Abstract
Dihydroartemisinin (DHA) is a semisynthetic derivative of artemisinin and has been used as an antimalarial drug. Recently, roles of artemisinin and its derivatives in treating diseases besides antimalarial effect were documented. Thus, this study was undertaken to investigate the role of DHA in indoxyl sulfate (IS)-promoted cell cycle progression in glomerular mesangial cells, as well as the potential mechanisms. Under the basal condition, DHA significantly retarded the cell cycle progression as shown by decreased cell percentage in S phase and increased cell percentage in G1/G0 phases in line with reduced cell cycle proteins cyclin A2 and cyclin D1. Interestingly, DHA also inactivated the COX-2/mPGES-1/PGE2 cascade which has been shown to play a critical role in promoting the mesangial cell cycle progression by our previous studies. Next, we investigated the role of DHA in IS-triggered cell cycle progression in this mesangial cell line. As expected, DHA treatment significantly retarded IS-induced cell cycle progression and inhibited the activation of COX-2/mPGES-1/PGE2 cascade induced by IS. In summary, these data indicated that DHA inhibited the cell cycle progression in glomerular mesangial cells under normal condition or IS challenge possibly through the inhibition of COX-2/mPGES-1/PGE2 cascade, suggesting a potential of DHA in treating glomerular diseases with mesangial cell proliferation.
Keywords: Dihydroartemisinin, mesangial cells, cell cycle progression, COX-2, PGE2
Introduction
Maintenance of the residual renal function (RRF) is of importance for both predialysis and dialysis patients with end stage renal disease (ESRD) due to its critical role in small-solute clearance, fluid balance, phosphorus control, and the removal of middle-molecular uremic toxins. More importantly, RRF also contributes to the removal of toxins relying on renal metabolism or tubular secretion such as indoxyl sulfate (IS). Evidence showed that serum concentration of IS was significantly elevated in ESRD patients [1]. However, indoxyl sulfate is difficult to be removed by conventional hemodialysis because of its binding to albumin in advanced chronic kidney disease (CKD) [2]. The enhanced IS could quicken renal cell injury and lead to subsequent fibrosis and inflammation, thereby acting as a nephrotoxin [1,3-5]. Studies also showed that IS could result in complex redox change in mesangial cells (MCs) and cell proliferation in vascular smooth muscle cells [6,7]. Our study also reported that IS can induce mesangial cell proliferation through COX-2/mPGES-1 pathway [8,9]. Recently, some traditional Chinese medicines were found to be effective in treating glomerular diseases, which triggered our interest to explore the role of dihydroartemisinin, a semisynthetic derivative of artemisinin, in IS-induced mesangial cell proliferation, as well as the potential mechanisms.
Artemisinin is a natural product of the plant artemisia annua and has been used as an antimalarial drug in the past decades [10]. In the recent years, besides the antimalaria effect, artemisinin and its derivatives also showed the efficacy in treating lupus nephritis and some kinds of cancers [11-13]. Dihydroartemisinin (DHA), a semisynthetic derivative of artemisinin (ARS), has been documented to have anticancer activity. Furthermore, DHA was also reported to be protective in some disease models including arthritis, allergic asthma, and myocardial infarction [14-16]. However, the role of DHA in the pathogenesis of glomerular diseases is still elusive. In the present study, we investigated the effect of DHA on the cell cycle progression of mesangial cells under the normal condition or IS challenge, as well as the potential mechanisms.
Materials and methods
Materials
IS was bought from Sigma Chemical Company (St. Louis, MO). Fetal bovine serum (FBS), penicillin-streptomycin, trypsin EDTA solution and Dulbecco’s modified Eagle’s medium (DMEM) were from Gibco company (Invitrogen, Grand Island, New York). Cyclin A2 rabbit polyclonal antibody and cyclin D1 mouse monoclonal antibody were purchased from Abcam company (Cambridge, MA). COX-2 and mPGES-1 antibodies were bought from Cayman Chemicals (Ann Arbor, Michigan) and Anti-GAPDH (ab9485) was supplied by Cell Signaling Technology (Danvers, MA). The PGE2 enzyme immunoassay kit was from Cayman Chemicals (Ann Arbor, Michigan). DHA was bought from Beyotime Biotechnology (Shanghai, China).
MC culture
The mouse MC line (SV40 MES 13) was purchased from the China Center for Type Culture Collection (CCTCC Wuhan, China). Cells were preserved at 37°C in humidified 5% CO2 atmosphere in DMEM which consists of 10% fetal bovine serum (FBS; GIBCO), 5.6 mM glucose, 100 U/mL penicillin, 44 mM NaHCO3, 100 mg/mL streptomycin and 14 mM 4-(2-hydroxy-ethyl)-1-piperazineethanesulfonic acid. After MCs were cultured to 60%-70% confluence, they were treated with IS for 24 h at doses of 0 µM, 250 µM and 500 µM with or without DHA treatment at a dose of 10 µM.
