Abstract
The objective of this study was to investigate whether the co-injection of human adipose stromal cells (hASCs) and rhBMP-2/fibrin gel into the bone tunnels of anterior cruciate-ligament reconstructions can effectively enhance tendon graft osteointegration. We performed bilateral reconstructions using autologous semitendinosus tendons in 45 New Zealand rabbits, which we divided into three groups. We injected the bone tunnels with fibrin gel for group 1, rhBMP-2 (1 μg/ml)/fibrin gel for group 2, and hASCs wrapped in rhBMP-2 (1 μg/ml)/fibrin gel for group 3. We sacrificed five rabbits (two for histological assessment and three for biomechanical tests) from each group at 2, 4, and 8 weeks post surgery. At 2 and 4 weeks post surgery, histological analysis showed that fibro-cartilage had appeared in the tendon-bone interface in group 2. At 4 weeks post surgery, mature bone cells could be seen in group 3. There was new bone formed between the host bone and the graft in groups 2 and 3 at 8 weeks post surgery. Biomechanical testing showed that at 4 and 8 weeks post surgery, the ultimate failure loads in group 3 were significantly higher than those in groups 1 and 2 (both P=0.01). The tendon stiffness in group 3 was significantly higher than that in the other groups at 4 weeks post surgery (P=0.01). Our results indicate that co-injection of hASCs and rhBMP-2/fibrin gel has the potential to promote tendon-bone healing after anterior cruciate-ligament reconstruction.
Keywords: Recombinant human bone morphogenetic protein-2, human adipose stromal cells, fibrin gel, tendon-bone healing
Introduction
The anterior cruciate ligament (ACL) is very important for the stability and normal movement of the knee joint [1,2]. Because the tendon-bone interface is the weakest link, it limits the rehabilitation of patients that undergo ACL reconstruction during the early rehabilitation process, which implies that the reestablishment of the anatomical structure in the tendon-bone attachment is the primary requirement for ACL reconstruction.
Many recent studies have focused on various growth factors to enhance tendon-bone healing [3-5]. Bone morphogenic protein 2 (BMP-2) has the ability to enhance tendon-bone healing [6-8]; however, it has some disadvantages, such as extensive ectopic soft-tissue swelling and a short half-life [9]. Hunziker [10] demonstrated that gradual liberation systems can effectively promote the capacity of BMP-2 to induce and sustain local bone formation. Fibrin gel has been used for many years as a biocompatible scaffold in regenerative and tissue-engineering medicine [11-13]. Therefore, we assume that BMP-2/fibrin gel scaffolds can not only degradation rate of grafts in vivo, but also reduce the overall side effects of reconstructive procedures.
BMP-2 has the potential to induce stem cells to differentiate into osteogenic and chondrogenic cells [10,14,15]. In recent years, studies have focused on bone marrow-derived mesenchymal stromal cells (BMSCs) to enhance tendon-bone healing because of their potential for multilineage differentiation [16,17]. ASCs have advantages over BMSCs, because they are more readily available and have a lower risk of donor-site morbidity [18,19]. Wang, B [20] demonstrated that co-injection of ASCs and basic fibroblast growth factor can improve cardiac remodeling and function in myocardial infarction. Although many studies have shown that ASCs can be induced into osteoblasts or chondrocytes [21-23], there is little information about whether the transplantation of ASCs together with recombinant human bone morphogenic protein-2 (rhBMP-2)/fibrin gel can enhance tendon-bone healing.
The purpose of this study was to investigate whether co-injection of human adipose stromal cells (hASCs) and rhBMP-2/fibrin gel can effectively enhance tendon-bone healing in a rabbit ACL reconstruction model. We hypothesized that the co-injection of hASCs and rhBMP-2/fibrin gel into the ACL reconstruction bone tunnel can improve tendon-bone healing.
Materials and methods
Study design
All animal experiments were performed according to the rules of the Southern Medical University Institutional Animal Care and Use Committee. We randomly divided 45 female New Zealand white rabbits weighing 2-2.5 kg, purchased from the Animal Experimental Center of Southern Medical University, into three groups. During ACL reconstruction, we injected the bone tunnels of group 1 with fibrin gel, those of group 2 with rhBMP-2/fibrin gel, and those of group 3 with hASCs and rhBMP-2/fibrin gel. We preformed the ACL reconstructions bilaterally using semitendinosus tendon autograft. We sacrificed five rabbits in each group by intravenous injection an overdose of sodium phenobarbital at 2, 4, and 8 weeks post surgery. We used three of the specimens in each sacrificed group for biomechanical tests and used the remaining specimens for hematoxylin and eosin (H&E) staining and Masson’s trichrome staining.
