Abstract
The present study investigated the radiosensitizing effect of placenta specific 8 (PLAC8) in nasopharyngeal carcinoma (NPC) cells. A PLAC8-knockout CNE2 cell line was constructed using the CRISPR/Cas9 system. The CCK-8 assay demonstrated knockout of PLAC8 significantly reduced cell proliferation and cell survival after irradiation compared to both control cells and non-irradiated PLAC8-knockout cells. The clonogenic assay showed knockout of PLAC8 enhanced the radiosensitivity of NPC cells. Flow cytometry revealed knockout of PLAC8 increased apoptosis and G2/M phase arrest after irradiation. Western blotting demonstrated knockout of PLAC8 was associated with increased levels of γHA2X, a higher BAX:BCL-2 ratio, and increased levels of phosphorylated-Akt and phosphorylated-GSK-3β. Overall, this study indicates PLAC8 contributes to radioresistance in NPC by inhibiting the PI3K/AKT/GSK3β pathway.
Keywords: Apoptosis, knockout, nasopharyngeal carcinoma, PI3K/Akt/GSK3β pathway, placenta specific 8, radiosensitivity
Introduction
Nasopharyngeal carcinoma (NPC) is one of the most common head and neck malignancies and has a low survival rate. The incidence of NPC is 20-30 per 100,000 in southeast Asia and 15-50 per 100,000 in southern China, but less than 1 per 100,000 in western countries [1]. Radiotherapy is the most effective treatment for NPC [2]; however, radioresistance plays a major role in treatment failure [3]. The ability to enhance the radiosensitivity of NPC cells may help to improve survival outcomes.
Previous genome-wide mRNA expression analysis of microdissected human pancreatic tissues identified placenta-specific 8 (PLAC8) mRNA is overexpressed in advanced preneoplastic lesions (pancreatic intraepithelial neoplasia grade 3; PanIN3) and invasive pancreatic ductal adenocarcinoma [4]. PLAC8, also known as onzin, is a 16 kDa protein originally described to be highly expressed in mouse placenta [5]. PLAC8 mRNA expression was subsequently detected in myeloid and lymphoid cells, as well as lung and intestinal epithelial cells. Knockout of PLAC8 in mice results in defects in innate immunity [6], impaired brown and white fat cell differentiation, late-onset obesity [7,8], and contact hypersensitivity [9]. Although evolutionarily highly conserved, PLAC8 does not contain any known functional protein domains and its molecular function remains unclear.
The cellular role of PLAC8 seems to be context-dependent, with highly variable and sometimes contradictory effects observed in different cell types, ranging from protection against apoptosis in fibroblasts [10], induction of apoptosis in human lymphocytes [11], inhibition of cell differentiation in primary acute myeloid leukemia cells, and induction of the epithelial-mesenchymal transition (EMT) in cultured colon cancer cells [12].
The PI3K/Akt/GSK3β signaling pathway plays pivotal roles in cancer cell proliferation, invasion, metastasis and apoptosis. The serine/threonine kinase Akt is the central downstream effector molecule of PI3K. Activated Akt phosphorylates glycogen synthase kinase 3β (GSK3β), another serine/threonine kinase, which regulates a variety of cellular functions, including apoptosis [13,14]. PLAC8 can regulate the expression of genes associated with cell growth, autophagy and the EMT [10-12,15]. Autophagy functions as a protective mechanism in irradiated cells [16]. Moreover, irradiation can induce autophagy in 5-8F and CNE2 cells [17]. However, the association between PLAC8 and radioresistance is unknown. In this study, we investigated the effect of knocking out the PLAC8 gene on the radiosensitivity of CNE2 NPC cells in vitro.
Materials and methods
Cell culture and generation of PLAC8-knockout cells
Human CNE2 NPC cells were obtained from Wuhan University (Wuhan, China) and cultured in RMPI-1640 media (Jenom, Hangzhou, China) supplemented with 10% fetal bovine serum (Gibco-BRL/Invitrogen Life Technologies, MA, USA) in a humidified atmosphere containing 5% CO2 at 37°C.
PLAC8-knockout CNE2 cells, hereafter called PLAC8(-), were established by Beijing Biocytogen Company (Beijing, China). Briefly, the intron sequence between the second and third exons of PLAC8 was replaced with Puro by homologous recombination using the CRISPR/CAS9 gene editing method to knockout PLAC8. Positive clones were isolated by screening for puromycin resistance and confirmed by PCR, sequencing and western blotting.
