Abstract
The FoxM1 (Forkhead Box M1) transcription factor plays a key role in regulation of cell growth, cell cycle, and transformation. Higher expression of FoxM1 has been observed in various types of human cancers including bladder cancer. However, the exact function of FoxM1 in bladder cancer has not been elucidated. To investigate the cellular and molecular function of FoxM1 in bladder cancer, we measured the consequences of downregulation and upregulation of FoxM1 in bladder cancer cells using MTT assay, wound healing assay, and invasion assay. We found that downregulation of FoxM1 inhibited cell growth, but induced apoptosis in bladder cancer cells. Moreover, we found that inhibition of FoxM1 retarded cell migration and invasion. In line with this, upregulation of FoxM1 led to cell growth promotion and inhibited cell apoptosis in bladder cancer cells. Consistently, upregulation of FoxM1 led to increased cell migration and invasion. Our Western blotting results identified that downregulation of FoxM1 increased p27 level and inhibited VEGF, while overexpression of FoxM1 reduced p27 level and increased VEGF. Our findings suggest that FoxM1 could be a useful target for the treatment of bladder cancer.
Keywords: FoxM1, bladder cancer, cell growth, apoptosis, invasion
Introduction
Bladder cancer is one of the common malignancies, which has about 400,000 new patients diagnosed yearly worldwide [1]. Tobacco smoking is one of main risk factors [2]. Non-muscle-invasive bladder cancer (NMIBC) is the main type of bladder cancer, which often has recurrence after transurethral resection [3]. Thus, some patients develop to muscle-invasive bladder cancer after multiple recurrences [4-6]. Therefore, it is required to determine the molecular insight onto bladder tumorigenesis and explore new therapeutic strategies to reduce recurrences in bladder cancer.
FoxM1 (Forkhead Box M1) signaling plays a critical role in governing cell proliferation and apoptosis [7,8]. FoxM1 (also named as HFH-11, MPP2, Win, and Trident) is a member of the Fox transcription factor family [9,10]. It has been reported that FoxM1 is a key cell cycle regulator through regulation of some cell cycle genes such as Cdc25A (cell division cycle 25A), Cdc25B, cyclin B, cyclin D1, p21cip1 and p27kip1 [11,12]. It has been known that dysfunction of FoxM1 is associated with tumorigenesis. Upregulation of FoxM1 has been observed in a variety of human cancers including lung cancer, prostate cancer, hepatocellular carcinoma, breast cancer, and pancreatic cancer [13-16]. These reports suggest that FoxM1 could be an oncoprotein in tumorigenesis [17]. However, the role of FoxM1 in bladder cancer has not been elucidated. Therefore, we sought to determine the function of FoxM1 in bladder cancer.
In the current study, we explored the consequence of down-regulation of FoxM1 by its siRNA on bladder cancer cell growth, apoptosis, and invasion. We also examined the effect of FoxM1 overexpression on the process of cell growth and invasion of bladder cancer cells. We found that inhibition of FoxM1 suppressed cell growth and induced apoptosis in bladder cancer cells. Moreover, down-regulation of FoxM1 inhibited cell migration and invasion of bladder cancer cells. Notably, overexpression of FoxM1 enhanced cell growth and invasion, but inhibited apoptosis in bladder cancer cells. Strikingly, down-regulation of FoxM1 increased p27 level and decreased VEGF (vascular endothelial growth factor) expression, whereas over-expression of FoxM1 reduced p27 level and increased VEGF expression in bladder cancer cells. Our findings demonstrated that inactivation of FoxM1 could represent a novel strategy for anti-cancer therapies for bladder cancer.