Culture and osteogenic differentiation of hASCs
We purchased hASCs from Cyagen Biosciences (Santa Clara, CA) and cultured the cells in Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Grand Island, NY) with 10% of fetal bovine serum (FBS; Gibco), 100 U/ml penicillin, and 100 μg/ml streptomycin (Gibco) at 37°C with 5% CO2. When the cultured cells reached 80% confluence, we detached them with 0.25% trypsin-EDTA (Gibco). We neutralized the enzyme activity in the cultures with a twofold volume of DMEM and centrifuged them for 5 min at 100 g/min. We then transferred the cell suspensions to a 100-mm culture dish (1) and incubated them at 37°C with 5% CO2.
To determine whether rhBMP-2 can promote osteogenic hASC differentiation in vitro, we cultured hASCs in DMEM containing 1 mg/ml rhBMP-2 for seven or 14 days. The control cells cultured in DMEM without rhBMP-2. We then used qRT-PCR to measure the gene expression of osteogenic proteins (OPN, RUNX2, ALP, and Collagen-1). The primers used for qRT-PCR are shown in Table 1.
Table 1.
Primers used for qRT-PCR
| Gene | Sequences (5’ to 3’) | Tm (°C) |
|---|---|---|
| OPN | F-GTGATTTGCTTTTGCCTCCT | 60 |
| R-GAGATGGGTCAGGGTTTAGC | ||
| RUNX2 | F-GAGACTACTGCCGACCAC | 60 |
| R-TACCTCTCCGAGGGCTACC | ||
| ALP | F-ACCATTCCCACGTCTTCACATTTG | 60 |
| R-AGACATTCTCTCGTTCACCGCC | ||
| Collagen-1 | F-AGGGCCAAGACGAAGACATC | 60 |
| RAGATCACGTCATCGCACAACA | ||
| GAPDH | F-CACATGGCCTCCAAGGAGTAA | 60 |
| R-GTACATGACAAGGTGCGGCTC |
ACL reconstruction model
We anesthetized the rabbits by intravenous injection of 3% sodium pentobarbital (30 mg/kg). We then fixed the animals on the operation table in the supine position with the knees being able to flex freely to 90°. We shaved the bilateral lower extremities and then sterilized them three times with 2.5% iodophors. We made a 5-cm medial parapatellar incision, dislocated the patella laterally, and excised the ACL from its femoral and tibial insertions (Figure 1A, 1B). We then harvested the semitendinosus tendon for autografting (Figure 1C, 1F). We used a 2.0-mm-diameter Kirschner wire to drill bone tunnels through the ACL footprint (Figure 1D). We then pulled the graft into the tunnels using a 1.0-mm-diameter guide wire. We fixed the graft using the intraosseous fixation technique (Figure 1E). We then irrigated the joint with fibrin gel (group 1), fibrin gel and 1 μg/ml rhBMP-2 (group 2), or fibrin gel/rhBMP-2/hASCs (group 3) into the bone tunnels. We then sutured the patellar tendon and skin using 2-0 wire sutures layer by layer. After the surgery, we allowed the animals to move freely in their cages without external fixation and applied prophylactic antibiotic (50 mg/kg ampicillin) intramuscularly once per day for 5 days. We inspected the local wound condition and the activities of the knees daily.
Figure 1.
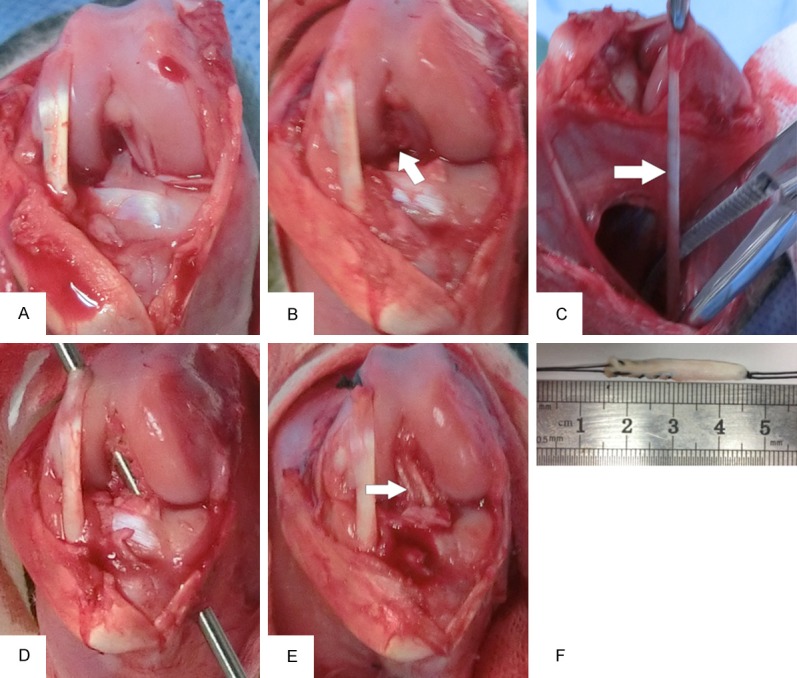
ACL reconstruction surgery in a rabbit model. A: Normal structure of the ACL. B: Cutting of the ACL for the footprint. C, F: Harvesting of autologous semitendinosus tendons. D: Formation the femur and tibial bone tunnels using a 2.0-mm Kirschner wire. E: Reconstruction of the ACL.
