Abstract
Chinese herbal medicine utilizes clinically effective adjuvants that can potentiate the effects of hepatectomy and molecule-targeted drugs for the treatment of hepatocellular carcinoma (HCC). The aim of this study was to investigate the possible molecular mechanisms underlying the antitumor effect of fufang yiliu yin (FYY) on HCC cells. We investigated the effects of FYY on the proliferation, migration, invasion, and apoptosis of SMMC-7721 cells in vitro and in mouse subcutaneous xenograft models in vivo. FYY significantly inhibited the proliferation of SMMC-7721 cells compared to that of normal hepatocytes; cell proliferation was blocked at the G2/M phase in accordance with reduced expression of proliferating cell nuclear antigen. FYY treatment resulted in the activation of caspase-8, caspase-3 and poly (ADP-ribose) polymerase, with reduced protein levels of tumor necrosis factor receptor-associated factor 2, indicating an induction of cell apoptosis. In addition, we observed decreases in the protein expression of matrix metalloproteinase-2 and -9 along with an inhibition of cell migration and invasion after FYY treatment. Furthermore, FYY treatment significantly inhibited the growth of tumors in vivo. These data demonstrate the strong inhibitory effects of FYY on SMMC-7721 cells, and we propose FYY as a novel potential anticancer adjuvant.
Keywords: Hepatocellular carcinoma, Chinese herbal medicine, anticancer
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common cancer worldwide [1], with a majority (55%) of the cases found in China [2]. Moreover, HCC is the third leading cause of cancer death, with 600,000 deaths per year and morbidity increasing on a yearly basis [1]. Although the best treatment for HCC is hepatectomy, the resection rate is low and the mortality and recurrence rates are high [3]. HCC has poor sensitivities to chemotherapy and radiotherapy, and molecular-targeted therapies are associated with high levels of drug intolerance. Therefore, new and effective cytotoxic drugs are urgently needed for the treatment of patients with HCC.
As an adjuvant to HCC treatments, Chinese herbal medicine (CHM) has been effective for relieving clinical symptoms, improving the quality of life and immune function, and increasing the long-term survival of patients [4].CHM incorporates many different chemical components, which act synergistically to increase the therapeutic efficacy [5], and frequently combines current scientific methodologies with other holistic approaches. Research on the antitumor activities of CHM has intensified in recent years, especially in China where it has been studied for the prevention and treatment of liver cancer [6,7].Although CHM is generally well accepted in Asian countries, the lack of understanding on the underlying molecular mechanisms limits its use elsewhere.
Here, we focused on the anticancer properties and underlying mechanisms offufang yiliu yin (FYY), which is used in CHM. FYY comprises the herbs and herb products Radix Astragali [8], Ganoderma lucidum [9], Semen Armeniacae amarum [10], Hedyotis diffusa Willd [11], Aconiti Lateralis Radix Praeparata [12], Glycyrrhiza Glabra Linne [13], Radix Panacis Quinquefolii [14], and Platycodi Radix [15], which reportedly possess anti-inflammatory and detoxifying activities. This study investigated the antitumor effects of FYY on HCC cells in vitro and in vivo. The individual contributions of each component in FYY were not investigated.
Materials and methods
Preparation of FYY
The primary composition of FYY was 2 g Radix Astragali, 1 g Ganoderma lucidum, 1 g Semen Armeniacae amarum, 3 g Hedyotis diffusa Willd, 2 g Aconiti Lateralis Radix Praeparata, 2 g Glycyrrhiza Glabra Linne, 1 g Radix Panacis Quinquefolii, and 1 g Platycodi Radix. These CHM products were purchased from Bai Caotang Pharmaceutical Co. Ltd. (Fujian, China), conforming with the provisions stated by the Chinese Pharmacopoeia, and prepared at the Weifang Hospital of Traditional Chinese Medicine. The components were boiled together in 100 ml of distilled water under reflux for 30 min, and the solution was filtered and freeze dried. The powder was then dissolved in 100 ml phosphate-buffered saline (PBS) for a final stock concentration of 120 mg/ml and stored at -20°C. This stock solution was diluted in cell culture medium in subsequent in vivo experiments.
Reagents and facilities
The cell cycle and apoptosis analysis kit and Hoechst 33258 staining kit were purchased from Beyotime Biotechnology (Shanghai, China). The Alexa Fluor 488 annexin V/dead cell apoptosis kit was purchased from Molecular Probes (Oregon, USA). Antibodies against matrix metalloproteinase-2 ([MMP-2] #87809), matrix metalloproteinase-9 ([MMP-9] #2270), tumor necrosis factor receptor-associated factor 2 ([TRAF2] #4712), caspase-8 (#9746), caspase-3 (#9662), poly (ADP-ribose) polymerase ([PARP] #9532), and glyceraldehyde-3-phosphate dehydrogenase ([GAPDH] #5174) were obtained from Cell Signaling Technology Inc. (Danvers, MA, USA). Antibodies against proliferating cell nuclear antigen ([PCNA] ab92552) as well as goat anti-rabbit and goat anti-mouse horseradish peroxidases ([HRPs] IgG H&L; ab6721 and ab6789, respectively) were obtained from Abcam (Cambridge, MA, USA).