Cell proliferation assay
Four groups of cells were prepared: PLAC8(+), PLAC8(-), IR (irradiated)-PLAC8(+), and IR-PLAC8(-). Briefly, 0.5 to 1 × 104 cells were seeded into 96-well culture plates, cultured for 12 h, then irradiated (or not) with a single dose of 6 Gy at 400 cGy/min using a linear accelerator (Trilogy, Austin, TX, USA) using 6 MV photons at room temperature. The cells were cultured for 24, 48 or 72 h, then cell numbers were assessed using the Cell Counting Kit-8 (CCK-8; Dojindo, Shanghai, China) according to the manufacturer’s instructions. Briefly, 10 μl of CCK-8 (5 g/L) was added, incubated for 1 h and absorbance was measured at 450 nm using a Victor3 micro-plate reader (Perkin-Elmer, Waltham, MA, USA). The morphological changes in PLAC8(+) and PLAC8(-) cells at 24 h and 48 h after irradiation were observed using an inverted microscope.
Clonogenic survival assay
Two groups of cells were prepared: IR-PLAC8(+) and IR-PLAC8(-). Briefly, 500, 1000, 3000 or 10000 cells were seeded into 6-well plates, incubated for 6 h, irradiated at 2, 4, 6 or 8 Gy, cultured for 14 days, and fixed and stained with methanol containing 1% crystal violet (Beijing Dingguo Changsheng Biotech Co., Ltd., Beijing, China). Colonies (≥ 50 cells) were counted under a light microscope (U-LH100L-3; Olympus Corporation, Tokyo, Japan) and colony formation efficiency was calculated as number of colonies/number of cells inoculated. GraphPad Prism 6.0 Software (GraphPad Software Inc., La Jolla, CA, USA) was used to calculate and fit the dose survival curve using the linear-quadratic model.
Apoptosis assays
Four groups of cells were prepared: PLAC8(+), PLAC8(-), IR-PLAC8(+) and IR-PLAC8(-). Cells were plated in 6-well plates at 3 × 105 cells per well, cultured overnight, irradiated (or not) at 6 Gy, cultured for 24 h, collected, stained with Annexin V-FITC and propidium iodide (PI), and then analyzed using a FACS Calibur flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA). Data were analyzed using BD FACS DIVA software (BD Biosciences).
Cell cycle analysis
Cells were treated as described above. At 24 h after irradiation, cells were trypsinized, washed with phosphate buffered saline (PBS) lacking magnesium and calcium, resuspended in 400 μL staining solution containing 0.5 μg/mL RNaseA and 50 μg/mL propidium iodide (PI; Sigma, St. Louis, MO, USA), incubated for 15 min in the dark at room temperature, and analyzed using a FACS Calibur flow cytometer with BD FACS DIVA software.
Western blot analysis
Cells were treated as described above. At 24 h after irradiation, cells were lysed in RIPA buffer containing protease inhibitors (Cell Biolabs Inc., San Diego, CA, USA) and protein concentrations were quantified using the BCA method (Cell Biolabs Inc., San Diego, CA, USA). Equal amounts of total protein (30-50 μg) were resolved by 10% SDS-PAGE (Bio-Rad Laboratories, Inc., Hercules, CA, USA), transferred onto PVDF membranes (Thermo Fisher Scientific, Inc.), blocked in 5% non-fat milk at room temperature for 1 h, then incubated with the following antibodies: rabbit monoclonal γH2AX (1:1,000; ab2893), primary rabbit polyclonal Akt (1:500; ab8805), rabbit monoclonal GSK-3β (1:500; ab32391), rabbit monoclonal phospho (p)Akt (Ser473) (1:500; ab81283), rabbit monoclonal phospho (p)-GSK-3β (Ser-9) (1:1000; ab75814; all Abcam, Cambridge, ST, USA), rabbit monoclonal BCL2 (1:1000; #2872), rabbit monoclonal BAX (1:1000; #2774), rabbit monoclonal cyclin D1 (1:1000; #2978) or rabbit monoclonal survivin (1:1000; #2803; all Cell Signaling Technology, ST, USA). The membranes were incubated with a secondary antibody (1:4,000, #9916, anti-rabbit IgG; CST) for 1 h at room temperature and the labeled proteins were detected using chemiluminescence reagent and automatic X-ray film. GAPDH was assessed as an internal loading control.
Statistical analysis
SPSS software version 22.0 (SPSS Inc., Chicago, IL, USA) was used for all statistical analyses. Quantitative data are presented as mean ± standard deviation of at least three independent replicates. The significance of the variances between each group was assessed using the Student’s t-test. P < 0.05 was considered to indicate statistical significance.