Materials and methods
Cell culture, reagents and antibodies
The human bladder cancer RT4 cells were incubated in a 5% CO2 humidified atmosphere at 37°C in DMEM (Dulbecco’s modified Eagle’s medium) medium (Gibco Company, Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS), 100 units/ml penicillin, and 100 µg/ml streptomycin. Anti-FoxM1 and anti-p27 antibodies were obtained from Cell Signaling Technology. Anti-tubulin antibody was bought from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Lipofectamine 2000 was purchased from Invitrogen (Carlsbad, CA). MTT [3-(4,5-dimethythiazol-2-yl)-2,5-diphenyl tetrazolium bromide] was obtained from Sigma (St. Louis, MO).
Transfection
Bladder cancer cells were seeded in six-well plates and transfected with control siRNA or FoxM1 siRNAs (Genepharma, Shanghai, China) using Lipofectamine 2000 according to manufacturer’s protocol. RT4 cells were also transfected with FoxM1 cDNA or pcDNA3.0 as control using Lipofectamine 2000 [18].
MTT assay
The transfected cells (5 × 103) were seeded in a 96-well culture plate. After 48 h and 72 h, cells were then incubated with MTT reagent (0.5 mg/ml) for 2 h at 37°C. Cell growth assay was conducted by determining the absorbance at 560 nm using a Benchmark Microplate Reader (Bio-Rad, Hercules, CA, USA). All values were normalized to those of the controls [19].
Apoptosis assay
The transfected cells were cultured in six-well plate for 48 h. Then, the cells were trypsinized, collected, and washed with PBS. Cells were collected by centrifugation and the pellets were resuspended in 500 μl binding buffer with 5 μl propidium iodide (PI) and 5 μl FITC-conjugated anti-Annexin V antibody. Apoptosis was analyzed by a FACScalibur flow cytometer [20].
Real-time reverse transcription-PCR analysis
Total RNA from the transfected cells was isolated with Trizol. One microgram of total RNA from each sample was reversed-transcribed into cDNA by TaqMan reverse transcription reagents kit (Applied Biosystems, Foster City, CA). RT reaction was conducted at 25°C for 10 min, followed by 48°C for 30 min and 95°C for 5 min. The primers used in the PCR reaction are FoxM1 forward primer (5’-AAC CGC TAC TTG ACA TTG G-3’) and reverse primer (5’-GCA GTG GCT TCA TCT TCC -3’); ß-actin forward primer (5’-CCA CAC TGT GCC CAT CTA CG-3’) and reverse primer (5’-AGG ATC TTC ATG AGG TAG TCA GTC AG-3’).
Western blotting analysis
Cells were lysed in lysis buffer [50 mmol/L Tris (pH 7.5), 100 mmol/L NaCl, 1 mmol/L EDTA, 0.5% NP40, 0.5% Triton X-100, 2.5 mmol/L sodium orthovanadate, 10 µL/mL protease inhibitor cocktail, and 1 mmol/L PMSF]. The protein concentrations were measured by Bio-Rad assay system. Equal amount of proteins were fractionated by SDS-PAGE (sodium dodecyl sulfate-polyacrylamide gel electrophoresis) and then transferred to nitrocellulose membranes. The membranes were immunoblotted by primary antibodies. The anti-FoxM1 (1:2000), anti-p27 (1:1000), anti-VEGF (1:2000), and anti-tubulin (1:4000) antibodies were used. The expression of tubulin was used as internal control.
Wound healing assay
Cells were seeded in 6-well plates and grown to almost confluency. Then, monolayers of cells were scratched with 200 μL small yellow pipette tips and washed twice with PBS. The scratched area was photographed with a microscope at 0 h and 20 h, respectively [21].
Transwell invasion assay
Cell invasion was assessed using BD BioCoat Matrigel invasion chambers. Briefly, tranfected cells were seeded in DMEM without serum in the upper chamber of the system. The bottom chamber was added with complete medium. After 20 hours of incubation, the non-invading cells were removed. The cells that had invaded through Matrigel matrix membrane were stained with Wright’s-Giemsa or 4 μg/ml Calcein AM in hanks buffered saline at 37°C for one hour. The labeled invasive cells were photographed under a microscope.