All experiments were conducted at the Department of Medical Research center and the medical animal laboratory of the Affiliated Hospital of Qingdao University, Qingdao, Shandong, P. R. China.
Cell lines and cell culture
The HCC cell line SMMC-7721 and normal hepatocyte cell line HL-7702 were purchased from the cell resource center of the Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences (Shanghai, China). Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) at 37°C with a 5% CO2 atmosphere in a humidified incubator (Thermo Fisher Scientific, Waltham, MA, USA).
Cell viability assay
The cells (5 × 103/well) were seeded in 96-well plates and incubated for 24 h. When the cell density reached 60-70%, SMMC-7721 cells were treated with different concentrations of FYY (1, 2, 4, 8, 12, and 16 mg/ml) for 24, 48, and 72 h. HL-7702 cells and PBS were used as the controls. Cell viability was assayed via an MTT [3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide] assay.
Colony formation assay
SMMC-7721 cells were treated with different concentrations of FYY (2, 4, 8, and 12 mg/ml) or PBS (as a control) for 24 h. The cells were then cultured in 6-well plates (3 × 103 cells/well), and the medium was changed every 2 d for 12-14 days. The colony-forming efficiency of single cells was calculated as the number of colonies/number of inoculated cells × 100 and is reported as a percentage.
Cell cycle and cell apoptosis analyses
A flow cytometry assay of propidium iodide-stained DNA fragments was used to determine cell-cycle progression. The Alexa Fluor 488 annexin V/dead cell apoptosis kit was used to identify apoptotic FYY-treated and untreated (PBS control) SMMC-7721 cells. The data were analyzed using FlowJo software (version 7.6). Cellular apoptosis was also detected with an apoptosis-Hoechst 33258 staining kit, and cells were visualized under a fluorescence microscope (Olympus IX50; Olympus Corp., Tokyo, Japan) at × 400 magnification.
Wound healing assays
SMMC-7721 cells were treated with different concentrations of FYY (2, 4, and 8 mg/ml) or PBS for 24 h. Cell monolayers cultured in DMEM with 1% FBS were wounded by scraping with a 200-μl pipette tip and incubated at 37°C for an additional 24 h. The areas of the wounds were measured and the wound healing rates were calculated using Image J software.
Transwell migration and invasion assays
Cell migration and invasion were assessed using Transwell polycarbonate membranes (8.0-μm pores; Corning Inc., Corning, NY, USA) placed in each well of a 24-well plate containing 600μl DMEM with 10% FBS. For migration, SMMC-7721 cells were treated with different concentrations of FYY (2, 4, and 8 mg/ml) or PBS for 24 h and then seeded (1.5 × 105 cells) on the membranes using serum-free DMEM for 48 h at 37°C. To assess invasion, the Transwell membranes were precoated with Matrigel (BD, Franklin Lakes, NJ, USA). After the 48-h incubation, the cells were fixed with methanol for 15 min and stained with 0.5% crystal violet for 15 min. The percentage of cells that had penetrated through the membrane was quantified under a microscope at × 200 magnification.
Western blotting
Total proteins were extracted from cells using RIPA lysis buffer (CWBIO, Beijing, China) and the concentrations were determined with a BCA (bicinchoninic acid) protein quantitation kit (Thermo Fisher Scientific). Equal amounts of protein from samples were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then transferred onto 0.45-μm polyvinylidene difluoride membranes (Bio-Rad Laboratories, Hercules, CA, USA). Subsequently, the membranes were blocked with 5% milk in PBS plus 0.1% Tween 20 (PBST) for 60 min. The membranes were incubated with primary antibodies (at the recommended dilutions) overnight at 4°C. The next day, the membranes were incubated for 60 min with the corresponding secondary antibodies. The bands were detected using an enhanced chemiluminescence reagent and visualized with a Fusion FX7 System (Vilber Lourmat, France). ImageJ software was used to calculate the intensity (gray value) of each protein band, which was normalized to that for GAPDH.
In vivo tumorigenicity assays
SMMC-7721 cells (5 × 106) were injected subcutaneously into the posterior flanks of male BALB/c nude mice (4-5 weeks old) purchased from the Beijing Vital River Laboratory Animal Technology Co. Ltd. (Beijing, China). The mice were divided into two groups: an FYY drug group and a control group (n = 6 mice/group). FYY (0.2 ml/10 g of body weight) or an equivalent volume of saline was intragastrically administered daily to the mice. Tumor sizes were measured every 3 d using calipers, and tumor volume (mm3) was calculated as the length × width2/2. The mice were killed by cervical dislocation on day 33, and the tumors were excised, weighed, and photographed. All animal studies were approved by the Animal Ethics Committee of Qingdao University.
Statistical analysis
Statistical analysis was performed in GraphPad Prism 6.0 software (San Diego, CA, USA). All experiments were performed in triplicates. Data were analyzed by one-way analyses of variance (ANOVAs) and are presented as means ± SDs. A P value of < 0.05 was considered as statistically significant.
