Abstract
Zhuangguguanjie formulation (ZG) can provide noticeable relief from joint pain in patients suffering from knee osteoarthritis (OA). However, the underlying mechanism has not been fully described. Male C57BL/6 mice were administered either ZG or normal saline (NS) following surgical destabilization of the medial meniscus (DMM). At weeks 4, 6 and 8 (post-surgery), knee joints were harvested and assessed with Safranin-O staining. Blood serum was collected and tested. In vitro analysis was carried out to evaluate the effects of ZG on the expression of the OA-related genes. DMM mice indicated reduced cartilage destruction and lower blood serum biomarkers of OA (COMP1 and CTX-1) following ZG treatment. Moreover, the femoral condyle and tibial plateau histological scores were significantly reduced following ZG treatment of the DMM mice. ZG could markedly downregulate the expression of OA-related genes namely, ADAMTS5, MMP3 and MMP13, while it simultaneously upregulated collagen II as demonstrated by in vitro assays. Moreover, chondrocyte apoptosis was significantly decreased following ZG treatment. These results may be caused by the up-regulation of p-AKT expression levels, since the anti-apoptotic effects of ZG can be blocked by treatment with an AKT inhibitor. ZG is capable of preventing and/or reducing the progression of OA by inhibiting chondrocyte apoptosis via the p-AKT/Caspase 3 pathway.
Keywords: Osteoarthritis, knee, zhuangguguanjie, chondrocyte, DMM model
Introduction
Osteoarthritis (OA) is a slowly progressing disease and is considered one of the most prevalent chronic health conditions that notably affect the elderly [1,2]. Approximately 10-12% of the global population have OA [3-5] and more than one-third of the world’s adult population exhibit radiologic signs of OA [6]. The treatment of OA mainly comprises joint reconstruction via an arthroplasty (either hip or knee). However, this type of treatment requires a modification of daily life habits postoperatively and is subject to surgery following a certain period of time [7]. Consequently, the application of less invasive interventions for the treatment of OA has been employed, including pharmaceutical agents such as analgesics and non-steroidal anti-inflammatory drugs [8,9]. Although the efficacy of these drugs has been demonstrated, they frequently cause serious adverse events in the gastrointestinal and/or the cardiovascular systems [10,11].
It has been reported that one out of three OA patients prefers complementary and alternative medical (CAM) treatment in order to avoid the expensive costs and the side effects of conventional non-steroidal anti-inflammatory therapy (NSAIDs) [11,12]. However, a limited number of clinical and basic research studies have been conducted. The traditional Chinese medicine, Zhuangguguanjie (ZG) is already used widely in OA patients in China [13]. Zhang et al. reported partial clinical success using ZG alone and/or in combination with celecoxib for the treatment of OA, lumbar strains, and other conditions [14]. The results indicated that ZG exerts therapeutic potential for OA, although the authors did not explore its underlying mechanism of action. Therefore, the present study aimed to investigate further the underlying mechanism of the preventive effects of ZG against the progression of OA. The data indicated reduced cartilage destruction and lower blood serum biomarkers of OA (COMP1 and CTX-1) in DMM mice following ZG treatment, whereas the mechanism of action involved downregulation of the expression of OA-related genes namely, ADAMTS5, MMP3 and MMP13, upregulation of collagen II, reduction of chondrocyte apoptosis and activation of the Akt signaling pathway.
Materials and methods
Animals
Ten-week-old male C57BL/6 mice, weighing 20 ± 2 g that were raised under pathogen free conditions, were purchased from the Model Animal Research Center of Nanjing University. The animals were maintained in a 12-h light/dark cycle. A total of 96 mice were equally divided to the following groups: Destabilization of the medial meniscus (DMM) + ZG (Sanjiu Medical & Pharmaceutical Co., Ltd., Beijing, China), DMM + Normal saline (NS), Sham + ZG and Sham + NS groups. A total of 24 mice were used per group. The mice were anesthetized by ketamine (50 mg/kg, i.p) and microsurgery was conducted in order to transect the medial meniscotibial ligament and achieve surgical destabilization of the medial meniscus (DMM) as described previously [15]. A similar incision was made to the sham mice, which was subsequently sutured, without any other procedures. Following surgery, both the DMM and the sham groups were administered ZG (468 mg/kg/day) or NS. All mice were allowed to rest for one-week following 4 weeks of ZG/NS treatment (Figure 1). The mice (8 mice for each time point) were sacrificed by cervical dislocation following 4, 6, and 8 weeks of continuous treatment. Surgically-manipulated knee joints were harvested and blood serum was collected for subsequent testing. The experimental protocols were reviewed and approved by the Animal Care Committee of the Nanjing University in accordance with the Institutional Animal Care and Use Committee guidelines.
Figure 1.
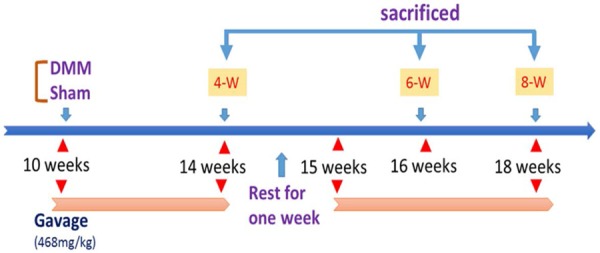
Schematic diagram depicting the time of treatment and the follow-up period of the animals. DMM mice were administered ZG and/or NS.
Specimen preparation and histological evaluation
The tibia and femur tissues with synovium were separated, fixed in 4% paraformaldehyde, washed with water and placed in a 5% EDTA decalcification solution. Following dehydration and clearing, the tissues were embedded in paraffin, and 5 mm thick sections of the knee joint were cut in serial incisions in the coronal plane. The sections were stained with Safranin-O and scored according to the Osteoarthritis Research Society International (OARSI) standards (Supplementary Table 1). The overall degree of degeneration was assessed by estimating the highest observed score (at both the medial femoral condyle and the medial tibial plateau).