Results
Confirmation of PLAC8-knockout CNE2 cells
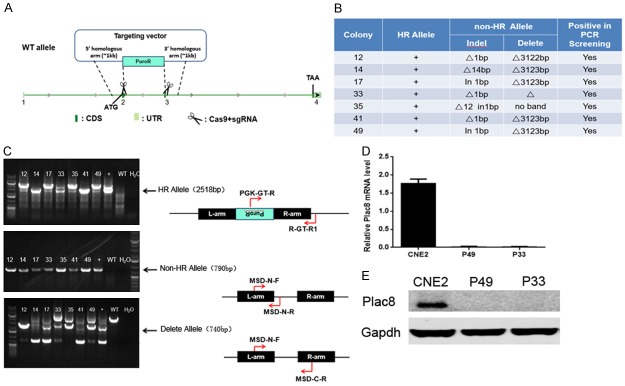
Selection of resistant gene cassettes was determined by antibiotic titration and karyotyping (Figure 1A). Genotyping suggested colonies 12, 14, 17, 33, 35, 41 and 49 were PLAC8-knockout CNE2 cells, termed PLAC8(-) (Figure 1B, 1C). Western blot analysis confirmed high level expression of PLAC8 in normal CNE2 cells and a lack of detectable PLAC8 in PLAC8(-) colonies P33 and P49 (Figure 1E). The P49 PLAC8(-) colony was used for all subsequent experiments.
Figure 1.
Construction of PLAC8-knockout CNE2 cells. A: The intron sequence between the second and third exons of the PLAC8 gene was replaced with Puro via homologous recombination using CRISPR/CAS9 gene editing. B, C: Genotyping indicated colonies 12, 14, 17, 33, 35, 41 and 49 were PLAC8-knockout cell lines. D: Relative mRNA expression of PLAC8 gene in colonies P49 and P33 compared to parental CNE-2 cells. All quantitative data shown represent the means ± SD of 3 independent replicates (P < 0.05). E: Western blotting confirmed PLAC8 protein expression was undetectable in PLAC8-knockout colonies P49 and P33.
Knockout of PLAC8 decreases NPC cell proliferation, survival and radiosensitivity
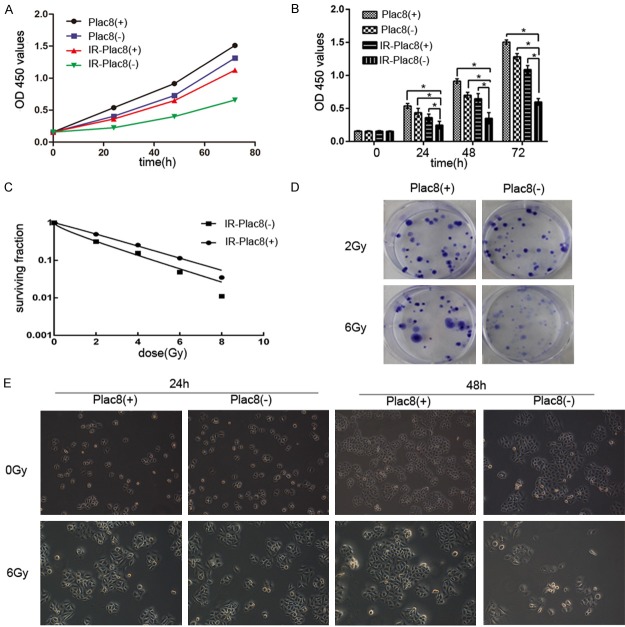
The CCK8 assay was used to evaluate the radiosensitivity of PLAC8(+) and PLAC8(-) CNE2 cells. As shown in Figure 2A and 2B, significant differences in the number of surviving cells were observed between irradiated (6 Gy) and non-irradiated PLAC8(+) and PLAC8(-) CNE2 cells at 24, 48 and 72 h (P < 0.05). Knockout of PLAC8 significantly reduced the survival of both non-irradiated and irradiated CNE2 cells compared to PLAC8(+) cells at all time-points (P < 0.05).
Figure 2.
Knockout of PLAC8 decreases proliferation and reduces the surviving fraction after irradiation in CNE2 cells. A, B: CCK-8 assay of the number of surviving PLAC8(+) and PLAC8(-) cells at 24, 48 and 72 h after 6 Gy irradiation. Knockout of PLAC8 significantly reduced the survival of both non-irradiated and irradiated CNE2 cells compared to PLAC8(+) cells at all time-points. All quantitative data shown represent the means ± SD of 3 independent replicates; significant differences were shown as *P < 0.05. C, D: IR-PLAC8(-) cells had significantly lower surviving fractions compared to IR-PLAC8(+) cells after irradiation at 2, 4, 6 and 8 Gy. E: The proliferation of IR-PLAC8(-) cells was significantly suppressed compared to IR-PLAC8(+) cells after irradiation at 24 h and 48 h.