Results
FYY inhibits the proliferation of HCC cells
To study the effect of FYY on the viability of hepatocytes, SMMC-7721 and HL-7702 cells were treated with different concentrations of FYY for 24, 48, and 72 h. FYY inhibited the proliferation of SMMC-7721 cells in a dose- and time-dependent manner without significantly altering the viability of normal hepatocytes (Figure 1A). We next investigated the anticancer effect of FYY on SMMC-7721 cells. The cloning formation assay showed that the number of cell colonies in the FYY-treated SMMC-7721 group was lower than that of the PBS control (Figure 1B). We found that FYY was the most effective at inhibiting cell colony formation at a concentration of 12 mg/ml. We also measured the expression of PCNA by Western blotting and found that protein levels were decreased in the FYY-treated SMMC-7721 cells (Figure 1C). Finally, we performed flow cytometry to assess cell-cycle progression. Compared with that in the PBS controls, the percentage of cells in the G2/M phase was significantly increased in FYY-treated SMMC-7721 cells (Figure 1D). However, there was no significant difference in the percentage of cells in S phase. These data suggest that FYY has an inhibitory effect on the proliferation of HCC cells by inducing cell-cycle arrest at the G2/M phase.
Figure 1.
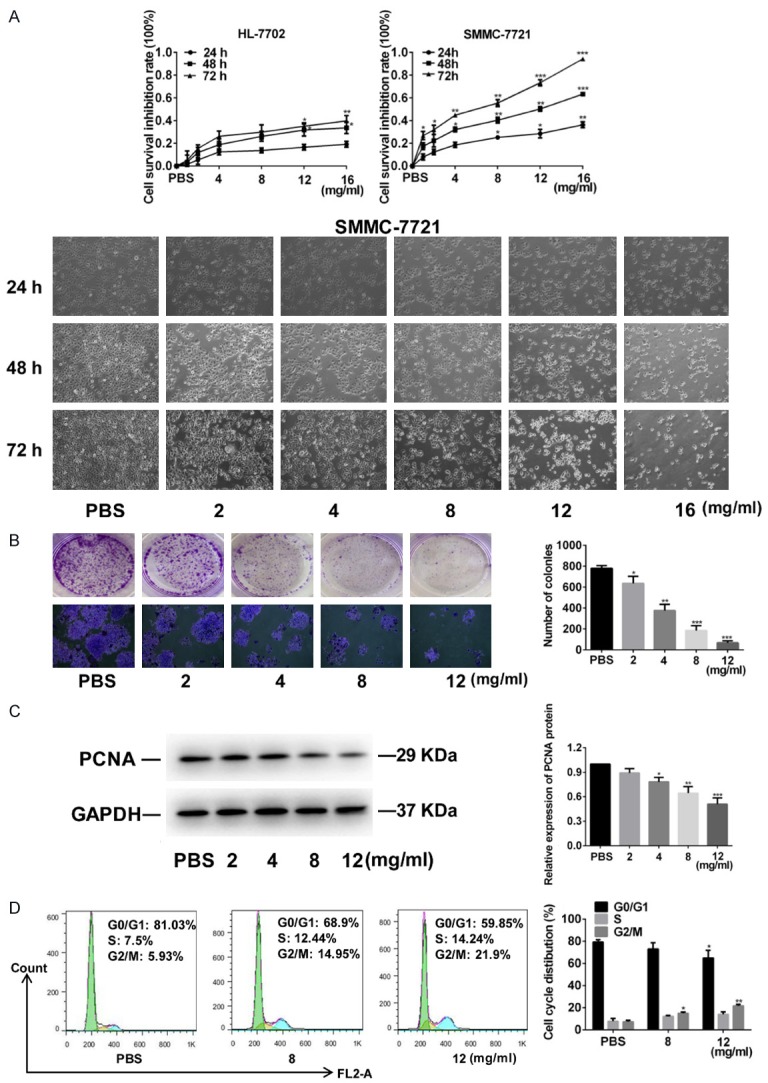
FYY inhibits the proliferation of SMMC-7721 cells. A. FYY inhibited the proliferation of SMMC-7721 cells, but not normal HL-7702 hepatocyte cells, treated for 24, 48, and 72 h in a dose- and time- dependent manner. Cell density and morphology changes are shown (× 100 magnification). B. The colony formation ability was decreased after treatment with FYY (× 100 magnification). C. Representative Western blot and quantitation of PCNA expression showing a dose-dependent decrease with FYY treatment. D. Cell-cycle progression was arrested at the G2/M phase by FYY. Data are expressed as the means ± SDs from three separate experiments. *P < 0.05, **P < 0.01, and ***P < 0.001 vs. PBS.
