Abstract
This research aimed to explore the effect of augmenter of liver regeneration (ALR) in acute pancreatitis (AP) of mice and the underlying mechanism. Caerulein were given to mice to get AP models. AP mice were given saline, ALR plasmids or negative control plasmids. Then, pancreas tissues were fixed and stained for histological examination. The levels of serum amylase, serum lipase, MPO, HMGB1, TNF-α, IL-1β as well as MCP-1 were detected by ELISA assay. The mRNA levels of TLR4, p65, IκBα, iNOS, COX-2 and GAPDH were examined by RT-qPCR. The protein levels of HMGB1, TLR4, MD2, MyD88, IκBα and GAPDH were detected by western blotting. ALR decreased serum amylase as well as lipase levels and alleviated the histopathological alterations of the pancreas in AP mice. ALR decreased the MPO activity of pancreas in AP Mice. ALR decreased the HMGB1/TLR4 signaling pathway in AP Mice. ALR decreased pancreas IL-1β and MCP-1 in AP mice, and also decreased plasma TNF-α and IL-1β in AP mice. ALR attenuated the cerulein-caused increase in p65 mRNA and protein levels, but had no effects on mRNA and protein levels of IκBα. The AP mice significantly promoted the mRNA levels of iNOS and COX-2 that was inhibited by ALR. HNE formation was also increased in AP mice, but it was decreased by ALR. ALR alleviates acute pancreatitis by inhibiting HMGB1/TLR4/NF-κB signaling pathway. It is promising to alleviate the syndromes of patients with acute via targeting ALR.
Keywords: Augmenter of liver regeneration (ALR), acute pancreatitis (AP), HMGB1, TLR4/NF-κB signaling pathway
Introduction
Pancreatitis is a kind of rare, early-onset genetic disease featured with pain in the epigastrium as well as various severe complications [1]. Acute pancreatitis is a disorder with different severity. Some patients with acute pancreatitis have mild and self-limited syndromes, but others have a more serious, highly morbid, as well as frequently deadly attack [2]. Acute pancreatitis is a life-threatening disorder and the correct mechanism remains unclear about how local pancreas inflammation can develop into systemic illness. Under normal circumstances, acinar cell damage is the early events of acute pancreatitis. Afterwards, the damage of acinar cells result in local immune activation of the pancreas, then leading to systemic illness of the patients [3].
Augmenter of liver regeneration (ALR) is a kind of sulfhydryl oxidase enzyme, which is expressed in all of mammalian tissues [4]. ALR exhibits strong cellular protection and pro-survival functions. More and more reports demonstrate that ALR is a protector of cells from apoptosis caused by H2O2, tumor cells in particular [5]. Subsequent evidences showed that ALR have far-reaching influence on basic biological processes like energy transduction, cell viability and regeneration, metabolic balance, iron metabolism, as well as maintenance of stem cells [6]. In addition, depletion of ALR in mitochondrial ALR raises oxidative stress, decreases adenosine triphosphate rapidly, as well as leads to cell death via apoptosis and necrosis [7]. However in some other conditions, ALR attenuates apoptosis in activated lymphocytes [8], renal proximal tubular cells, neuroblastoma cells, glioma, and skeletal muscle cells [9]. Moreover, in primary hepatocytes, ALR protects against apoptosis from various stimuli [10].
In consideration of the cellular protection and prosurvival functions of ALR, we hypothesized that ALR could alleviates acute pancreatitis in mice. This research aimed to investigate the role of ALR in acute pancreatitis of mice and the underlying mechanism.
Materials and methods
Animal model and experiment grouping design
A total of 32 three-week old adult SD mice which weigh 120-170 g were purchased from the Laboratory Animal Center of the Xi’an Jiaotong University (Xi’an, China). All mice were treated and fed as previously reported [11]. To get acute pancreatitis model, 6 doses of cerulein (50 μg/kg, MedChem Express, NJ, USA) at 1-hour intervals were intraperitoneally injected to mice. Mice were divided into 4 groups at random: control group, AP group, AP+NC group, AP+ALR group. In control group, mice were treated with 0.9% NaCl intraperitoneally rather than cerulein. In AP+ALR group, the ALR plasmid (10 mg/kg, Genepharm Company, Shanghai, China), was injected into the tail vein of mice 6 hours before cerulein treatment. In AP+NC group, a negative control plasmid (10 mg/kg, Genepharm Company, Shanghai, China) was used in the same way as the ALR plasmid.
Sample collection and preparation
Animals were sacrificed under anesthesia by intraperitoneal injection of pentobarbital sodium; their pancreas was dissected immediately. Blood samples were gathered before and 12 and 24 hours after first cerulein injection. After mice sacrifice, pancreas were collected for further detection: a part of the pancreas were fixed with 4% paraformaldehyde, others were stored at -80°C for protein or RNA investigation.
Serum amylase as well as lipase assay
Blood was obtained by retroorbital bleed. Serum amylase as well as lipase were determined via enzymatic methods. Activity of lipase and amylase was determined using lipase kits (Nanjing Jiancheng Corp., Nanjing, China) and amylase kits (Zhongsheng Beikong Bio-Technology, Beijing, China). All procedures were done on the basis of the kit instructions.