The colony survival assay is a well-established method of determining radiosensitivity. A lower surviving fraction indicates the cells are more radiosensitive. IR-PLAC8(-) cells had significantly lower surviving fractions compared to IR-PLAC8(+) cells after irradiation at 2, 4, 6 and 8 Gy (Figure 2C and 2D). Furthermore, the proliferation of IR-PLAC8(-) cells was significantly suppressed compared to IR-PLAC8(+) cells after irradiation at 24 h and 48 h (Figure 2E).
Knockout of PLAC8 increases apoptosis and alters cell cycle distribution in irradiated CNE2 cells
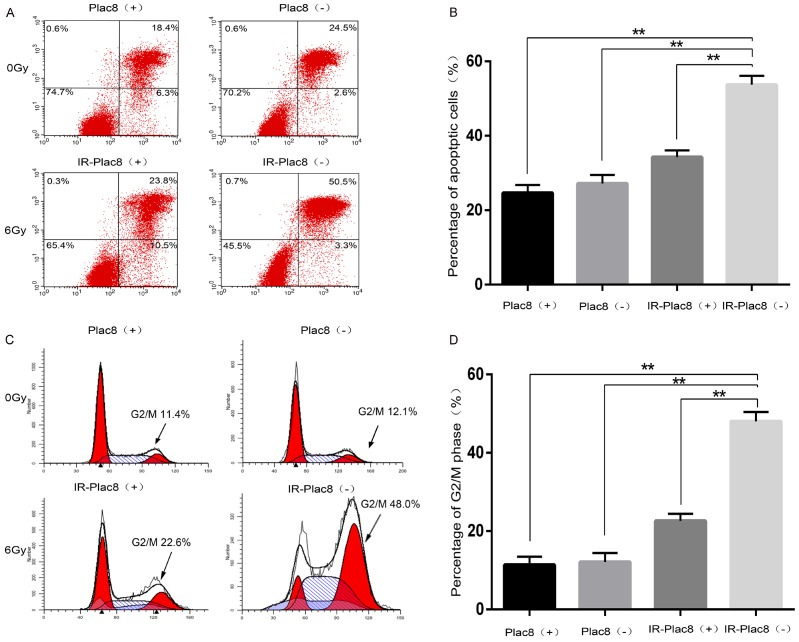
At 24 h after irradiation, the apoptotic rates of PLAC8(+), PLAC8(-), IR-PLAC8(+), and IR-PLAC8(-) CNE-2 cells were 24.68 ± 0.90, 27.17 ± 1.60, 34.28 ± 1.59, and 53.71 ± 0.75%, respectively. The rate of apoptosis was significantly higher in IR-PLAC8(-) cells than IR-PLAC8(+) cells (P < 0.05; Figure 3A and 3B).
Figure 3.
Knockout of PLAC8 induces apoptosis and G2/M phase arrest in CNE2 cells. A: Detection of apoptosis by flow cytometry after Annexin V-FITC/propidium iodide (PI) staining of PLAC8(+) and PLAC8(-) cells at 48 h after irradiation at 0 or 6 Gy. B: The rate of apoptosis was significantly higher in IR-PLAC8(-) cells than PLAC8(+), PLAC8(-) and IR-PLAC8(+) cells. C: Cell cycle analysis of PLAC8(+) and PLAC8(-) cells at 48 h after irradiation at 0 or 6 Gy. D: The rate of G2/M cell cycle arrest was significantly higher in IR-PLAC8(-) cells than PLAC8(+), PLAC8(-) and IR-PLAC8(+) cells; All quantitative data shown represent the means ± SD of 3 independent replicates; significant differences were shown as **P < 0.01.
As shown in Figure 3C, the majority of unirradiated PLAC8(+) and PLAC8(-) cells were in the G0/G1 phase. Irradiation significantly increased the proportions of PLAC8(+) and PLAC8(-) cells in the G2/M phase, though knockout of PLAC8 significantly increased the proportion of G2/M phase cells after 6 Gy irradiation compared to IR-PLAC8(+) cells (Figure 3C and 3D).