Histological analysis
We sacrificed two rabbits from each group by intravenous injection of an overdose of sodium phenobarbital at 2, 4, and 8 weeks post surgery (n=18) and used H&E and Masson trichrome staining to evaluate the formation of new bone and collagen fibers at the tendon-bone interface. We harvested the entire joints, including the proximal tibia and distal femur, fixed them in 4% paraformaldehyde for 72 h at room temperature, and then decalcified them in 10% EDTA for 3 or 4 weeks. We dehydrated the specimens using a graded ethanol series, embedded them in paraffin, and serially cut them into 4-µm-thick sections. We stained the sections using H&E and Masson trichrome staining kits.
Biomechanical testing
We harvested a total of 27 rabbits (54 femur-ACL graft-tibia constructs) for biomechanical testing. We removed all of the soft tissues except for the ACL graft. Before the tensile test, we rigidly fixed the femur-ACL graft-tibia complexes to a custom-made clamp. After preloading with a static preload of 1 N, we measured the ultimate failure load with a displacement rate of 5 mm/min. The test was completed when the graft was ruptured or pulled out of the bone tunnel.
Statistical analysis
We performed statistical analysis using the Statistical Package for Social Science (SPSS) 13.0 (SPSS Inc, Chicago, IL). We performed comparisons of numerical data among the groups by one-way analyses of variance. If the test of Homogeneity of Variances is equal, Bonferroni test was used for multiple comparisons. If not, the Dunnett’s T3 test was used. We analyzed the qRT-PCR results using t-tests for two independent samples. We considered differences to be statistically significant when the p-value was less than 0.05.
Results
qRT-PCR
Compared with control cells, hASCs cultured in the presence of 1 μg/ml rhBMP-2 had higher expression levels of OPN, RUNX2, ALP, and collagen-1 after 7 and 14 days (P=0.001; Figure 2).
Figure 2.
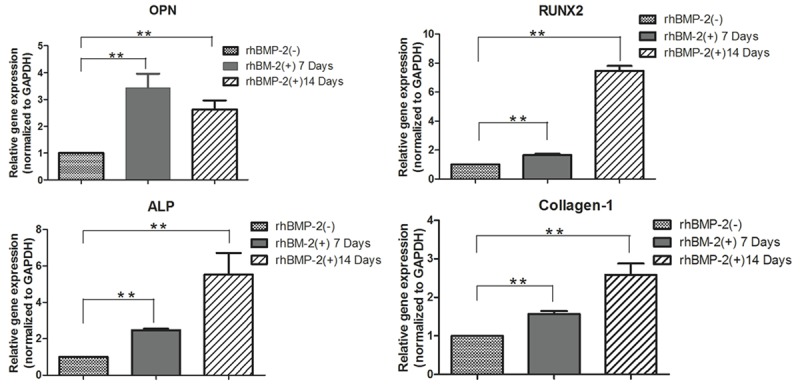
qRT-PCR results showing the expression of osteogenic proteins in human adipose stromal cells cultured in rhBMP-2 differentiation medium and in a control medium lacked rhBMP-2 for 7 or 14 days. **P<0.01.
Histological findings
At 2 weeks post surgery, we observed extensive infiltration by inflammatory cells at the tendon-bone interface in group 1 (Figure 3A). At the same time point in groups 2 and 3, we observed cartilage tissue in the tendon-bone interface (Figure 3B, 3C). Group 1 showed a distinct and broad fiber-tissue interface zone and no cartilage or bone formation at 4 and 8 weeks post surgery (Figure 3D, 3G). In group 2, we observed a fibro-cartilage zone in the tendon-bone interface at 4 weeks post surgery (Figure 3E). At the same time point in group 3, we observed mature bone cells at the interface (Figure 3F). At 8 weeks post surgery, we observed new bone formation between the host bone and tendon graft in groups 2 and 3 (white arrows in Figure 3H, 3I). Masson’s trichrome staining showed collagen fibers in the tendon-bone interface at 4 weeks post surgery in group 2 (Figure 4E); however, at 8 weeks post surgery, group 3 displayed substantially more collagen fibers in the tendon-bone interface than the other two groups (Figure 4I). We did not observe typical direct healing in the tendon-bone interface in any of the animals at any time point.
Figure 3.
