Abstract
Small cell lung cancer (SCLC) is a malignant human cancer and patients have very limited benefit from traditional anticancer treatments, with a poor five-year survival rate being 10% less. In present study, we observed that Notch signalling activation induced SCLC cell growth suppression via overexpressing Notch active fragments (ICN1, ICN2, ICN3 and ICN4), implying its tumor suppressive role. The histone deacetylase (HDAC) inhibitors also displayed their suppressive effects. Valproic acid (VPA) as a HDAC inhibitor was found to suppress SCLC cell growth and cell cycle arrest at phase G1, and observed to decrease HDAC4 and increase acetylation of histone H4 (AcH4) while activating Notch signalling with an increase of Notch1, Notch target gene HES1 and p21. Meanwhile, we also observed that VPA greatly stimulated the expression of somatostatin receptor type II (SSTR2) that is usually overexpressed in many cancer cells and is used as a target for anticancer drug development, providing a combination therapy with VPA and the SSTR2-targeting cytotoxins. Thus, VPA was investigated in combination with SSTR2-targeted cytotoxins captothecine-somatostatin conjugate (CPT-SST) and colchicine-somatostatin conjugate (COL-SST). Our assays showed that these combination treatments strongly led to a greater suppression as compared to each alone. In conclusion, we found that VPA suppressed SCLC cell growth and increased the expression of SSTR2. These may provide a novel clinical opportunity for enhanced anticancer therapy using the combination strategy of Notch signalling regulator and SSTR2-targeting cytotoxins in SCLC treatments.
Keywords: Small cell lung cancer, cell proliferation, HDAC inhibitor, valproic acid, somatostatin receptor, Notch signaling
Introduction
Lung cancer is the leading cause of cancer-related death worldwide, with small cell lung cancer (SCLC) and non-SCLC accounting for approximately 15% and 80% of all lung cancers, respectively [1-3]. Most SCLC patients are strongly associated with their smoking histories [2]. An increase of SCLC cases was observed in China and other developing countries, with a decrease in the developed countries. SCLC is extremely malignant and lethal lung cancer, with rapid tumor growth and metastasis, and also drug resistance. Patients could get very limited clinical benefits from most current treatments, with a poor five-year survival rate being 10% less [3]. Thus, a new effective and specific anti-SCLC therapeutic is urgently needed.
Notch signalling has been demonstrated involved in tumor growth and progression. Notch signalling is critically involved in many biological processes and modulates various different cellular functions [4,5]. Notch signalling is highly conserved and determines cell destiny. Notch signalling is involved in the progression of many human cancers and is highly cell- and context-specific [6]. Notch signalling acts as a tumor suppressor in some cancers such as lung cancer, cervical cancer and medullary thyroid cancer [7-9], or as an oncogene in some others such as leukemia, liver cancer [10-13]. Thus, to target Notch signalling may be a potential anti-cancer therapeutic strategy.
However, The Notch gene family encodes single-pass, heterodimeric type I transmembrane receptors [4]. These receptors and their ligands are all large protein molecules that are limited for drug development. Therefore, to search small molecules has been catching attentions from scientists in the fields of Notch signalling. Certain HDAC inhibitors have been found to modulate Notch signalling and play different roles in different types of cancers. Valproic acid (VPA), a HDAC inhibitor and a classic anti-convulsant drug, has been found to suppress tumor cell growth and also observed to up-regulate Notch signalling in certain cancer cells or down-regulate it in certain others [10,14].
In our previous studies, besides resulting in tumor suppression, Notch1 signaling activation in cervical cancer cells up-regulated certain G protein-coupled receptors such somatostatin (SST) receptors, bombesin receptors [14]. These receptors have been found to aberrantly express in many cancer cells such as lung cancer, pancreatic cancer, neuroblastoma, carcinoid [15], and applied as drug targets during anticancer drug development [16,17]. Thus, a Notch signalling regulator like VPA in combination with receptor-targeted drug could further enhance the antitumor efficacy of drugs targeting these receptors [8]. The specific antitumor advantages of this combination strategy have been confirmed in our previous studies of cervical cancer, carcinoid and liver cancer [8,10,14].
Our previous study showed that Notch signaling might be a tumor suppressor in SCLC cells and a potential anti-SCLC target. Thus, in the present study, we further investigated the effects of Notch signaling in SCLC DMS53 cells and the potential applications of Notch signaling-targeted drugs. We found that Notch signaling activation via the transfection of Notch active fragments (ICN1, ICN2, ICN3 and ICN4) suppressed SCLC cell growth. The HDAC inhibitor VPA could stimulate Notch signaling as expected and also greatly increase the expression of SSTR2, besides suppressing SCLC cell growth. VPA in combination with the SSTR2-targeting cytotoxic SST conjugates [17] was also investigated and found to enhance suppressive efficacy in SCLC cells, supporting the combination therapy could be a novel potential therapeutic strategy in SCLC treatments.