FYY promotes apoptosis of HCC cells
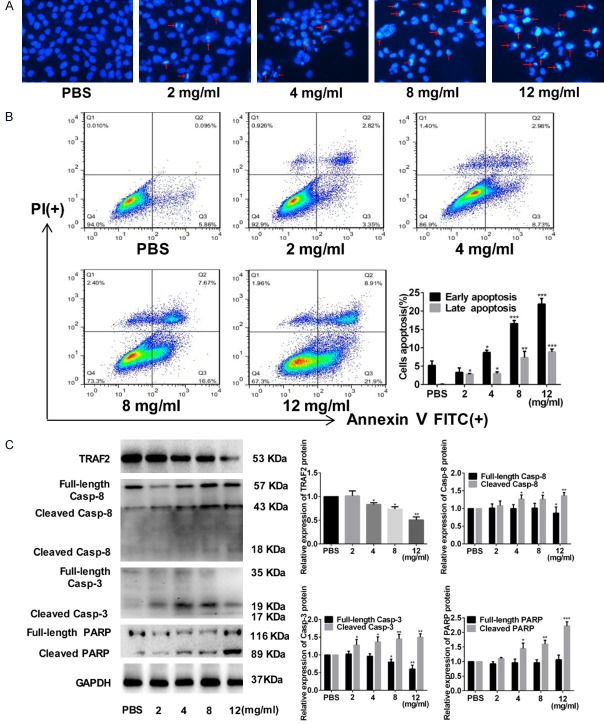
FYY significantly induced cell apoptosis after 24 h of treatment as determined by Hoechst staining and flow cytometry (Figure 2A and 2B). We also investigated the expression of apoptosis-related proteins by Western blotting and observed increases in caspase-8, caspase-3, and PARP, with a decrease in TRAF2 by FYY treatment (Figure 2C). The results suggest that FYY promotes both early and late stage apoptosis in FYY-treated SMMC-7721 cells.
Figure 2.
FYY induces apoptosis of SMMC-7721 cells. Hoechst staining (A) and flow cytometric analysis (B) indicate that FYY induced cellular apoptosis in a dose-dependent manner. (C) Expression levels of apoptosis-related proteins (TRAF2, caspase-8, caspase-3, and PARP) were altered by FYY treatment as determined by Western blotting. *P < 0.05, **P < 0.01, and ***P < 0.001 vs. PBS.
FYY inhibits the migration and invasion of HCC cells
After treating SMMC-7721 cells with different concentrations of FYY for 24 h, the wound healing rate was lower than that of the PBS controls (Figure 3A). Moreover, cell migration (Figure 3B) and cell invasion (Figure 3C) were decreased in FYY-treated SMMC-7721 cells compared with that in PBS controls. These decreases corresponded with reduced expression of the migration- and invasion-related proteins MMP-2 and MMP-9 (Figure 3D). Thus, FYY dose-dependently inhibits HCC invasion and migration.
Figure 3.
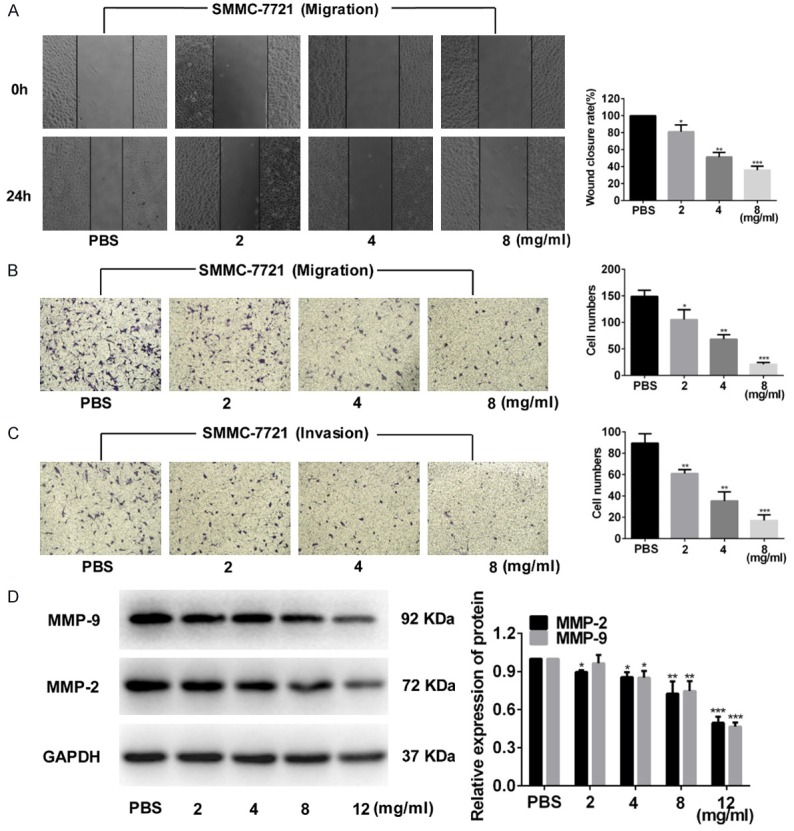
FYY inhibits SMMC-7721 cell migration and invasion. Wound healing (A) and Transwell invasion (B) assays indicate that FYY inhibited SMMC-7721 cell migration. (C) FYY also inhibited the invasion of SMMC-7721 cells into Matrigel. (D) Expression levels of extracellular matrix degradation-related proteins MMP-2 and MMP-9 were dose-dependently decreased with FYY treatment as determined by Western blotting. *P < 0.05, **P < 0.01, and ***P < 0.001 vs. PBS.
