws concerning animal welfare (RD 233/88, MAPA). This study was approved by the Ethical Committee for Animal Research of Castilla‐La Mancha University (PR‐2016‐05‐13).
The mice were anesthetized using an intraperitoneal injection of ketamine (75 mg kg−1) and xylazine (10 mg kg−1), and then transcardially perfused with cold saline solution and 4% paraformaldehyde. The brains were dissected from the skull, post‐fixed in the same fixative for 24 h and then cut using a freezing microtome at 50 μm. Each brain was split in half and cut coronally (left hemisphere) and sagittally (right hemisphere). Every fifth section was immediately mounted on a gelatine‐coated slide for Nissl staining.
Other sections were processed for immunohistochemical (IHC) detection of vitamin transporters (SVCT2 and αTTP), GFAP (RRID: AB_10013382) or NeuN (RRID: AB_2651140) (single detection) or for double immunolabeling for SVCT2‐αTTP, SVCT2‐GFAP, αTTP‐GFAP, SVCT2‐NeuN or αTTP‐NeuN.
The free‐floating IHC protocol used during this study to perform single‐ and double‐labeling was similar to methods previously described by our group (Marcos et al. 2013; Cebada‐Sanchez et al. 2014). Single IHC reactions were performed after peroxidase activity was reduced using methanol and hydrogen peroxide (2 : 1). The sections were then extensively rinsed in Tris buffer saline (TBS) 0.1 m (pH 7.6). Blocking buffer containing 1% horse serum, 0.3% Triton X‐100 was used to dilute each of the antibodies used for IHC. The sections were then incubated for 5 min in blocking buffer plus 0.5% bovine serum albumin. They were then incubated overnight at 4 °C with primary antibodies that were diluted as follows: 1/100 for the vitamin transporters, 1/300 for NeuN, and 1/200 for GFAP (Supporting Information Table S1). After the sections were rinsed in TBS 0.1 m (pH 7.6) and blocking buffer, they were incubated at room temperature for 90 min with the appropriate biotinylated secondary antibodies (anti‐goat or anti‐rabbit), which were diluted 1/2000, and then with peroxidase‐conjugated streptavidin (diluted 1/2000) for 90 min. The color reactions were then developed using the 3,3′‐diaminobenzidine (DAB) method. The progress of reaction was monitored under a light microscope.
Two methods were used to perform the double‐immunolabeling experiments. For SVCT2/αTTP, because both primary antibodies were raised in the same species, we first performed IHC to detect SVCT2 (developed using DAB) and then performed the immunoreaction to detect αTTP on the same sections (developed using a previously described 4‐chloro‐1‐naphtol method, Marcos et al. 2013). This same methodology was used to double‐label cells for SVCT2/GFAP, αTTP/GFAP, SVCT2/NeuN and αTTP/NeuN. To confirm the results obtained using primary antibodies raised in different species, a double immunofluorescence protocol was performed. In these cases, the incubation with the primary antibodies was performed at 4 °C for 48 h. Secondary biotinylated antibodies and streptavidin coupled to Alexa 488 were used to detect the vitamin transporters, and donkey anti‐rabbit coupled to Alexa 568 secondary antibodies were used to detect GFAP or NeuN.
The primary goat anti‐αTTP antibody (αTTP (C‐14), RRID: AB_2651137; Santa Cruz Biotechnology, Inc.) used in this study is an affinity‐purified polyclonal antibody that was raised against a peptide that maps to the C‐terminus of human αTTP. The goat anti‐SVCT2 antibody (SVCT2 (S‐19), RRID: AB_661205; Santa Cruz Biotechnology, Inc.) is an affinity‐purified antibody that was raised against a peptide that maps near the N‐terminus of rat SVCT2. The usual controls were performed to assure the specificity of both primary antibodies. No labeling was observed when brain sections were incubated with pre‐immune serum. No immunoreactions were observed when anti‐rat or anti‐rabbit secondary antibodies were used. When the first antibody was preabsorbed, no immunostaining was detected (Supporting Information Fig. S1).
Sections were mounted on gelatin‐coated slides, dehydrated (for DAB‐reacted sections) and coverslipped using DPX. DAB/chloronaphthol‐reacted sections were coverslipped in a mixture of glycerol/phosphate buffer (3 : 1), and fluorescent sections were coverslipped with DABCO.
All results were analyzed using a Nikon Eclipse 80‐i microscope equipped with appropriate filters. Immunofluorescence was also examined using a LSM 710 Zeiss confocal microscope. ‘The Mouse brain in stereotaxic coordinates’ (Franklin & Paxinos, 2008) was used as a reference for mapping. Adobe photoshop software was used to adjust only the contrast and brightness of the pictures shown in this article.
Results
The distribution of immunoreactivity (IR) in both cell bodies and fibers was analyzed throughout whole mouse brains separately for the two vitamin transporters. A similar distribution pattern was observed between the vitamin transporters at each studied age and in each genotype, and no variation in mapping was noted between the groups.
αTTP is more widely distributed than SVCT2, and many fiber tracts were αTTP‐IR‐positive but not SVCT2‐IR‐positive. For both transporters, cortical areas displayed only pericellular immunoreactivity, whereas in other brain regions, immunolabeling was clearly observed in cell bodies.
αTTP
Distribution of αTTP
αTTP‐IR was distributed throughout the entire rostrocaudal length of the brain (Table 1). IR‐positive cell bodies were especially abundant in the core of the accumbens, the piriform cortex, the ventral pallidum (Fig. 1A and B), the lateral division of the bed nucleus of the stria terminalis, the globus pallidus, the entopeduncular nucleus, the reticular nucleus of the thalamus (Fig. 1B), the dorsal subiculum, the postsubiculum and parasubiculum, and the medial entorhinal cortex. In the brainstem, numerous positive cell bodies were observed in the red nucleus and paratrochlear nucleus, the external cortex and central nucleus of the inferior colliculus, the ventral and dorsal nuclei of the lateral lemniscus, the tegmental nucleus, the mesencephalic trigeminal nucleus, the reticulotegmental nucleus of the pons, the deep and intermediate gray layers of the superior colliculus, and the facial nucleus.
Table 1.
Results of immunostaining for both vitamin transporters classified by anatomical regions
| Cortical region | αTTP | SVCT2 |
|---|---|---|
| AuC | − | + |
| Cg | + | + |
| FrA | + | + |
| IC | − | + |
| MC | + | + |
| OC | + | + |
| Pir | + | + |
| PoC | − | + |
| PrL | − | + |
| RSC | + | + |
| SC | + | + |
| VC | + | + |
| Telencephalic regions | αTTP | SVCT2 |
|---|---|---|
| AcN | + | + |
| DTT | − | + |
| LSN | + | − |
| OB | + | + |
| st | + | − |
| STLD | + | − |
| Tu | − | + |
| VTT | − | + |
| Basal ganglia | αTTP | SVCT2 |
|---|---|---|
| CPu | − | + |
| GP | + | − |
| VP | + | − |
| En | + | − |
| Amygdaloid complex | αTTP | SVCT2 |
|---|---|---|
| AA | − | + |
| ACo | − | + |
| AHiAL | + | + |
| BL | + | + |
| BM | − | + |
| Ce | + | − |
| LAN | − | + |
| PMCo | − | + |
| Hippocampal formation | αTTP | SVCT2 |
|---|---|---|
| DS | + | + |
| EC | + | + |
| Hip | + | + |
| MF | + | − |
| PaS | + | − |
| Post | + | − |
| PrS | + | − |
| VS | + | + |
| Fx | + | − |
| Diencephalon | αTTP | SVCT2 |
|---|---|---|
| Thalamus | ||
| AVTN | − | + |
| AMTN | − | + |
| DLG | − | + |
| LDT | − | + |
| LPT | − | + |
| PG | + | − |
| Po | − | + |
| Rt | + | − |
| Hypothalamus | ||
| StHy | + | − |
| MPA | + | − |
| MPO | + | − |
| VLPO | + | − |
| Subthalamus | ||
| ZI | + | + |
| Brainstem | αTTP | SVCT2 |
|---|---|---|
| Midbrain | ||
| 3n | + | − |
| BIC | + | + |
| CIC | + | + |
| cp | + | − |
| DLL | + | − |
| DpG | + | + |
| ECIC | + | + |
| InG | + | − |
| InWH | − | + |
| IPN | − | + |
| LL | + | − |
| Me5 | + | − |
| Mo5 | + | − |
| NST | + | − |
| Pa4 | + | − |
| PAG | − | + |
| R | + | − |
| Sag | + | − |
| SN | + | + |
| Pons | ||
| 7N | + | − |
| 7n | + | − |
| DC | + | − |
| TZ | − | + |
| PnC | − | + |
| RtTg | + | − |
| tz | + | − |
| VLL | + | + |
| Medulla oblongata | ||
| IRt | − | + |
| Sp | + | − |
| sp5 | + | − |
| Ve | + | − |
| Cerebellum | αTTp | SVCT2 |
|---|---|---|
| CoP | + | − |
The distribution of both vitamin transporters with each classified according to anatomical region. −: absence, +: presence.