Histological examination
Paraffin sections of pancreas tissues were treated with hematoxylin and eosin, then detected via a light microscope. Histopathological analysis of slides was made by two pathologists blindly.
Myeloperoxidase (MPO) assay
To carry out the assays, pancreas tissue samples were thawed and homogenized in normal saline. The tissue homogenate was assayed for MPO activity with test kits. All procedures were performed on the basis of the instructions of test kits (Nanjing Jiancheng Corp, Nanjing, China).
Immunohistochemistry
Paraffin sections of pancreas tissues was deparaffinized as well as rehydrated with graded ethanol. After antigen retrieval using citric acid, the tissues were washed using PBS for three tomes. Then tissues were blocked with blocking solution at room temperature for 20 minutes. Afterwards, slides were put into primary antibody overnight at 4°C. On the following day, slides were put into the secondary antibody for 45 minutes at 37°C. In the end, slides were counterstained with hematoxylin before being observed via a light microscope.
Enzyme-linked immunosorbent assays (ELISA)
In order to detect the extents of NF-κB p65, TNF-α, IL-1β as well as MCP-1, enzyme-linked immunosorbent assays (ELISA) kits (Cell Signaling Technology, USA, for NF-κB p65; RayBiotech®, GA, USA, for TNF-α, IL-1β as well as MCP-1) were used.
Real-time reverse transcriptase-PCR (RT-PCR)
Total RNA was isolated from cells as well as frozen tissues via Trizol reagent (Invitrogen, Carlsbad, CA). Each measurement was performed in triplicate and a dissociation curve analysis was conducted for each PCR. GAPDH served as internal standards for miRNA and mRNA, respectively. Relative quantification of RNA expression was calculated via the 2-ΔΔCt method. The primer sequences used to amplify mRNAs were shown in Table 1.
Table 1.
RT-PCR primers’ sequence details
| Gene name | Forward primers (3’-5’) | Reverse primer (5’-3’) |
|---|---|---|
| TLR4 | ACATCAGAGGAAGAACAAGAAGCA | CGGAAATTGTAAACATAATGGGTTT |
| p65 | CATGCGTTTCCGTTACAAGTGCGA | TGGGTGCGTCTTAGTGGTATCTGT |
| IκBα | TGGCCTTCCTCAACTTCCAGAACA | TCAGGATCACAGCCAGCTTTCAGA |
| iNOS | GGACCACCTCTATCAGGAA | CCTCATGATAACGTTTCTGGC |
| COX-2 | CTTCGGGAGCACAACAGAG | GCGGATGCCAGTGATAGAG |
| GAPDH | TGCACCACCAACTGCTTAGC | GGCATGGACTGTGGTCATGAG |
[TLR4: Toll-like receptor 4; p65: subunit p65 of nuclear factor kappa B; IκBα: I kappa B alpha protein; iNOS: inducible oxide nitric synthase; COX-2: cyclooxygenase-2; GAPDH: glyceraldehyde-3-phosphate dehydrogenase].
Western blot (WB)
Pancreatic tissues were homogenized with RIPA buffer (Beyotime Biotechnology, Beijing, China) containing PMSF (Beyotime Biotechnology, Beijing, China) as well as protease inhibitors (Roche, Shanghai, China). After detection of protein concentrations, a total of 50 μg proteins were separated via 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis before transferred to PVDF membranes. PVDF membranes were incubated with 5% none-fat milk at room temperature for 1 hour and put into primary antibody at 4°C overnight. On the following day, PVDF membranes were put into secondary antibody for 2 hour at 4°C. In the end, PVDF membranes were developed via the ECL detection system.
Statistical analyses
Statistical analysis was done via the SPSS 16.0 software. Results were showed as mean ± SD. One-way ANOVA or Student’s t test were applied for analysis of data. P < 0.05 was considered as statistically significant.
Results
ALR decreased serum amylase as well as lipase levels and alleviated the histopathological alterations of the pancreas in AP mice
Serum amylase as well as lipase are most frequently regarded as serum markers of AP, which can represent the severity of AP. As shown in Figure 1, ALR significantly decreased serum amylase as well as lipase levels. Then we examined the histopathological alterations of the pancreas. Normal pancreatic architectures were seen in the control group. Compared with the AP group, we found that ALR plasmids notably improved the histological features of pancreatic injury, featured by lower degree of edema, less inflammatory cell infiltration, as well as alleviated acinar cell necrosis (Figure 2).
Figure 1.
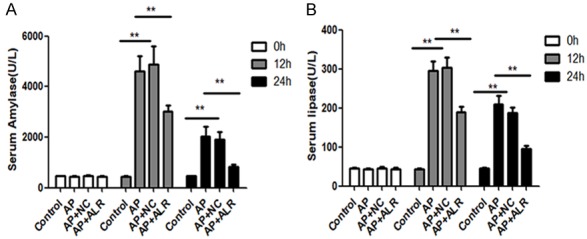
Effect of ALR (augmenter of liver regeneration) on serum amylase and lipase levels in AP (acute pancreatitis). Blood samples were collected before and 12 and 24 hours after first caerulein injection for amylase and lipase analysis. Data are represented as mean ± SD (n = 8 per group). **p < 0.01.