FYY inhibits growth of tumors in vivo
Subcutaneous xenograft tumors in twelve mice were examined to investigate the antitumor effect of FYY in vivo (Figure 4A). Palpable tumors were present after 6 d in all mice and we started FYY or saline treatment. The tumors size in the saline-treated control mice was larger than those in the FYY-treated mice after 18 days of treatment. The tumor volumes in the control group and FYY-treated group were 314.948 ± 60.569 mm3 and 188.549 ± 54.342 mm3, respectively, after 27 days of treatment (P < 0.05) (Figure 4B). The weights of the tumors from the control and FYY-treated groups were 255.0 ± 78.7 mg and 125 ± 50.0 mg, respectively (P < 0.05) (Figure 4C).
Figure 4.
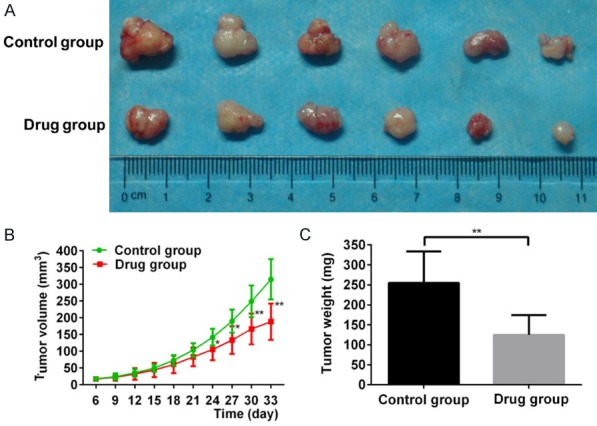
FYY inhibits the growth of SMMC-7721 cells in vivo. (A) Subcutaneous xenograft tumors after 33 d. Animals treated with FYY had tumors with significantly smaller volumes (B) and lower weights (C). *P < 0.05 and **P < 0.01 vs. control (saline-treated animals).
Discussion
CHM provides clinically effective anticancer treatments with few side effects [16,17]. Here, we demonstrate the antitumor effects of FYY on apoptosis, proliferation, migration, and invasion of HCC cells in vitro, consistent with results from previous studies [18,19]. Moreover, we demonstrate that FYY can significantly inhibit xenograft HCC tumor growth in vivo.
Our results show that FYY induces cell-cycle arrest in HCC cells at the G2/M phase and reduced their proliferation, corresponding to a loss of PCNA protein expression. Four of the eight components comprising FYY, namely, Radix Astragali, Ganoderma lucidum, Platycodi Radix, and Hedyotis diffusa Willd, were previously shown to inhibit cancer cell proliferation. Radix Astragali, the dried root of Astragalus membranaceus Bge var. mongholicus, has been reported to act as an immunomodulatory adjunctive cancer therapeutic [20] and shows antiproliferative effects [21]. This ingredient primarily comprises polysaccharides, saponins, and flavonoids, for which all have demonstrated anticancer effects in preclinical studies [22]. The anticancer effects of Ganoderma lucidum, an extract from Reishi mushrooms, are largely attributed to its bioactive polysaccharides, which are immunomodulatory via the activation of certain cytokines [23,24], and triterpenoids, which can induce G2 phase cell-cycle arrest in HCC cells [25]. Platycodi Radix, from the root of Platycodon grandiflorus, contains platycodin D, which has been shown to induce apoptosis in several cancer cell types [26,27] and inhibit the proliferation and cell-cycle progression (at the G2/M phase) of HepG2 cells [28]. Hedyotis diffusa Willd belongs to the family Rubiaceae and contains quercetin, kaempferol, and rutin, which also exhibit antiproliferative and apoptotic effects in ovarian cells [29] and in colorectal cancer cells by inhibiting STAT3 signaling and regulating PI3K/AKT signaling, respectively [30,31].
We found the FYY treatment also increased the percentages of early and late apoptotic HCC cells. The induction of tumor cell apoptosis is a documented attribute of CHM [32] and has been documented for three components of FYY, namely, Semen Armeniacae amarum, Platycodi Radix, and Hedyotis diffusa Willd. Semen Armeniacae amarum contains hydrogen cyanide and amygdalin, which induces cell apoptosis via the upregulation of Bax and downregulation Bcl-2, resulting in caspase-3 activation [33]. FYY treatment in our study was associated with a dose-dependent activation of caspase-3 as well as a reduction in the expression of TRAF2, a member of the TRAF family of proteins associated with cell proliferation, apoptosis, and inflammatory reactions [34] and which plays a crucial role in internal and external apoptotic pathways [35]. TRAF2-induced caspase-8 activation triggers the proteolytic cascade leading to the activation of caspase-3 [36], of which PARP is a substrate [37] that is commonly used as biological marker of apoptosis. Another component of FYY, Glycyrrhiza Glabra Linne, has been shown to enhance caspase-3 activity and PARP cleavage, leading to cell apoptosis via extrinsic and intrinsic pathways [38]. Consistent with these observations, we found increases in caspase-3, caspase-8, and PARP cleavage in SMMC-7721 cells treated with FYY.