3n, oculomotor nerve; 7N, facial nucleus; AA, anterior amygdaloid area; ac, anterior commissure; AcN, Accumbens nucleus; ACo, anterior cortical amygdaloid area; AHiAL, amygdalohippocampal area; AuC, auditory cortex; AMTN, anteromedial thalamic nucleus; AVTN, anteroventral thalamic nucleus; BIC, nucleus of Brachium of the inferior colliculus; BL, basolateral amygdaloid nucleus; BM, basomedial amygdaloid nucleus; CA1, Cornu Ammonis, Ammon's horn (field of the hippocampus); CA3, Cornu Ammonis, Ammon`s horn (field of the hippocampus); Ce, central amygdaloid nucleus; Cg, cingulate cortex; CIC, central nucleus of the inferior colliculus; CoP, cerebellum, layer of Purkinje cells; cp, cerebral peduncle; CPu, caudate and putamen (striatum); DC, dorsal cochlear nucleus; DG, dentate gyrus; DLG, dorsal lateral geniculate nucleus; DLL, dorsal nucleus of the lateral lemniscus; DpG, deep gray layer of the superior colliculus; DS, dorsal subiculum; DTT, dorsal tenia tecta; EC, entorhinal cortex; ECIC, external cortex of the inferior colliculus; eml, external medullary lamina; En, entopeduncular nucleus; FrA, frontal association cortex; fmj, forceps major of the corpus callosum; gl, granular layer; GP, globus pallidus; hf, hippocampal fissure; Hip, hippocampus (dentate gyrus and Ammon's horn); IC, insular cortex; Ien, intermediate endopiriform claustrum; IGL, intergeniculate leaflet; ic, internal capsule; InG, intermediate gray layer of superior colliculus; InWH, intermediate white layer of the superior colliculus; IPN, interpeduncular nucleus; IRt, intermediate reticular nucleus; LAN, lateral amygdaloid nucleus; LDT, laterodorsal thalamic nucleus; LL, lateral lemniscus; LPLR, lateral posterior thalamic nucleus, laterorostral part; LPT, lateral posterior thalamic nucleus; LSN, lateral septal nucleus; LV, lateral ventricle; MC, motor cortex; Me5, mesencephalic trigeminal nucleus; MEC, medial entorhinal cortex; mf, mossy fibers; ml, molecular layer; MPA, medial preoptic area; Mo5, Motor root of the trigeminal nerve; MPO, medial preoptic nucleus; MVPO, medioventral periolivary nucleus; NST, nigrostriatal tract; OB, olfactory bulb; OC, orbital cortex; opt, optic tract; Pa4, paratrochlear nucleus; PAG, periaqueductal gray substance; PaS, parasubiculum; PG, pregeniculate nucleus; pl, polymorphic layer; PMCo, posteriomedial cortical amygdaloid area; Pn, pontine nuclei; PnC, pontine reticular nucleus; Po, posterior thalamic nuclear group; PoC, postrhinal cortex; Post, postsubiculum; Pr5, principal sensory trigeminal nucleus; PrCnF, precuneiform area; PrL, prelimbic cortex; PrS, presubiculum; py, pyramidal tract; pyl, pyramidal layer; R, red nucleus; RSC, retrosplenial cortex; Rt, reticular nucleus; RtTg, reticulotegmental nucleus of the pons; rs, rubrospinal tract; RSGc, retrosplenial granular cortex, c region; S1BF, primary somatosensory cortex, barrel field; Sag, sagulum nucleus; SC, somatosensory cortex; scc, splenium of the corpus callosum; SN, substantia nigra; Sp, spinal trigeminal nucleus; sp5, spinal trigeminal tract; StHy, striohypothalamic nucleus; sl, stratum lucidum; slm, stratum lacunosum‐moleculare; so, stratum oriens; SPO, superior paraolivary nucleus; sr, stratum radiatum; st, stria terminalis; STLD, bed nucleus of the stria terminalis, lateral division; SubG, superficial gray layer of the superior colliculus; tfp, transverse fibers of the pons; Tu, olfactory tubercle; TZ, nucleus of the trapezoid body; tz, trapezoid body; VC, visual cortex; Ve, vestibular nucleus; VL, ventrolateral nucleus of the thalamus; VLL, ventral nucleus of the lateral lemniscus; VLPO, ventrolateral preoptic nucleus; VP, ventral pallidum; VPL, ventral posterolateral thalamic nucleus; VPM, ventral posteromedial thalamic nucleus; VS, ventral subiculum; VTT, ventral tenia tecta; ZI, zona incerta.
Figure 1.
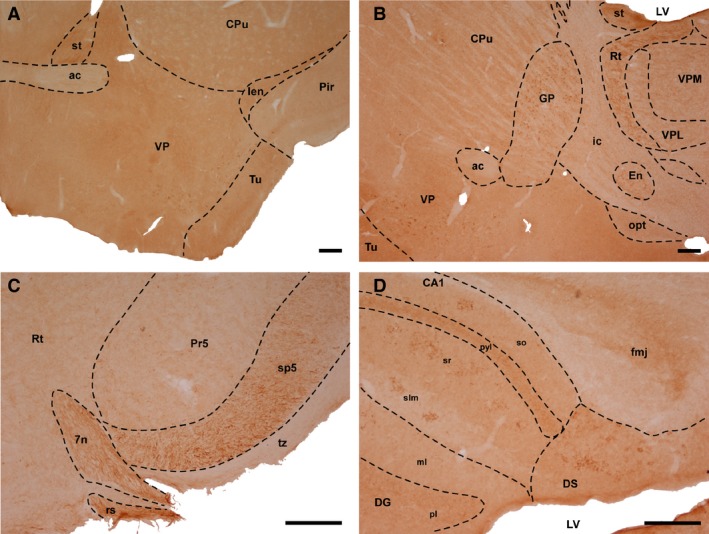
Immunohistochemistry for αTTP. (A) Coronal section at the level of the anterior commissure (wt, 4 months old). (B) Sagittal section showing thalamic nuclei and the basal ganglia (wt, 12 months old). (C) Coronal section of the brainstem (wt, 24 months old). (D) Sagittal section including different components of the hippocampal formation (wt, 12 months old). Scale bar: 200 μm. 7n, facial nucleus; ac, Anterior commissure; CA1, Cornu Ammonis, Ammon's horn (field of the hippocampus); CPu, caudate and Putamen (striatum); DG, dentate gyrus; DS, dorsal subiculum; En, entopeduncular nucleus; fmj, forceps major of the corpus callosum; GP, globus pallidus; Ien, intermediate endopiriform claustrum; ic, internal capsule; LV, lateral ventricle; ml, molecular layer; opt, optic tract; Pir, piriform cortex; pl, polymorphic layer; Pr5, principal sensory trigeminal nucleus; pyl, pyramidal layer; Rt, reticular nucleus; rs, rubrospinal tract; slm, stratum lacunosum‐moleculare; so, stratum oriens; sp5, spinal trigeminal tract; sr, stratum radiatum; st, stria terminalis; Tu, olfactory tubercle; tz, trapezoid body; VP, ventral pallidum; VPL, ventral posterolateral thalamic nucleus; VPM, ventral posteromedial thalamic nucleus.
αTTP‐IR was also found in the neuropil of the external plexiform layer of the olfactory bulb and the piriform cortex, lateral septal nucleus, core of the accumbens, lateral division of the bed nucleus of the stria terminalis, nigrostriatal tract, fornix, entopeduncular nucleus, dorsal hippocampal commissure, cerebral peduncle, substantia nigra, dorsal entorhinal cortex, pyramidal cell layer of the hippocampus, postsubiculum and dorsal cochlear nucleus.
Fiber tracts, including the stria terminalis and cingulum, the mossy fibres of the hippocampus and the lateral lemniscus, spinal trigeminal tract and trapezoid body (Fig. 1C), as well as motor cranial nerves, including the oculomotor and facial nerves (Fig. 1C) and the motor root of the trigeminal nerve, displayed strong immunoreactivity for αTTP. These fibers were longitudinal, although in some processes dot‐like puncta were also detected, including in the strata radiatum and lacunosum moleculare of the hippocampus, where they were mainly located in CA1 (Fig. 1D), and the piriform cortex and Purkinje cell layer of the cerebellum.
In cortical regions, αTTP‐IR was only visualized in the pericellular area. The strongest cortical labeling was observed in the deep layers of the motor cortex, the retrosplenial granular and dysgranular cortices, and the primary somatosensorial cortex in the barrel fields. Other cortical areas, such as the lateral orbital, frontal association cortex, secondary visual, cingulate or primary somatosensory cortex, had a less pericellular profile of αTTP‐IR.