Cell cycle analysis
MCs were treated with vehicle, DHA with or without IS in serum-free DMEM for 24 h. Then the cells were rinsed twice with PBS, digested with 0.25% trypsin and fixed in 70% ethanol for at least 2 h at 4°C. Next, the cells were collected by centrifugation, treated with RNase and stained with propidium iodide using the cell cycle detection kit (KeyGEN, Shanghai, China). The number of cells in G1, S and G2/M cell cycle phases were detected by flow cytometry (BD FACS Calibur flow cytometer, Bedford, MA) and data analysis was implemented with modfit 3.0 software.
Quantitative real-time PCR (qRT-PCR)
The total RNA was extracted from cultured MCs using a TRIzol reagent (TaKaRa) according to the manufacturer’s protocol. Reverse transcription was carried out using a PrimeScript RT reagent Kit (TaKaRa) following the manufacturer’s instruction. Oligonucleotides (cyclin D1: forward, 5’-CGCCCTCCGTTTCTTACTTC-3’, and reverse, 5’-GCAGTCAGGGGAATGGTCT-3’; cyclin A2: forward, 5’-AAGATGCCCTGGCTTTTAGTG-3’, and reverse, 5’-TAACATTCACTGGCTTTTCGTCT-3’; Cyclooxygenase-2: forward, 5’-AGGACTCTGCTCACGAAGGA-3’, and reverse, 5’-TGACATGGATTGGAACAGCA-3’; and GAPDH: forward, 5’-GTCTTCACTACCATGGAGAAGG-3’, and reverse, 5’-TCATGGATGACCTTGGCCAG-3’) were designed using Primer 5 software (online at http://frodo.wi.mit.edu/) and produced by Invitrogen. By using SYBR Premix Ex Taq (TaKaRa), the real-time PCR amplification was operated using the ABI 7500 Real-Time PCR Detection System (Foster City, CA). The cycling program consisted of a preliminary denaturation (95°C for 10 min), followed by 40 cycles (95°C for 15 s and 60°C for 1 min). The relative gene expression level was calculated through Delta-delta Ct method and GAPDH was used as the internal control.
Western blotting analysis
At the recommended time, MCs were washed with ice-cold PBS and lysed on ice in lysis buffer with protease inhibitors. After centrifugation, the protein concentration was measured using a micro BCA protein assay kit with bovine serum albumin as a standard (Pierce, Thermo). Then, 60 µg of cellular protein was separated by SDS-PAGE and transferred onto PVDF membranes (Bio-Rad). TBS-T (0.1% Tween 20 in TBS) containing 5% nonfat milk was used to block the membranes for 1 hour at room temperature, and then incubated with primary antibodies against cyclin D1 (1:1000), cyclin A2 (1:500), COX-2 (1:1000), mPGES-1 (1:1000), and GAPDH (1:2000) overnight at 4°C. Eventually HRP-labeled secondary antibodies were added (at room temperature for 1 h). GAPDH was used as a loading control. Band intensity was measured using Image J software (NIH, Bethesda, MD, USA).
Enzyme immunoassay
Centrifugation of cell culture medium for 5 min at 12,000×g was done. According to the manufacturers’ instructions, the concentration of PGE2 in the medium was measured by enzyme immunoassay (Cayman Chemical).
Data analysis
Data are presented as means ± SE and statistical analysis was completed using ANOVA analysis followed by a Bonferroni posttest. P<0.05 means statistically significant.
Results
DHA inhibited cell cycle progression in MCs
In order to define the role of DHA in cell cycle progression in normal mesangial cells, we measured the cell cycle by flow cytometry in MCs exposed to different doses of DHA. As shown by the data, DHA caused a moderate but significant increase of cell number in G1/G0 phases and a decrease of cell number in S phase (Figure 1A-F). These data suggested that DHA could directly retard cell cycle progression in MCs.
Figure 1.