Figure 2.
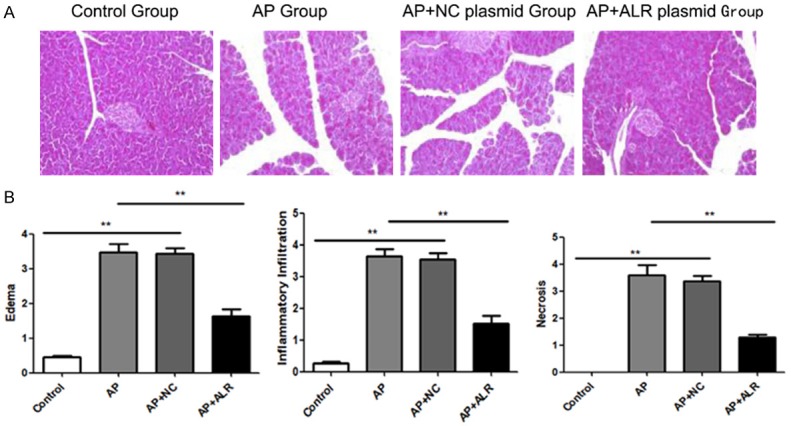
Effect of ALR (augmenter of liver regeneration) on pancreas histology in AP (acute pancreatitis). Pancreas was dissected 24 hours after first caerulein injection. (A) Representative HE staining and (B) histological scores of pancreas are shown. Data are represented as mean ± SD (n = 8 per group). **p < 0.01.
ALR reduced the MPO level of pancreas in AP mice
MPO level was detected to judge the neutrophil infiltration into the damaged pancreas tissues. As shown in Figure 3, mice with AP showed increased MPO activity in pancreas; however, ALR significantly reduced the MPO activity in pancreas (Figure 3C). Similar changes were also observed at the immunohistochemical staining of MPO in pancreatic tissues (Figure 3A and 3B).
Figure 3.
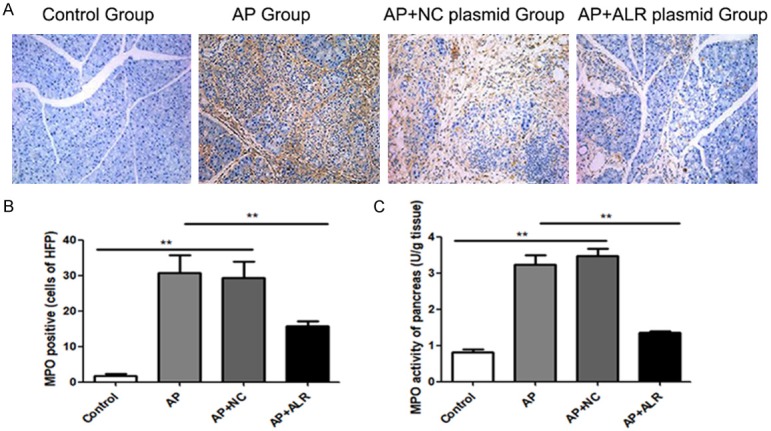
Effect of ALR (augmenter of liver regeneration) on the neutrophil infiltration in pancreas. MPO activity in the pancreas was examined to evaluate the neutrophil infiltration into the damaged tissue. A. Representative Immunohistochemistry images for Myeloperoxidase (MPO) in the pancreas. B. The frequencies of MPO positive cell in pancreas. C. MPO activity of pancreas. Data are represented as mean ± SD (n = 8 per group). **P < 0.01.
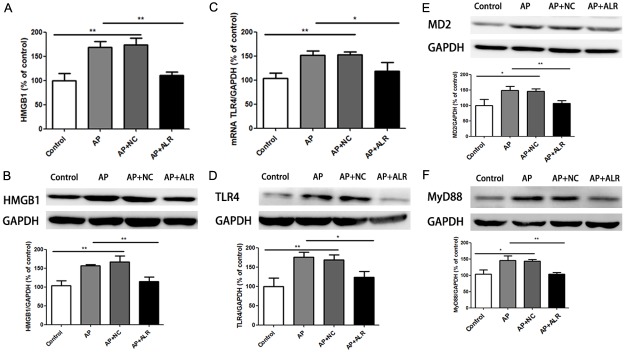
ALR decreased the HMGB1/TLR-4 signaling pathway in AP mice
HGMB1, a kind of danger signaling cytokine, activates TLR4 endogenously. Firstly, we detected main influences of cerulein exposure as well as ALR plasmid in HMGB1 expression. Higher expression of HMGB1 in AP mice was attenuated by ALR group (Figure 4A and 4B). Meantime, there was an increase in both TLR4 mRNA and protein expression in AP group, while ALR reduced the effect on TLR-4 mRNA and protein expression (Figure 4C and 4D). Myeloid differentiation protein-2 (MD2) is the co-receptor of TLR4, and the myeloid differentiation factor 88 (MyD88) works as an adapter protein of the TLR4 signaling pathway in the cell. Meanwhile, we detected the expression of MD2 and MyD88. Cerulein exposure increased the protein expression of MD2, while ALR reduced the cerulein-induced upregulation in MD2 protein expression (Figure 4E). For MyD88 protein expression, ALR had the same effect (Figure 4F).