TUNEL staining
TUNEL assay was conducted with an in situ cell death detection kit (G3250, Promega, USA) according to the manufacturer’s protocol. Briefly, paraffin-embedded sections were deparaffinized, washed with PBS and pretreated with proteinase K (20 µg/ml) for 15 min. The equilibration buffer was applied directly to each section and incubated for 10 s. Sections were then incubated with the rTdT enzyme in a humidified chamber at 37°C for 1 h. Following labeling, the sections were washed and mounted on coverslips. DNA fragmentation was detected by selecting four fields at 100 × magnification in each section. All images were analyzed by Adobe Photoshop CS2 in order to quantify the signals. The data were expressed as the number of apoptotic cells per high-power field.
Enzyme linked immunosorbent assay (ELISA)
A total of 500 μl of blood was collected from the hearts of the mice following anesthesia. Serum samples were obtained by centrifugation of blood at 3,000 rpm for 10 min and they were stored at -80°C prior to analysis. An ELISA assay was used to measure the plasma concentration of the biomarkers COMP, CTX-1, and MMP-13 and TNF-α (Xinfan, Shanghai, China) according to the manufacturer’s instructions. The optical absorbance at 450 and 570 nm was determined using a microplate absorbance reader (Model 680 Microplate Reader; Bio-Rad).
Chondrocyte cell lines and primary chondrocyte cell culture
SW1353 human chondrosarcoma cells (HTB-94, American Type Culture Collection, USA) were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum and penicillin (100 U/ml). The cells were maintained at 37°C in a humidified incubator under 5% CO2 in air. SW1353 cells were plated in 12-well plates at a density of 1.8 × 105/ml. Following incubation of 10 ng/μl and 20ng/μl for 12 h (the concentration was determined by the Cell Counting Kit (CCK)-8 test, data not shown), total RNA was extracted according to the protocol described.
The primary chondrocyte cells were isolated as described previously [16]. Briefly, femoral heads and tibial plateaus from 12 male mice (C57Bl/6 mice, ages 6 days) were dissected and incubated for 45 min with collagenase D (3 mg/ml in DMEM) at 37°C in 5% CO2. The cartilage samples were incubated overnight with collagenase D (0.5 mg/ml) at 37°C. The resulting chondrocytes were suspended in culture medium (DMEM-Glutamax containing 1 g/L glucose, Gibco) in the presence of 10% fetal calf serum (FCS) and antibiotics. The chondrocytes were subsequently seeded in 10-cm dishes (designated passage 0, P0). The primary chondrocyte cells were plated in 6-well and 12-well plates at a density of 1.8 × 105/ml. Following incubation of 1 μg/ml for 12 and 24 h, respectively (the concentration was determined by the (CCK-8 test, data not shown)), total RNA and protein were extracted.
Flow cytometry
FITC-annexin V/PI double-fluorescence labeling was used in order to monitor the changes in the induction of apoptosis. The assay was conducted by flow cytometry. Primary chondrocytes were collected following treatment with ZG at 0, 1, 5 μg/ml both in the control and/or IL-1β induced chondrocyte groups. Flow cytometry was conducted according to the apoptosis detection kit (Bimake, Houston, USA) protocol that was provided by the manufacturer. Briefly, primary chondrocytes (1 × 106/mL) were harvested by centrifugation, and subsequently incubated in buffer containing FITC-annexin V and PI for 15 min. The number of apoptotic cells was measured by a flow cytometer (FACSCalibur BD, San Jose, CA) within 30 min.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was extracted from the cultured chondrocytes using TRIzol (Takara, Shiga, Japan). cDNA was subsequently synthesized from total RNA using a reverse transcriptase cDNA synthesis kit (Takara, Shiga, Japan), according to the manufacturer’s instructions. Real time PCR was carried out by incubation of cDNA, H2O, primers and SYBR Premix Ex Taq (Takara, Shiga, Japan). The reagents comprised a total volume of 20 μl per reaction. The samples were subjected to 40 cycles of amplification according to the following steps: denaturation at 95°C for 1 min, annealing at 57°C for 30 s and extension at 72°C for 30 s. The PCR reactions were carried out in a 7500 Real-Time PCR System (Applied Biosystems, Waltham, MA, USA). All data were normalized to β-actin by the 2-ΔΔCt method and the assays were carried out in triplicate. The sequences of the forward and reverse primers that were used are indicated in Table 1.
Table 1.