Statistical analysis
The data were presented as mean ± SD. Student’s t-test was performed to evaluate statistical significance. The value of P (< 0.05) was considered as significance.
Results
Downregulation of FoxM1 by its siRNA inhibited cell growth
In order to ascertain the function of FoxM1 in the progression of bladder cancer, we conducted a series of experiments to achieve our goal. The bladder cancer cells were transfected with FoxM1 siRNA to down-regulate the expression of FoxM1. The efficacy of FoxM1 for knockdown by siRNA was validated by real-time RT-PCR and Western blotting in bladder cancer cells. Our RT-PCR results showed that FoxM1 mRNA was significantly inhibited in FoxM1 siRNA transfected cells, compared with control siRNA transfected cells (Figure 1A). We also observed that FoxM1 protein expression was barely detectable in FoxM1 siRNA transfected cells (Figure 1B and Supplementary Figure 1). MTT was performed to measure cell viability in FoxM1 siRNA transfected cells. Our MTT data showed that downregulation of FxoM1 expression led to cell growth inhibition in bladder cancer cells (Figure 2A).
Figure 1.
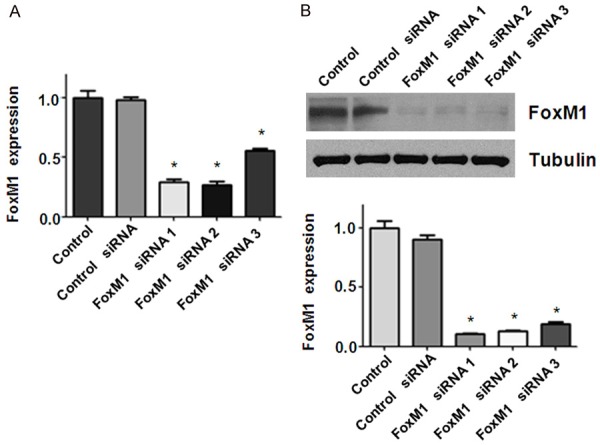
Down-regulation of FoxM1 by its siRNA in bladder cancer cells. A. Real-time RT-PCR analysis was used to determine the efficacy of FoxM1 siRNA in RT4 bladder cancer cells. *P < 0.01 vs Control siRNA. B. Top panel: Western blot analysis was used to measure the FoxM1 expression in RT4 bladder cancer cells transfected with different FoxM1 siRNAs. Bottom panel: Quantitative results for Top panel. *P < 0.01, vs Control siRNA.
Figure 2.
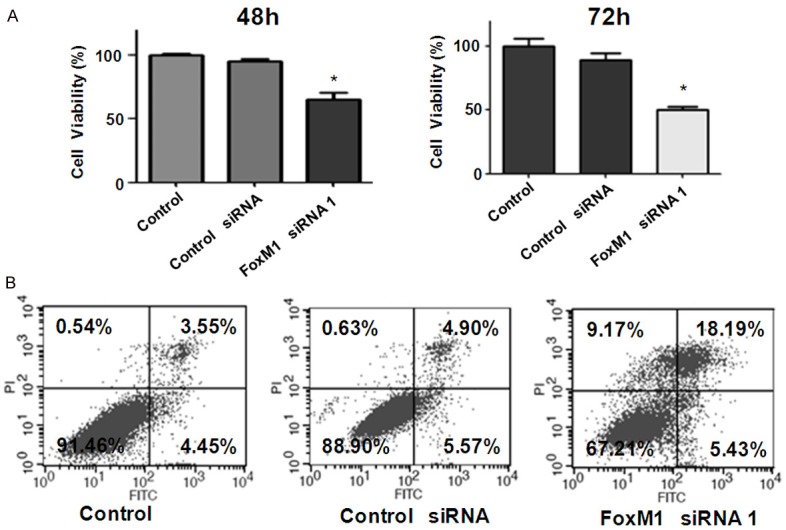
Down-regulation of FoxM1 inhibited cell proliferation and induced apoptosis. A. MTT assay was used to measure cell proliferation in RT4 bladder cancer cells after FoxM1 siRNA transfection. The transfected cells (5 × 103) were seeded in a 96-well plate. After 48 h and 72 h, cells were incubated with MTT reagent (0.5 mg/ml) for 2 h at 37°C. Cell growth was determined by measuring absorbance at 560 nm. All values were normalized to those of the controls. *P < 0.05 vs Control siRNA. B. Flow cytometry was used to measure cell apoptosis in RT4 bladder cancer cells after FoxM1 siRNA transfection. The transfected cells were cultured in the 6-well plate for 48 h. Then, the cells were collected by centrifugation and resuspended in binding buffer with 5 μl propidium iodide and 5 μl FITC-conjugated anti-Annexin V antibody. Apoptosis was analyzed by a FACScalibur flow cytometer.