Knockout of PLAC8 alters the expression of proteins related to DNA repair, apoptosis and the cell cycle
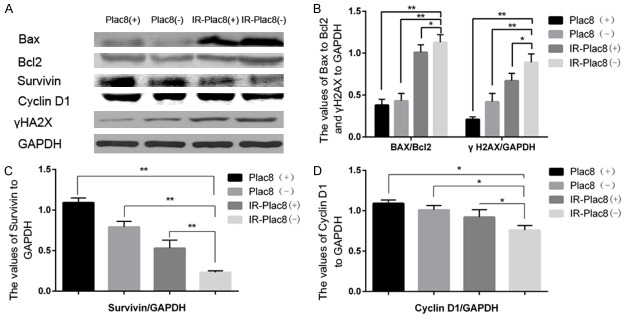
The levels of γH2AX, a sensitive indicator of DNA double strand break (DSB) repair, correlate positively with apoptosis and radiosensitivity [18]. Western blotting revealed γH2AX protein levels were markedly increased in IR-PLAC8(-) cells compared to IR-PLAC8(+) cells, PLAC8(+) cells and PLAC8(-) cells.
Furthermore, knocking out PLAC8 in combination with irradiation significantly increased the ratio between the pro-apoptotic protein BAX and anti-apoptotic protein BCL-2, and reduced the protein levels of survivin, an inhibitor of apoptosis, as well as the cell cycle-related protein cyclin D1 (Figure 4).
Figure 4.
Knockout of PLAC8 alters the expression of proteins related to apoptosis and the cell cycle. Western blots of PLAC8(+) and PLAC8(-) cell lysates at 48 h after irradiation at 0 or 6 Gy. A: BAX, BCL-2 and γH2AX protein expression increased and cyclin D1 and survivin expression decreased in IR-PLAC8(-) cells compared to PLAC8(+), PLAC8(-) and IR-PLAC8(+) cells. B: Quantification of the BAX:BCL2 ratio and γH2AX levels. C: Quantification of survivin expression in IR-PLAC8(-) cells. D: Quantification of cyclin D1 expression. All quantitative data shown represent the means ± SD of 3 independent replicates; significant differences were shown as *P < 0.05. **P < 0.01.
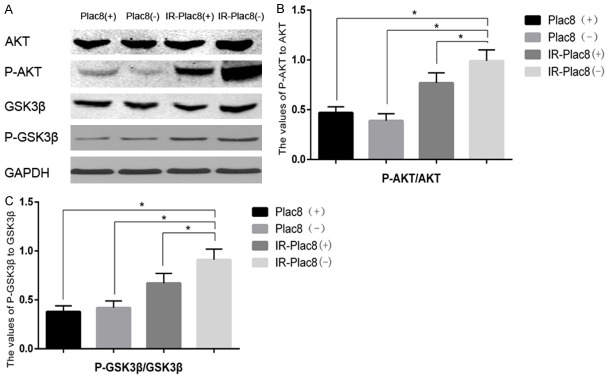
Knockout of PLAC8 activates the PI3K/AKT/GSK3β pathway
Next, we elucidated the signaling pathway associated with increased apoptosis in irradiated PLAC8(-) cells. Western blotting revealed knocking out PLAC8 in combination with irradiation increased the levels of phospho-Akt and phospho-GSK-3β, but not total Akt or total GSK-3β, compared to PLAC8(+) cells, IR-PLAC8(+) cells and IR-PLAC8(-) cells (Figure 4B). The phospho-Akt:total Akt ratio was 0.48, 0.39, 0.89 and 1.12, and the phospho-GSK-3β:GSK-3β ratio was 0.36, 0.43, 0.67 and 0.87 in PLAC8(+), PLAC8(-), IR-PLAC8(+) and IR-PLAC8(-) cells, respectively; the differences between each treatment were significantly different for both phospho-AKT and phospho-GSK-3β (Figure 5).
Figure 5.
Knockout of PLAC8 activates the PI3K/AKT/GSK3β pathway. Western blots of PLAC8(+) and PLAC8(-) cell lysates at 48 h after irradiation at 0 or 6 Gy. A: Phospho-Akt and phospho-GSK-3β protein expression increased but total Akt and GSK-3β remained unchanged in IR-PLAC8(-) cells compared to PLAC8(+), PLAC8(-) and IR-PLAC8(+) cells. B: Quantification of the phospho-Akt:AKT ratio. C: Quantification of the phospho-GSK-3β: GSK-3β ratio. All quantitative data shown represent the means ± SD of 3 independent replicates; significant differences were shown as *P < 0.05. **P < 0.01.
Discussion
Radiotherapy is an effective component of treatment for NPC, and radioresistance is the main risk factor for poor outcome [19]. Radioresistance can develop during primary radiation therapy, and surviving cells can become more resistant to subsequent radiation treatment, thereby leading to the failure of radiotherapy [20,21]. The molecules and signaling pathways involved in radioresistance need to be identified in order to develop targeted therapies and enhance the efficacy of radiation. The present study reveals that knockout of the PLAC8 gene enhanced the radiosensitivity of CNE2 cells in vitro.