Figure 4.
Effects of ALR (augmenter of liver regeneration) in the HMGB1/TLR4 signaling pathway in AP (acute pancreatitis) mice. A. HMGB1 levels measured by enzyme-linked immunosorbent assays. B. HMGB1 levels by western blot. C. Relative mRNA levels of TLR4. D. Protein levels of TLR4 levels by western blot. E. Protein levels of MD2 by western blot. F. Protein levels of MyD88 by western blot. Data are represented as mean ± SD (n = 8 per group). *P < 0.05; **P < 0.01.
Effects of ALR in plasma and pancreas TNF-α, IL-1β as well as MCP-1
The levels of TNF-α protein were higher in pancreas of mice under cerulein treatment, but were not inhibited by ALR (Figure 5A). But we found a noticeable increase of TNF-α levels in plasma of AP mice, which could be inhibited by ALR (Figure 5B). AP mice had an increase in pancreas IL-1β protein levels, which was attenuated by ALR (Figure 5C). In plasma, cerulein also increased plasma IL-1β levels, an effect that was inhibited by ALR (Figure 5D). Regarding MCP-1 levels, AP mice showed higher levels of MCP-1 in pancreas and ALR attenuated this effect (Figure 5E).
Figure 5.
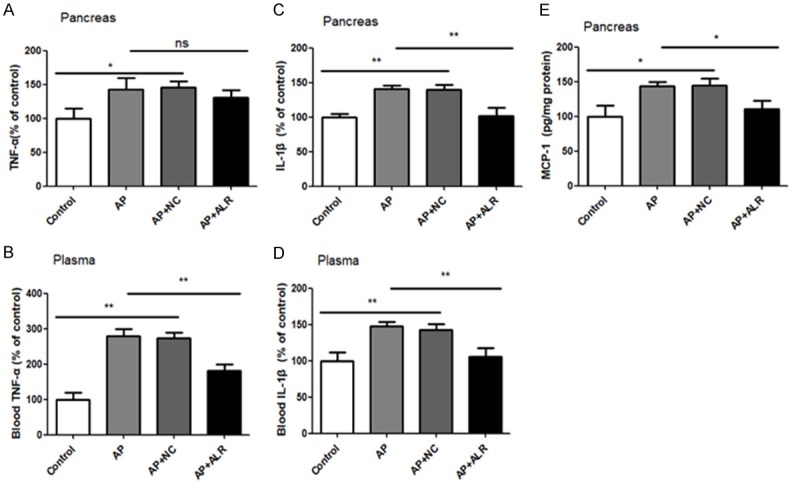
Release of TNF-α, IL-1β and MCP-1 in pancreas and/or plasma after individual treatments. Enzyme-linked immunosorbent assay (ELISA) data of pancreas of TNF-α (A), IL-β (C) and MCP-1 (E). ELISA-detected plasma levels of TNF-α (B) and IL-β (D). Data are represented as mean ± SD (n = 8 per group). *P < 0.05; **P < 0.01; ns, none significant.
Effect of ALR in NF-κB signaling pathway in AP mice
In the pancreas of AP mice, there was an increase of NF-κB p65 mRNA levels, and this increase could be partially inhibited by ALR (Figure 6A). The NF-κB p65 activity was also increased in the pancreas of AP mice, which could be counteracted by ALR (Figure 6B). Meanwhile, AP mice showed increased mRNA and protein expression of IκBα. However, ALR could not inhibit the increase in mRNA and protein levels of IκBα (Figure 6C and 6D).
Figure 6.
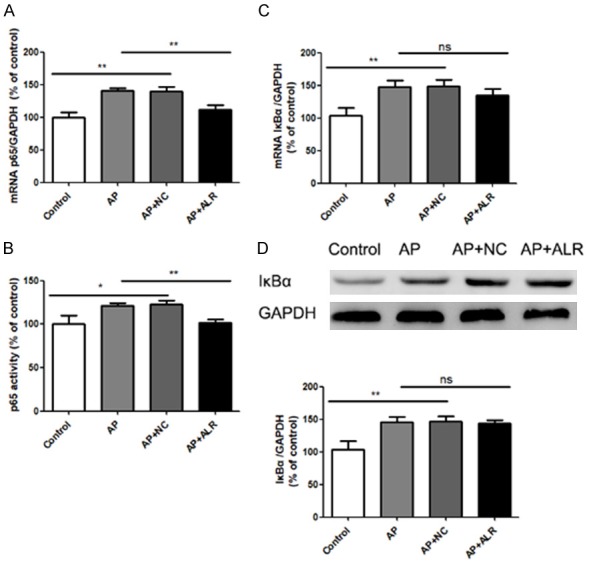
Activation of pancreas NFκB in AP (acute pancreatitis) mice and effects of ALR (augmenter of liver regeneration) treatment. A. Relative mRNA levels of NFκB p65. B. NFκB p65 activity measured by an ELISA-based kit. C. Relative mRNA levels of IκBα. D. Protein levels of IκBα measured by western blot. Data are represented as mean ± SD (n = 8 per group). *P < 0.05; **P < 0.01; ns, none significant.