Primer Sequences used for qPCR
| Primer | Forward (5’-3’) | Reverse (5’-3’) |
|---|---|---|
| COL2α1 | TGGACGATCAGGCGAAACC | GCTGCGGATGCTCTCAATCT |
| MMP-1 | AAAATTACACGCCAGATTTGCC | GGTGTGACATTACTCCAGAGTTG |
| MMP-3 | AGTCTTCCAATCCTACTGTTGCT | TCCCCGTCACCTCCAATCC |
| MMP-13 | ACTGAGAGGCTCCGAGAAATG | GAACCCCGCATCTTGGCTT |
| ADAMTS5 | GAACATCGACCAACTCTACTCCG | CAATGCCCACCGAACCATCT |
| Aggrecan | ACTCTGGGTTTTCGTGACTCT | ACACTCAGCGAGTTGTCATGG |
| Bad | AAGTCCGATCCCGGAATCC | GCTCACTCGGCTCAAACTCT |
| Caspase 1 | ACAAGGCACGGGACCTATG | TCCCAGTCAGTCCTGGAAATG |
| Caspase 3 | ATGGAGAACAACAAAACCTCAGT | TTGCTCCCATGTATGGTCTTTAC |
| Caspase 6 | GGAAGTGTTCGATCCAGCCG | GGAGGGTCAGGTGCCAAAAG |
| β-actin | CATGTACGTTGCTATCCAGGC | CTCCTTAATGTCACGCACGAT |
Western blot analysis
The cells were homogenized and lysed in RIPA buffer. Total proteins were extracted by centrifugation at 4°C for 15 min at 13,000 g. Subsequently, total proteins were subjected to 10% SDS-PAGE and transferred onto PVDF membranes (Bio-Rad, Richmond, CA). The membranes were blocked in 4% non-fat milk, and primary antibodies of Collagen II (Abcam, Cambridge, USA), Adamts 5 (Santa Cruz Biotechnology, USA), Akt (Abcam, Cambridge, USA), p-Akt (Abcam, Cambridge, USA), Caspase 3 (Santa Cruz Biotechnology, USA), Cleaved-Caspase 3 (Santa Cruz Biotechnology, USA) and β-actin (Santa Cruz Biotechnology, USA) were added and incubated at 4°C overnight. Goat anti-Rabbit HRP-labeled secondary antibodies (Santa Cruz Biotech) were added and incubated at room temperature for 1 h. Finally, the membranes were scanned with Licor Odyssey equipment (LI-COR Biosciences, USA).
Statistical analysis
The data are expressed as mean ± standard deviation. The Kolmogorov-Smirnov test was used to assess normality of the data. The differences among the 4 groups for the ordinal variables were evaluated by the Chi-squared test, whereas the differences for the continuous variables among the 4 groups of animal treatment were evaluated by the Kruskal Wallis test. The differences for the in vitro experiments that contained 2 groups of treatment were compared by the paired T test and/or Wilcoxon matched pairs test. The analysis was performed using GraphPad Prism 7.0 software (GraphPad Software, La Jolla, CA, USA). A P-value of less than 0.05 was considered statistically significant.
Results
The histological score in OA mice was decreased following ZG treatment
The results indicated that at 4 weeks, the normal saline treatment group (NS-DMM) exhibited increased degeneration at the femoral condyle compared with the sham control group (NS-Sham) (Supplementary Figure 1A). The treatment group that had undergone destabilization of the medial meniscus (ZG-DMM) exhibited significantly lower femoral condyle and tibial plateau maximal histological scores compared with the normal saline treatment group (NS-DMM) (Supplementary Figure 1B, 1C). The difference was higher when the femoral condyle parameter was investigated (approximately 10-fold reduction, Supplementary Figure 1B). No significant differences were noted for the sham groups (NS-Sham and ZG-Sham) with regard to the aforementioned histological scores (Supplementary Figure 1B, 1C). At the 6th week, the NS-DMM group demonstrated increased degeneration compared with the sham control group, with erosion of cartilage at the femoral condyle extending to 25% of the articular surface, and vertical clefts at the tibial plateau extending to less than 25% of the articular surface (Figure 2A). The OARSI scores at femoral condyle and tibial plateau indicated a significant decrease of approximately 50% in the ZG-DMM group of the destabilized medial meniscus compared with the NS-DMM group (Figure 2B, 2C). In contrast to the DMM groups, no significant differences were noted with regard to the sham groups for the OARSI scores (Figure 2B, 2C).
Figure 2.
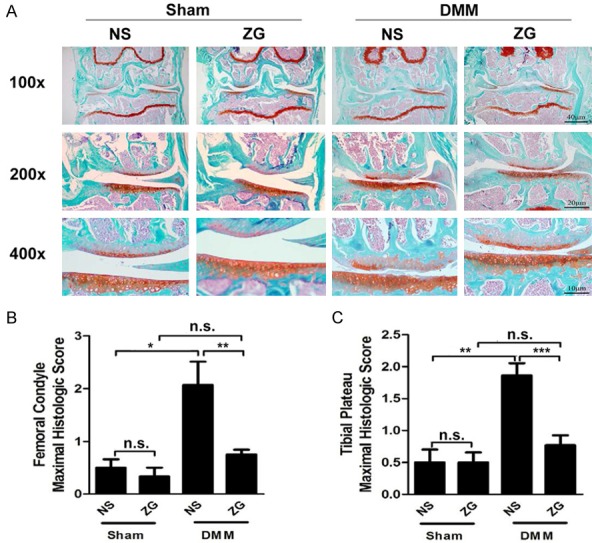
ZG inhibits cartilage degeneration in DMM mice at the 6th week. A. Joint histology in the DMM model. Knee joint tissue sections were derived from DMM mice following 6 weeks of ZG and NS treatments (NS). Joint sections were stained with Safranin-O/fast green. Representative images of mouse joints are shown (n=8 mice per time point). B. Maximal histological scores across the femoral condyle. C. Maximal histological scores across the tibial plateau. *P<0.05, **P<0.01, ***P<0.001, n.s, not statistically significant.
Similar results were noted for the 8th week time period (Figure 3). The normal saline treatment group exhibited increased degeneration at the tibial plateau than the sham control group, with vertical fibrillations extending to greater than 50% of the articular surface. The OARSI scores of the femoral condyle and tibial plateau indicated a significant decrease similar to that noted at the 6th week period in the ZG-DMM group of the destabilized medial meniscus compared with the NS-DMM group (Figure 3B, 3C). The protective effect mediated by ZG treatment was higher for the tibial plateau histological score compared with the femoral condyle (Figure 3B, 3C). In contrast to the DMM groups, no significant differences were noted with regard to the sham groups for the OARSI scores (Figure 3B, 3C).
Figure 3.