Double‐labeling of αTTP/GFAP and αTTP/NeuN
An analysis of sections that were double‐immunolabeled for αTTP and GFAP revealed that this transporter was not located in glial cells (Fig. 2A and B). However, images from confocal microscopy showed that some of the dot‐like accumulations of αTTP‐IR were located in the Purkinje cell layer of the cerebellum, in which GFAP was also observed. This suggests that αTTP is present in Bergmann glial cells (Fig. 2C and D). In contrast, virtually all of the cell bodies with αTTP‐IR contained NeuN‐positive nuclei, demonstrating that αTTP is located in neurons (Fig. 3A–F).
Figure 2.
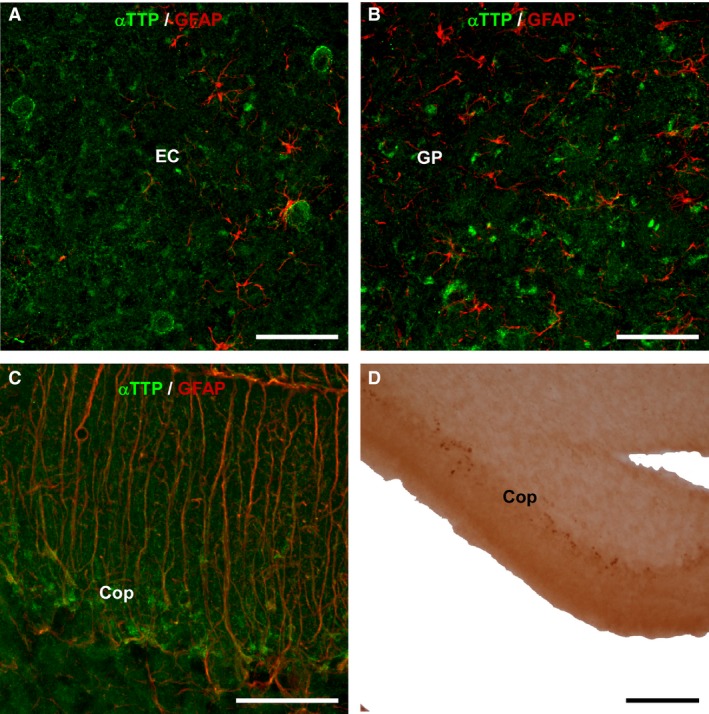
Double immunohistochemistry for GFAP/αTTP. The images were obtained using laser confocal microscopy and show double immunohistochemistry for GFAP (red) and αTTP (green) in 24‐month‐old KO mice. (A) Sagittal section showing the entorhinal cortex (EC). (B) Globus pallidus. (C) The Purkinje cell layer of the cerebellum contained some double‐labeled cells that might represent Bergmann's glia. Otherwise, no double labeling was detected. (D) Coronal section of the cerebellum showing single labeling for αTTP (18 months old, heterozygote). Thick points can be observed in the layer of Purkinje cells. Scale bars: 50 μm (A, B, C), 100 μm (D). CoP, cerebellum, layer of Purkinje cells; EC, entorhinal cortex; GP, globus pallidus.
Figure 3.
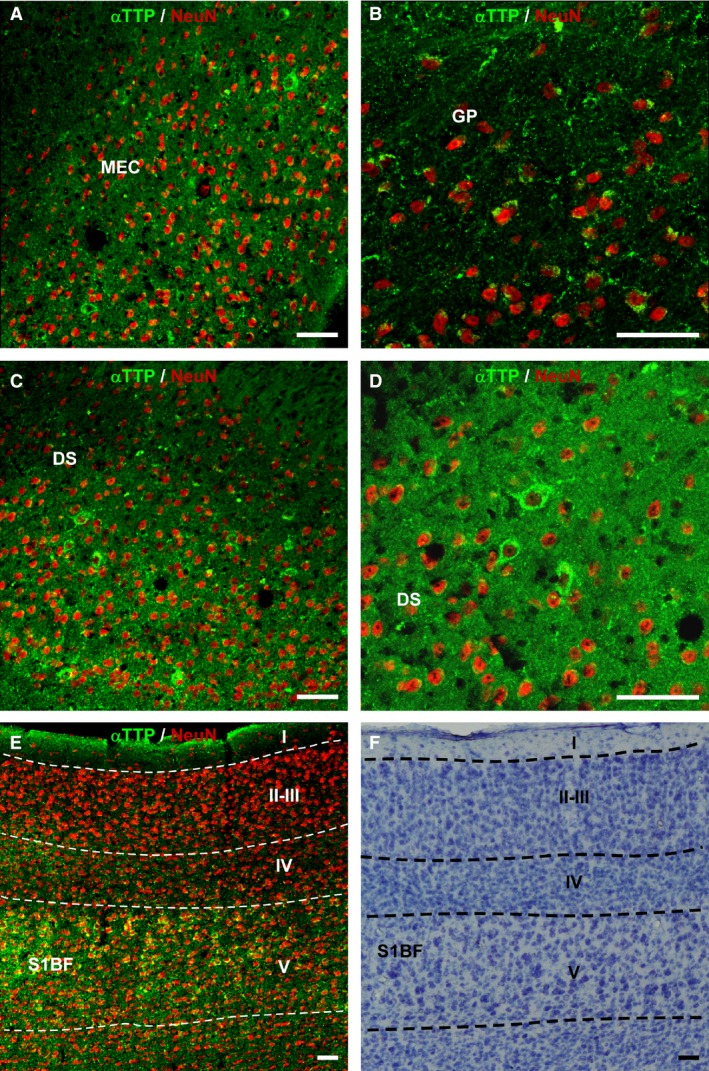
Double labeling for αTTP (green) and NeuN (red) in 24‐month‐old heterozygote mice. (A) Sagittal section containing the medial entorhinal cortex. (B) Globus pallidus. (C) Dorsal subiculum. (D) Same region as that shown in (C) but at a higher magnification. (E) Somatosensory cortex, including the barrel fields. Cell bodies containing the transporter are also shown that were immunoreactive for NeuN. (F) Nissl‐staining in a section adjacent to (E). Scale bar: 50 μm. I‐V, cortical layers; DS, dorsal subiculum; GP, globus pallidus; MEC, medial entorhinal cortex; S1BF, primary somatosensory cortex, barrel, field.
SVCT2
Distribution of SVCT2
SVCT2‐IR‐positive cell bodies were widely distributed in the brain of these mouse models (Table 1), with the highest expression found in the thalamic nuclei (Fig. 4A), striatum (Fig. 4B), cortex and hippocampus (Fig. 4C and D). Although SVCT2‐IR labeling was widely distributed in the thalamic nuclei, the strongest labeling was found in the dorsomedial part of the laterodorsal thalamic nucleus, the mediodorsal and laterodorsal parts of the lateral posterior thalamic nucleus, the anteroventral and anteromedial thalamic nuclei, the posterior thalamic nuclear group, and the dorsal lateral geniculate nucleus (Fig. 4A). All hippocampal fields contained cell bodies with SVCT2‐IR. Specifically, all layers of the dentate gyrus (mainly in the subgranular area) (Fig. 4C) and all CAs (mostly stratum oriens and the pyramidal layer) contained SVCT2‐IR. The dorsal and ventral subiculum also exhibited strong SVCT2‐IR. The entire rostrocaudal extent of the striatum (including the caudate and putamen) contained a homogeneous distribution of cell bodies with SVCT2‐IR.
Figure 4.
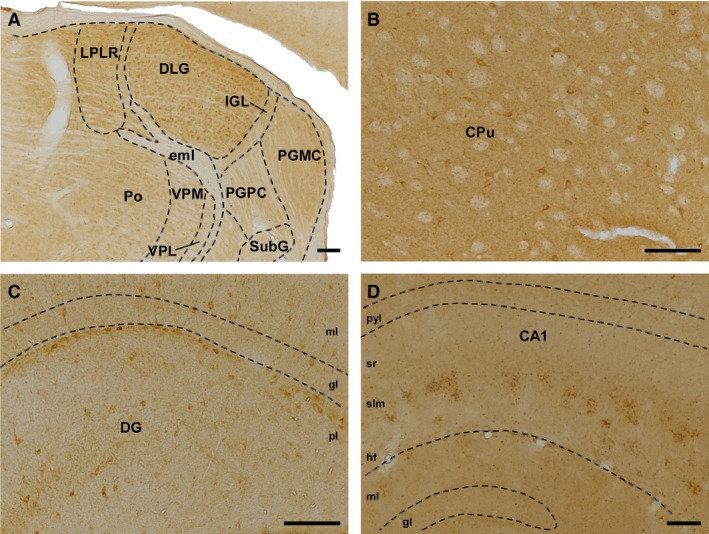
Immunolabeling for SVCT2. (A) Coronal section of thalamic nuclei (wt, 18 months old). (B) Immunoreactivity for SVCT2 in the caudate and putamen (wt, 4 months old). (C) Sagittal section showing the different layers of the dentate gyrus, in which cell bodies are located mainly in the polymorphic layer (wt, 4 months old). (D) Sagittal section of CA1, in which thick SVCT2‐positive profiles arranged in clusters are located in the stratum lacunosum moleculare (24 months old, KO). Scale bar: 100 μm. CA1, Cornus Ammonis, Ammon's horn (field of the hippocampus); CPu, caudate putamen; DG, dentate gyrus; DLG, dorsal lateral geniculate nucleus; eml, external medullary lamina; gl, granular layer; hf, hippocampal fissure; IGL, intergeniculate leaflet; LPLR, lateral posterior thalamic nucleus; ml, molecular layer; PGMC, pregeniculate nucleus, magnocellular part; PGPC, pregeniculate nucleus, parvocellular part; pl, polymorphic layer; Po, posterior thalamic nuclear group; pyl, pyramidal layer; slm, stratum lacunosum‐moleculare; sr, stratum radiatum; SubG, superficial gray layer of the superior colliculus; VPL, ventral posterolateral thalamic nucleus; VPM, ventral posteriomedial thalamic nucleus.