Methods and materials
Materials
Valproic acid sodium salt (valporic acid, VPA), trichostatin A (TSA), valpromide (VPM) and dibenzazepine (DBZ) were purchased from Sigma (St. Louis, MO). Antibodies of p21 (Cat. No.: sc-756), p63 (sc-8343), SSTR2 (sc-11609), histone 4 (H4, sc-10810), HDAC4 (sc-11418), acetylation of histone 4 (AcH4, sc-8660-R), Notch1 (sc-23299), HES1 (sc-25392) and β-actin (sc-1616-HRP), were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The plasmids expressing the intracellular domains (active Notch fragments) of the four Notch receptors (ICN1, ICN2, ICN3, and ICN4) were gifts from Dr. Wu (University of Florida). The cytotoxic conjugates camptothecin-somatostatin conjugate (CPT-SST) and colchicine-somatostatin conjugate (COL-SST) were synthesized in our laboratories.
Cell culture and cell transfection
Human small cell lung cancer (SCLC) DMS53 cells were maintained in Way mouth’s medium, supplemented with 10% fetal bovine serum (FBS) and 1% penicilin/streptomycin, and with incubation at 37°C in a 5% CO2 atmosphere. For cell transfection assay to evaluate the effects of Notch signaling on SCLC cell growth, 500 μl of cells (2×105 cells/ml) were plated in each well of 24-well plates. Two μl of LipofectamineTM 2000 (Lipo-2000) and 0.8 μg of plasmid DNA carrying Notch active fragment ICN1, ICN2, ICN3 or ICN4, respectively, were added separately into a vial with 50 μl Opti-MEM transfection medium, and combined together after a 5-10 minute incubation. The DNA/Lipo2000 complexes were continuously incubated for 20-30 minutes and then added to each well. The transfected cells were continued a 3-day incubation for the cell proliferation assay as described below. The experiments were done separately three times. The data were statistically analyzed with nonparametric t test.
Real-time PCR
This assay was run to evaluate the expression of certain genes at mRNA levels. All primers were from previously published reports, including SSTR2 primers (Forward (F): 5’ GAG AAG AAG GTC ACC CGA ATG G 3’; Reverse (R): 5’ TTG TCC TGC TTA CTG TCA CTC CGC 3’), p53 primers (F: 5’ CAG CAT CTT ATC CGA GTG GAA GG 3’; R: 5’ CAC AAA CAC GCA CCC AAA GC 3’), p21 primers (F: 5’ TGA TGC GCT AAT GGC GGG CT 3’; R: 5’ TGC TGG TCT GCC GCC GTT TT 3’), p63 primers (F: 5’ TCC TCA GGG AGC TGT TAT CC 3’; R: 5’ ACA TAC TGG GCA TGG CTG TT 3’), BCL-2 primers (F: 5’ TGA TTC CTG CCG CCC AGC TT 3’; R: 5’ TGT AAC CGC AGT GGC GCC TT 3’), PCNA primers (F: 5’ AGC ACG CAC CCT GCC ACA AT 3’; R: 5’ ACA GCC CAG CAG CAG CAT GA 3’), MMP2 primers (F: 5’ AAC GAC CGC AAC CGC ATC GT 3’; R: 5’ AAA GTG GGC AAC GCC CGT GT 3’) [14]. Real-time PCR assays were performed on a Bio-Rad iCycler (Hercules CA) using iScriptTM cDNA Synthesis Kit and iQTM SYBR Green Supermix (Bio-Rad) as described [14]. The cDNA synthesis was run for one cycle of 25°C for 5 min, 42°C for 30 min, 85°C for 5 min and held at 4°C. PCR reactions were run on a Rotor-Gene 3000 Real-Time PCR Thermal Cycler (Corbett Research). b-actin was used as the internal control and results were calculated by applying 2-ΔΔCT methods. The experiments were done separately three times. The data were statistically analyzed with nonparametric t test.
Western blot analysis
This assay was performed for the expression of certain genes of interest at protein levels and as described in the instructions of the protocol (Santa Cruz). Briefly, cells were harvested, re-suspended in RIPA buffer with cocktail inhibitors, homogenized with a 21-gauge needle, mixed with the loading buffer and heated for 7 minutes in the boiling water. Supernatants were loaded to run on a 8-16% Tris-glycine gel after centrifugation at 12,000× g for 10 minutes. Protein was transferred to a nitrocellulose membrane and blocked with a 5% dried milk/TBST solution, then washed and incubated with primary antibody. The membrane was washed again and incubated with second antibody (Santa Cruz). Membranes were developed according to the ECL system protocol (Amersham Biosciences, England). The experiments were done separately three times.
Cell cycle analysis
For cell cycle analysis assay to evaluate effects of tested compounds on SCLC cell cycle arrest, cells were plated on 6-well plates and incubated overnight. The tested compounds were added. Cells were incubated as required and then harvested for the assay. The Coulter DNA Prep reagents kit (Cat. No.: PN 6607055) from Beckman Coulter (Fullerton, CA) was used. Analysis was done on a Beckman-Coulter Epics FC500 analyzer using CXP software for acquisition and the ModFit LT v3.1 (Verity Software) for cell cycle modeling. Experiments were separatedly repeated three times.