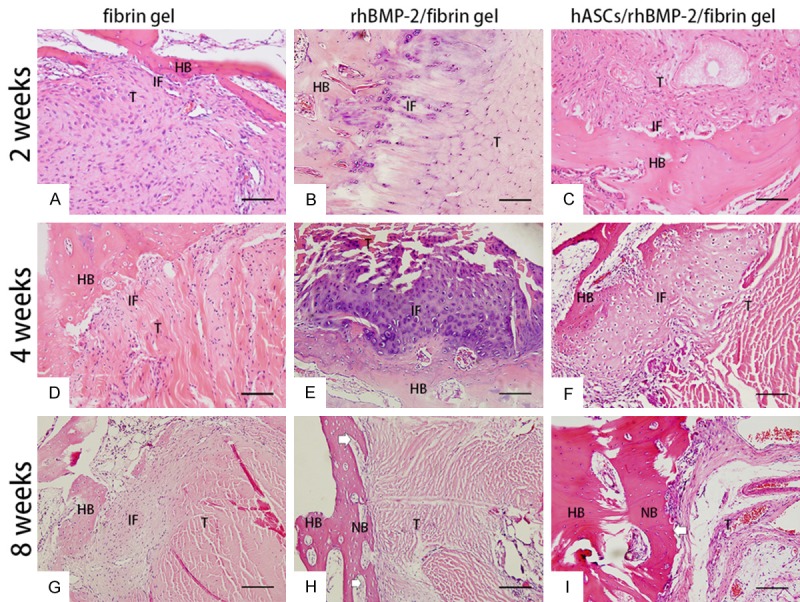
Histological findings in each group at 2, 4, and 8 weeks post surgery. A: Many inflammatory cells gathered at the tendon-bone interface in the rh-BMP/fibrin gel group at 2 weeks post surgery. B, C: Cartilage tissues could be seen in the rhBMP-2/fibrin gel group and the hASC/rhBMP-2/fibrin gel group at the tendon-bone interface. D, G: No cartilage or bone formation was found at 4 weeks or 8 weeks post surgery in the fibrin gel group. E: A fibro-like cartilage zone could be seen in the tendon-bone interface at 4 weeks post surgery in the rhBMP-2/fibrin gel group. F: Some mature bone cells could be seen at the interface in the hASCs/rhBMP-2/fibrin gel group at 4 weeks post surgery. H, I: New bone (white arrows) formation between the host bone and tendon graft was found at 8 weeks post surgery in the rhBMP-2/fibrin gel group and the hASC/rhBMP-2/fibrin gel group. H&E staining, original magnification 200×. Scale bar: 100 μm. HB: host bone; IF: interface; T: tendon; NB: new bone.
Figure 4.
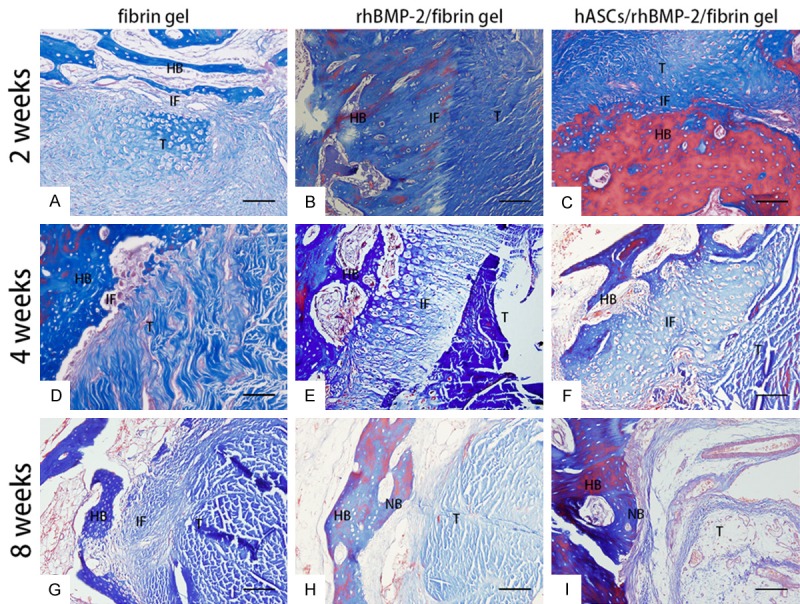
Masson’s trichrome staining in each group at 2, 4, and 8 weeks post surgery. A-D: Collagen fibrous and fibro-cartilage could not be seen in the tendon-bone interface. E, F: Collagen fbrous and fbrocartilage in the tendon-bone interface could be seen in the rhBMP-2/fbrin gel group and hASC/rhBMP-2/fibrin gel group at 4 weeks post surgery. G-I: Abundant collagen fbers in the tendon-bone interface were found in the hASC/rhBMP-2/fbrin gel group at 8 weeks post surgery compared with the rhBMP-2/fbrin gel group and the fbrin gel group. Original magnifcation 200×. Scale bar: 100 μm. HB: host bone; IF: interface; T: tendon; NB: new bone.