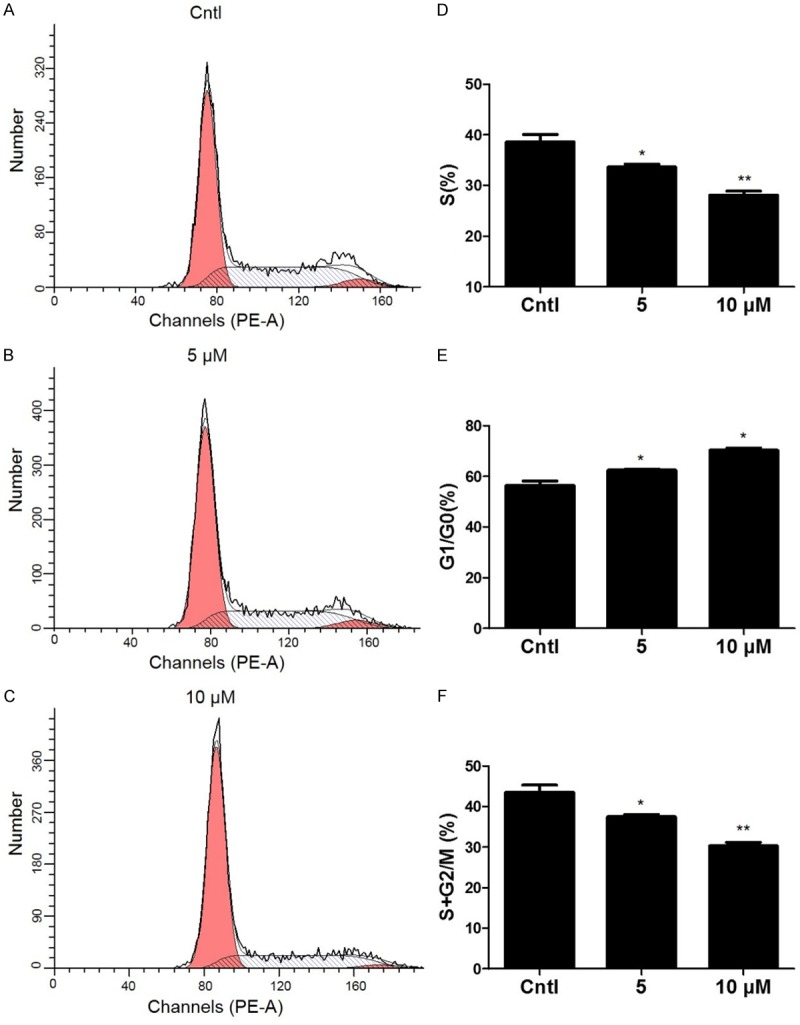
The effect of DHA on cell cycle progression in MCs. The percentage of cells at different cell cycle phases was detected by flow cytometry after MCs were treated with the indicated doses of DHA for 24 h. A-C. Representative images of cell cycle with different doses of DHA. D-F. Percentage of cells at S, G1/G0, and (S+G2)/M phases. Values are means ± SE; n = 6 for each group. *P<0.05 vs. control, **P<0.01 vs. control.
DHA downregulated cyclin D1 and cyclin A2 in MCs
To further validate DHA effect on cell cycle progression in MCs, we detected the expressions of key cell cycle-related proteins including cyclin D1 and cyclin A2. Here we found that DHA decreased the mRNA levels of cyclin D1 and cyclin A2 in dose dependent manner as determined by qRT-PCR (Figure 2A, 2B). By Western blotting, we observed a similar pattern of the protein expressions of cyclin D1 and cyclin A2 (Figure 2C, 2D). These data further confirmed the DHA effect on cell cycle progression in MCs.
Figure 2.
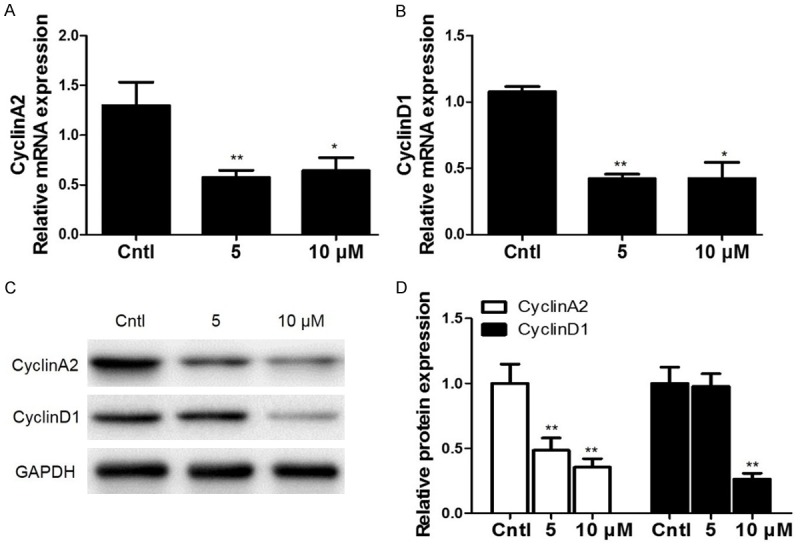
Effects of DHA on the expressions of cyclin D1 and cyclin A2 in MCs. (A, B) After MCs were treated with DHA (5 and 10 μM) for 24 h, a dose-dependent increase of cyclin A2 (A) and cyclin D1 (B) mRNA levels was observed. (C) Representative images of cyclin A2 and cyclin D1 Western blots in dose-dependent experiments. (D) Quantification of the Western bots in (C). All values are means ± SE; n = 6 for each group. *P<0.05 vs. control, **P<0.01 vs. control.
DHA reduced COX-2/mPGES-1 expressions and decreased PGE2 production in MCs
Previously, we reported that COX-2/mPGE-1/PGE2 cascade played an important role during the cell cycle progression and cell proliferation in MCs. To study the potential mechanism mediating the DHA effect on retarding cell cycle progression of MCs, COX-2 and mPGES-1 expressions were detected by qRT-PCR and Western blotting. As shown by the data, COX-2 and mPGES-1 expressions were all strikingly decreased at both mRNA and protein levels after DHA treatment (Figure 3A-D). To further examine the efficacy of DHA inhibition on COX-2/mPGES-1/PGE2 cascade in this experimental setting, we measured PGE2 production in the medium. As shown in Figure 3E, PGE2 level was significantly blocked by DHA. These results demonstrated that COX-2/mPGES-1/PGE2 cascade could be directly inhibited by DHA in MCs.
Figure 3.