Cell proliferation assay
The cell proliferation assay (Promega, Madison, WI) was performed as described previously (38). Briefly, 50 μl aliquots of medium with different concentrations of compounds were added to 96-well plates. All concentrations were tested in triplicate. Fifty μl of the DMS53 cell stock (1×105 cells/ml) was dispensed into each tested well and the plates were incubated at 37°C in a CO2 incubator for 3 days. Following the incubation period, 15 μl of the dye solution was added to each well and the plates were continuously incubated at 37°C for 4 hours, followed by the addition of 100 µl per well of the solubilization solution. The plates were incubated at 37°C until the contents in each well became a uniform-colored solution. The absorbance was measured and recorded at 570 nm by a Victor Plate Reader (PerkinElmer, Boston, MA).
Results
Our previous studies showed that Notch signaling played a suppressive or oncogenic role in different types of cancer cells, with the HDAC inhibitors inhibited cancer cell growth while activating or inactivating Notch signaling [9,10]. Notch signaling is aberrantly expressed in SCLC cells and plays a critical role in SCLC progression. Herein, we further investigated the effects of Notch signaling activation on SCLC cell growth. Also, we evaluated the effects of the HDAC inhibitor VPA on SCLC cell growth and Notch signaling.
Notch signaling activation suppressed cell proliferation
Firstly, we evaluated the effects of Notch signaling in SCLC DMS53 cells. Notch signaling was activated in SCLC DMS53 cells via transfecting cells with the plasmids carrying Notch active fragments ICN1, ICN2, ICN3 and ICN4, respectively. We found that Notch activation could suppress SCLC cell proliferation. As shown in Figure 1, the suppressive effects of all ICN1, ICN2, ICN3 and ICN4 were in a dose-dependent manner, with the inhibitory rates being 9% (ICN1: 200 ng) and 18% (ICN1: 400 ng), 17% (ICN2: 200 ng) and 29% (ICN2: 400 ng), 13% (ICN3: 200 ng) and 21% (ICN3: 400 ng), 9% (ICN4: 200 ng) and 17% (ICN4: 400 ng), respectively (Figure 1). These results support that Notch signaling possibly act as a tumor suppressor in SCLC cells.
Figure 1.
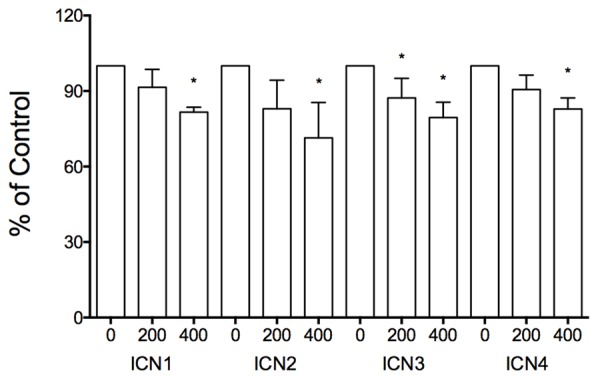
In vitro cell proliferation assays showed the suppressive effects of Notch signaling activation on SCLC DMS53 cell growth. All the four Notch active intracellular domains (ICN1, ICN2, ICN3, and ICN4) displayed their suppression of SCLC cell proliferation in a dose-dependent manner (ICN: 0, 200, 400 ng). *showed significant change with P value < 0.05.
HDAC inhibitors suppressed cell proliferation and activated Notch signaling
In our previous study, HDAC inhibitors were identified to suppress tumor cell proliferation and tumor growth in many cancers while they modulated Notch signaling [10,14]. Therefore, we tested the effects of certain HDAC inhibitors VPA, TSA, VPM (the VPA analogue), and Notch signaling inhibitor (DBZ) on cell growth and Notch signaling. We found that all these molecules displayed anti-proliferative abilities in a dose-dependent manner. As shown in Figure 2, VPA significantly induced cell proliferation suppression, with the suppressive rates being 51.5% (VPA: 4 mM), 28.1% (2 mM), 9.3% (1 mM) and 7.6% (0.5 mM), respectively (Figure 2). Real-time PCR analysis also showed that VPA-induced suppression of cell proliferation marker PCNA (0.3-fold), cell apoptotic marker BCL-2 (0.4-fold) and MMP2 (0.4-fold) (Figure 3). The other two HDAC inhibitors TSA and VPM also displayed the suppressive effect on cell growth. Particularly, TSA displayed a much more potent suppression, with TSA-induced suppression rates being 98% (TSA: 2 mM), 97% (1 mM) and 71.7% (0.1 µM), respectively. And compared to VPA, its analogue VPM also showed a more suppressive ability, with the suppressive rates being 98.6% (VPM: 4 mM), 74.6% (2 mM), 51.6% (1 mM) and 7.6% (0.5 mM), respectively. Meanwhile, DBZ also inhibited SCLC cell growth with the inhibitory rates being 36.7% (DBZ: 40 mM), 15.9% (20 mM) and 6.2% (10 µM), respectively (Figure 2).