Biomechanical testing
The mean ultimate failure loading and the stiffness of the tendon graft are shown in Table 2. At 2 weeks post surgery, there was no significant difference among the three groups in ultimate failure loading (P=0.200). At 4 and 8 weeks post surgery, the ultimate failure loads in group 3 were significantly higher than those in groups 1 and 2 (both P=0.01). Multiple comparisons by Dunnett’s T3 test showed significant differences between groups 1 and 2 (P=0.047), groups 1 and 3 (P<0.001), and groups 2 and 3 (P=0.024) at 4 weeks post surgery. A Bonferroni test showed that there were significant differences between all three groups at 8 weeks post surgery (all P=0.001; Figure 5A).
Table 2.
Ultimate failure load and stiffness
| Time point | Group | Failure Load (N) | p-value | Stiffness (N/mm) | p-value |
|---|---|---|---|---|---|
|
|
|
||||
| Mean ± SD | Mean ± SD | ||||
| 2 weeks (n=6) | Group 1 | 12.36 ± 7.67 | 0.20 | 7.85 ± 4.11 | 0.244 |
| Group 2 | 20.71 ± 10.67 | 16.14 ± 13.27 | |||
| Group 3 | 21.02 ± 8.30 | 9.76 ± 5.07 | |||
| 4 weeks (n=6) | Group 1 | 14.76 ± 4.10a**,b** | <0.001 | 8.98 ± 4.12a*,b** | 0.001 |
| Group 2 | 48.41 ± 24.1a**,c** | 15.17 ± 8.91a*,c** | |||
| Group 3 | 87.52 ± 10.29b**,c** | 27.80 ± 7.24b**,c** | |||
| 8 weeks (n=6) | Group 1 | 47.0 ± 12.81a**,b** | <0.001 | 30.28 ± 23.86 | 0.478 |
| Group 2 | 85.53 ± 17.42a**,c** | 44.85 ± 25.82 | |||
| Group 3 | 126.31 ± 12.85b**,c** | 37.18 ± 5.62 |
Denotes significant difference (P<0.05) between groups 1 and 2.
Denotes significant difference (P<0.01) between groups 1 and 2.
Denotes significant difference (P<0.01) between groups 1 and 3.
Denotes significant difference (P<0.01) between groups 2 and 3.
Figure 5.
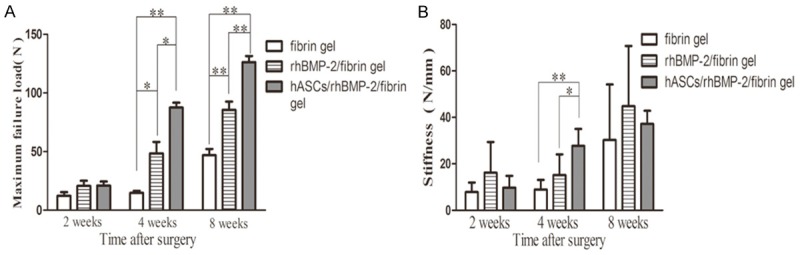
Biomechanical testing of the reconstructed ACL to measure pull-out strength and stiffness. A: The maximum failure load in the hASC/rhBMP-2/fibrin gel group was significantly higher than that in the rhBMP-2/fibrin gel group and the fibrin gel group at 4 weeks and 8 weeks post surgery. B: The stiffness in the hASC/rhBMP-2/fibrin gel group was significantly higher than that in the rhBMP-2/fibrin gel group and the fibrin gel group at 4 weeks post surgery. *P<0.05, **P<0.01.
There was no significant difference in stiffness among the three groups at 2 or 8 weeks post surgery, although group 2 appeared to have a greater mean stiffness than groups 1 and 3. There was, however, a significant difference in stiffness at 4 weeks after surgery (P=0.001). A Bonferroni test showed that there were significant differences between groups 1 and 3 (P=0.001) and groups 2 and 3 (P=0.022; Figure 5B).
Discussion
We found that co-injection of hASCs and rhBMP-2/fibrin gel into the bone tunnels during ACL reconstruction can enhance tendon-bone healing and pull-out force of the graft tendon by the formation cartilage and bone in the tendon-bone interface.
hASCs can be induced to form cartilage or bone in vitro and in vivo [24,25]. To the best of our knowledge, only a few studies have investigated whether the transplantation of hASCs and rhBMP-2/fibrin gel into the tendon-bone interface can enhance tendon-bone healing [26]. Our aim was to evaluate how the co-injection of hASCs and rhBMP-2/fibrin gel into the anchor hole during ACL reconstruction affects tendon-bone healing and bone formation. Our results showed that the co-injection of hASCs and rhBMP-2/fibrin gel resulted in more bone formation at the tendon-bone interface at 8 weeks post surgery compared with the injection of rhBMP-2/fibrin gel alone. Furthermore, the pull-out force of the graft tendon was significantly higher after co-injection of hASCs and rhBMP-2/fibrin gel than after injection of fibrin gel or rhBMP-2/fibrin gel at 4 and 8 weeks post surgery. Those results suggest that the transplantation of hASCs and rhBMP-2/fibrin gel scaffold may be a new way to enhance tendon-bone healing.