As was the case for αTTP, small grouped dots of SVCT2‐IR were observed in specific locations within the mouse brain. These structures were especially evident in the strata radiatum and lacunosum moleculare of CA fields (Fig. 4D), although some of them were observed in the stratum oriens of CA1. These dot‐like structures were also detected in the piriform cortex, surrounding the lateral olfactory tract, in the olfactory tubercle and the anterior olfactory area, and dorsal to the rhinal fissure.
In the cortex, as for αTTP, structures with pericellular SVCT2‐IR were observed. The areas with the strongest labeling were the retrosplenial cortex (mainly the granular part) and the cingulate, motor, orbital and frontal association cortices. In the somatosensory, visual and auditory cortices, the expression of SVCT2 seemed to be stronger in the superficial and deep layers and weaker in intermediate layers.
Double‐labeling for SVCT2/GFAP and SVCT2/NeuN
The analysis of double‐immunolabeling for SVCT2 and GFAP showed that SVCT2 was not detected in astrocytes (Fig. 5A–D). In contrast, double‐immunohistochemistry for SVCT2 and NeuN showed that SVCT2 is located in neurons (Fig. 6A–F).
Figure 5.
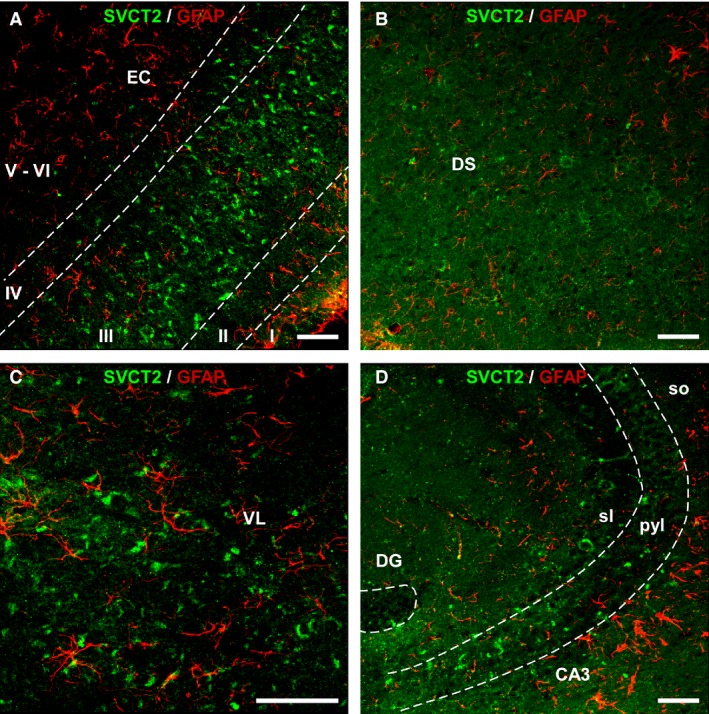
Double immunohistochemistry for SVCT2 and GFAP. Images were obtained using laser confocal microscopy and show co‐localization between SVCT2 (green) and GFAP (red) in 24‐month‐old wt mice. (A) Sagittal section showing the entorhinal cortex. (B) Dorsal subiculum. (C) Ventrolateral nucleus of the thalamus. (D) CA3 field of the hippocampal formation. No double labeling was found. Scale bar: 50 μm. I‐VI, cortical layers; CA3, Cornus Ammonis, Ammon's horn (field of the hippocampus); DG, dentate gyrus; DS, dorsal subiculum; EC, entorhinal cortex; pyl, pyramidal layer; sl, stratum lucidum; so, stratum oriens; VL, ventrolateral nucleus of the thalamus.
Figure 6.
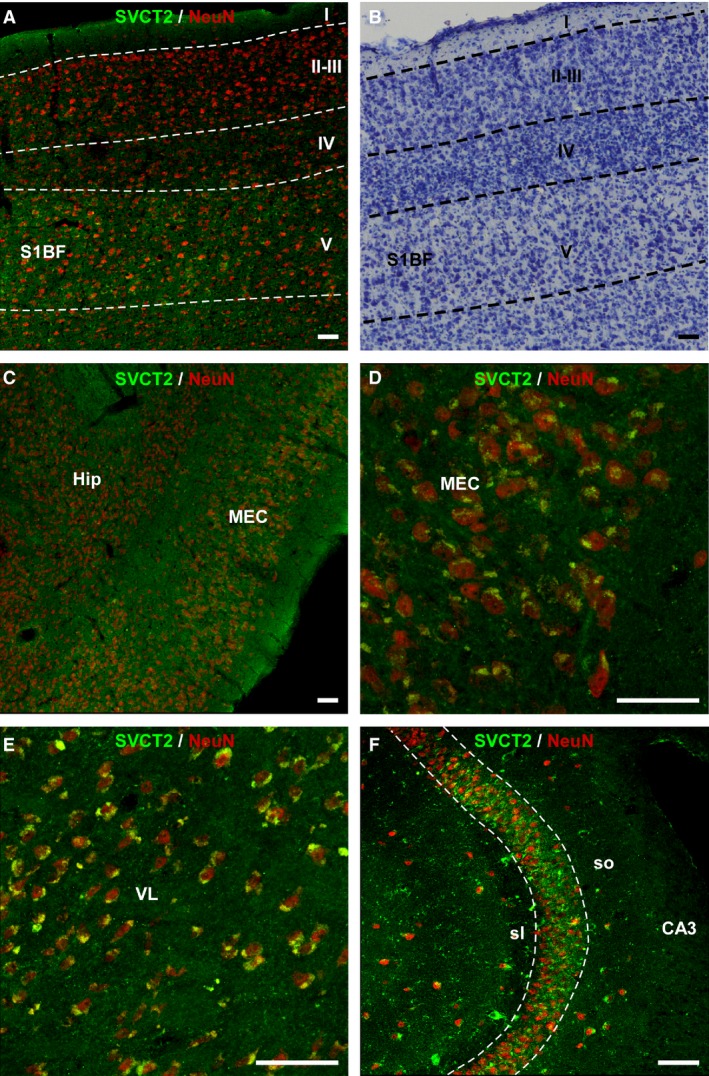
Double‐immunolabeling for SVCT2 (green) and NeuN (red) in 18‐month‐old heterozygote mice. (A) Somatosensory cortex including the barrel fields. (B) Nissl‐stained section adjacent to the section shown in (A). (C) Sagittal section showing the medial entorhinal cortex. (d) Higher magnification of the image shown in (C). (E) Ventrolateral nucleus of the thalamus. (F) The CA3 field of the hippocampal formation. Cell bodies with SVCT2‐immunoreactivity also contained NeuN. Scale bar: 50 μm. I‐V, cortical layers; CA3, Cornus Ammonis, Ammon's horn (field of the hippocampus); Hip, hippocampus; MEC, medial entorhinal cortex; sl, stratum lucidum; so, stratum oriens; VL, ventrolateral nucleus of the thalamus.
Double‐immunohistochemistry for αTTP/SVCT2
Individual mapping of αTTP and SVCT2 suggested that, in general, they have complementary distribution patterns in the mouse brain. This complementarity was clearly evident in some regions. For example, SVCT2‐positive cell bodies were detected in the hippocampus (Fig. 7A), whereas αTTP‐IR was observed mostly in mossy fibers but in only a few cell bodies (Fig. 7B, C). While both transporters were detected in the basal ganglia, SVCT2 was also found in the striatum, and αTTP was also found in the globus pallidus and entopeduncular nucleus. In the auditory system, SVCT2 was present in the neurons of the nucleus of the trapezoid body, whereas the trapezoid body itself displayed immunoreactivity for αTTP (Fig. 7D). In the thalamus, αTTP was found only in the neurons of the reticular nucleus, but the SVCT2 transporter was not. However, the remaining thalamic nuclei contained SVCT2‐IR neurons.
Figure 7.