Figure 2.
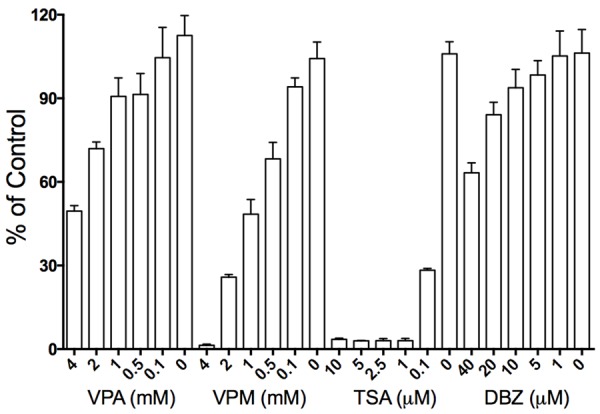
In vitro cell proliferation assays showed the suppressive effects of four small molecules on SCLC DMS53 cell growth. The three HDAC inhibitors (VPA, VPM and TSA) and Notch signaling inhibitor DBZ displayed their suppressive effects in a dose-dependent manner, with TSA and VPM having more potent efficacy and DBZ having less.
Figure 3.
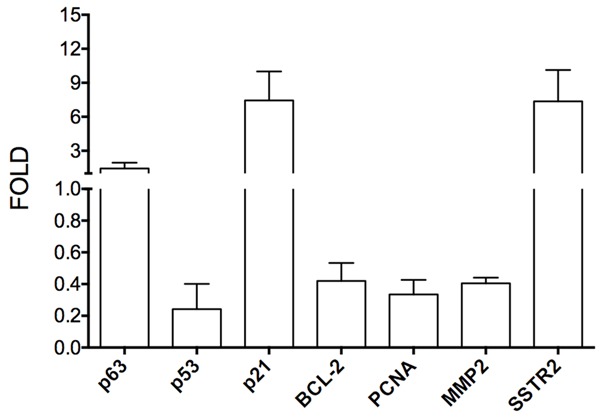
Real-time PCR assays showed the effects of the HDAC inhibitor VPA on the expression of certain genes at mRNA level. VPA induced a decrease of BCL-2 (0.4-fold), PCNA (0.3-fold), MMP2 (0.4-fold) and p53 (0.2-fold), an increase of p21 (7.4-fold) and SSTR2 (7.4-fold) and insignificant change of p63 (1.4-fold).
We further investigated and confirmed the effects of these molecules on histone acetylation/deacetylation in SCLC DMS53 cells. Western blot analysis showed that the two HDAC inhibitors VPA and TSA reduced the expression of HDAC4 and induced the acetylation of histone H4 (AcH4), with no obvious change of histone H4 (Figure 4A). Both HDAC inhibitors were also observed to obviously stimulate the expression of Notch1, Notch target genes HES1 and p21 (Figure 4A). The time course assay showed that VPA induced the increase of AcH4. But VPA induced a transient increase of HES1, with a quick increase in a short incubation (3, 6 and 18 h) and then a decrease with incubation being extended (36 and 72 h) (Figure 4B). VPM (the VPA analogue) and The Notch inhibitor DBZ suppressed the expression of HDAC4, but not to H4 and AcH4. Moreover, VPM did not affect Notch1, HES1 and p21 as well. DBZ induced a slight increase of p21 and a slight decrease of HES1, but not to Notch1 (Figure 4A).
Figure 4.
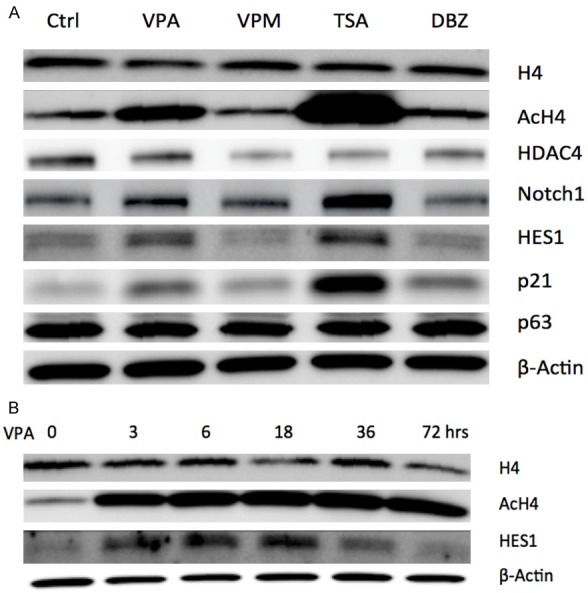
Western blot assays showed the effects of small molecules on the expression of certain genes in SCLC DMS53 cells. A: The two HDAC inhibitors VPA and TSA reduced the expression of HDAC4, induced the acetylation of histone H4 (AcH4), and increased the expression of Notch1, Notch target genes HES1 and p21, with no obvious change of histone H4 and p63. VPM (the VPA analogue) and the Notch inhibitor DBZ suppressed the expression of HDAC4, but not to H4 and AcH4. VPM did not affect Notch1, HES1, p63 and p21 as well. DBZ induced a slight increase of p21 and a slight decrease of HES1, but not to Notch1 and p63. B: The time course assay showed that VPA induced an increase of AcH4. And VPA induced a transient increase of HES1, with an increase in a short incubation (3, 6 and 18 h) and then a decrease with incubation being extended (36 and 72 h).