An important effect of anticancer therapeutics is their ability to prevent metastasis. The migration and invasion of cancer cells are influenced by the extracellular matrix. Ganoderma lucidum was previously shown to inhibit HCC metastasis by downregulating the expression of MMP-2 and MMP-9 [39], which are proteolytic enzymes involved in extracellular matrix modification, cell migration and invasion, and the cleavage of cytokines [40]. Consistent with this, we found that FYY treatment reduced the expression of both MMP-2 and MMP-9 and suppressed the migratory and invasive properties of SMMC-7721 cells in vitro.
Taken together, we show that FYY is an effective anticancer agent that inhibits the proliferation, migration, and invasion while promoting apoptosis of HCC cells. These effects were validated in the observed reduction in tumor size in a xenograft model following the administration of FYY. Therefore, FYY represents a potential adjuvant therapy for HCC.
Acknowledgements
This research was supported by institutional funding from the Affiliated Hospital of Qingdao University to C. Sun and C. Zhu.
Disclosure of conflict of interest
None.
References
- 1.Caldwell S, Park SH. The epidemiology of hepatocellular cancer: from the perspectives of public health problem to tumor biology. J Gastroenterol. 2009;44(Suppl 19):96–101. doi: 10.1007/s00535-008-2258-6. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 3.El-Serag HB, Davila JA. Surveillance for hepatocellular carcinoma: in whom and how? Therap Adv Gastroenterol. 2011;4:5–10. doi: 10.1177/1756283X10385964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chung VC, Wu X, Hui EP, Ziea ET, Ng BF, Ho RS, Tsoi KK, Wong SY, Wu JC. Effectiveness of Chinese herbal medicine for cancer palliative care: overview of systematic reviews with meta-analyses. Sci Rep. 2015;5:18111. doi: 10.1038/srep18111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qiao X, Wang Q, Wang S, Miao WJ, Li YJ, Xiang C, Guo DA, Ye M. Compound to extract to formulation: a knowledge-transmitting approach for metabolites identification of gegen-qinlian decoction, a traditional Chinese medicine formula. Sci Rep. 2016;6:39534. doi: 10.1038/srep39534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qi F, Li A, Inagaki Y, Gao J, Li J, Kokudo N, Li XK, Tang W. Chinese herbal medicines as adjuvant treatment during chemo- or radio-therapy for cancer. Biosci Trends. 2010;4:297–307. [PubMed] [Google Scholar]
- 7.Konkimalla VB, Efferth T. Evidence-based Chinese medicine for cancer therapy. J Ethnopharmacol. 2008;116:207–210. doi: 10.1016/j.jep.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 8.Deng P, Li X, Wei Y, Liu J, Chen M, Xu Y, Dong B, Zhu L, Chai L. The herbal decoction modified Danggui Buxue Tang attenuates immune-mediated bone marrow failure by regulating the differentiation of T lymphocytes in an immune-induced aplastic anemia mouse model. PLoS One. 2017;12:e0180417. doi: 10.1371/journal.pone.0180417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taofiq O, Heleno SA, Calhelha RC, Alves MJ, Barros L, Gonzalez-Paramas AM, Barreiro MF, Ferreira I. The potential of ganoderma lucidum extracts as bioactive ingredients in topical formulations, beyond its nutritional benefits. Food Chem Toxicol. 2017;108:139–147. doi: 10.1016/j.fct.2017.07.051. [DOI] [PubMed] [Google Scholar]
- 10.Chang HK, Yang HY, Lee TH, Shin MC, Lee MH, Shin MS, Kim CJ, Kim OJ, Hong SP, Cho S. Semen Armeniacae amarum extract suppresses lipopolysaccharide-induced expressions of cyclooxygenase [correction of cycloosygenase] -2 and inducible nitric oxide synthase in mouse BV2 microglial cells. Biol Pharm Bull. 2005;28:449–454. doi: 10.1248/bpb.28.449. [DOI] [PubMed] [Google Scholar]
- 11.Chen Y, Lin Y, Yachan LI, Candong LI. Total flavonoids of Hedyotis diffusa Willd inhibit inflammatory responses in LPS-activated macrophages via suppression of the NF-κB and MAPK signaling pathways. Exp Ther Med. 2016;11:1116–1122. doi: 10.3892/etm.2015.2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu C, Zhu C, Wang G, Xu R, Zhu Y. Higenamine regulates Nrf2-HO-1-Hmgb1 axis and attenuates intestinal ischemia-reperfusion injury in mice. Inflamm Res. 2015;64:395–403. doi: 10.1007/s00011-015-0817-x. [DOI] [PubMed] [Google Scholar]