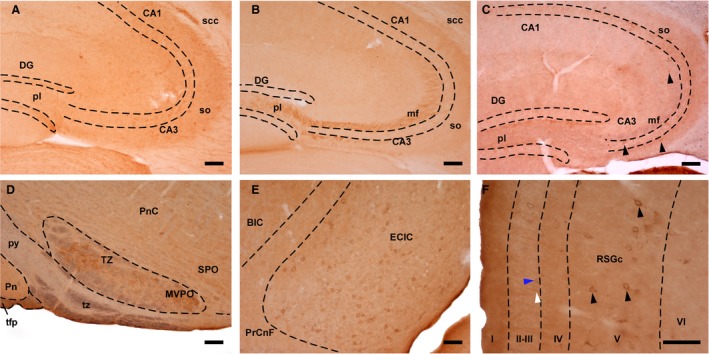
Double‐immunostaining for SVCT2 and αTTP. (A) SVCT2‐immunoreactive profiles in the hippocampal formation (heterozygote, 4 months old). (B) Mossy fibers displayed TTPα‐immunoreactivity (wt, 24 months old). (C) Coronal section of the hippocampus, in which SVTC2 (brown precipitate) and αTTP (blue color) were observed (wt, 24 months old). Arrowheads: SVCT2‐positive neurons. (D) Sagittal section of the brainstem displaying the trapezoid body (tz), which was positive for αTTP (blue color), and the nucleus of the trapezoid body (TZ), which contained SVCT2‐immunoreactive (brown precipitate) neurons (heterozygote, 4 months old). (E) Sagittal section of the inferior colliculus showing neurons exhibiting double immunostaining (wt, 24 months old). (F) Coronal section of the granular part of the retrosplenial cortex, in which pericellular immunostaining for SVCT2 (white arrowhead) and αTTP (blue arrowhead) as well as double‐labeled cells (black arrowheads) were observed (wt, 24 months). Scale bar: 100 μm. I‐VI, cortical layers; BIC, nucleus of the brachium of the inferior colliculus; CA1 and CA3, Cornus Ammonis, Ammon's horn (fields of the hippocampus); DG, dentate gyrus; ECIC, external cortex of the inferior colliculus; mf, mossy fibers; MVPO, medioventral periolivary nucleus; pl, Polymorphic layer; Pn, pontine nuclei; PnC, pontine reticular nucleus; PrCnf, precuneiform area; py, pyramidal tract; RSGc, retrosplenial granular cortex, c region; scc, splenium of the corpus callosum; so, stratum oriens; SPO, superior paraolivary nucleus; tfp, transverse fibers of the pons; TZ, nucleus of the trapezoid body; tz, trapezoid body.
Some degree of colocalization was observed in the inferior colliculus (Fig. 7E), dorsal subiculum and medial entorhinal cortex, and the deep layers of several cortical areas. Both vitamin transporters were detected in these regions, and sequential immunohistochemistry performed using the chromogens DAB and chloronaphthol showed that some pericellular structures were clearly double‐labeled (Fig. 7F).
Discussion
αTTP and/or SVCT2 are transporters that play fundamental roles in the internalization of VitE and VitC in the CNS. We analyzed their neuroanatomical distribution patterns across different ages and genotypes of a mouse model of aging. The results showed that αTTP and/or SVCT2 immunoreactivity was similar across the different animal groups, supporting the physiological actions of these vitamins in the CNS.
Distribution of αTTP
To our knowledge, this is the first detailed mapping of IHC‐detected αTTP in the rodent brain. αTTP has been detected in the rat brain using other techniques, such as Northern blot, RT‐PCR and in situ hybridization (Hosomi et al. 1998). The results of in situ hybridization showed that the mRNA for αTTP is expressed only in the cerebellar cortex, probably in Bergmann glial cells (Hosomi et al. 1998), and the results obtained in the present work using confocal microscopy and double‐immunohistochemistry for αTTP/GFAP support this notion (Fig. 2C). Bergmann glial cells are important for Purkinje cell metabolism, and Ulatowski et al. (2014) demonstrated that VitE is essential for optimal Purkinje cell functions. Our findings provide a neuroanatomical basis for how VitE might be supplied to Purkinje neurons (Copp et al. 1999; Brigelius‐Flohe, 2006; Patel et al. 2011; Johnson et al. 2013; Marin et al. 2014; Mocchegiani et al. 2014; Ulatowski et al. 2014). Moreover, Northern blot analysis showed that αTTP is expressed in the cerebral cortex at very low levels (Hosomi et al. 1998), and in the present study we also detected this transporter in cortical regions as well as in many other regions of the mouse brain.
IHC has been used to detect αTTP in human brains to compare controls and patients with Alzheimer's disease, Down syndrome, and AVED (Copp et al. 1999). In normal human brains, no immunostaining was found. However, patients with each of these three pathologies showed immunoreactivity for αTTP in the soma and dendrites of Purkinje cells. In addition, some pyramidal neurons in CA2 were αTTP‐positive in patients with Alzheimer's disease or Down syndrome. Our results are not in agreement with those of Copp et al. (1999) because we observed a wider and different distribution pattern for αTTP. However, this could be because of differences in species and/or methodologies.
Other approaches have focused primarily on the analysis of vitamin concentrations (mainly α‐tocopherol) in plasma and a variety of tissues, including the brain. In rats, Vatassery et al. (1984) reported that the highest concentration of α‐tocopherol was found in the gray matter of the frontal cortex, followed by the thalamus and pons. Lower concentrations were detected in the medulla, caudate/putamen and hypothalamus, and the spinal cord and cerebellum exhibited the lowest levels. However, the authors noted that the cerebellum is a particularly active metabolizer of VitE because it showed high levels of α‐tocopherol uptake. Hence, the vitamin concentration found in a particular cerebral region does not necessary match its uptake level. The restricted distribution of αTTP that was observed in the cerebellum in the present study also supports this notion.
It has been previously reported that VitE plays a double role as both an antioxidant against brain oxidative stress and an anti‐inflammatory agent against the inflammation associated with the pathogenesis of dementia and other neurodegenerative diseases (Mocchegiani et al. 2014). Moreover, mice deficient in αTTP exhibit anxiety and deficits in memory as they age (Gohil et al. 2004), suggesting that the limbic system, and mainly the hippocampus, is affected by the depletion of VitE. In the present study, we detected αTTP in memory‐related brain regions and their connections, supporting the role of VitE as a lipid‐soluble antioxidant that protects lipids and membranes from oxidative damage (Brigelius‐Flohe, 2006) since this transporter was observed not only in neurons but also in fiber tracts. Specifically, dot‐like IR for αTTP was observed in the thick fibers of the strata radiatum and lacunosum‐moleculare as well as in the mossy fibers that travel from the dentate gyrus to CA3 (Tables 1 and 2). This projection is an important component of the hippocampal circuit, which is essential for good memory functions. In addition, other brain regions that are also implicated in learning and memory processes, such the entorhinal cortex (which is the starting point of the perforant pathway to the dentate gyrus), exhibited strong αTTP immunostaining.
Table 2.
Results of immunostaining for both vitamin transporters classified by functional regions
| Limbic system | αTTP | SVCT2 |
|---|---|---|
| Cg | + | + |
| PrL | + | + |
| RSC | + | + |
| DS | + | + |
| EC | + | + |
| Hip | + | + |
| MF | + | − |
| PaS | + | − |
| Post | + | − |
| PrS | + | − |
| VS | + | + |
| AA | − | + |
| ACo | − | + |
| AHiAL | + | + |
| BL | + | + |
| BM | − | + |
| Ce | + | − |
| LAN | − | + |
| PMCo | − | + |
| AcN | + | + |
| LSN | + | − |
| ST | + | − |
| STLD | + | − |
| AVTN | − | + |
| AMTN | − | + |
| Fx | + | − |
| Visual system | αTTP | SVCT2 |
|---|---|---|
| DLG | − | + |
| LPT | − | + |
| PG | + | − |
| VC | + | + |
| Motor functions | αTTP | SVCT2 |
|---|---|---|
| MC | + | + |
| CPu | − | + |
| GP | + | − |
| VP | + | − |
| En | + | − |
| ZI | + | + |
| 3n | + | − |
| Me5 | + | − |
| Mo5 | + | − |
| NST | + | − |
| Pa4 | + | − |
| R | + | − |
| SN | + | + |
| 7N | + | − |
| 7n | + | − |
| PnC | − | + |
| RtTg | + | − |
| CoP | + | − |
| cp | + | − |
| Hormonal control | αTTP | SVCT2 |
|---|---|---|
| StHy | + | − |
| MPA | + | − |
| MPO | + | − |
| Auditory system | αTTP | SVCT2 |
|---|---|---|
| AuC | − | + |
| BIC | + | + |
| CIC | + | + |
| DLL | + | − |
| ECIC | + | + |
| DC | + | − |
| TZ | − | + |
| LL | + | − |
| tz | + | − |
| VLL | + | + |
| Sag | + | − |
| Somatosensorial | αTTP | SVCT2 |
|---|---|---|
| SC | + | + |
| Po | − | + |
| Rt | + | − |
| InG | + | − |
| InWH | − | + |
| Sp | + | − |
| sp5 | + | − |
| Ve | + | − |
| Olfactory system | αTTP | SVCT2 |
|---|---|---|
| DTT | − | + |
| OB | + | + |
| Tu | − | + |
| VTT | − | + |
| Pir | + | + |
| Other functions | αTTP | SVCT2 | Function |
|---|---|---|---|
| FrA | + | + | Associative learning, stimulus integration |
| OC | + | + | Motivation, social behavior |
| PoC | − | + | Memory, spacial navigation |
| DpG | + | + | Multiple sensorial‐eye movement |
| IPN | − | + | REM sleep |
| PAG | − | + | Pain modulation |
| IRt | − | + | Motor, sleep, pain modulation, CVC regulation |
| VLPO | + | − | Non‐REM sleep |
The distribution of both vitamin transporters with each classified according to functional region. −, absence, +, presence.