VPA induced cell cycle arrest
We also evaluated the effects of these molecules on the cell cycle progression. As seen on Table 1, TSA at 1 mM and under an 18-hour incubation was found to significantly induce cell cycle arrest at phase G2 (20.1%) and cell death (18.6%) compared to control (9.9% and 7.1%, respectively). However, VPA (1 mM), VPM (0.5 mM) and DBZ (20 mM) slightly induced cell cycle arrest at phase G1, with slight increase of cell debris (Table 1).
Table 1.
The effects of different agents on SCLC DMS53 cell cycle progression
| Agents | Phases | Debris | Changes | ||
|---|---|---|---|---|---|
|
| |||||
| G1 | G2 | S | |||
| Control | 58.9±0.7 | 9.9±0.4 | 31.7±1.7 | 7.1±0.85 | |
| VPA | 62.4±0.9 | 10.2±1.2 | 27.6±0.8 | 8.5±1.6 | G1 |
| VPM | 64.1±1.4 | 11.2±0.9 | 24.3±1.1 | 7.6±0.9 | G1 |
| TSA | 54.7±1.5 | 20.1±1.9 | 25.2±1.5 | 18.6±3.9 | G2 |
| DBZ | 63.0±1.6 | 9.7±1.8 | 27.3±1.9 | 8.1±1.2 | G1 |
The time course analysis further showed the VPA at 4 mM and under different incubation time (0, 3, 18, 72 hours) induced an increase at phase G1 with time extension. VPA under 72-hour incubation induced a significant increase at phase G1 (84.7%) compared to control (71.1%). We also observed an increase of cell debris (13.4%) compared to control (5.3%), (Figure 5). VPA also induced an increase of the tumor repressor p21 at the mRNA level by real-time PCR (Figure 3) and at the protein level by western blot analysis (data not shown), but not p63 (Figure 3). VPA could induce cell morphological change in many cancer cells, but we did not observe its effects to SCLC DMS53 cells and other lung cancer cells (data not shown).
Figure 5.
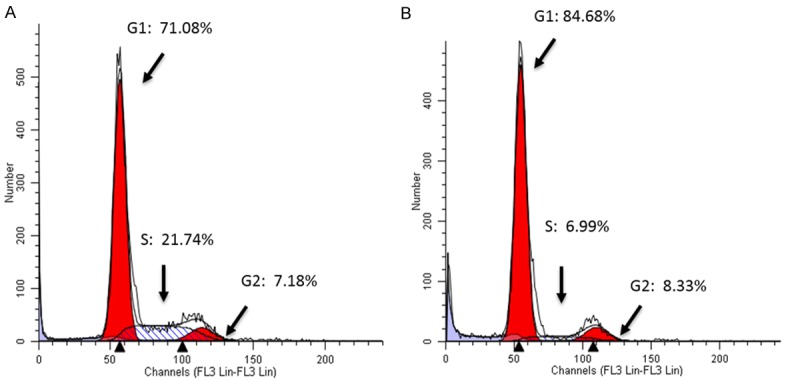
Cell cycle analysis showed the effects of VPA on cell cycle progression in SCLC DMS53 cells. VPA at 4 mM and with a 72-h incubation induced cell cycle arrest at phase G1 with the rate being 84.68% (B) compared to 71.08% of control (A).
The application of VPA in combination with SSTR2-targeting cytotoxins
Due to its effects on certain GPCR members that are aberrantly expressed in many cancer cells and have been used for receptor-targeting therapeutics [15,16], VPA was then used in combination therapy with receptor-targeting anticancer drugs. Thus, presently, VPA also was evaluated for its effects on certain GPCR members such as the SST, BN and PACAP receptors (SSTR1-5, GRPR, BRS3, NMBR, PAC1, VPAC1 and VPAC2) in SCLC cells. We observed a high expression of SSTR1, SSTR2, SSTR3, BRS3, NMBR and VPAC1 in SCLC DMS53 cells, with a trace of SSTR4, SSTR5, GRPR, PAC1 and VPAC2 (data not shown). We also treated DMS53 cells with VPA and found VPA significantly increased the expression of SSTR2 at the mRNA level by real-time PCR (Figure 3) and at the protein level by Western blot analysis (Figure 6).
Figure 6.