Effect of ALR in iNOS and COX-2 expression as well as lipid peroxidation in AP mice
NF-κB activation leads to the generation of inflammatory mediators, like iNOS and COX-2. AP mice showed a vital promotion in the iNOS mRNA level, which was attenuated by ALR (Figure 7A). At the same time, COX-2 expression had the same trend (Figure 7B). On account of an over-activation of pro-inflammatory signaling pathways, lipid peroxides generated because of activated cellular oxidative stress have a bad influence on cell viability. HNE adduct is a reactive compound produced via lipid peroxides decomposition. AP mice showed an increase in the HNE formation in pancreas, while ALR decreased the formation of HNE (Figure 7C).
Figure 7.
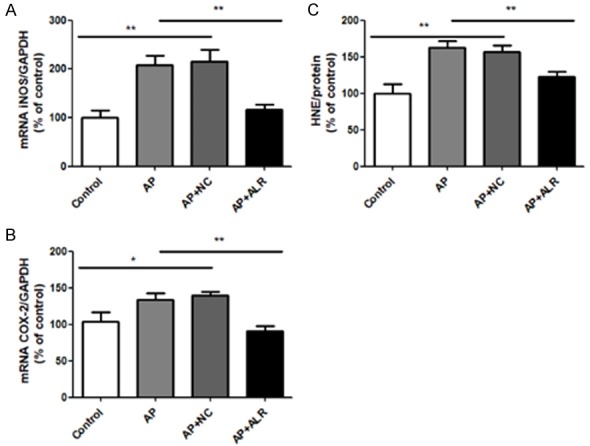
Role of ALR (augmenter of liver regeneration) in iNOS and COX-2 expressions, and lipid peroxidation in AP (acute pancreatitis) mice. A. Relative mRNA levels of iNOS. B. Relative mRNA levels of COX-2. C. Relative levels of 4-hydroxynonenal (4-HNE), a natural product of lipid peroxidation. Data are represented as mean ± SD (n = 8 per group). *P < 0.05; **P < 0.01.
Discussions
In this study, we for the first time confirmed that ALR could remarkably alleviate the severity of acute pancreatitis (AP) via inhibiting HMGB1/TLR4/NF-κB signaling pathway. In mice model, AP induced by caerulein injection is similar to the clinical manifestations of human AP [12,13]. In this current research, we detected the effect of ALR in AP of mice. Our research showed that ALR notably improved the pancreatic injury in AP, as characterized by the results of histological characteristics, MPO activity, and serum amylase and lipase levels. In addition, ALR may regulate the severity of acute pancreatitis via inhibiting HMGB1/TLR4/NF-κB signaling pathway.
Augmenter of liver regeneration (ALR) was first recognized in regenerating rat livers [14], thereby destining the protein for inclusion in an ever-expanding constellation of hepatic growth factors [15]. However, the later identification of ALR as a protein with significant homology to yeast Erv1 [16,17] hinted at a more elemental function. Subsequent studies revealed short (15 kDa) and long (22 kDa) forms of this ancient protein, with profound effects on fundamental processes such as energy transduction, cell survival and regeneration, metabolic homeostasis, iron metabolism, and stem cell maintenance. In this study, data showed that ALR acted as a protector of cerulean-induced acute pancreatitis in mice, which was chiefly reflected in improvements of histological features, MPO activity, as well as serum amylase and lipase levels after ALR treatment (Figures 1, 2 and 3).
The high-motility group box protein 1 (HMGB1) was firstly regarded as a non-histone DNA-binding nuclear protein, connecting with intracellular as well as extracellular activities [18-22]. Extracellular HMGB1 has cytokine-like properties [19,22]. It can trigger the release of many other cytokines, like tumor necrosis factor-α (TNF-α), interleukin (IL)-1β as well as IL-6, which are involved with mediating the inflammatory action [23,24]. It has been previously reported that HMGB1 increases in pancreatic tissue of acute pancreatitis. Moreover, HMGB1 elevation is tightly connected to the severity of acute pancreatitis [25-28]. These reports prove that HMGB1 plays an important part in the occurrence and development of AP.