RhBMP-2 belongs to the TGF-β family and has been reported to enhance tendon-bone healing in the early stages of ACL reconstruction. Rodeo [27] demonstrated that rhBMP-2 can accelerate the healing process. Kim [8] performed ACL reconstruction in a rabbit patellar tendon model using rhBMP-2 and observed new bone formation at 4 and 8 weeks post surgery. Another recent study reported that rhBMP-2 regenerated direct insertion morphology at the tendon-bone junction at 8 or 12 weeks post surgery [28]. In our study, histological analysis indicated more abundant fibrocartilage at the tendon-bone interface at 4 and 8 weeks post surgery both in the rhBMP-2/fibrin gel group and in the hASC/rhBMP-2/fibrin gel group. At 8 weeks post surgery, we observed abundant collagen fibers and new bone between the graft and bone interface in the hASC/rhBMP-2/fibrin gel group.
In terms of tendon-bone healing, stem cells, such as BMSCs, have become increasingly attractive. Kosaka [26] reported that adipose-derived regenerative cells can enhance the failure load and stiffness of grafts. Kanaya [16] found that the injection of MSCs into the intra-articular joint significantly increased the ultimate failure load of the femur-ACL-tibial complex at 4 weeks post surgery. Dong [29] reported that BMSCs with exogenous BMP-2 on the gastrocnemius tendon improved the biomechanical properties of grafts in the bone tunnel. Our study showed that the maximum failure load after co-injection of hASCs and rhBMP-2/fibrin gel was significantly higher than that after the injection of fibrin gel or rhBMP-2/fibrin gel at 4 and 8 weeks post ACL reconstruction.
ASCs have several advantages over BMSCs in terms of their availability, potential sources, multilineage differentiation potential, and donor site morbidity. Many previous studies have reported that ASCs and BMP-2 can promote bone formation [30-32]. Jeon [33] and Levi [34] both reported that hASCs can differentiate into osteoblasts without the need for pre-differentiation. In our study, hASCs enhanced tendon healing at 4 and 8 weeks post surgery by forming fibrocartilage cells and bone at the tendon-bone interface without pre-differentiation, thus demonstrating the potential of hASCs without pre-differentiation to promote tendon-bone healing. Those results suggest a novel method to enhance tendon-bone healing in ACL reconstructions.
Our study has several limitations. First, the number of biomechanical experimental animals was not enough to avoid selection bias. Second, we observed only three time points (2, 4, and 8 weeks post surgery) and did not assess healing over a longer period, because our purpose was to detect the early healing of tendon grafts. Third, the mechanism by which hASCs differentiate into osteogenic and chondrogenic cells on the tendon-bone interface is still unknown. Zhang, X [35] reported that the bone morphogenetic proteins signaling pathway plays a major role in ASC osteogenesis. Uysal [36] reported that ASCs can enhance primary tendon repair by increasing collagen type I, fibroblast growth factor and vascular endothelial growth factor levels and decreasing TGF-β levels in a rabbit model, although their study did not explain the complex interaction among those proteins.
Our results indicate that co-injection of hASCs and rhBMP-2/fibrin gel has the potential to promote tendon-bone healing after anterior cruciate-ligament reconstruction. Further research should be carried out to investigate the mechanism by which hASCs and rhBMP-2 promote tendon-bone healing.
Acknowledgements
This work was supported by a grant (2013B021800150) from the Guang Dong Provincial Science and Technology Plan Project.
Disclosure of conflict of interest
None.
References
- 1.Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35:1756–1769. doi: 10.1177/0363546507307396. [DOI] [PubMed] [Google Scholar]
- 2.Spindler KP, Wright RW. Clinical practice. Anterior cruciate ligament tear. N Engl J Med. 2008;359:2135–2142. doi: 10.1056/NEJMcp0804745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang X, Ma Y, Fu X, Liu Q, Shao Z, Dai L, Pi Y, Hu X, Zhang J, Duan X, Chen W, Chen P, Zhou C, Ao Y. Runx2-modified adipose-derived stem cells promote tendon graft integration in anterior cruciate ligament reconstruction. Sci Rep. 2016;6:19073. doi: 10.1038/srep19073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwarting T, Schenk D, Frink M, Benolken M, Steindor F, Oswald M, Ruchholtz S, Lechler P. Stimulation with bone morphogenetic protein-2 (BMP-2) enhances bone-tendon integration in vitro. Connect Tissue Res. 2016;57:99–112. doi: 10.3109/03008207.2015.1087516. [DOI] [PubMed] [Google Scholar]