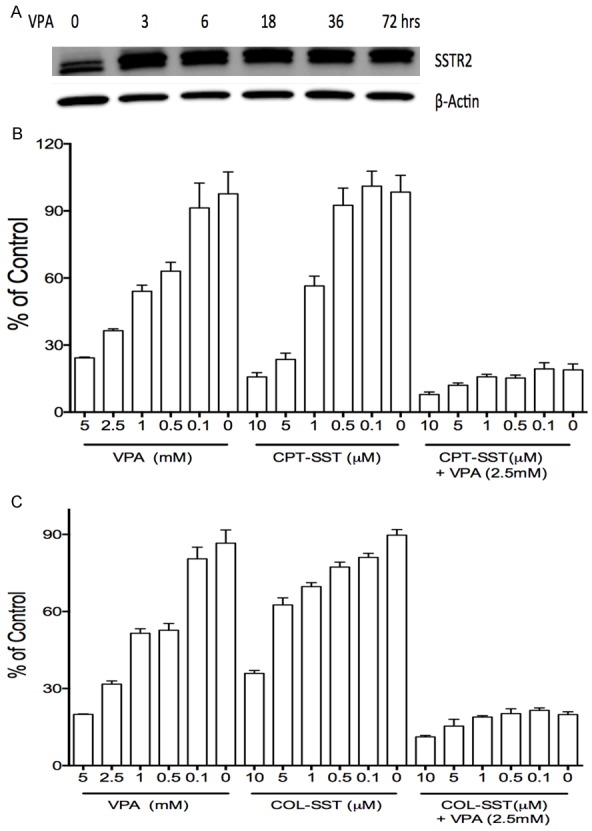
VPA in combination treatments enhanced the proliferative suppression of SSTR2-targeted cytotoxins CPT-SST and COL-SST in SCLC DMS53 cells. VPA enhanced the expression of SSTR2 (A), VPA in combination with the cytotoxins CPT-SST (B) and COL-SST (C) displayed more potent suppressive efficacy compared to each alone.
The aberrant expression and VPA-induced increase of SSTR2 in SCLC cells provide a potential strategy to develop a novel anti-SCLC targeted drug using VPA in combination with SSTR2-targeting cytotoxins. We therefore evaluated the effects of the combination treatments in DMS53 cells. Our in vitro proliferation assays identified that VPA, CPT-SST and COL-SST suppressed cell proliferation in a dose-dependent manner. Moreover, VPA in combination with these SSTR2-targeting cytotoxins (CPT-SST or COL-SST) could greatly enhance cell growth suppression compared to each alone (Figure 6). The next in vivo antitumor assays are under our schedule.
Discussion
Lung cancer is one of the aggressive human cancers and a tough challenge for scientists to develop targeted and efficacious drugs [1]. Notch signaling can behave as an oncogene in some cancers such as leukemia, hepatoCellular cancer and a tumor suppressor in some others such as neuroendoCrine tumor, cervical cancer [6,14,18]. Presently, we found that the Notch signaling activation via overexpressing each Notch active fragment (ICN1, ICN2, ICN3 and ICN4) from all Notch receptors induced suppression of SCLC cell growth. These support that activated Notch signaling executed a suppressive role in SCLC DMS53 cells and predict the possibility of developing Notch signaling-targeted anti-SCLC drugs. Certain HDAC inhibitors were found to modulate Notch signaling and subsequently induce tumor cell growth arrest [8,10,19,20]. We found that VPA, a HDAC inhibitor, suppressed Notch signaling and induced proliferation suppression in some cancers such as liver cancer, leukemia and ovarian cancer, in which Notch signaling plays an oncogenic role (data not shown). In the present study, we found that the HDAC inhibitors VPA and TSA induced proliferation suppression and activated Notch signaling in the investigated SCLC DMS53 cancer cells.
Many HDAC inhibitors have been demonstrated to mediate Notch signaling and affect the expression of the Notch1 downstream genes with a decrease of genes such as ASCL1, CgA and Cyclin D1 (BCL-1) and an increase of genes such as p21, p27 and caspase-3 [14,19,21,22]. Presently, we also observed that VPA induced an increase of Notch1, Notch target gene HES1 and p21, and a decrease of BCL-2, PCNA and MMP2. These gene changes were observed in other cancer cells [10,14]. However, we found that p53 is slightly decreased by VPA in DMS53 and other tested cancer cells. Gene p21 is usually under p53 control [23]. The change in VPA-mediated p21 (a Notch target gene) showed that it might be p53-independent in these VPA-treated cancer cells, or p53 might be mutated. The change of p21 seems assoCiated with the change of Notch signaling in these cancer cells. Notch signaling increases concomitantly with an increase of p21 in DMS-53 cells. Both ICN1 and VPA were found to induce EMT in certain cancer cells such cervical cancer Hela cells, with that the epithelial marker E-cadherin was repressed and the mesenchymal markers N-cadherin, fibronectin and MMP2 were increased [24,25]. However, we did not observe ICN- and VPA-induced morphological change in SCLC DMS53 cells and other lung cancer cells. However, we indeed observed VPA-induced cell cycle arrest at phase G1.