Downregulation of FoxM1 induced apoptosis in bladder cancer cells
To further investigate whether the growth inhibitory effect of FoxM1 knockdown is due to apoptosis, we conducted apoptosis assay by Annexin V-FITC/PI method in RT4 cells after FoxM1 siRNA transfection. We found that downregulation of FoxM1 increased the percentage of apoptotic cells in RT4 cells transfected with FoxM1 siRNA. Specifically, cell apoptosis was increased from 10.47% in control siRNA treatment group to 23.62% in FoxM1 siRNA treatment group in RT4 cells (Figure 2B). This finding indicated that depletion of FoxM1 triggered apoptosis, which might contribute to cell growth inhibition in bladder cancer cells.
Downregulation of FoxM1 retarded bladder cancer cell migration and invasion
FoxM1 is thought to play a role in the processes of tumor cell migration and invasion. Therefore, we examined the effects of FoxM1 down-regulation on bladder cancer cell migration and invasion. Our data showed that downregulation of FoxM1 inhibited bladder cancer cell invasion (Figure 3A). Specifically, our Matrigel invasion assay results demonstrated that FoxM1 siRNA transfected cells exhibited a lower level of penetration through the matrigel-coated membrane. The numbers of invaded cells was reduced about 3-4 folds compared with the control group (Figure 3A and 3B). The Wound healing assay results illustrated that FoxM1 downregulation inhibited cell migration in bladder cancer cells (Figure 3C).
Figure 3.
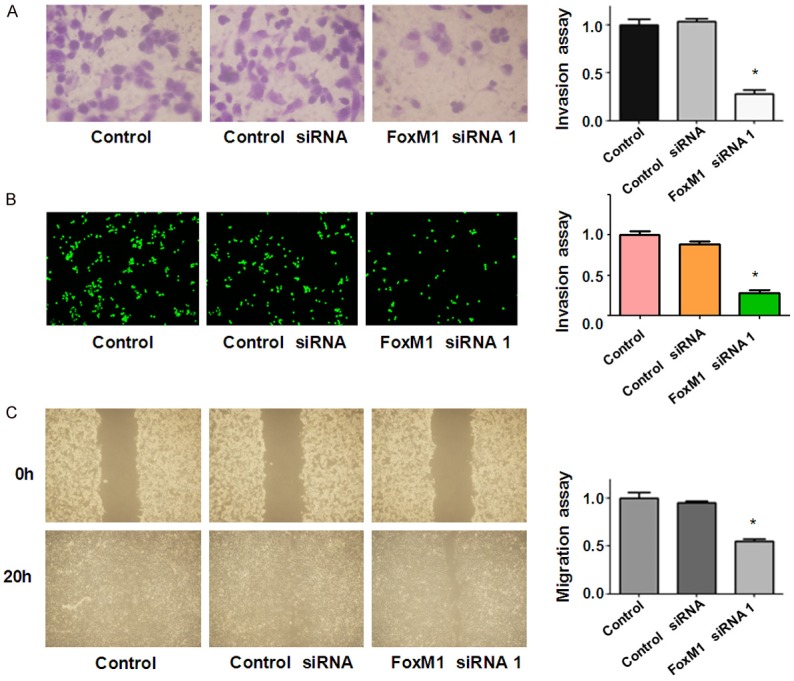
Down-regulation of FoxM1 inhibited motility span>activity in bladder cancer cells. (A, B) Invasion assays were conducted to detect the invasive capacity in RT4 cells after FoxM1 siRNA transfection. The tranfected cells were seeded in DMEM without serum in the upper chamber. The bottom chamber was added with complete medium. After 20 h of incubation, the cells that had invaded through Matrigel matrix membrane were stained with Wright’s-Giemsa (A) or 4 μg/ml Calcein AM (B) in hanks buffered saline at 37°C for 1 h. The labeled invasive cells were photographed under a microscope. (C) Wound healing assays was performed to measure cell migration in RT4 after FoxM1 siRNA transfection. Cells were seeded in 6-well plates and grown to almost confluency. Then, monolayers of cells were scratched with 200 μL small yellow pipette tips and washed twice with PBS. The scratched area was photographed with a microscope at 0 h and 20 h, respectively.