See abbreviations in footnotes to Table 1.
Other authors (Johnson et al. 2013) have detected α‐tocopherol in many cortical areas in humans, and the results of our study provide a more detailed description of the distribution of αTTP in different cortical layers and its wider distribution throughout the cortex. The distribution of cortical immunolabeling was different from that in other brain areas because it was pericellular and not cytoplasmatic. This may help to balance excessive glutamate concentrations, which can be neurotoxic and lead to neurodegeneration and were observed in extracellular spaces. This finding is in agreement with previous studies that reported that VitE plays a prophylactic role in preventing glutamate‐induced injury and cell death (Rati Selvaraju et al. 2014).
Distribution of SVCT2
The distribution of the VitC transporter SVCT2 was previously mapped in the rat brain using immunohistochemistry (Mun et al. 2006). In addition, SVCT2 has been detected in embryonic mouse neurons (Figueroa‐Mendez & Rivas‐Arancibia, 2015) and in mouse hypothalamic glial cells (Garcia Mde et al. 2005). The results obtained in the present study agree with these and other previous studies (Rice, 2000; Harrison & May, 2009; Harrison et al. 2010) showing that SVCT2 is present in neurons but not in astrocytes. Garcia Mde et al. (2005) restricted their analysis to the hypothalamus, and they did find that SVCT2 was present in GFAP‐positive tanycytes. Although this finding is not totally in line with our results, both studies detected SVCT2 in the GFAP‐negative ependymal cells of the ventricles. Because it has been suggested that tanycytes are not enough to carry a vitamin from the cerebrospinal fluid to the brain interstitium, the presence of SVCT2 in these cells supports the notion that this transporter plays a role in the uptake of VitC by choroid plexus cells (for review, see Harrison & May, 2009).
The distribution pattern of SVCT2 in the rat brain was described by Mun et al. (2006) but we found that it was different in the mouse brain. In the rat brain, only cell bodies were positive for SVCT2, whereas we observed SVCT2‐IR in cellular processes and cell bodies. Mun et al. (2006) did not find immunolabeling in striatum, nucleus accumbens or any thalamic nucleus, whereas we found that in mice, these regions displayed strong immunolabeling. Conversely, no immunoreactivity was observed in the hypothalamus or the cerebellum in the present study, whereas intense SVCT2‐IR was observed in many hypothalamic nuclei and in the Purkinje cell layer in the rat. Similar results were obtained by Oyarce et al. (2017) in mouse Purkinje cells. Finally, in both species, strong immunostaining was observed in neurons of cortex and hippocampus, although the patterns were not perfectly aligned.
Thus, the distribution of SVCT2 varies across brain regions, and some authors have suggested that the distribution of VitC mirrors the distribution of its transporter (see Harrison et al. 2010). However, Harrison et al. (2010) reported that the level of VitC in a tissue does not always depend on the level of SVCT2. This mismatch is potentially explained by which of the two possible mechanisms is used to internalize ascorbate into neurons. SVCT2‐mediated transport is the main route used by neurons (Garcia‐Krauss et al. 2016); however, when HPLC is used to measure VitC levels in brain extracts, the results include all VitC that is obtained via both mechanisms (Harrison et al. 2010). Thus, a higher level of VitC than SVCT2 is expected. Furthermore, because SVCT2 was absent from astrocytes (Castro et al. 2001; Garcia Mde et al. 2005; Harrison et al. 2010; Harrison & May, 2009; present study), the level of VitC should be higher in areas with a high concentration of neurons, such as the cortex or hippocampus. Numerous studies have focused on describing the distribution and/or concentration of VitC and/or ascorbate anion in the mammalian brain, including humans (Oke et al. 1987). For example, a previous study using immunohistochemistry in the macaque monkey found that the only cell bodies containing VitC were located in the primary somatosensory cortex (Coveñas et al. 2011), whereas in a immunohistochemical study carried out in children, VitC was observed in several brainstem regions (Covenas et al. 2015). Our results are partially in agreement with these reports, although the distribution of SVCT2 described in the present study does not totally match the pattern described for VitC in children's brainstem. Differences in the level of VitC across brain regions are related to which cell types are located within each area. For example, in multiple species, brain ascorbate levels increase linearly as neuronal density increases in adult cerebral cortex (Rice, 2000). This finding is consistent with the fact that oxidative metabolism occurs at a higher rate in neurons than in glial cells, and in agreement with the results of previous studies of the distribution and retention of VitC in the mouse brain (Harrison et al. 2010). This is also in agreement with the results obtained in the present study, in which we found that SVCT2 is present in regions with a high neuronal density, such is the cortex, thalamus, striatum and hippocampus.
Double immunohistochemistry for αTTP/SVCT2
In general, both vitamin transporters are present in similar brain regions. They are widely distributed in limbic structures, and the visual and auditory systems contain numerous areas that express one or both of these transporters. However, αTTP was more widely expressed than SVCT2 in motor and somatosensorial regions and was the only one that was detected in the hypothalamic nuclei that are associated with hormonal control (Table 2). Additionally, SVCT2 was more widely distributed than αTTP in olfactory structures and in regions implicated in functions including REM sleep, pain modulation, and spatial navigation (Table 2).
The results obtained in the present study show that there was a complementary distribution pattern between these vitamin transporters in some brain areas. In the basal ganglia, SVCT2 was detected in the striatum, and αTTP was detected in the globus pallidus. In the auditory pathway, αTTP was observed in the trapezoid body, whereas SVCT2 was found in the nucleus of the trapezoid body. In addition, some brain regions, such as the inferior colliculus, entorhinal cortex, dorsal subiculum and cortex, contain neurons that were double‐labeled for αTTP and SVCT2. These separate but coincident distribution patterns could be a morphological reflection of interactions between the two vitamins. VitC works synergistically with VitE to protect neurons against the oxidative stress damage caused by free radicals (Rice, 2000; Harrison et al. 2010). In the mouse cerebral cortex, lower levels of VitC are clearly associated with higher levels of lipid peroxidation products (Harrison et al. 2010). Interestingly, VitC can intercept free radicals in the aqueous phase before they attack lipids (Harrison et al. 2014), and VitE is the first line of defence against the peroxidation of lipid membranes (Brigelius‐Flohe, 2006; Patel et al. 2011; Mocchegiani et al. 2014). The antioxidant activity of α‐tocopherol prevents the uncontrolled propagation of lipid peroxidation and produces a tocopheroxyl radical, which requires ascorbate to be regenerated back to reduced tocopherol (for review, see Dolu et al. 2015; Mocchegiani et al. 2014). In cortical regions, both transporters were pericellularly localized, suggesting that they are important in regulating the homeostasis of the extracellular compartment. The level of vitamins in the extracellular fluid is important for regulating synaptic communication, and in this sense, ascorbate may be a potential candidate extracellular neuromodulator (Rebec & Pierce, 1994; Rice, 2000).
Animal model and aging
Because allelic variations can alter the efficiency of the metabolic pathways that regulate vitamin storage and removal (Baxter et al. 2012; Harrison et al. 2014; Mocchegiani et al. 2014), the genetic background of a model system is important. In fact, there is increasing interest in studies that explore the influence of gene expression on these two vitamins (Brigelius‐Flohe, 2006; Harrison et al. 2014; Mocchegiani et al. 2014; Oyarce et al. 2017). During aging, the expression of genes involved in stress‐response pathways increases. However, not all genes exhibit altered expression in the same way in all organisms. In the aging mouse brain, no age‐related changes in expression have been found in genes implicated in inhibitory interneuron function or in myelin‐related or glial genes, all of which are altered in humans during aging (for review, see Bishop et al. 2010).