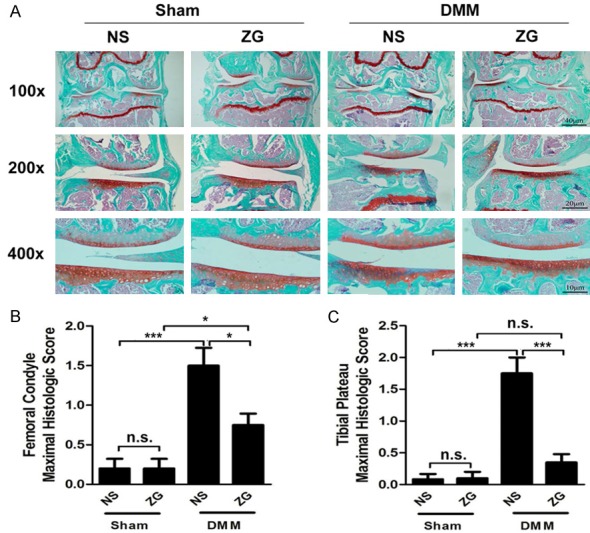
ZG inhibits cartilage degeneration in DMM mice at the 8th week. A. Joint histology in the DMM model. Knee joint tissue sections were derived from DMM mice following 8 weeks of ZG and NS treatments. Joint sections were stained with Safranin-O/fast green. Representative images of mouse joints are shown (n=8 mice per time point). B. Maximal histological scores across the femoral condyle. C. Maximal histological scores across the tibial plateau. *P<0.05, **P<0.01, ***P<0.001, n.s, not statistically significant.
ZG treatment decreases the serum concentration levels of OA biomarkers in mice
To further explore the effects of ZG on OA, we measured the expression of OA-related markers in the blood serum. The serum TNF-α increased following DMM. However, no elevation was detected in the ZG treatment group (Figure 4A). The serum concentration of COMP was significantly higher in the NS-DMM group compared with the NS-Sham group, whereas no significant difference was noted in the ZG-DMM and ZG-Sham groups (Figure 4B). However, the ZG-DMM group exhibited significantly lower levels of COMP compared with the NS-DMM group (Figure 4B). Similar changes were noted in the subchondral bone damage biomarker, CTX-I (Figure 4C), although the expression levels of MMP-13 indicated no significant changes among the three groups (Figure 4D).
Figure 4.
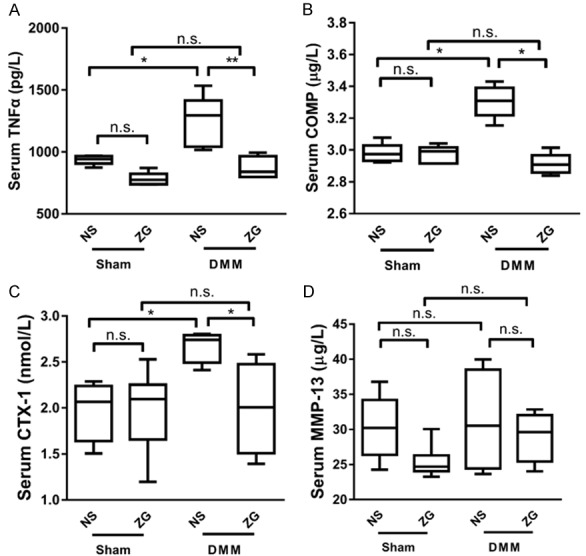
ZG reduces blood serum concentration of OA biomarkers. The serum concentration levels of the OA biomarkers in DMM mice were significantly lower in the treatment group (ZG) compared with the normal saline group (NS) (P<0.05) with the exception of MMP-13, where no significant difference was noted. (n=8 mice per time point). TNF-α (A), COMP (B), CTX-1 (C), and MMP-13 (D). *P<0.05, **P<0.01, ***P<0.001, n.s, not statistically significant.
ZG inhibits chondrocyte production of matrix degrading enzymes
Furthermore, the effects of different ZG concentrations on OA-related genes in SW1353 cells were measured. At 10 ng/µl of ZG treatment no significant changes were noted with regard to collagen II expression compared with the control group, although the expression levels were approximately 2-times higher when the concentration was increased to 20 ng/µl (Figure 5A). However, there was no statistically significant difference in the levels of Aggrecan, following 10 and/or 20 ng/μl of ZG treatment compared with the control group (Figure 5B).
Figure 5.
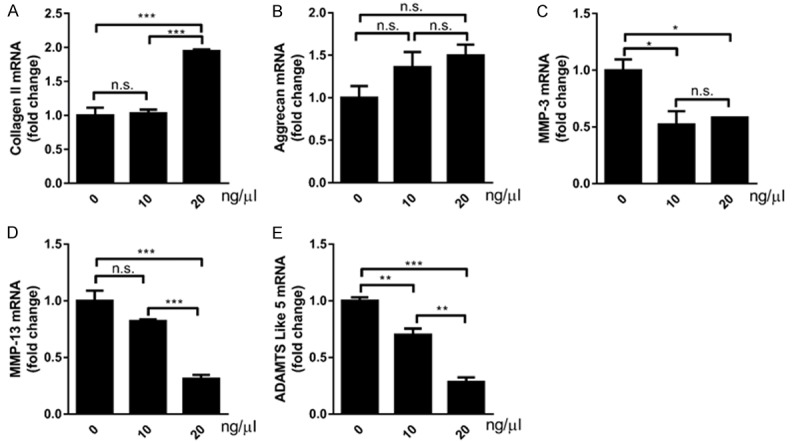
ZG reduces the expression of OA biomarkers and promotes chondrocyte matrix production. The measurement of cell biomarkers and cartilaginous matrix components was conducted by qPCR. (A) The expression levels of the biomarkers collagen II, Aggrecan, MMP-1, MMP-3, MMP-13 and ADAMTS5 mRNA were evaluated in chondrocytes. (A) Collagen II, (B) Aggrecan, (C) MMP-3, (D) MMP-13, (E) ADAMTS5. *P<0.05, **P<0.01, ***P<0.001, n.s, not statistically significant.