- 13.Krausse R, Bielenberg J, Blaschek W, Ullmann U. In vitro anti-helicobacter pylori activity of extractum glycyrrhiza glabra linne, glycyrrhizin and its metabolites. J Antimicrob Chemother. 2004;54:243–246. doi: 10.1093/jac/dkh287. [DOI] [PubMed] [Google Scholar]
- 14.Liu H, Shi D, Wang W, Zhang C, Fu M, Ge J. Panax quinquefolium saponins inhibited immune maturation of human monocyte-derived dendritic cells via blocking nuclear factor-kappaB pathway. J Ethnopharmacol. 2012;141:982–988. doi: 10.1016/j.jep.2012.03.029. [DOI] [PubMed] [Google Scholar]
- 15.Lee JH, Choi YH, Kang HS, Choi BT. An aqueous extract of Platycodi radix inhibits LPS-induced NF-kappaB nuclear translocation in human cultured airway epithelial cells. Int J Mol Med. 2004;13:843–847. [PubMed] [Google Scholar]
- 16.Sun Z, Liang ST, Zhai XF, Lang QB, Zhou QH, Yue XQ, He J, Xu J, Zhu Y, Ling CQ. A traditional Chinese herbal medicine compound preparation versus interventional therapy after resection of small hepatocellular carcinoma: 22-year follow-up. J Tradit Chin Med. 2012;32:156–163. doi: 10.1016/s0254-6272(13)60005-9. [DOI] [PubMed] [Google Scholar]
- 17.Ma L, Wang B, Long Y, Li H. Effect of traditional Chinese medicine combined with Western therapy on primary hepatic carcinoma: a systematic review with meta-analysis. Front Med. 2017;11:191–202. doi: 10.1007/s11684-017-0512-0. [DOI] [PubMed] [Google Scholar]
- 18.Cao ZY, Chen XZ, Liao LM, Peng J, Hu HX, Liu ZZ, Du J. Fuzheng Yiliu Granule inhibits the growth of hepatocellular cancer by regulating immune function and inducing apoptosis in vivo and in vitro. Chin J Integr Med. 2011;17:691–697. doi: 10.1007/s11655-011-0847-3. [DOI] [PubMed] [Google Scholar]
- 19.Huang XY, Wang L, Huang ZL, Zheng Q, Li QS, Tang ZY. Herbal extract “Songyou Yin” inhibits tumor growth and prolongs survival in nude mice bearing human hepatocellular carcinoma xenograft with high metastatic potential. J Cancer Res Clin Oncol. 2009;135:1245–1255. doi: 10.1007/s00432-009-0566-8. [DOI] [PubMed] [Google Scholar]
- 20.Yoshida Y, Wang MQ, Liu JN, Shan BE, Yamashita U. Immunomodulating activity of Chinese medicinal herbs and Oldenlandia diffusa in particular. Int J Immunopharmacol. 1997;19:359–370. doi: 10.1016/s0192-0561(97)00076-3. [DOI] [PubMed] [Google Scholar]
- 21.Ye MN, Chen HF, Zhou RJ, Liao MJ. [Effects of Astragalus polysaccharide on proliferation and Akt phosphorylation of the basal-like breast cancer cell line] . Zhong Xi Yi Jie He Xue Bao. 2011;9:1339–1346. doi: 10.3736/jcim20111210. [DOI] [PubMed] [Google Scholar]
- 22.Jung Y, Jerng U, Lee S. A systematic review of anticancer effects of radix astragali. Chin J Integr Med. 2016;22:225–236. doi: 10.1007/s11655-015-2324-x. [DOI] [PubMed] [Google Scholar]
- 23.Weng CJ, Yen GC. The in vitro and in vivo experimental evidences disclose the chemopreventive effects of ganoderma lucidum on cancer invasion and metastasis. Clin Exp Metastasis. 2010;27:361–369. doi: 10.1007/s10585-010-9334-z. [DOI] [PubMed] [Google Scholar]
- 24.Ruan W, Wei Y, Popovich DG. Distinct responses of cytotoxic ganoderma lucidum triterpenoids in human carcinoma cells. Phytother Res. 2015;29:1744–1752. doi: 10.1002/ptr.5426. [DOI] [PubMed] [Google Scholar]
- 25.Lin SB, Li CH, Lee SS, Kan LS. Triterpene-enriched extracts from ganoderma lucidum inhibit growth of hepatoma cells via suppressing protein kinase C, activating mitogen-activated protein kinases and G2-phase cell cycle arrest. Life Sci. 2003;72:2381–2390. doi: 10.1016/s0024-3205(03)00124-3. [DOI] [PubMed] [Google Scholar]
- 26.Park JC, Lee YJ. In vivo and in vitro antitumor effects of platycodin d, a saponin purified from platycodi radix on the h520 lung cancer cell. Evid Based Complement Alternat Med. 2014;2014:478653. doi: 10.1155/2014/478653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim JY, Park KW, Moon KD, Lee MK, Choi J, Yee ST, Shim KH, Seo KI. Induction of apoptosis in HT-29 colon cancer cells by crude saponin from Platycodi Radix. Food Chem Toxicol. 2008;46:3753–3758. doi: 10.1016/j.fct.2008.09.067. [DOI] [PubMed] [Google Scholar]