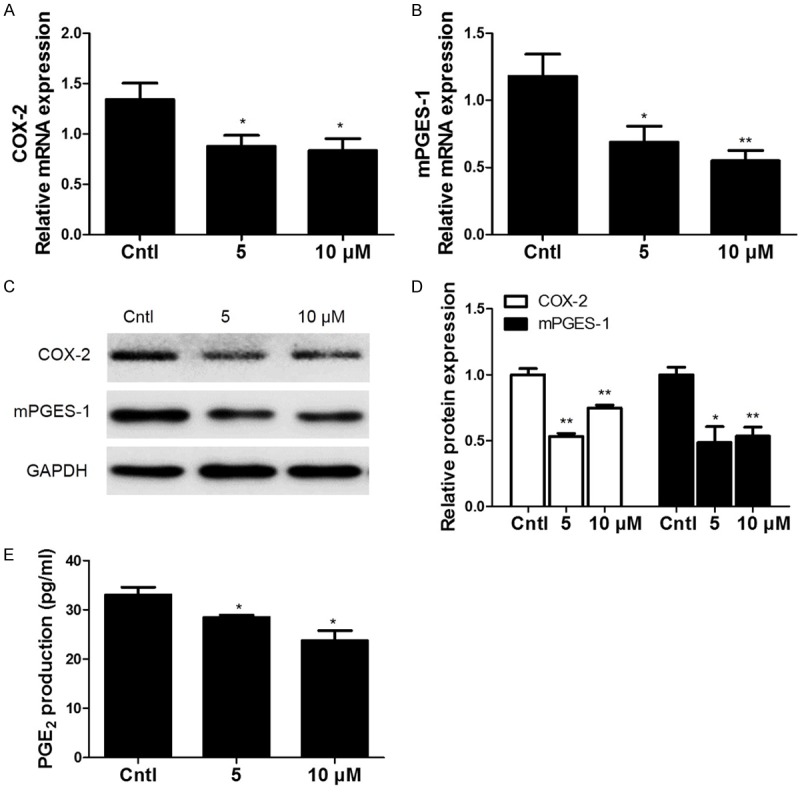
Expressions of COX-2 and mPGES-1 in DHA-treated MCs. MCs were treated with the indicated doses of DHA (5, 10 μM) for 24 h and then COX-2 and mPGES-1 mRNA and protein expressions (A-D) were analyzed by qRT-PCR and Western blotting. (E) Enzyme immunoassay of PGE2 in the medium. Values are means ± SE; n = 6 for each group. *P<0.05 vs. control, **P<0.01 vs. control.
DHA blocked IS-Induced cell cycle progression in MCs
Furthermore, we examined the effect of DHA treatment on IS-induced cell cycle progression in MCs. As expected, DHA treatment decreased the cell percentage in S phase and increased the cell percentage in G1/G0 phases in MCs challenged with IS (Figure 4A-H). These data demonstrated that DHA could be of importance in retarding IS-promoted cell cycle progression in MCs.
Figure 4.
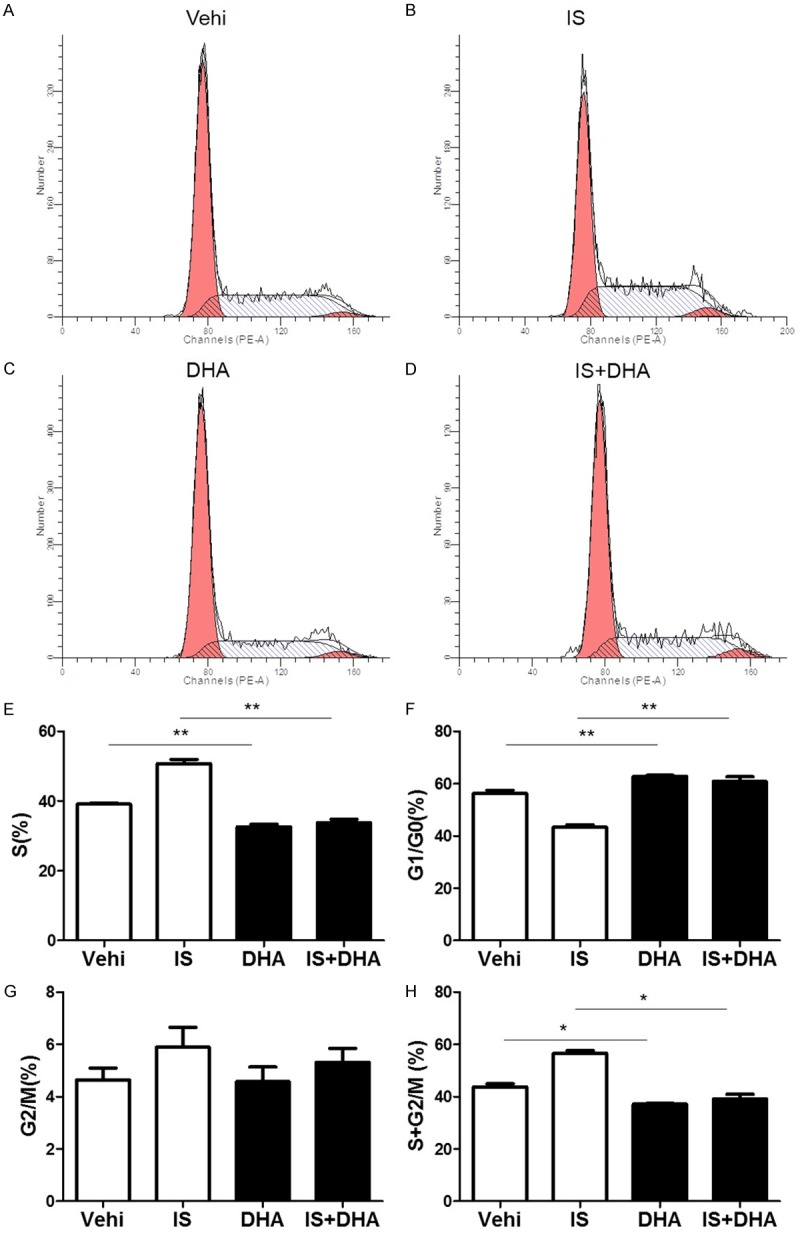
Effects of DHA on cell cycle progression in MCs after IS treatment. The cells were treated with IS (500 μM) for 24 h, then DHA (10 μM) was added to the cells with IS for another 24 h. (A-D) Representative images of cell cycle following IS challenge with or without DHA treatment. (E-H) Percentage of cells at S (E), G1/G0 (F), G2/M (G), and (S+G2)/M (H) following IS challenge with or without DHA treatment. Values are means ± SE; n = 6 in each group. *P<0.05 vs. control, **P<0.01 vs. control.