Constantly dividing and renewing cell populations have higher radiosensitivity than non-dividing cells. If a cell population which is at a low rate of proliferation or an undifferentiated stage become activated, then radiosensitivity may increase [22]. In this study, knockout of PLAC8 significantly decreased the proliferation and colony orming ability of irradiated CNE2 cells, indicating PLAC8 confers to radioresistance in NPC.
Induction of cellular apoptosis and cell cycle changes are major effects of irradiation. However, cancer cells develop mechanisms to alter signaling pathways, evade host immune surveillance and prevent cell death, which facilitates long-term survival [23]; such changes can lead to tumor progression and metastasis. In this study, we observed knockout of PLAC8 significantly increased the levels of apoptosis in irradiated CNE2 cells.
DNA damage induced by UV, irradiation or DNA damaging agents activates checkpoint pathways and delays G1, S or G2 phase cell cycle progression [24]. Knockout of PLAC8 significantly increased apoptosis in irradiated CNE2 cells, as revealed by a significant increase in the proportions of cells in the G2/M and S phases and a decrease in the proportion of G1/G0 phase cells. The S phase is a critical stage when DNA replication occurs, and DNA synthesis is tightly controlled to guarantee that replication origins are not activated more than once per cell cycle [25,26], while G2/M is the most radiosensitive phase [9]. Therefore, these data indicate that knockout of PLAC8 increased G2/M phase arrest and induced more DNA damage.
The BCL-2 protein family are well-characterized executioners of apoptosis [27]. Amyloid beta-protein induces expression of pro-apoptotic BAX and downregulates anti-apoptotic BCL-2, which directly activates the mitochondrial death pathway. Apoptotic signals can be initiated by external stimuli/ligands and or cellular stress such as gamma/UV radiation and cytotoxic drugs, which in turn leads to altered mitochondrial permeability [28]. Knockout of PLAC8 significantly increased the levels of apoptosis and the BAX:BCL-2 ratio in irradiated CNE2 cells, confirming that PLAC8(-) promoted the radiosensitivity of CNE2 cells.
The PI3K/Akt/BCL anti-apoptotic and survival signaling pathway plays a crucial role in apoptosis [28,29]. Once activated, Akt translocates to cell membrane where it phosphorylates and activates the multifunctional serine/threonine kinase GSK-3β. In cancer cells, increased activation of GSK-3β is pro-apoptotic, whereas suppression of GSK-3β exerts an anti-apoptotic effect [30]. The levels of phosphorylated AKT (Ser473) and phosphorylated-GSK-3β (Ser-9) reflect AKT and GSK-3β activity, respectively. We observed knockout of PLAC8 reduced the level of phosphorylated-AKT (Ser-473), whereas knockout of PLAC8 combined with irradiation increased phosphorylated-AKT (Ser-473). Reduced levels of phosphorylated-AKT are associated with autophagy, while increased levels of phosphorylated-AKT promote apoptosis. Furthermore, the increased levels of phosphorylated-GSK-3β in the IR-PLAC8(-) cells confirmed that knockout of PLAC8 in combination with irradiation activated the PI3K/Akt/GSK3β pathway.
Overexpression of PLAC8 promotes apoptosis in human ovarian cancer cells, leukemia cells and peripheral blood lymphocytes, whereas knockout of PLAC8 inhibits apoptosis [10]. However, a relationship between knockout of the PLAC8 gene and the radioresistance of cancer cells has not previously been reported.
The present study has a number of limitations. Our experiments were mainly performed on a single cell line, and we only assessed the effects of PLAC8 in vitro. In subsequent work, we would like to assess PLAC8 mRNA/protein expression in normal nasopharyngeal epithelium and NPC tissues. Furthermore, animal experiments are being planned to investigate the molecular mechanism of action of PLAC8 in NPC and radiosensitivity in vivo.
Taken together, these results indicate PLCA8 contributes to radioresistance in NPC by inhibiting the PI3K/AKT/GSK3Β pathway. These findings may help to identify a therapeutic strategy to enhance radiosensitivity and improve treatment outcomes in NPC.
Acknowledgements
This work was supported by the National Natural Science Foundation of China [grant number 81172569]. We would like to thank the native English-speaking scientists of Elixigen Company (Huntington Beach, California) for editing our manuscript.