In our previous studies, Notch1 activation could upregulate SST signaling in cervical cancer Hela cells and carcinoid BON cells [8,9], indicating a correlation between Notch signaling and SST signaling. We found that VPA could activate SSTRs and certain other GPCRs in SCLC cells. This implies a connection VPA-mediated Notch signaling and SSTR/GPCR signaling, and a potential combination therapeutic of VPA and SSTR-targeted cytotoxins. On the other hand, traditional chemotherapy can lead to severe toxic side effects in cancer patients [17]. Thus, a targeted therapeutics is critically important. SSTR2 was found highly expressed in SCLC cells. This provides a new strategy to specifically target these receptors and quickly internalize chemical agents via conjugating these cytotoxic agents with peptide/antibody-based drug vehicles. For instance, short peptide ligands or monoClonal antibodies for these receptors were used as drug delivery vehicles by coupling cytotoxic agents to them [26-28]. The new cytotoxic conjugates have been demonstrated to increase antitumor efficacy and decrease toxic side effects [17,26]. In our previous study, some small molecules such as VPA were found to modulate Notch signaling and also upregulate the expression of certain GPCRs such as SST receptors and bombesin receptors in certain cancer cells [14]. Here, we confirmed that the combination therapy of both VPA and the SSTR2-targeted cytotoxic conjugate (CPT-SST or COL-SST) had a much more potent anti-proliferative efficacy than did each alone. By taking advantage of VPA’s direct tumor-suppressive ability and SSTR2-activating ability, the combination therapy of VPA and receptor-targeted cytotoxic conjugates could be possibly applied to improve anti-SCLC efficacy and benefit SCLC patients.
Acknowledgements
This work was supported by The Zhishan Plan Program Fund from The Third Xiangya HospitaI of Central South University ([2017]15) and the Tulane Peptide Research Fund.
Disclosure of conflict of interest
None.
References
- 1.Gazdar AF, Bunn PA, Minna JD. Small-cell lung cancer: what we know, what we need to know and the path forward. Nat Rev Cancer. 2017;17:725–737. doi: 10.1038/nrc.2017.87. [DOI] [PubMed] [Google Scholar]
- 2.Rahal Z, El Nemr S, Sinjab A, Chami H, Tfayli A, Kadara H. Smoking and lung cancer: a geo-regional perspective. Front Oncol. 2017;7:194. doi: 10.3389/fonc.2017.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang S, Tang J, Sun T, Zheng X, Li J, Sun H. Survival changes in patients with small cell lung cancer and disparities between different sexes, socioeconomic statuses and ages. Sci Rep. 2017;7:1339. doi: 10.1038/s41598-017-01571-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu L, Griffin JD. Modulation of Notch signaling by mastermind-like (MAML) transcriptional co-activators and their involvement in tumorigenesis. Semin Cancer Biol. 2004;14:348–356. doi: 10.1016/j.semcancer.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 5.Andersson ER, Lendahl U. Therapeutic modulation of Notch signalling--are we there yet? Nat Rev Drug Discov. 2014;13:357–378. doi: 10.1038/nrd4252. [DOI] [PubMed] [Google Scholar]
- 6.Aster JC, Pear WS, Blacklow SC. The varied roles of notch in cancer. Annu Rev Pathol. 2017;12:245–275. doi: 10.1146/annurev-pathol-052016-100127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun L, Liu M, Sun GC, Yang X, Qian Q, Feng S. Notch signaling activation in cervical cancer cells induces cell growth arrest with the involvement of the nuclear receptor NR4A2. J Cancer. 2016;7:1388–1395. doi: 10.7150/jca.15274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun L, Qian Q, Sun G, Mackey LV, Fuselier JA, Coy DH. Valproic acid induces NET cell growth arrest and enhances tumor suppression of the receptor-targeted peptide-drug conjugate via activating somatostatin receptor type II. J Drug Target. 2016;24:169–177. doi: 10.3109/1061186X.2015.1066794. [DOI] [PubMed] [Google Scholar]
- 9.Tsai C, Leslie JS, Franko-Tobin LG, Prasnal MC, Yang T, Vienna Mackey L, Sun LC. Valproic acid suppresses cervical cancer tumor progression possibly via activating Notch1 signaling and enhances receptor-targeted cancer chemotherapeutic via activating somatostatin receptor type II. Arch Gynecol Obstet. 2013;288:393–400. doi: 10.1007/s00404-013-2762-7. [DOI] [PubMed] [Google Scholar]