DHA blocked IS-induced expressions of cyclin A2 and cyclin D1 in MCs
In addition to the effect on the cell number in different phases of cycle, we also found that DHA significantly blocked IS-induced upregulation of cyclin D1 and cyclin A2 at mRNA and protein levels (Figure 5A-D). These data further confirmed that DHA could block IS-triggered cell cycle progression in MCs.
Figure 5.
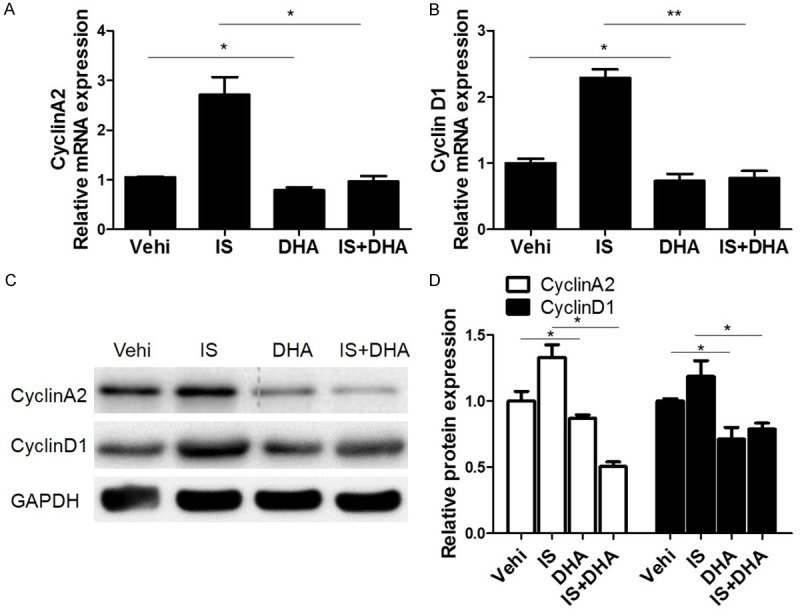
DHA blocked IS-induced upregulation of cyclin A2 and cyclin D1 in MCs. The cells were treated with IS (500 μM) for 24 h. Then DHA (10 μM) was added to the cells with IS for another 24 h. (A, B) qRT-PCR analyses of cyclin A2 (A) and cyclin D1 (B). (C) Representative images of the Western blots of cyclin A2 and cyclin D1. (D) Quantification of the Western blots of cyclin A2 and cyclin D1. Values are means ± SE; n = 6 in each group. *P<0.05 vs. control, **P<0.01 vs. control.
DHA blocked IS-induced activation of COX-2/mPGES-1/PGE2 cascade in MCs
Finally, we examined the effect of DHA on the activation of COX-2/mPGES-1/PGE2 cascade induced by uremic toxin IS. As expected, DHA attenuated the upregulation of COX-2 and mPGES-1 (Figure 6A-D) and the increment of PGE2 (Figure 6E) after IS treatment. These data suggested that the DHA effect on retarding IS-induced cell cycle progression in MCs could be through modulating COX-2/mPGES-1/PGE2 cascade to some extent.
Figure 6.