Disclosure of conflict of interest
None.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Rottey S, Madani I, Deron P, Van Belle S. Modern treatment for nasopharyngeal carcinoma: current status and prospects. Curr Opin Oncol. 2011;23:254–258. doi: 10.1097/CCO.0b013e328344f527. [DOI] [PubMed] [Google Scholar]
- 3.Buchholz M, Braun M, Heidenblut A, Kestler HA, Kloppel G, Schmiegel W, Hahn SA, Luttges J, Gress TM. Transcriptome analysis of microdissected pancreatic intraepithelial neoplastic lesions. Oncogene. 2005;24:6626–6636. doi: 10.1038/sj.onc.1208804. [DOI] [PubMed] [Google Scholar]
- 4.Galaviz-Hernandez C, Stagg C, de Ridder G, Tanaka TS, Ko MS, Schlessinger D, Nagaraja R. Plac8 and Plac9, novel placental-enriched genes identified through microarray analysis. Gene. 2003;309:81–89. doi: 10.1016/s0378-1119(03)00508-0. [DOI] [PubMed] [Google Scholar]
- 5.Ledford JG, Kovarova M, Koller BH. Impaired host defense in mice lacking ONZIN. J Immunol. 2007;178:5132–5143. doi: 10.4049/jimmunol.178.8.5132. [DOI] [PubMed] [Google Scholar]
- 6.Jimenez-Preitner M, Berney X, Uldry M, Vitali A, Cinti S, Ledford JG, Thorens B. Plac8 is an inducer of C/EBPbeta required for brown fat differentiation, thermoregulation, and control of body weight. Cell Metab. 2011;14:658–670. doi: 10.1016/j.cmet.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 7.Jimenez-Preitner M, Berney X, Thorens B. Plac8 is required for white adipocyte differentiation in vitro and cell number control in vivo. PLoS One. 2012;7:e48767. doi: 10.1371/journal.pone.0048767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ledford JG, Kovarova M, Jania LA, Nguyen M, Koller BH. ONZIN deficiency attenuates contact hypersensitivity responses in mice. Immunol Cell Biol. 2012;90:733–742. doi: 10.1038/icb.2011.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rogulski K, Li Y, Rothermund K, Pu L, Watkins S, Yi F, Prochownik EV. Onzin, a c-Myc-repressed target, promotes survival and transformation by modulating the Akt-Mdm2-p53 pathway. Oncogene. 2005;24:7524–7541. doi: 10.1038/sj.onc.1208897. [DOI] [PubMed] [Google Scholar]
- 10.Mourtada-Maarabouni M, Watson D, Munir M, Farzaneh F, Williams GT. Apoptosis suppression by candidate oncogene PLAC8 is reversed in other cell types. Curr Cancer Drug Targets. 2012;13:80–91. [PubMed] [Google Scholar]
- 11.Wu SF, Huang Y, Hou JK, Yuan TT, Zhou CX, Zhang J, Chen GQ. The downregulation of onzin expression by PKCepsilon-ERK2 signaling and its potential role in AML cell differentiation. Leukemia. 2010;24:544–551. doi: 10.1038/leu.2009.280. [DOI] [PubMed] [Google Scholar]
- 12.Li C, Ma H, Wang Y, Cao Z, Graves-Deal R, Powell AE, Starchenko A, Ayers GD, Washington MK, Kamath V, Desai K, Gerdes MJ, Solnica-Krezel L, Coffey RJ. Excess PLAC8 promotes an unconventional ERK2-dependent EMT in colon cancer. J Clin Invest. 2014;124:2172–2187. doi: 10.1172/JCI71103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu W, Xiao L, Cao C, Hua S, Wu D. UBE2T promotes nasopharyngeal carcinoma cell proliferation, invasion, and metastasis by activating the AKT/GSK3beta/beta-catenin pathway. Oncotarget. 2016;7:15161–15172. doi: 10.18632/oncotarget.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lv J, Wei Y, Chen Y, Zhang X, Gong Z, Jiang Y, Gong Q, Zhou L, Wang H, Xie Y. Dexmedetomidine attenuates propofol-induce neuroapoptosis partly via the activation of the PI3k/Akt/GSK3beta pathway in the hippocampus of neonatal rats. Environ Toxicol Pharmacol. 2017;52:121–128. doi: 10.1016/j.etap.2017.03.017. [DOI] [PubMed] [Google Scholar]
- 15.Kaistha BP, Lorenz H, Schmidt H, Sipos B, Pawlak M, Gierke B, Kreider R, Lankat-Buttgereit B, Sauer M, Fiedler L, Krattenmacher A, Geisel B, Kraus JM, Frese KK, Kelkenberg S, Giese NA, Kestler HA, Gress TM, Buchholz M. PLAC8 localizes to the inner plasma membrane of pancreatic cancer cells and regulates cell growth and disease progression through critical cell-cycle regulatory pathways. Cancer Res. 2016;76:96–107. doi: 10.1158/0008-5472.CAN-15-0216. [DOI] [PubMed] [Google Scholar]