- 10.Zhu B, Sun L, Luo W, Li M, Coy DH, Yu L. Activated Notch signaling augments cell growth in hepatoCellular carcinoma via up-regulating the nuclear receptor NR4A2. Oncotarget. 2017;8:23289–23302. doi: 10.18632/oncotarget.15576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun L, Sun G, Yu Y, Coy DH. Is notch signaling a specific target in hepatoCellular carcinoma? Anticancer Agents Med Chem. 2015;15:809–815. doi: 10.2174/1871520615666150202102809. [DOI] [PubMed] [Google Scholar]
- 12.Vadillo E, Dorantes-Acosta E, Pelayo R, Schnoor M. T cell acute lymphoblastic leukemia (T-ALL): New insights into the cellular origins and infiltration mechanisms common and unique among hematologic malignancies. Blood Rev. 2017 doi: 10.1016/j.blre.2017.08.006. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 13.Zhang J, Kuang Y, Wang Y, Xu Q, Ren Q. Notch-4 silencing inhibits prostate cancer growth and EMT via the NF-kappaB pathway. Apoptosis. 2017;22:877–884. doi: 10.1007/s10495-017-1368-0. [DOI] [PubMed] [Google Scholar]
- 14.Franko-Tobin LG, Mackey LV, Huang W, Song X, Jin B, Luo J, Sun LC. Notch1-mediated tumor suppression in cervical cancer with the involvement of SST signaling and its application in enhanced SSTR-targeted therapeutics. Oncologist. 2012;17:220–232. doi: 10.1634/theoncologist.2011-0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reubi JC. Peptide receptors as molecular targets for cancer diagnosis and therapy. Endocr Rev. 2003;24:389–427. doi: 10.1210/er.2002-0007. [DOI] [PubMed] [Google Scholar]
- 16.Sun L, Coy DH. Editorial: GPCR-targeted drug development. Curr Drug Targets. 2016;17:486–487. doi: 10.2174/138945011705160303154500. [DOI] [PubMed] [Google Scholar]
- 17.Sun LC, Coy DH. Somatostatin receptor-targeted anti-cancer therapy. Curr Drug Deliv. 2011;8:2–10. doi: 10.2174/156720111793663633. [DOI] [PubMed] [Google Scholar]
- 18.Koch U, Radtke F. Notch and cancer: a double-edged sword. Cell Mol Life Sci. 2007;64:2746–62. doi: 10.1007/s00018-007-7164-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jang S, Janssen A, Aburjania Z, Robers MB, Harrison A, Dammalapati A. Histone deacetylase inhibitor thailandepsin-A activates Notch signaling and suppresses neuroendoCrine cancer cell growth in vivo. Oncotarget. 2017;8:70828–70840. doi: 10.18632/oncotarget.19993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sholler GS, Currier EA, Dutta A, Slavik MA, Illenye SA, Mendonca MC. PCI-24781 (abexinostat), a novel histone deacetylase inhibitor, induces reactive oxygen species-dependent apoptosis and is synergistic with bortezomib in neuroblastoma. J Cancer Ther Res. 2013;2:21. doi: 10.7243/2049-7962-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greenblatt DY, Cayo MA, Adler JT, Ning L, Haymart MR, Kunnimalaiyaan M, Chen H. Valproic acid activates Notch1 signaling and induces apoptosis in medullary thyroid cancer cells. Ann Surg. 2008;247:1036–1040. doi: 10.1097/SLA.0b013e3181758d0e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ning L, Greenblatt DY, Kunnimalaiyaan M, Chen H. Suberoyl bis-hydroxamic acid activates Notch-1 signaling and induces apoptosis in medullary thyroid carcinoma cells. Oncologist. 2008;13:98–104. doi: 10.1634/theoncologist.2007-0190. [DOI] [PubMed] [Google Scholar]
- 23.Melnik BC. p53: key conductor of all anti-acne therapies. J Transl Med. 2017;15:195. doi: 10.1186/s12967-017-1297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mao S, Lu G, Lan X, Yuan C, Jiang W, Chen Y. Valproic acid inhibits epithelialmesenchymal transition in renal cell carcinoma by decreasing SMAD4 expression. Mol Med Rep. 2017;16:6190–6199. doi: 10.3892/mmr.2017.7394. [DOI] [PubMed] [Google Scholar]
- 25.Bonyadi Rad E, Hammerlindl H, Wels C, Popper U, Ravindran Menon D, Breiteneder H. Notch4 signaling induces a mesenchymal-epithelial-like transition in melanoma cells to suppress malignant behaviors. Cancer Res. 2016;76:1690–1697. doi: 10.1158/0008-5472.CAN-15-1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Engel JB, Tinneberg HR, Rick FG, Berkes E, Schally AV. Targeting of peptide cytotoxins to LHRH receptors for treatment of cancer. Curr Drug Targets. 2016;17:488–494. doi: 10.2174/138945011705160303154717. [DOI] [PubMed] [Google Scholar]
- 27.Borcoman E, Le Tourneau C. Antibody drug conjugates: the future of chemotherapy? Curr Opin Oncol. 2016;28:429–436. doi: 10.1097/CCO.0000000000000310. [DOI] [PubMed] [Google Scholar]
- 28.Schally AV, Engel JB, Emons G, Block NL, Pinski J. Use of analogs of peptide hormones conjugated to cytotoxic radicals for chemotherapy targeted to receptors on tumors. Curr Drug Deliv. 2011;8:11–25. doi: 10.2174/156720111793663598. [DOI] [PubMed] [Google Scholar]