According to the ‘free radical theory of aging’ developed by Harman in 1956 (Harman, 1972; Haigis & Yankner, 2010; Yeoman et al. 2012), a common feature of all organisms is that oxidative stress increases with aging. However, more recently it has been reported that this increase is not the principle determinant of the aging process, and it seems that altered redox‐sensitive signaling pathways are more important for CNS aging (Yeoman et al. 2012). Hence, Pol μ−/− mice, which exhibit fewer signs of brain aging and higher resistance to oxidative damage (Lucas et al. 2009; Escudero et al. 2014), are an excellent animal model for studying the distribution patterns of these two important antioxidant transporters. The data currently available for this animal model indicate that the Pol μ−/− phenotype is strongly dependent on oxidative stress levels, shows a more efficient mitochondrial function, a lower level of apoptosis in the liver and brain, and above‐normal genomic stability (Escudero et al. 2014). In addition, Pol μ−/− mice provide advantages related to the two most important age‐associated changes, which are learning and memory functions, and motor performance. Significantly better sensorimotor coordination was observed in the rota‐rod test in mice lacking DNA polymerase μ than in controls, even at older ages, and the KO mice maintained the ability to learn at older ages, whereas the wild‐type mice did not (Lucas et al. 2009). Our results provide an anatomical basis for these behaviors. The distribution pattern observed for each vitamin transporter was constant across the genetic groups and between groups with different ages. This ability to maintain the antioxidant levels during aging may support the maintenance of the above described capacities in older mice. Some characteristics have been previously reported to be unchanged in this animal model. For example, there was no difference in the mossy fibers (in which we detected αTTP) traveling from the dentate gyrus to CA3 between 4‐month‐old wild‐type and Pol μ−/− mice (Lucas et al. 2009). On the other hand, the consistency in these distribution patterns could be related to the animals’ age. In guinea pigs and rats (Lykkesfeldt & Moos, 2005), the reduction in VitC status that occurs with age begins early in life and is a phenomenon that is associated with maturation rather than aging. In these species, VitC concentrations significantly declined in young animals (i.e. those under 6 months of age in guinea pigs and under 3 months in rats), but there was no further significant decline in older animals, suggesting that degenerative oxidation processes might begin earlier than is presently acknowledged. A similar pattern was observed in αTTP‐deficient mice, which lose their synapses early but in older ages exhibit minimal differences with matched‐age wild‐type animals (Gohil et al. 2004). This was also the case in the present study, but further studies are required because we did not include animals younger than 4 months old.
Another age‐related feature, neuroinflammation and subsequent astrogliosis, was not detected in our studies. The sizes and shapes of astrocytes remained the same across all of the experimental groups, and there was no apparent difference in glial density. The preservation of glial cells during aging also contributed to the maintenance of brain functions in this animal model (Lucas et al. 2009). These results support previous data demonstrating that α‐tocopherol and αTTP are required for the normal functions of glial cells (Gohil et al. 2004).
Functional correlation: antioxidant activity
The effects of antioxidants on learning have been widely reported in the literature and it is currently thought that antioxidant nutrients exert beneficial effects by preventing neurodegeneration and dementia (Rice, 2000; Hansen et al. 2014; Harrison et al. 2014; Mocchegiani et al. 2014; Dolu et al. 2015; Warner et al. 2015). In aged mice, the administration of ascorbate in combination with VitE improved performance on a passive avoidance task (for review, see Harrison & May, 2009). Our results provide anatomical support for these data because we found that these two vitamin transporters are present in the hippocampus and other regions of the hippocampal formation, such as the entorhinal cortex and the dorsal subiculum.
On the other hand, it seems that the administration of high doses of these vitamins did not produce remarkable effects except in cases of previous deficiency (Brigelius‐Flohe, 2006; Harrison & May, 2009; Figueroa‐Mendez & Rivas‐Arancibia, 2015). Because the two transporters of these vitamins are saturable, treatment with high doses of these vitamins results in a plateau in their concentration in the brain. Although the results of studies exploring the benefits that vitamin supplementation provides to learning and memory functions and motor performance in normal subjects have been inconclusive, it is widely reported that a deficit in VitE or VitC causes harmful effects, mainly because the loss of their neuroprotective effects results in massive neuronal death. These deficits also lead to an increase in oxidative stress that is not homogeneously distributed throughout the brain, suggesting that some regions are more resistant to this type of stress. Thus, a region containing VitE and/or VitC transporters could be more resistant to oxidation, neurotoxicity and further neuronal death. Our results indicate that each of these vitamin transporters has a widespread and constant distribution pattern. Furthermore, double‐labeling experiments showed that the neuronal profiles of these transporters overlap in regions with high neuronal density and a high metabolic rate, where antioxidant substances are especially important.
Conclusions
Our results show that αTTP and SVCT2 are widely distributed throughout the entire brain in wt, heterozygous and Pol μ−/− mice. These distribution patterns remain similar with age. The presence of these transporters in regions implicated in learning and memory as well as in motor control may explain the maintenance of these abilities that is observed in these animals when they reach old age. Vitamins E and C may be taken up into neurons by their respective carriers, whereupon they exert their antioxidant properties in addition to performing other physiological functions. Our results provide an anatomical basis that may help to explain their higher resistance to brain oxidative stress, which allows them to achieve better motor performance and learning abilities in old age.
Conflict of interest
None.
Author contributions
P. Marcos: concept/design, acquisition of data, data analysis and interpretation, drafting of the manuscript, critical revision of the manuscript, approval of the article. J. González‐Fuentes: concept/design, acquisition of data, data analysis and interpretation, drafting of the manuscript, critical revision of the manuscript, approval of the article. L. Castro‐Vázquez: data interpretation, drafting of the manuscript, critical revision of the manuscript, approval of the article. M. V. Lozano: data interpretation, drafting of the manuscript, critical revision of the manuscript, approval of the article. M. J. Santander‐Ortega: data interpretation, drafting of the manuscript, critical revision of the manuscript, approval of the article. V. Rodríguez‐Robledo: data interpretation, drafting of the manuscript, critical revision of the manuscript, approval of the article. N. Villaseca‐González: data interpretation, drafting of the manuscript, critical revision of the manuscript, approval of the article. M. M. Arroyo‐Jiménez: concept/design, data analysis and interpretation, drafting of the manuscript, critical revision of the manuscript, approval of the article.
Non‐author contributors: D. Lucas and A. Bernad: provided the Pol μ mice. M. J. Lagartos and M. Íñiguez de Onzoño: PCR analysis. J. R. Marín Tebar: technical assistance with confocal microscopy.
Supporting information
Figure S1. Control of primary antibodies.
Table S1. Antibodies used in this study.
Acknowledgements
This work was supported by Junta de Comunidades de Castilla‐La Mancha, Spain (grants PEII‐2014‐003 and PEII‐2014‐040‐P) and University of Castilla‐La Mancha (UCLM, Spain) grants. We extend many thanks to D. Lucas and A. Bernad for providing the Pol μ mice, to M. J. Lagartos and M. Íñiguez de Onzoño for performing the PCR analysis, and J. R. Marín Tebar for his technical assistance with confocal microscopy.