- 5.Mutsuzaki H, Fujie H, Nakajima H, Fukagawa M, Nomura S, Sakane M. Effect of calcium phosphate-hybridized tendon graft in anatomic single-bundle ACL reconstruction in goats. Orthop J Sports Med. 2016;4:2325967116662653. doi: 10.1177/2325967116662653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kohno T, Ishibashi Y, Tsuda E, Kusumi T, Tanaka M, Toh S. Immunohistochemical demonstration of growth factors at the tendon-bone interface in anterior cruciate ligament reconstruction using a rabbit model. J Orthop Sci. 2007;12:67–73. doi: 10.1007/s00776-006-1088-8. [DOI] [PubMed] [Google Scholar]
- 7.Chen CH, Liu HW, Tsai CL, Yu CM, Lin IH, Hsiue GH. Photoencapsulation of bone morphogenetic protein-2 and periosteal progenitor cells improve tendon graft healing in a bone tunnel. Am J Sports Med. 2008;36:461–473. doi: 10.1177/0363546507311098. [DOI] [PubMed] [Google Scholar]
- 8.Kim JG, Kim HJ, Kim SE, Bae JH, Ko YJ, Park JH. Enhancement of tendon-bone healing with the use of bone morphogenetic protein-2 inserted into the suture anchor hole in a rabbit patellar tendon model. Cytotherapy. 2014;16:857–867. doi: 10.1016/j.jcyt.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 9.Carragee EJ, Hurwitz EL, Weiner BK. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine J. 2011;11:471–491. doi: 10.1016/j.spinee.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 10.Hunziker EB, Enggist L, Kuffer A, Buser D, Liu Y. Osseointegration: the slow delivery of BMP-2 enhances osteoinductivity. Bone. 2012;51:98–106. doi: 10.1016/j.bone.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 11.Wu X, Ren J, Li J. Fibrin glue as the cell-delivery vehicle for mesenchymal stromal cells in regenerative medicine. Cytotherapy. 2012;14:555–562. doi: 10.3109/14653249.2011.638914. [DOI] [PubMed] [Google Scholar]
- 12.Kaipel M, Schutzenberger S, Schultz A, Ferguson J, Slezak P, Morton TJ, Van Griensven M, Redl H. BMP-2 but not VEGF or PDGF in fibrin matrix supports bone healing in a delayed-union rat model. J Orthop Res. 2012;30:1563–1569. doi: 10.1002/jor.22132. [DOI] [PubMed] [Google Scholar]
- 13.Breidenbach AP, Dyment NA, Lu Y, Rao M, Shearn JT, Rowe DW, Kadler KE, Butler DL. Fibrin gels exhibit improved biological, structural, and mechanical properties compared with collagen gels in cell-based tendon tissue-engineered constructs. Tissue Eng Part A. 2015;21:438–450. doi: 10.1089/ten.tea.2013.0768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Q, He QF, Zhang TH, Yu XL, Liu Q, Deng FL. Improvement in the delivery system of bone morphogenetic protein-2: a new approach to promote bone formation. Biomed Mater. 2012;7:045002. doi: 10.1088/1748-6041/7/4/045002. [DOI] [PubMed] [Google Scholar]
- 15.An C, Cheng Y, Yuan Q, Li J. IGF-1 and BMP-2 induces differentiation of adipose-derived mesenchymal stem cells into chondrocytes-like cells. Ann Biomed Eng. 2010;38:1647–1654. doi: 10.1007/s10439-009-9892-x. [DOI] [PubMed] [Google Scholar]
- 16.Kanaya A, Deie M, Adachi N, Nishimori M, Yanada S, Ochi M. Intra-articular injection of mesenchymal stromal cells in partially torn anterior cruciate ligaments in a rat model. Arthroscopy. 2007;23:610–617. doi: 10.1016/j.arthro.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 17.Lim JK, Hui J, Li L, Thambyah A, Goh J, Lee EH. Enhancement of tendon graft osteointegration using mesenchymal stem cells in a rabbit model of anterior cruciate ligament reconstruction. Arthroscopy. 2004;20:899–910. doi: 10.1016/j.arthro.2004.06.035. [DOI] [PubMed] [Google Scholar]
- 18.Toma JG, Akhavan M, Fernandes KJ, Barnabe-Heider F, Sadikot A, Kaplan DR, Miller FD. Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nat Cell Biol. 2001;3:778–784. doi: 10.1038/ncb0901-778. [DOI] [PubMed] [Google Scholar]
- 19.Kern S, Eichler H, Stoeve J, Kluter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294–1301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- 20.Wang B, Ma X, Zhao L, Zhou X, Ma Y, Sun H, Yang Y, Chen B. Injection of basic fibroblast growth factor together with adipose-derived stem cell transplantation: improved cardiac remodeling and function in myocardial infarction. Clin Exp Med. 2016;16:539–550. doi: 10.1007/s10238-015-0383-0. [DOI] [PubMed] [Google Scholar]