Toll-like receptor (TLR) 4 is a kind of pattern-recognition receptors, which can be activated by HMGB1 [29,30]. Extracellular HMGB1 that bind to TLR4 leads to the activation of nuclear factor-κ-B (NF-κB) mediated by MyD88. Activated NF-κB is translocated from the cytoplasm to the nucleus, and this translocation causes the increase of inflammatory factors, like TNF-α, IL-1β as well as IL-6 [31-33]. Extracellular HMGB1 plays a role as a damage-related molecular, and it triggers pro-inflammatory signal pathways via the activation of pattern recognition receptors, like TLR2/4 [34,35]. It has been reported that TLR4 plays a vital part in the pathogenesis of acute lung injury, which is mediated by HMGB1 [18,36]. Meanwhile, TLR4 is widely expressed in pancreas tissues, and is involved with pancreatic damage during AP [37-39]. The activation of HMGB1/TLR4/ NF-κB signaling pathway results in the expression of various inflammatory factors [18,36]. Superfluous inflammation responses mediated by inflammatory cytokines plays a vital role in the occurrence and development of AP. In this study, results showed that HMGB1/TLR4 signaling pathway was activated in AP mice, just as reported before, while ALR treatment provided a protective effect in AP mice via decreasing HMGB1/TLR4 signaling pathway.
Meanwhile, we demonstrated that ALR inhibited the expression as well as the activity of NF-κB p65 subunit, which participates a majority of the transcriptional activity of NF-κB, but ALR did not affect the expression of IkBα, which is the NF-κB inhibitory protein. NF-κB p65 subunit translocation from cytoplasm to the nucleus triggers the transcription of various pro-inflammatory cytokines. These released cytokines play a role in the cytoplasm and triggers NF-κB again in a way of amplified inflammatory responses. Though lkBα is the NF-κB inhibitory protein, noteworthy persistent increases in IkBα may result in the reducing of proinflammatory gene induction later on, as IkBα inhibits NF-κB in the cytoplasm. MCP-1 is produced via NF-κB transcriptional activity, and it mediates the migration of immune cells to damage cells. We demonstrated that ALR decreases the production of IL-1β, TNF-α as well as MCP-1. In AP model, increased NF-κB activity also promoted the expression of COX-2 and iNOS, as well as the production of 4-HNE, and these promotion were attenuated by ALR pretreatment.
In a word, in this research we illuminate that ALR takes effect in alleviating acute pancreatitis and provide related mechanisms, which is notably favorable for the prevention and treatment for AP. In years to come, combination therapy including ALR may provide a new possible molecular treatment for patients with AP.
Acknowledgements
This work was supported by Scientific Research Fund Project of Shaanxi Provincial Health and Family Planning Commission (no. 2016D015), Science and Technology Planning Project of Xi’an (no. 2016045SF/YX01(2)) and Shaanxi Province Key Scientific and Technological Project (no. 2016SF-232).
Disclosure of conflict of interest
None.
References
- 1.Besselink MG, van Santvoort HC, Buskens E, Boermeester MA, van Goor H, Timmerman HM, Nieuwenhuijs VB, Bollen TL, van Ramshorst B, Witteman BJ, Rosman C, Ploeg RJ, Brink MA, Schaapherder AF, Dejong CH, Wahab PJ, van Laarhoven CJ, van der Harst E, van Eijck CH, Cuesta MA, Akkermans LM, Gooszen HG Dutch Acute Pancreatitis Study Group. Probiotic prophylaxis in predicted severe acute pancreatitis: a randomised, double-blind, placebo-controlled trial. Lancet. 2008;371:651–659. doi: 10.1016/S0140-6736(08)60207-X. [DOI] [PubMed] [Google Scholar]
- 2.Banks PA, Freeman ML Practice Parameters Committee of the American College of Gastroenterology. Practice guidelines in acute pancreatitis. Am J Gastroenterol. 2006;101:2379–2400. doi: 10.1111/j.1572-0241.2006.00856.x. [DOI] [PubMed] [Google Scholar]
- 3.Granger J, Remick D. Acute pancreatitis: models, markers, and mediators. Shock. 2005;24(Suppl 1):45–51. doi: 10.1097/01.shk.0000191413.94461.b0. [DOI] [PubMed] [Google Scholar]
- 4.Hagiya M, Francavilla A, Polimeno L, Ihara I, Sakai H, Seki T, Shimonishi M, Porter KA, Starzl TE. Cloning and sequence analysis of the rat augmenter of liver regeneration (ALR) gene: expression of biologically active recombinant ALR and demonstration of tissue distribution. Proc Natl Acad Sci U S A. 1994;91:8142–8146. doi: 10.1073/pnas.91.17.8142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kallergi E, Kalef-Ezra E, Karagouni-Dalakoura K, Tokatlidis K. Common players in mitochondria biogenesis and neuronal protection against stress-induced apoptosis. Neurochem Res. 2014;39:546–555. doi: 10.1007/s11064-013-1109-x. [DOI] [PubMed] [Google Scholar]