Contributor Information
P. Marcos, Email: Pilar.Marcos@uclm.es
M. M. Arroyo‐Jiménez, Email: Mariamar.Arroyo@uclm.es
References
- Baxter LL, Marugan JJ, Xiao J, et al. (2012) Plasma and tissue concentrations of alpha‐tocopherol and delta‐tocopherol following high dose dietary supplementation in mice. Nutrients 4, 467–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop NA, Lu T, Yankner BA (2010) Neural mechanisms of ageing and cognitive decline. Nature 464, 529–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigelius‐Flohe R (2006) Bioactivity of vitamin E. Nutr Res Rev 19, 174–186. [DOI] [PubMed] [Google Scholar]
- Castro M, Caprile T, Astuya A, et al. (2001) High‐affinity sodium‐vitamin C co‐transporters (SVCT) expression in embryonic mouse neurons. J Neurochem 78, 815–823. [DOI] [PubMed] [Google Scholar]
- Cebada‐Sanchez S, Insausti R, Gonzalez‐Fuentes J, et al. (2014) Distribution of peptidergic populations in the human dentate gyrus (somatostatin [SOM‐28, SOM‐12] and neuropeptide Y [NPY]) during postnatal development. Cell Tissue Res 358, 25–41. [DOI] [PubMed] [Google Scholar]
- Cecchini T, Ciaroni S, Ferri P, et al. (2003) Alpha‐tocopherol, an exogenous factor of adult hippocampal neurogenesis regulation. J Neurosci Res 73, 447–455. [DOI] [PubMed] [Google Scholar]
- Copp RP, Wisniewski T, Hentati F, et al. (1999) Localization of alpha‐tocopherol transfer protein in the brains of patients with ataxia with vitamin E deficiency and other oxidative stress related neurodegenerative disorders. Brain Res 822, 80–87. [DOI] [PubMed] [Google Scholar]
- Covenas R, Gonzalez‐Fuentes J, Rivas‐Infante E, et al. (2015) Developmental study of vitamin C distribution in children's brainstems by immunohistochemistry. Ann Anat 201, 65–78. [DOI] [PubMed] [Google Scholar]
- Coveñas R, Mangas A, Bodet D, et al. (2011) Vitamin C in the monkey brain In: Vitamin C: Nutrition, Side Effects and Supplements. (ed. Jackson CME.), pp. 275–288: Nova Science. [Google Scholar]
- Dolu N, Khan A, Dokutan S (2015) Effect of vitamin E administration on learning of the young male rats. J Exp Neurosci 9, 81–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escudero B, Lucas D, Albo C, et al. (2014) Polμ deficiency increases resistance to oxidative damage and delays liver aging. PLoS ONE 9, e93074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa‐Mendez R, Rivas‐Arancibia S (2015) Vitamin C in health and disease: its role in the metabolism of cells and redox state in the brain. Front Physiol 6, 397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G (2008) The Mouse Brain in Stereotaxic Coordinates. Amsterdam; Oxford: Elsevier. [Google Scholar]
- Garcia Mde L, Salazar K, Millan C, et al. (2005) Sodium vitamin C cotransporter SVCT2 is expressed in hypothalamic glial cells. Glia 50, 32–47. [DOI] [PubMed] [Google Scholar]
- Garcia‐Krauss A, Ferrada L, Astuya A, et al. (2016) Dehydroascorbic acid promotes cell death in neurons under oxidative stress: a protective role for astrocytes. Mol Neurobiol 53, 5847–5863. [DOI] [PubMed] [Google Scholar]
- Gohil K, Godzdanker R, O'Roark E, et al. (2004) Alpha‐tocopherol transfer protein deficiency in mice causes multi‐organ deregulation of gene networks and behavioral deficits with age. Ann N Y Acad Sci 1031, 109–126. [DOI] [PubMed] [Google Scholar]
- Haigis MC, Yankner BA (2010) The aging stress response. Mol Cell 40, 333–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen SN, Tveden‐Nyborg P, Lykkesfeldt J (2014) Does vitamin C deficiency affect cognitive development and function? Nutrients 6, 3818–3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harman D (1972) Free radical theory of aging: dietary implications. Am J Clin Nutr 25, 839–843. [DOI] [PubMed] [Google Scholar]
- Harrison FE, May JM (2009) Vitamin C function in the brain: vital role of the ascorbate transporter SVCT2. Free Radic Biol Med 46, 719–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison FE, Green RJ, Dawes SM, et al. (2010) Vitamin C distribution and retention in the mouse brain. Brain Res 1348, 181–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison FE, Bowman GL, Polidori MC (2014) Ascorbic acid and the brain: rationale for the use against cognitive decline. Nutrients 6, 1752–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosomi A, Goto K, Kondo H, et al. (1998) Localization of alpha‐tocopherol transfer protein in rat brain. Neurosci Lett 256, 159–162. [DOI] [PubMed] [Google Scholar]
- Johnson EJ, Vishwanathan R, Johnson MA, et al. (2013) Relationship between serum and brain carotenoids, alpha‐tocopherol, and retinol concentrations and cognitive performance in the oldest old from the georgia centenarian study. J Aging Res 2013, 951786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas D, Escudero B, Ligos JM, et al. (2009) Altered hematopoiesis in mice lacking DNA polymerase mu is due to inefficient double‐strand break repair. PLoS Genet 5, e1000389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lykkesfeldt J, Moos T (2005) Age‐dependent change in Vitamin C status: a phenomenon of maturation rather than of ageing. Mech Ageing Dev 126, 892–898. [DOI] [PubMed] [Google Scholar]
- Mangas A, Covenas R, Bodet D, et al. (2009) Vitamins in the monkey brain: An immunocytochemical study. J Chem Neuroanat 38, 1–8. [DOI] [PubMed] [Google Scholar]
- Manor D, Morley S (2007) The alpha‐tocopherol transfer protein. Vitam Horm 76, 45–65. [DOI] [PubMed] [Google Scholar]
- Marcos P, Arroyo‐Jimenez MM, Lozano G, et al. (2013) Mapping of tyrosine hydroxylase in the diencephalon of alpaca (Lama pacos) and co‐distribution with somatostatin‐28 (1‐12). J Chem Neuroanat 50–51, 66–74. [DOI] [PubMed] [Google Scholar]
- Marin T, Contreras P, Castro JF, et al. (2014) Vitamin E dietary supplementation improves neurological symptoms and decreases c‐Abl/p73 activation in Niemann‐Pick C mice. Nutrients 6, 3000–3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A, Janigian D, Shukitt‐Hale B, et al. (1999) Effect of vitamin E intake on levels of vitamins E and C in the central nervous system and peripheral tissues: implications for health recommendations. Brain Res 845, 50–59. [DOI] [PubMed] [Google Scholar]
- Mocchegiani E, Costarelli L, Giacconi R, et al. (2014) Vitamin E‐gene interactions in aging and inflammatory age‐related diseases: implications for treatment. A systematic review. Ageing Res Rev 14, 81–101. [DOI] [PubMed] [Google Scholar]
- Mun GH, Kim MJ, Lee JH, et al. (2006) Immunohistochemical study of the distribution of sodium‐dependent vitamin C transporters in adult rat brain. J Neurosci Res 83, 919–928. [DOI] [PubMed] [Google Scholar]
- Oke AF, May L, Adams RN (1987) Ascorbic acid distribution patterns in human brain. A comparison with nonhuman mammalian species. Ann N Y Acad Sci 498, 1–12. [DOI] [PubMed] [Google Scholar]
- Oyarce K, Silva‐Alvarez C, Ferrada L, et al. (2017) SVCT2 is expressed by cerebellar precursor cells, which differentiate into neurons in response to ascorbic acid. Mol Neurobiol 54, 1–14. https://doi.org/10.1007/s12035-016-0366-5 [DOI] [PubMed] [Google Scholar]
- Patel V, Rink C, Khanna S, et al. (2011) Tocotrienols: the lesser known form of natural vitamin E. Indian J Exp Biol 49, 732–738. [PMC free article] [PubMed] [Google Scholar]
- Qian J, Wilson K, Nava P, et al. (2004) Intracellular localization of alpha‐tocopherol transfer protein and alpha‐tocopherol. Ann N Y Acad Sci 1031, 330–331. [DOI] [PubMed] [Google Scholar]
- Qiu S, Li L, Weeber EJ, et al. (2007) Ascorbate transport by primary cultured neurons and its role in neuronal function and protection against excitotoxicity. J Neurosci Res 85, 1046–1056. [DOI] [PubMed] [Google Scholar]
- Rati Selvaraju T, Khaza'ai H, Vidyadaran S, et al. (2014) Cytoprotective effect of tocotrienol‐rich fraction and alpha‐tocopherol vitamin E isoforms against glutamate‐induced cell death in neuronal cells. Int J Vitam Nutr Res 84, 140–151. [DOI] [PubMed] [Google Scholar]
- Rebec GV, Pierce RC (1994) A vitamin as neuromodulator: ascorbate release into the extracellular fluid of the brain regulates dopaminergic and glutamatergic transmission. Prog Neurobiol 43, 537–565. [DOI] [PubMed] [Google Scholar]
- Rice ME (2000) Ascorbate regulation and its neuroprotective role in the brain. Trends Neurosci 23, 209–216. [DOI] [PubMed] [Google Scholar]
- Selvaraju TR, Khaza'ai H, Vidyadaran S, et al. (2014) The neuroprotective effects of tocotrienol rich fraction and alpha tocopherol against glutamate injury in astrocytes. Bosn J Basic Med Sci 14, 195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector R, Johanson CE (2007) Vitamin transport and homeostasis in mammalian brain: focus on Vitamins B and E. J Neurochem 103, 425–438. [DOI] [PubMed] [Google Scholar]
- Ulatowski L, Parker R, Warrier G, et al. (2014) Vitamin E is essential for Purkinje neuron integrity. Neuroscience 260, 120–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatassery GT, Angerhofer CK, Knox CA (1984) Effect of age on vitamin E concentrations in various regions of the brain and a few selected peripheral tissues of the rat, and on the uptake of radioactive vitamin E by various regions of rat brain. J Neurochem 43, 409–412. [DOI] [PubMed] [Google Scholar]
- Venkateshappa C, Harish G, Mythri RB, et al. (2012) Increased oxidative damage and decreased antioxidant function in aging human substantia nigra compared to striatum: implications for Parkinson's disease. Neurochem Res 37, 358–369. [DOI] [PubMed] [Google Scholar]
- Warner TA, Kang JQ, Kennard JA, et al. (2015) Low brain ascorbic acid increases susceptibility to seizures in mouse models of decreased brain ascorbic acid transport and Alzheimer's disease. Epilepsy Res 110, 20–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeoman M, Scutt G, Faragher R (2012) Insights into CNS ageing from animal models of senescence. Nat Rev Neurosci 13, 435–445. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Control of primary antibodies.
Table S1. Antibodies used in this study.