The expression levels of the matrix metalloproteinase enzymes MMP-1 and MMP-13 were further assessed by qRT-PCR assays. Administration of 10 and 20 ng/mL of ZG caused a significant decrease to MMP-1 expression levels compared with the control group (Figure 5C). At 20 ng/µl of ZG treatment, MMP-13 expression levels demonstrated a significant decrease compared with that noted in the normal saline treated samples (Figure 5D). At 10 ng/μl, the reduction noted with regard to MMP-13 levels was not significantly different compared with the control samples (Figure 5D). Finally, the biomarker ADAMTS5 that is involved in cartilaginous matrix degradation, demonstrated a significant decrease at 10 ng/µl of ZG treatment, and an even higher decrease when the concentration of ZG was increased to 20 ng/mL (Figure 5E).
The effect of ZG treatment on articular chondrocyte apoptosis is mediated via the AKT/caspase 3 pathway
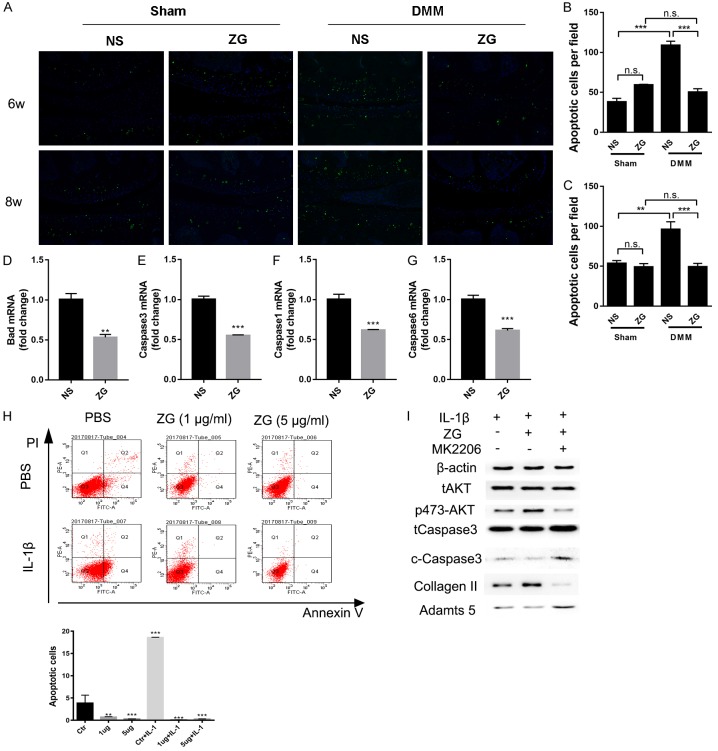
To further explore the protective mechanism of ZG, articular chondrocyte apoptosis was evaluated using Tunnel-staining. Representative slides indicated that the Tunnel-positive chondrocytes of the ZG-DMM group were significantly decreased compared with the NS-DMM group following treatment of ZG for 6 and 8 weeks (Figure 6A). Moreover, the data demonstrated that the positive number of chondrocytes per field in the NS-DMM group was significantly increased compared with the NS-Sham group (65.6% for 6-weeks, 44.13% for 8-weeks). The number of apoptotic chondrocytes was estimated to approximately 53.86% at the 6th week (Figure 6B) and 48.87% at the 8th week, respectively (Figure 6C). These percentages were noted in the ZG-DMM group.
Figure 6.
ZG reduces chondrocyte apoptosis possibly via the AKT/caspase 3 pathways. Tunnel staining of represented slides (A) and semi-quantification of the percentage of apoptosis (B, C) from the 6-week and 8-week groups. qPCR analysis of the expression of the apoptotic genes, like Bad (D), caspase 1 (F), caspase 3 (E) and caspase 6 (G). (H) Annexin-V- and propidium iodide-stained cells were analyzed for cell apoptosis using flow cytometry. (I) Western blot detection of the expression of Collagen II, Adamts 5, AKT, p-AKT, total caspase 3 and cleaved-caspase 3 proteins *P<0.05, **P<0.01, ***P<0.001, n.s, not statistically significant.
Moreover, the administration of 1 µg/ml of ZG in primary chondrocytes caused a significant decrease in the expression levels of the apoptotic genes. The expression levels of bad, caspase 1, caspase 3 and caspase 6 genes were significantly decreased in the ZG compared with the control groups (Figure 6D-G). In addition, Annexin V/PI staining was used to evaluate the percentage of apoptosis in primary chondrocytes. Treatment of primary chondrocytes with different concentrations of ZG significantly decreased the percentage of Annexin V/PI-double positive cells compared with cells treated with PBS both in the normal and the IL-1β (10 ng/ml)-induced primary chondrocyte inflammation models (Figure 6H). Western blot analysis indicated that the protein levels of collagen II and of Adamts 5 were increased and decreased, respectively, following treatment with 1 µg/ml of ZG. Moreover, the levels of the phosphorylated form of AKT (S473) were increased following treatment with ZG, which may have caused the decrease in the levels of the cleaved-caspase 3 following administration of different concentrations of ZG (Figure 6I). The anti-apoptotic effects of ZG could be blocked by administration of 1 μM of the AKT inhibitor MK2206 (Selleck, USA) (Figure 6I).
Discussion
In the present study, the potential of ZG formulation to prevent degeneration of osteoarthritis was investigated. The data suggested that ZG exerts significant therapeutic implications with regard to the treatment of OA and that it may be considered a promising agent for the treatment of this disease. The mechanism of action of ZG involved the downregulation of the ADAMTS5, MMP3 and MMP13 genes and the upregulation of collagen II. Furthermore, the reduction in chondrocyte apoptosis that was induced by ZG, was mediated via the activation of the Akt signaling pathway.