- 16.Tam SY, Wu VW, Law HK. Influence of autophagy on the efficacy of radiotherapy. Radiat Oncol. 2017;12:57. doi: 10.1186/s13014-017-0795-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun Q, Liu T, Zhang T, Du S, Xie GX, Lin X, Chen L, Yuan Y. MiR-101 sensitizes human nasopharyngeal carcinoma cells to radiation by targeting stathmin 1. Mol Med Rep. 2015;11:3330–3336. doi: 10.3892/mmr.2015.3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sayed AE, Igarashi K, Watanabe-Asaka T, Mitani H. Double strand break repair and gamma-H2AX formation in erythrocytes of medaka (Oryzias latipes) after gamma-irradiation. Environ Pollut. 2017;224:35–43. doi: 10.1016/j.envpol.2016.11.050. [DOI] [PubMed] [Google Scholar]
- 19.Zhang G, Wang W, Yao C, Ren J, Zhang S, Han M. Salinomycin overcomes radioresistance in nasopharyngeal carcinoma cells by inhibiting Nrf2 level and promoting ROS generation. Biomed Pharmacother. 2017;91:147–154. doi: 10.1016/j.biopha.2017.04.095. [DOI] [PubMed] [Google Scholar]
- 20.Chen ZT, Liang ZG, Zhu XD. A review: proteomics in nasopharyngeal carcinoma. Int J Mol Sci. 2015;16:15497–15530. doi: 10.3390/ijms160715497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin H, Chen ZT, Zhu XD, Li L, Qu S, Wei Z, Su F, Wei JN, Liang ZG, Mo QY, Wu JB, Meng HL. Serum CD166: a novel biomarker for predicting nasopharyngeal carcinoma response to radiotherapy. Oncotarget. 2017;8:62858–62867. doi: 10.18632/oncotarget.16399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gerweck LE, Wakimoto H. At the crossroads of cancer stem cells, radiation biology, and radiation oncology. Cancer Res. 2016;76:994–998. doi: 10.1158/0008-5472.CAN-15-2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wong RS. Apoptosis in cancer: from pathogenesis to treatment. J Exp Clin Cancer Res. 2011;30:87. doi: 10.1186/1756-9966-30-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tan EP, Duncan FE, Slawson C. The sweet side of the cell cycle. Biochem Soc Trans. 2017;45:313–322. doi: 10.1042/BST20160145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma X, Wang L, Huang , Li Y, Yang D, Li T, Li F, Sun L, Wei H, He K, Yu F, Zhao D, Hu L, Xing S, Liu Z, Li K, Guo J, Yang Z, Pan X, Li A, Shi Y, Wang J, Gao P, Zhang H. Polo-like kinase 1 coordinates biosynthesis during cell cycle progression by directly activating pentose phosphate pathway. Nat Commun. 2017;8:1506. doi: 10.1038/s41467-017-01647-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Machida YJ, Hamlin JL, Dutta A. Right place, right time, and only once: replication initiation in metazoans. Cell. 2005;123:13–24. doi: 10.1016/j.cell.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 27.Tao X, Xu L, Yin L, Han X, Qi Y, Xu Y, Song S, Zhao Y, Peng J. Dioscin induces prostate cancer cell apoptosis through activation of estrogen receptor-beta. Cell Death Dis. 2017;8:e2989. doi: 10.1038/cddis.2017.391. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Wang T, Li Y, Tuerhanjiang A, Wang W, Wu Z, Yuan M, Maitituoheti M, Wang S. Twist2 contributes to cisplatin-resistance of ovarian cancer through the AKT/GSK-3beta signaling pathway. Oncol Lett. 2014;7:1102–1108. doi: 10.3892/ol.2014.1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zeng KW, Wang XM, Ko H, Kwon HC, Cha JW, Yang HO. Hyperoside protects primary rat cortical neurons from neurotoxicity induced by amyloid beta-protein via the PI3K/Akt/Bad/Bcl(XL)-regulated mitochondrial apoptotic pathway. Eur J Pharmacol. 2011;672:45–55. doi: 10.1016/j.ejphar.2011.09.177. [DOI] [PubMed] [Google Scholar]
- 30.Li J, Xing M, Zhu M, Wang X, Wang M, Zhou S, Li N, Wu R, Zhou M. Glycogen synthase kinase 3beta induces apoptosis in cancer cells through increase of survivin nuclear localization. Cancer Lett. 2008;272:91–101. doi: 10.1016/j.canlet.2008.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]