- 6.Nalesnik MA, Gandhi CR, Starzl TE. Augmenter of liver regeneration: a fundamental life protein. Hepatology. 2017;66:266–270. doi: 10.1002/hep.29047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thirunavukkarasu C, Wang LF, Harvey SA, Watkins SC, Chaillet JR, Prelich J, Starzl TE, Gandhi CR. Augmenter of liver regeneration: an important intracellular survival factor for hepatocytes. J Hepatol. 2008;48:578–588. doi: 10.1016/j.jhep.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang N, Sun H, Shen Y, Li XF, Pan T, Liu GL, Liu Q. Augmenter of liver regeneration inhibits apoptosis of activated human peripheral blood lymphocytes in vitro. Immunopharmacol Immunotoxicol. 2013;35:257–263. doi: 10.3109/08923973.2013.764502. [DOI] [PubMed] [Google Scholar]
- 9.Polimeno L, Rossi R, Mastrodonato M, Montagnani M, Piscitelli D, Pesetti B, De Benedictis L, Girardi B, Resta L, Napoli A, Francavilla A. Augmenter of liver regeneration, a protective factor against ROS-induced oxidative damage in muscle tissue of mitochondrial myopathy affected patients. Int J Biochem Cell Biol. 2013;45:2410–2419. doi: 10.1016/j.biocel.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 10.Ilowski M, Kleespies A, de Toni EN, Donabauer B, Jauch KW, Hengstler JG, Thasler WE. Augmenter of liver regeneration (ALR) protects human hepatocytes against apoptosis. Biochem Biophys Res Commun. 2011;404:148–152. doi: 10.1016/j.bbrc.2010.11.083. [DOI] [PubMed] [Google Scholar]
- 11.Pan LF, Yu L, Wang LM, He JT, Sun JL, Wang XB, Bai ZH, Wang H, Yan TL, Pei HH. The Toll-like receptor 4 antagonist TAK-242 protects against chronic pancreatitis in rats. Mol Med Rep. 2017;16:3863–3868. doi: 10.3892/mmr.2017.7105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mareninova OA, Sung KF, Hong P, Lugea A, Pandol SJ, Gukovsky I, Gukovskaya AS. Cell death in pancreatitis: caspases protect from necrotizing pancreatitis. J Biol Chem. 2006;281:3370–3381. doi: 10.1074/jbc.M511276200. [DOI] [PubMed] [Google Scholar]
- 13.Gukovskaya AS, Pandol SJ. Cell death pathways in pancreatitis and pancreatic cancer. Pancreatology. 2004;4:567–586. doi: 10.1159/000082182. [DOI] [PubMed] [Google Scholar]
- 14.LaBrecque DR, Pesch LA. Preparation and partial characterization of hepatic regenerative stimulator substance (SS) from rat liver. J Physiol. 1975;248:273–284. doi: 10.1113/jphysiol.1975.sp010973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Starzl TE, Jones AF, Terblanche J, Usui S, Porter KA, Mazzoni G. Growth-stimulating factor in regenerating canine liver. Lancet. 1979;1:127–130. doi: 10.1016/s0140-6736(79)90519-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hagiya M, Francavilla A, Polimeno L, Ihara I, Sakai H, Seki T, Shimonishi M, Porter KA, Starzl TE. Cloning and sequence analysis of the rat augmenter of liver regeneration (ALR) gene: expression of biologically active recombinant ALR and demonstration of tissue distribution. Proc Natl Acad Sci U S A. 1995;92:3076. doi: 10.1073/pnas.92.7.3076d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lisowsky T, Weinstat-Saslow DL, Barton N, Reeders ST, Schneider MC. A new human gene located in the PKD1 region of chromosome 16 is a functional homologue to ERV1 of yeast. Genomics. 1995;29:690–697. doi: 10.1006/geno.1995.9950. [DOI] [PubMed] [Google Scholar]
- 18.Yang Z, Deng Y, Su D, Tian J, Gao Y, He Z, Wang X. TLR4 as receptor for HMGB1-mediated acute lung injury after liver ischemia/reperfusion injury. Lab Invest. 2013;93:792–800. doi: 10.1038/labinvest.2013.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li J, Wang H, Mason JM, Levine J, Yu M, Ulloa L, Czura CJ, Tracey KJ, Yang H. Recombinant HMGB1 with cytokine-stimulating activity. J Immunol Methods. 2004;289:211–223. doi: 10.1016/j.jim.2004.04.019. [DOI] [PubMed] [Google Scholar]
- 20.Andersson U, Erlandsson-Harris H, Yang H, Tracey KJ. HMGB1 as a DNA-binding cytokine. J Leukoc Biol. 2002;72:1084–1091. [PubMed] [Google Scholar]
- 21.Dumitriu IE, Baruah P, Manfredi AA, Bianchi ME, Rovere-Querini P. HMGB1: guiding immunity from within. Trends Immunol. 2005;26:381–387. doi: 10.1016/j.it.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 22.Yang H, Wang H, Czura CJ, Tracey KJ. HMGB1 as a cytokine and therapeutic target. J Endotoxin Res. 2002;8:469–472. doi: 10.1179/096805102125001091. [DOI] [PubMed] [Google Scholar]