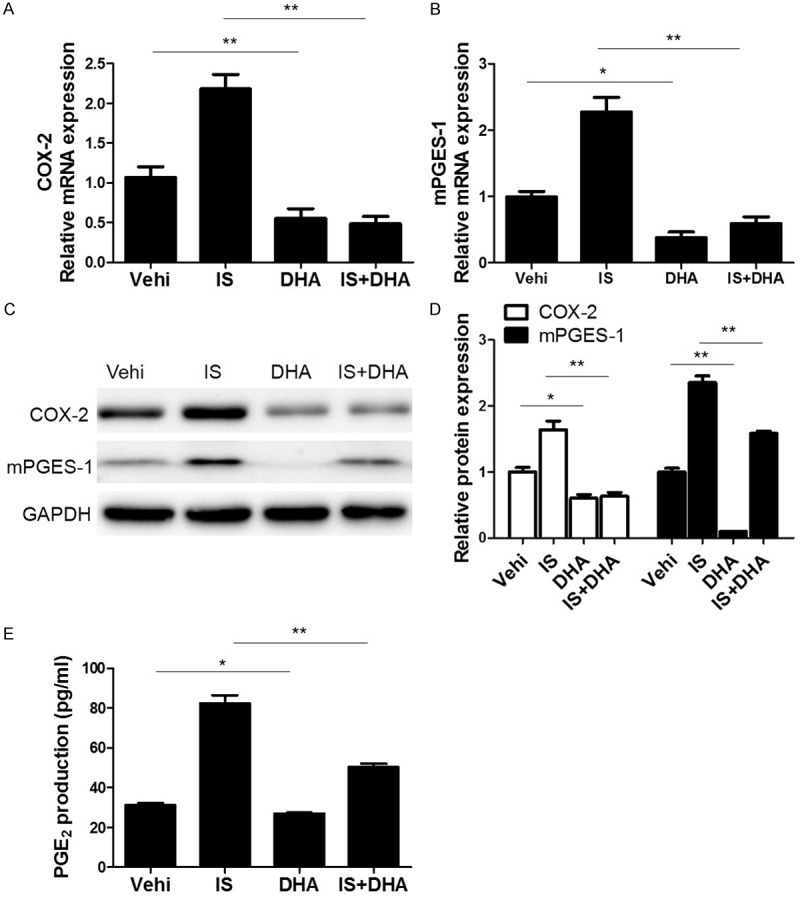
DHA blocked IS-induced activation of COX-2/mPGES-1/PGE2 cascade in MCs. (A, B) The mRNA analyses of COX-2 (A) and mPGES-1 (B). Cells were pretreated with IS for 24 h and then were treated with DHA for another 24 h at a dose of 10 μM. (C) Representative images of Western blots of COX-2 and mPGES-1 after DHA treatment. (D) Quantification of the Western blots of COX-2 and mPGES-1. (E) Enzyme immunoassay of PGE2 in the medium. Values are means ± SE; n = 6. *P<0.05 vs. control, **P<0.01 vs. control.
Discussion
RRF is of importance in both predialysis and dialysis patients. Accumulating evidence demonstrated that the loss of RRF is a critical factor for mortality and morbidity in peritoneal dialysis (PD) patients [17-19]. RRF is pretty valuable for the clearance of uremic toxins with middle and large molecular weights [17,20]. Indoxyl sulfate is prominently accumulated in the circulation of dialysis patients and quickens the disease progression [21]. In this study, we found that DHA can hinder the cell cycle progression (a key process of cell proliferation) in MCs under normal condition or IS challenge.
DHA is valuable as an anti-malarial medication [10,22-24]. Evidence has demonstrated that artemisinin is effective against cancer [25]. Recently, investigations have affirmed that DHA plays an essential role in anti-inflammation [26,27]. Here our study demonstrated that DHA significantly blocked the cell cycle progression and reduced cyclin A2 and cyclin D1 expression in MCs. The results suggested that DHA could serve as a modulator of cell cycle progression and cell expansion in MCs.
Our previous study demonstrated that COX-2 and mPGES-1 mediated IS-induced mesangial cell proliferation [9,28]. In the present study, we found that DHA downregulated the expression of COX-2 and mPGES-1 at both mRNA and protein levels in MCs under the normal condition or IS challenge in parallel with the reduced PGE2 production. Considering the established role of COX-2/mPGES-1/PGE2 cascade in MC proliferation and MC injury [8,9,28,29], the DHA effect on retarding the cell cycle progression of MCs could be through the blockade of this prostaglandin cascade at least to some extent.
Based on the results of this experiment, DHA was suggested to be an essential candidate in protecting MCs via inactivating COX-2/mPGES-1/PGE2 cascade in residual nephrons. Thus, DHA might be effective in protecting the RRF by inhibiting the MC proliferation and subsequent glomerular sclerosis. Moreover, we also observed that DHA could directly retard the cell cycle progression of MCs, indicating that the application of DHA could be extended to other primary and secondary glomerular diseases with mesangial cell proliferation because MC activation served as a key factor leading to the glomerular damage and final loss of renal function. In the future, the in vivo experiments in testing the efficacy of DHA and other artemisinin derivatives on treating glomerular diseases with mesangial cell proliferation will be required.
Acknowledgements
This work was supported by Grants from the National Natural Science Foundation of China (Nos. 81370802, 81770740, 81300591, 81670647, 81600557, 81600352, 81600532, 81770690 and 81570616), the National Key Research and Development Program (No. 2016YFC0906103), Youth Medical Talents Project of Jiangsu Province Science and Education Qiang Wei Project (No. QNRC2016091), and Project of Nanjing National Commission on Health and Family Planning (No. ZKX16059 and ZKX16057).