- 21.Hicok KC, Du Laney TV, Zhou YS, Halvorsen YD, Hitt DC, Cooper LF, Gimble JM. Human adipose-derived adult stem cells produce osteoid in vivo. Tissue Eng. 2004;10:371–380. doi: 10.1089/107632704323061735. [DOI] [PubMed] [Google Scholar]
- 22.Yoon E, Dhar S, Chun DE, Gharibjanian NA, Evans GR. In vivo osteogenic potential of human adipose-derived stem cells/poly lactide-co-glycolic acid constructs for bone regeneration in a rat critical-sized calvarial defect model. Tissue Eng. 2007;13:619–627. doi: 10.1089/ten.2006.0102. [DOI] [PubMed] [Google Scholar]
- 23.Sheykhhasan M, Qomi RT, Ghiasi M. Fibrin scaffolds designing in order to human adipose-derived mesenchymal stem cells differentiation to chondrocytes in the presence of TGF-beta3. Int J Stem Cells. 2015;8:219–227. doi: 10.15283/ijsc.2015.8.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang XF, Song Y, Liu YS, Sun YC, Wang YG, Wang Y, Lyu PJ. Osteogenic differentiation of three-dimensional bioprinted constructs consisting of human adipose-derived stem cells in vitro and in vivo. PLoS One. 2016;11:e0157214. doi: 10.1371/journal.pone.0157214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin CC, Fu SJ. Osteogenesis of human adipose-derived stem cells on poly(dopamine)-coated electrospun poly(lactic acid) fiber mats. Mater Sci Eng C Mater Biol Appl. 2016;58:254–263. doi: 10.1016/j.msec.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 26.Kosaka M, Nakase J, Hayashi K, Tsuchiya H. Adipose-derived regenerative cells promote tendon-bone healing in a rabbit model. Arthroscopy. 2016;32:851–859. doi: 10.1016/j.arthro.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 27.Rodeo SA, Suzuki K, Deng XH, Wozney J, Warren RF. Use of recombinant human bone morphogenetic protein-2 to enhance tendon healing in a bone tunnel. Am J Sports Med. 1999;27:476–488. doi: 10.1177/03635465990270041201. [DOI] [PubMed] [Google Scholar]
- 28.Takigami J, Hashimoto Y, Yamasaki S, Terai S, Nakamura H. Direct bone-to-bone integration between recombinant human bone morphogenetic protein-2-injected tendon graft and tunnel wall in an anterior cruciate ligament reconstruction model. Int Orthop. 2015;39:1441–1447. doi: 10.1007/s00264-015-2774-y. [DOI] [PubMed] [Google Scholar]
- 29.Dong Y, Zhang Q, Li Y, Jiang J, Chen S. Enhancement of tendon-bone healing for anterior cruciate ligament (ACL) reconstruction using bone marrow-derived mesenchymal stem cells infected with BMP-2. Int J Mol Sci. 2012;13:13605–13620. doi: 10.3390/ijms131013605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fan J, Park H, Lee MK, Bezouglaia O, Fartash A, Kim J, Aghaloo T, Lee M. Adipose-derived stem cells and BMP-2 delivery in chitosan-based 3D constructs to enhance bone regeneration in a rat mandibular defect model. Tissue Eng Part A. 2014;20:2169–2179. doi: 10.1089/ten.tea.2013.0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keibl C, Fugl A, Zanoni G, Tangl S, Wolbank S, Redl H, van Griensven M. Human adipose derived stem cells reduce callus volume upon BMP-2 administration in bone regeneration. Injury. 2011;42:814–820. doi: 10.1016/j.injury.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 32.Lin CY, Chang YH, Li KC, Lu CH, Sung LY, Yeh CL, Lin KJ, Huang SF, Yen TC, Hu YC. The use of ASCs engineered to express BMP2 or TGF-beta3 within scaffold constructs to promote calvarial bone repair. Biomaterials. 2013;34:9401–9412. doi: 10.1016/j.biomaterials.2013.08.051. [DOI] [PubMed] [Google Scholar]
- 33.Jeon O, Rhie JW, Kwon IK, Kim JH, Kim BS, Lee SH. In vivo bone formation following transplantation of human adipose-derived stromal cells that are not differentiated osteogenically. Tissue Eng Part A. 2008;14:1285–1294. doi: 10.1089/ten.tea.2007.0253. [DOI] [PubMed] [Google Scholar]
- 34.Levi B, James AW, Nelson ER, Vistnes D, Wu B, Lee M, Gupta A, Longaker MT. Human adipose derived stromal cells heal critical size mouse calvarial defects. PLoS One. 2010;5:e11177. doi: 10.1371/journal.pone.0011177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang X, Guo J, Zhou Y, Wu G. The roles of bone morphogenetic proteins and their signaling in the osteogenesis of adipose-derived stem cells. Tissue Eng Part B Rev. 2014;20:84–92. doi: 10.1089/ten.teb.2013.0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Uysal CA, Tobita M, Hyakusoku H, Mizuno H. Adipose-derived stem cells enhance primary tendon repair: biomechanical and immunohistochemical evaluation. J Plast Reconstr Aesthet Surg. 2012;65:1712–1719. doi: 10.1016/j.bjps.2012.06.011. [DOI] [PubMed] [Google Scholar]