In the present study, the OARSI scores of the ZG treatment group were decreased and the animals indicated no noticeable degeneration at either the femoral condyle and/or the tibial plateau, suggesting that ZG treatment prevented the progression of the disease. The data are in agreement with previous reports [13,17,18] and confirm the therapeutic effects of ZG with regard to OA.
In addition to the beneficial effects of ZG in the prevention of OA progression, the present study examined the expression of specific molecular biomarkers that are widely used in osteoarthritis monitoring. MMP3 and MMP13 contribute to the degradation of the cartilage matrix, whereas ADAMTS-5 and/or aggrecanase-2 act on proteoglycans known as Aggrecans, which are components of the connective tissues [19,20]. During the progression of OA, the expression levels of the aforementioned proteins are deregulated [20]. It has been shown that MMP-3 and MMP-13 degrade type II collagen, whereas their expression levels are regulated by numerous factors such as the enzymes of the TIMP family [21]. Although the effects of ZG on MMP enzymes in OA models have not been reported to date, the MMP-3 and MMP-13 levels were downregulated following treatment of IL-1β induced chondrocytes with the chinese herbal preparation SiMiaoFang (SMF) [22]. This effect was further noted in vivo in the joints of SMF treated rats [22]. Similarly SMF markedly decreased ADAMTS-4 and -5 expression in cruciate ligament transaction and medial meniscus rat joints [22]. Moreover, a recent study suggested that double knockout of the ADAMTS-4 and -5 expression in mice significantly protected against proteoglycan degradation and decreased the severity of OA induced in mice [23]. The results reported in the present study are consistent with these findings as ZG treatment resulted in decreased expression levels of ADAMTS-5 and MMP-3 and 13 in vitro. The in vitro experiments further confirmed that the exposure to low doses of a solution containing ZG for a certain period of time could promote chondrocyte cell viability and increase collagen II and Aggrecan levels.
OA is a multifactorial disease that is characterized by degradation of the extracellular matrix and destruction of articular cartilage [24]. The human articular cartilage is composed of chondrocytes that are considered key elements in the cartilage degeneration associated with OA [24]. Various biological processes that are associated with cell death, namely apoptosis, necroptosis, autophagic cell death and mitotic catastrophe, have been demonstrated in chondrocytes [25-29]. The pathogenesis of OA has been shown to be related to chondrocyte death by apoptosis [24]. The induction of apoptosis in chondrocytes can be triggered by several stimuli, such as nitric oxide (NO), prostaglandin E2, tumor necrosis factor-α (TNF-α) and interleukin 1ß, while the prevention of chondrocyte death has been suggested as a therapeutic strategy to limit cartilage damage [24].
Previous reports have suggested that the matrix synthesis and chondrocyte survival could be regulated by PI3K/AKT signaling [30-32]. For example, the expression of constitutively active Akt results in significant elevation in proteoglycan synthesis and cell survival in human articular chondrocytes [30]. In the present study, p-AKT protein was increased following ZG treatment in primary chondrocytes. Moreover, PI3K/AKT signaling was considered as the key regulator in cell apoptosis [33]. Leber et al. demonstrated that the phosphorylation of Bad by Akt triggered its dissociation from the Bcl-2/Bcl-xl complex and led to the loss of its pro-apoptotic function [34]. Furthermore, increased chondrocyte apoptosis is considered a key pathological feature of OA [35,36] and the use of chondrocyte apoptosis inhibitors has been investigated as a potential application for the treatment of osteoarthritis [37]. The data indicated that the number of positive-cells identified by Tunnel staining in the ZG-DMM group was significantly decreased compared with that noted in the NS-DMM group (Figure 6A-H). Following the administration of ZG in primary chondrocytes, the protein levels of cleaved-caspase 3 were decreased. This effect could be blocked by addition of the AKT inhibitor HK2206. It is likely that ZG could decrease primary chondrocyte apoptosis and inhibit the process of osteoarthritis via modulation of the AKT/Caspase 3 pathways.
A previous study has highlighted the contribution of the Akt and PKCα proteins in chondrocyte apoptosis of the human knee OA cartilage [38]. Akt and PKCα were involved in OA progression, along with the increase of cell apoptosis. Specifically, during OA, the expression levels of Akt decreased in cartilage tissues, whereas the opposite was noted for PKCα [38]. This suggested that Akt and PKCα are related to increased chondrocyte apoptosis in human OA cartilage [38]. The data presented in the current study are in agreement with the latter study, as the decrease in the induction of apoptosis by ZG was accompanied by activation of Akt, which demonstrated an opposite association between apoptosis and Akt signaling.
Taken together, the data suggest that ZG may be an effective therapeutic formulation that can be applied for OA treatment. However, further analysis is required in order to isolate the single active compound and/or the compound combinations that are responsible for the biological effects of ZG.