Disclosure of conflict of interest
None.
References
- 1.Pham NM, Recht NS, Hostetter TH, Meyer TW. Removal of the protein-bound solutes indican and p-cresol sulfate by peritoneal dialysis. Clin J Am Soc Nephrol. 2008;3:85–90. doi: 10.2215/CJN.02570607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Niwa T, Takeda N, Tatematsu A, Maeda K. Accumulation of indoxyl sulfate, an inhibitor of drug-binding, in uremic serum as demonstrated by internal-surface reversed-phase liquid chromatography. Clin Chem. 1988;34:2264–2267. [PubMed] [Google Scholar]
- 3.Niwa T, Ise M. Indoxyl sulfate, a circulating uremic toxin, stimulates the progression of glomerular sclerosis. J Lab Clin Med. 1994;124:96–104. [PubMed] [Google Scholar]
- 4.Niwa T, Yazawa T, Ise M, Sugano M, Kodama T, Uehara Y, Maeda K. Inhibitory effect of oral sorbent on accumulation of albumin-bound indoxyl sulfate in serum of experimental uremic rats. Nephron. 1991;57:84–88. doi: 10.1159/000186222. [DOI] [PubMed] [Google Scholar]
- 5.Niwa T, Emoto Y, Maeda K, Uehara Y, Yamada N, Shibata M. Oral sorbent suppresses accumulation of albumin-bound indoxyl sulphate in serum of haemodialysis patients. Nephrol Dial Transplant. 1991;6:105–109. doi: 10.1093/ndt/6.2.105. [DOI] [PubMed] [Google Scholar]
- 6.Gelasco AK, Raymond JR. Indoxyl sulfate induces complex redox alterations in mesangial cells. Am J Physiol Renal Physiol. 2006;290:F1551–1558. doi: 10.1152/ajprenal.00281.2004. [DOI] [PubMed] [Google Scholar]
- 7.Yisireyili M, Saito S, Abudureyimu S, Adelibieke Y, Ng HY, Nishijima F, Takeshita K, Murohara T, Niwa T. Indoxyl sulfate-induced activation of (pro)renin receptor promotes cell proliferation and tissue factor expression in vascular smooth muscle cells. PLoS One. 2014;9:e109268. doi: 10.1371/journal.pone.0109268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li S, Sun Z, Ding G, Gong W, Yu J, Xia W, Huang S, Zhang A, Zhang Y, Jia Z. mPGES-1-derived PGE2 contributes to indoxyl sulfate-induced mesangial cell proliferation. Cell Physiol Biochem. 2017;43:271–281. doi: 10.1159/000480369. [DOI] [PubMed] [Google Scholar]
- 9.Li S, Cheng S, Sun Z, Mungun HK, Gong W, Yu J, Xia W, Zhang Y, Huang S, Zhang A, Jia Z. Indoxyl sulfate induces mesangial cell proliferation via the induction of COX-2. Mediators Infl amm. 2016;2016:5802973. doi: 10.1155/2016/5802973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klayman DL. Qinghaosu (artemisinin): an antimalarial drug from China. Science. 1985;228:1049–1055. doi: 10.1126/science.3887571. [DOI] [PubMed] [Google Scholar]
- 11.Beekman AC, Woerdenbag HJ, Van Uden W, Pras N, Konings AW, Wikstrom HV. Stability of artemisinin in aqueous environments: impact on its cytotoxic action to Ehrlich ascites tumour cells. J Pharm Pharmacol. 1997;49:1254–1258. doi: 10.1111/j.2042-7158.1997.tb06080.x. [DOI] [PubMed] [Google Scholar]
- 12.Beekman AC, Wierenga PK, Woerdenbag HJ, Van Uden W, Pras N, Konings AW, el-Feraly FS, Galal AM, Wikstrom HV. Artemisinin-derived sesquiterpene lactones as potential antitumour compounds: cytotoxic action against bone marrow and tumour cells. Planta Med. 1998;64:615–619. doi: 10.1055/s-2006-957533. [DOI] [PubMed] [Google Scholar]
- 13.Steely AM, Willoughby JA Sr, Sundar SN, Aivaliotis VI, Firestone GL. Artemisinin disrupts androgen responsiveness of human prostate cancer cells by stimulating the 26S proteasome-mediated degradation of the androgen receptor protein. Anticancer Drugs. 2017;28:1018–1031. doi: 10.1097/CAD.0000000000000547. [DOI] [PubMed] [Google Scholar]
- 14.Wang YY, Liu YX, Xie QB, Liu G. [Effects of dihydroartemisinin on collagen II-induced arthritis in rats model] . Sichuan Da Xue Xue Bao Yi Xue Ban. 2012;43:851–854. [PubMed] [Google Scholar]
- 15.Wei M, Xie X, Chu X, Yang X, Guan M, Wang D. Dihydroartemisinin suppresses ovalbumin-induced airway infl ammation in a mouse allergic asthma model. Immunopharmacol Immunotoxicol. 2013;35:382–389. doi: 10.3109/08923973.2013.785559. [DOI] [PubMed] [Google Scholar]