- 28.Qin H, Du X, Zhang Y, Wang R. Platycodin D, a triterpenoid saponin from Platycodon grandiflorum, induces G2/M arrest and apoptosis in human hepatoma HepG2 cells by modulating the PI3K/Akt pathway. Tumour Biol. 2014;35:1267–1274. doi: 10.1007/s13277-013-1169-1. [DOI] [PubMed] [Google Scholar]
- 29.Zhang L, Zhang J, Qi B, Jiang G, Liu J, Zhang P, Ma Y, Li W. The anti-tumor effect and bioactive phytochemicals of Hedyotis diffusa willd on ovarian cancer cells. J Ethnopharmacol. 2016;192:132–139. doi: 10.1016/j.jep.2016.07.027. [DOI] [PubMed] [Google Scholar]
- 30.Li Q, Lai Z, Yan Z, Peng J, Jin Y, Wei L, Lin J. Hedyotis diffusa Willd inhibits proliferation and induces apoptosis of 5FU resistant colorectal cancer cells by regulating the PI3K/AKT signaling pathway. Mol Med Rep. 2018;17:358–365. doi: 10.3892/mmr.2017.7903. [DOI] [PubMed] [Google Scholar]
- 31.Cai Q, Lin J, Wei L, Zhang L, Wang L, Zhan Y, Zeng J, Xu W, Shen A, Hong Z, Peng J. Hedyotis diffusa Willd inhibits colorectal cancer growth in vivo via inhibition of STAT3 signaling pathway. Int J Mol Sci. 2012;13:6117–6128. doi: 10.3390/ijms13056117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kwon SB, Kim MJ, Yang JM, Lee HP, Hong JT, Jeong HS, Kim ES, Yoon DY. Cudrania tricuspidata stem extract induces apoptosis via the extrinsic pathway in SiHa cervical cancer cells. PLoS One. 2016;11:e0150235. doi: 10.1371/journal.pone.0150235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang HK, Shin MS, Yang HY, Lee JW, Kim YS, Lee MH, Kim J, Kim KH, Kim CJ. Amygdalin induces apoptosis through regulation of Bax and Bcl-2 expressions in human DU145 and LNCaP prostate cancer cells. Biol Pharm Bull. 2006;29:1597–1602. doi: 10.1248/bpb.29.1597. [DOI] [PubMed] [Google Scholar]
- 34.Liu J, Yan J, Jiang S, Wen J, Chen L, Zhao Y, Lin A. Site-specific ubiquitination is required for relieving the transcription factor Miz1-mediated suppression on TNF-alpha-induced JNK activation and inflammation. Proc Natl Acad Sci U S A. 2012;109:191–196. doi: 10.1073/pnas.1105176108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dai Y, Lawrence TS, Xu L. Overcoming cancer therapy resistance by targeting inhibitors of apoptosis proteins and nuclear factor-kappa B. Am J Transl Res. 2009;1:1–15. [PMC free article] [PubMed] [Google Scholar]
- 36.Matsushita K, Wu Y, Qiu J, Lang-Lazdunski L, Hirt L, Waeber C, Hyman BT, Yuan J, Moskowitz MA. Fas receptor and neuronal cell death after spinal cord ischemia. J Neurosci. 2000;20:6879–6887. doi: 10.1523/JNEUROSCI.20-18-06879.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vizetto-Duarte C, Custodio L, Gangadhar KN, Lago JH, Dias C, Matos AM, Neng N, Nogueira JM, Barreira L, Albericio F, Rauter AP, Varela J. Isololiolide, a carotenoid metabolite isolated from the brown alga Cystoseira tamariscifolia, is cytotoxic and able to induce apoptosis in hepatocarcinoma cells through caspase-3 activation, decreased Bcl-2 levels, increased p53 expression and PARP cleavage. Phytomedicine. 2016;23:550–557. doi: 10.1016/j.phymed.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 38.He SH, Liu HG, Zhou YF, Yue QF. Liquiritin (LT) exhibits suppressive effects against the growth of human cervical cancer cells through activating Caspase-3 in vitro and xenograft mice in vivo. Biomed Pharmacother. 2017;92:215–228. doi: 10.1016/j.biopha.2017.05.026. [DOI] [PubMed] [Google Scholar]
- 39.Weng CJ, Chau CF, Yen GC, Liao JW, Chen DH, Chen KD. Inhibitory effects of ganoderma lucidum on tumorigenesis and metastasis of human hepatoma cells in cells and animal models. J Agric Food Chem. 2009;57:5049–5057. doi: 10.1021/jf900828k. [DOI] [PubMed] [Google Scholar]
- 40.Elkington PT, Friedland JS. Matrix metalloproteinases in destructive pulmonary pathology. Thorax. 2006;61:259–266. doi: 10.1136/thx.2005.051979. [DOI] [PMC free article] [PubMed] [Google Scholar]