Acknowledgements
This work was supported by the Projects of International Cooperation and Exchanges NSFC (81420108021), National Key Technology Support Program (2015BAI08B02), Excellent Young Scholars NSFC (81622033), the Natural Science Foundation of Jiangsu Province (Grants No. BK20141088), Jiangsu Provincial Key Medical Center Foundation, Jiangsu Provincial Medical Talent Foundation and Jiangsu Provincial Medical Outstanding Talent Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Alcalde GE, Fonseca AC, Boscoa TF, Goncalves MR, Bernardo GC, Pianna B, Carnavale BF, Gimenes C, Barrile SR, Arca EA. Effect of aquatic physical therapy on pain perception, functional capacity and quality of life in older people with knee osteoarthritis: study protocol for a randomized controlled trial. Trials. 2017;18:317. doi: 10.1186/s13063-017-2061-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rahman MM, Kopec JA, Goldsmith CH, Anis AH, Cibere J. Validation of administrative osteoarthritis diagnosis using a clinical and radiological population-based cohort. Int J Rheumatol. 2016;2016:6475318. doi: 10.1155/2016/6475318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shen L, Yuan T, Chen S, Xie X, Zhang C. The temporal effect of platelet-rich plasma on pain and physical function in the treatment of knee osteoarthritis: systematic review and meta-analysis of randomized controlled trials. J Orthop Surg Res. 2017;12:16. doi: 10.1186/s13018-017-0521-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hunter DJ. Osteoarthritis. Best Pract Res Clin Rheumatol. 2011;25:801–814. doi: 10.1016/j.berh.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 5.Suri P, Morgenroth DC, Hunter DJ. Epidemiology of osteoarthritis and associated comorbidities. PM R. 2012;4:S10–19. doi: 10.1016/j.pmrj.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 6.Talic-Tanovic A, Hadziahmetovic Z, Madjar-Simic I, Papovic A. Comparison of clinical and radiological parameters at knee osteoarthritis. Med Arch. 2017;71:48–51. doi: 10.5455/medarh.2017.71.48-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhathal A, Spryszak M, Louizos C, Frankel G. Glucosamine and chondroitin use in canines for osteoarthritis: a review. Open Vet J. 2017;7:36–49. doi: 10.4314/ovj.v7i1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.da Costa BR, Reichenbach S, Keller N, Nartey L, Wandel S, Juni P, Trelle S. Effectiveness of non-steroidal anti-inflammatory drugs for the treatment of pain in knee and hip osteoarthritis: a network meta-analysis. Lancet. 2017;390:e21–e33. doi: 10.1016/S0140-6736(17)31744-0. [DOI] [PubMed] [Google Scholar]
- 9.Wielage RC, Myers JA, Klein RW, Happich M. Cost-effectiveness analyses of osteoarthritis oral therapies: a systematic review. Appl Health Econ Health Policy. 2013;11:593–618. doi: 10.1007/s40258-013-0061-x. [DOI] [PubMed] [Google Scholar]
- 10.Davies PS, Graham SM, MacFarlane RJ, Leonidou A, Mantalaris A, Tsiridis E. Disease-modifying osteoarthritis drugs: in vitro and in vivo data on the development of DMOADs under investigation. Expert Opin Investig Drugs. 2013;22:423–441. doi: 10.1517/13543784.2013.770837. [DOI] [PubMed] [Google Scholar]
- 11.De Luigi AJ. Complementary and alternative medicine in osteoarthritis. PM R. 2012;4(Suppl):S122–133. doi: 10.1016/j.pmrj.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 12.Xu Q, Chen B, Wang Y, Wang X, Han D, Ding D, Zheng Y, Cao Y, Zhan H, Zhou Y. The effectiveness of manual therapy for relieving pain, stiffness, and dysfunction in knee osteoarthritis: a systematic review and meta-analysis. Pain Physician. 2017;20:229–243. [PubMed] [Google Scholar]
- 13.Wang HM, Ge JR, Yin HB. [Effect of compound duzhong jiangu granule on knee joint osteoarthritis: a report of 400 cases] . Zhongguo Zhong Xi Yi Jie He Za Zhi. 2005;25:489–491. [PubMed] [Google Scholar]
- 14.Zhang XL, Yang J, Yang L, Liu JG, Cai XY, Fan WM, Yun XQ, Ma JZ, Weng XS. Efficacy and safety of zhuanggu joint capsules in combination with celecoxib in knee osteoarthritis: a multi-center, randomized, double-blind, double-dummy, and parallel controlled trial. Chin Med J (Engl) 2016;129:891–897. doi: 10.4103/0366-6999.179789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glasson SS, Blanchet TJ, Morris EA. The surgical destabilization of the medical meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthritis Cartilage. 2007;15:1061–1069. doi: 10.1016/j.joca.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 16.Gosset M, Berenbaum F, Thirion S, Jacques C. Primary culture and phenotyping of murine chondrocytes. Nat Protoc. 2008;3:1253–1260. doi: 10.1038/nprot.2008.95. [DOI] [PubMed] [Google Scholar]
- 17.Hu B. The clinical trial of the therapy of 60 subjects’ knee osteoarthritis by Zhuanggu capsule. China Pract Med. 2015;10:201–203. [Google Scholar]
- 18.Chen SX, Kang L, Chen HQ, Situ J, Zhao CD, Ding LJ, Liu HG. [Clinical randomized controlled trial on splint external fixation combined with Chinese herbs in treating distal radius fractures of elderly patients] . Zhongguo Gu Shang. 2008;21:181–183. [PubMed] [Google Scholar]