- 16.Khan AI, Kapoor A, Che J, Martin L, Rogazzo M, Mercier T, Decosterd L, Collino M, Thiemermann C. The anti-malarial drug artesunate attenuates cardiac injury in a rodent model of myocardial infarction. Shock. 2017 doi: 10.1097/SHK.0000000000000963. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 17.Tam P. Peritoneal dialysis and preservation of residual renal function. Perit Dial Int. 2009;29(Suppl 2):S108–110. [PubMed] [Google Scholar]
- 18.Bargman JM, Thorpe KE, Churchill DN CANUSA Peritoneal Dialysis Study Group. Relative contribution of residual renal function and peritoneal clearance to adequacy of dialysis: a reanalysis of the CANUSA study. J Am Soc Nephrol. 2001;12:2158–2162. doi: 10.1681/ASN.V12102158. [DOI] [PubMed] [Google Scholar]
- 19.Szeto CC, Wong TY, Leung CB, Wang AY, Law MC, Lui SF, Li PK. Importance of dialysis adequacy in mortality and morbidity of chinese CAPD patients. Kidney Int. 2000;58:400–407. doi: 10.1046/j.1523-1755.2000.00179.x. [DOI] [PubMed] [Google Scholar]
- 20.Paniagua R, Amato D, Vonesh E, Correa-Rotter R, Ramos A, Moran J, Mujais S Mexican Nephrology Collaborative Study Group. Effects of increased peritoneal clearances on mortality rates in peritoneal dialysis: ADEMEX, a prospective, randomized, controlled trial. J Am Soc Nephrol. 2002;13:1307–1320. doi: 10.1681/ASN.V1351307. [DOI] [PubMed] [Google Scholar]
- 21.Niwa T. Uremic toxicity of indoxyl sulfate. Nagoya J Med Sci. 2010;72:1–11. [PMC free article] [PubMed] [Google Scholar]
- 22.Adam I, Tarning J, Lindegardh N, Mahgoub H, McGready R, Nosten F. Pharmacokinetics of piperaquine in pregnant women in Sudan with uncomplicated plasmodium falciparum malaria. Am J Trop Med Hyg. 2012;87:35–40. doi: 10.4269/ajtmh.2012.11-0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoglund RM, Adam I, Hanpithakpong W, Ashton M, Lindegardh N, Day NP, White NJ, Nosten F, Tarning J. A population pharmacokinetic model of piperaquine in pregnant and non-pregnant women with uncomplicated Plasmodium falciparum malaria in Sudan. Malar J. 2012;11:398. doi: 10.1186/1475-2875-11-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benjamin JM, Moore BR, Salman S, Page-Sharp M, Tawat S, Yadi G, Lorry L, Siba PM, Batty KT, Robinson LJ, Mueller I, Davis TM. Population pharmacokinetics, tolerability, and safety of dihydroartemisinin-piperaquine and sulfadoxine-pyrimethamine-piperaquine in pregnant and nonpregnant Papua New Guinean women. Antimicrob Agents Chemother. 2015;59:4260–4271. doi: 10.1128/AAC.00326-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang X, Xie Z, Liu F, Han C, Zhang D, Wang D, Bao X, Sun J, Wen C, Fan Y. Dihydroartemisinin inhibits activation of the toll-like receptor 4 signaling pathway and production of type I interferon in spleen cells from lupus-prone MRL/lpr mice. Int Immunopharmacol. 2014;22:266–272. doi: 10.1016/j.intimp.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 26.Huang XJ, Ma ZQ, Zhang WP, Lu YB, Wei EQ. Dihydroartemisinin exerts cytotoxic effects and inhibits hypoxia inducible factor-1alpha activation in C6 glioma cells. J Pharm Pharmacol. 2007;59:849–856. doi: 10.1211/jpp.59.6.0011. [DOI] [PubMed] [Google Scholar]
- 27.Gao X, Luo Z, Xiang T, Wang K, Li J, Wang P. Dihydroartemisinin induces endoplasmic reticulum stress-mediated apoptosis in HepG2 human hepatoma cells. Tumori. 2011;97:771–780. doi: 10.1177/030089161109700615. [DOI] [PubMed] [Google Scholar]
- 28.Li S, Sun Z, Zhang Y, Ruan Y, Chen Q, Gong W, Yu J, Xia W, He JC, Huang S, Zhang A, Ding G, Jia Z. COX-2/mPGES-1/PGE2 cascade activation mediates uric acid-induced mesangial cell proliferation. Oncotarget. 2017;8:10185–10198. doi: 10.18632/oncotarget.14363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu X, Yang Y, Yuan H, Wu M, Li S, Gong W, Yu J, Xia W, Zhang Y, Ding G, Huang S, Jia Z, Zhang A. Inhibition of COX-2/PGE2 cascade ameliorates cisplatin-induced mesangial cell apoptosis. Am J Transl Res. 2017;9:1222–1229. [PMC free article] [PubMed] [Google Scholar]