- 23.Lotze MT, Zeh HJ, Rubartelli A, Sparvero LJ, Amoscato AA, Washburn NR, Devera ME, Liang X, Tor M, Billiar T. The grateful dead: damage-associated molecular pattern molecules and reduction/oxidation regulate immunity. Immunol Rev. 2007;220:60–81. doi: 10.1111/j.1600-065X.2007.00579.x. [DOI] [PubMed] [Google Scholar]
- 24.Kim JY, Park JS, Strassheim D, Douglas I, Diaz del Valle F, Asehnoune K, Mitra S, Kwak SH, Yamada S, Maruyama I, Ishizaka A, Abraham E. HMGB1 contributes to the development of acute lung injury after hemorrhage. Am J Physiol Lung Cell Mol Physiol. 2005;288:L958–965. doi: 10.1152/ajplung.00359.2004. [DOI] [PubMed] [Google Scholar]
- 25.Yuan H, Jin X, Sun J, Li F, Feng Q, Zhang C, Cao Y, Wang Y. Protective effect of HMGB1 a box on organ injury of acute pancreatitis in mice. Pancreas. 2009;38:143–148. doi: 10.1097/MPA.0b013e31818166b4. [DOI] [PubMed] [Google Scholar]
- 26.Zhang ZW, Zhang QY, Zhou MT, Liu NX, Chen TK, Zhu YF, Wu L. Antioxidant inhibits HMGB1 expression and reduces pancreas injury in rats with severe acute pancreatitis. Dig Dis Sci. 2010;55:2529–2536. doi: 10.1007/s10620-009-1073-0. [DOI] [PubMed] [Google Scholar]
- 27.Sawa H, Ueda T, Takeyama Y, Yasuda T, Shinzeki M, Nakajima T, Kuroda Y. Blockade of high mobility group box-1 protein attenuates experimental severe acute pancreatitis. World J Gastroenterol. 2006;12:7666–7670. doi: 10.3748/wjg.v12.i47.7666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luan ZG, Zhang XJ, Yin XH, Ma XC, Zhang H, Zhang C, Guo RX. Downregulation of HMGB1 protects against the development of acute lung injury after severe acute pancreatitis. Immunobiology. 2013;218:1261–1270. doi: 10.1016/j.imbio.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 29.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol. 2007;81:1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- 30.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 31.Noreen M, Shah MA, Mall SM, Choudhary S, Hussain T, Ahmed I, Jalil SF, Raza MI. TLR4 polymorphisms and disease susceptibility. Inflamm Res. 2012;61:177–188. doi: 10.1007/s00011-011-0427-1. [DOI] [PubMed] [Google Scholar]
- 32.Wullaert A. Role of NF-kappaB activation in intestinal immune homeostasis. Int J Med Microbiol. 2010;300:49–56. doi: 10.1016/j.ijmm.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 33.Luo H, Guo P, Zhou Q. Role of TLR4/NF-kappaB in damage to intestinal mucosa barrier function and bacterial translocation in rats exposed to hypoxia. PLoS One. 2012;7:e46291. doi: 10.1371/journal.pone.0046291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hori O, Brett J, Slattery T, Cao R, Zhang J, Chen JX, Nagashima M, Lundh ER, Vijay S, Nitecki D, et al. The receptor for advanced glycation end products (RAGE) is a cellular binding site for amphoterin. Mediation of neurite outgrowth and co-expression of rage and amphoterin in the developing nervous system. J Biol Chem. 1995;270:25752–25761. doi: 10.1074/jbc.270.43.25752. [DOI] [PubMed] [Google Scholar]
- 35.Park JS, Svetkauskaite D, He Q, Kim JY, Strassheim D, Ishizaka A, Abraham E. Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J Biol Chem. 2004;279:7370–7377. doi: 10.1074/jbc.M306793200. [DOI] [PubMed] [Google Scholar]
- 36.Deng Y, Yang Z, Gao Y, Xu H, Zheng B, Jiang M, Xu J, He Z, Wang X. Toll-like receptor 4 mediates acute lung injury induced by high mobility group box-1. PLoS One. 2013;8:e64375. doi: 10.1371/journal.pone.0064375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sharif R, Dawra R, Wasiluk K, Phillips P, Dudeja V, Kurt-Jones E, Finberg R, Saluja A. Impact of toll-like receptor 4 on the severity of acute pancreatitis and pancreatitis-associated lung injury in mice. Gut. 2009;58:813–819. doi: 10.1136/gut.2008.170423. [DOI] [PubMed] [Google Scholar]
- 38.Ding SQ, Li Y, Zhou ZG, Wang C, Zhan L, Zhou B. Toll-like receptor 4-mediated apoptosis of pancreatic cells in cerulein-induced acute pancreatitis in mice. Hepatobiliary Pancreat Dis Int. 2010;9:645–650. [PubMed] [Google Scholar]
- 39.Li Y, Zhou ZG, Xia QJ, Zhang J, Li HG, Cao GQ, Wang R, Lu YL, Hu TZ. Toll-like receptor 4 detected in exocrine pancreas and the change of expression in cerulein-induced pancreatitis. Pancreas. 2005;30:375–381. doi: 10.1097/01.mpa.0000160959.21580.41. [DOI] [PubMed] [Google Scholar]