- 19.Yang CY, Chanalaris A, Troeberg L. ADAMTS and ADAM metalloproteinases in osteoarthritis - looking beyond the ‘usual suspects’. Osteoarthritis Cartilage. 2017;25:1000–1009. doi: 10.1016/j.joca.2017.02.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldring MB, Otero M, Plumb DA, Dragomir C, Favero M, El Hachem K, Hashimoto K, Roach HI, Olivotto E, Borzi RM, Marcu KB. Roles of inflammatory and anabolic cytokines in cartilage metabolism: signals and multiple effectors converge upon MMP-13 regulation in osteoarthritis. Eur Cell Mater. 2011;21:202–220. doi: 10.22203/ecm.v021a16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He Z, Leong DJ, Zhuo Z, Majeska RJ, Cardoso L, Spray DC, Goldring MB, Cobelli NJ, Sun HB. Strain-induced mechanotransduction through primary cilia, extracellular ATP, purinergic calcium signaling, and ERK1/2 transactivates CITED2 and downregulates MMP-1 and MMP-13 gene expression in chondrocytes. Osteoarthritis Cartilage. 2016;24:892–901. doi: 10.1016/j.joca.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 22.Xu Y, Liu Q, Liu ZL, Lim L, Chen WH, Lin N. Treatment with SiMiaoFang, an anti-arthritis chinese herbal formula, inhibits cartilage matrix degradation in osteoarthritis rat model. Rejuvenation Res. 2013;16:364–376. doi: 10.1089/rej.2013.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Majumdar MK, Askew R, Schelling S, Stedman N, Blanchet T, Hopkins B, Morris EA, Glasson SS. Double-knockout of ADAMTS-4 and ADAMTS-5 in mice results in physiologically normal animals and prevents the progression of osteoarthritis. Arthritis Rheum. 2007;56:3670–3674. doi: 10.1002/art.23027. [DOI] [PubMed] [Google Scholar]
- 24.Lee SW, Rho JH, Lee SY, Yoo SH, Kim HY, Chung WT, Yoo YH. Alpha B-crystallin protects rat articular chondrocytes against casein kinase II inhibition-induced apoptosis. PLoS One. 2016;11:e0166450. doi: 10.1371/journal.pone.0166450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee SW, Song YS, Lee SY, Yoon YG, Lee SH, Park BS, Yun I, Choi H, Kim K, Chung WT, Yoo YH. Downregulation of protein kinase CK2 activity facilitates tumor necrosis factor-alpha-mediated chondrocyte death through apoptosis and autophagy. PLoS One. 2011;6:e19163. doi: 10.1371/journal.pone.0019163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee SW, Rho JH, Lee SY, Kim JH, Cheong JH, Kim HY, Jeong NY, Chung WT, Yoo YH. Leptin protects rat articular chondrocytes from cytotoxicity induced by TNF-alpha in the presence of cyclohexamide. Osteoarthritis Cartilage. 2015;23:2269–2278. doi: 10.1016/j.joca.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 27.Coustry F, Posey KL, Liu P, Alcorn JL, Hecht JT. D469del-COMP retention in chondrocytes stimulates caspase-independent necroptosis. Am J Pathol. 2012;180:738–748. doi: 10.1016/j.ajpath.2011.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hashimoto S, Ochs RL, Komiya S, Lotz M. Linkage of chondrocyte apoptosis and cartilage degradation in human osteoarthritis. Arthritis Rheum. 1998;41:1632–1638. doi: 10.1002/1529-0131(199809)41:9<1632::AID-ART14>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 29.Kim HA, Lee YJ, Seong SC, Choe KW, Song YW. Apoptotic chondrocyte death in human osteoarthritis. J Rheumatol. 2000;27:455–462. [PubMed] [Google Scholar]
- 30.Beier F, Loeser RF. Biology and pathology of Rho GTPase, PI-3 kinase-Akt, and MAP kinase signaling pathways in chondrocytes. J Cell Biochem. 2010;110:573–580. doi: 10.1002/jcb.22604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cravero JD, Carlson CS, Im HJ, Yammani RR, Long D, Loeser RF. Increased expression of the Akt/PKB inhibitor TRB3 in osteoarthritic chondrocytes inhibits insulin-like growth factor 1-mediated cell survival and proteoglycan synthesis. Arthritis Rheum. 2009;60:492–500. doi: 10.1002/art.24225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oh CD, Chun JS. Signaling mechanisms leading to the regulation of differentiation and apoptosis of articular chondrocytes by insulin-like growth factor-1. J Biol Chem. 2003;278:36563–36571. doi: 10.1074/jbc.M304857200. [DOI] [PubMed] [Google Scholar]
- 33.Xu S, Wu L, Zhang Q, Feng J, Li S, Li J, Liu T, Mo W, Wang W, Lu X, Yu Q, Chen K, Xia Y, Lu J, Xu L, Zhou Y, Fan X, Guo C. Pretreatment with propylene glycol alginate sodium sulfate ameliorated concanavalin A-induced liver injury by regulating the PI3K/Akt pathway in mice. Life Sci. 2017;185:103–113. doi: 10.1016/j.lfs.2017.07.033. [DOI] [PubMed] [Google Scholar]
- 34.Leber B, Lin J, Andrews DW. Embedded together: the life and death consequences of interaction of the Bcl-2 family with membranes. Apoptosis. 2007;12:897–911. doi: 10.1007/s10495-007-0746-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.D’Lima DD, Hashimoto S, Chen PC, Colwell CW Jr, Lotz MK. Human chondrocyte apoptosis in response to mechanical injury. Osteoarthritis Cartilage. 2001;9:712–719. doi: 10.1053/joca.2001.0468. [DOI] [PubMed] [Google Scholar]
- 36.Lopez-Armada MJ, Carames B, Lires-Dean M, Cillero-Pastor B, Ruiz-Romero C, Galdo F, Blanco FJ. Cytokines, tumor necrosis factor-alpha and interleukin-1beta, differentially regulate apoptosis in osteoarthritis cultured human chondrocytes. Osteoarthritis Cartilage. 2006;14:660–669. doi: 10.1016/j.joca.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 37.D’Lima D, Hermida J, Hashimoto S, Colwell C, Lotz M. Caspase inhibitors reduce severity of cartilage lesions in experimental osteoarthritis. Arthritis Rheum. 2006;54:1814–1821. doi: 10.1002/art.21874. [DOI] [PubMed] [Google Scholar]
- 38.Chen Q, Zhang B, Yi T, Xia C. Increased apoptosis in human knee osteoarthritis cartilage related to the expression of protein kinase B and protein kinase Cα in chondrocytes. Folia Histochem Cytobiol. 2012;50:137–143. doi: 10.2478/18709. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.