Downregulation of FoxM1 decreased VEGF expression and increased p27 level
To further explore the mechanism of FoxM1-mediated tumor progression, we investigated whether FoxM1 siRNA treatment could regulate VEGF and p27 in bladder cancer cells. Our Western blotting data showed that p27 level was dramatically increased in the FoxM1 siRNA transfected cells (Figure 4). We also found that VEGF expression was remarkably decreased in cells after FoxM1 transfection in bladder cancer cells (Figure 4A and Supplementary Figure 1). Our findings indicated that downregulation of FoxM1 exerts its anti-tumor function partly via inhibition of VEGF and upregulation of p27 in bladder cancer cells.
Figure 4.
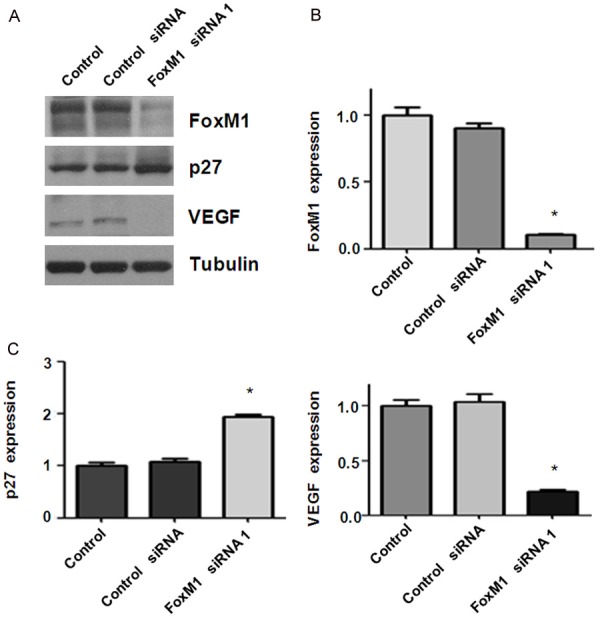
Down-regulation of FoxM1 increased p27 and decreased VEGF levels. A. Western blot analysis was performed to detect the expression of FoxM1, p27, and VEGF in RT4 bladder cancer cells after FoxM1 siRNA transfection for 48 h. B, C. Quantitative results for Western blotting analysis in panel A. *P < 0.01, vs control siRNA.
Over-expression of FoxM1 enhanced cell growth and inhibited apoptosis
To further validate the function of FoxM1 in bladder cancer cells, RT4 cells were transfected human FoxM1 vector or empty vector alone. We found that over-expression of FoxM1 by its cDNA transfection promoted bladder cancer cell growth (Figure 5A). Moreover, over-expression of FoxM1 inhibited cell apoptosis in bladder cancer cells (Figure 5B). Taken together, FoxM1 was critically involved cell growth in bladder cancer cells.
Figure 5.
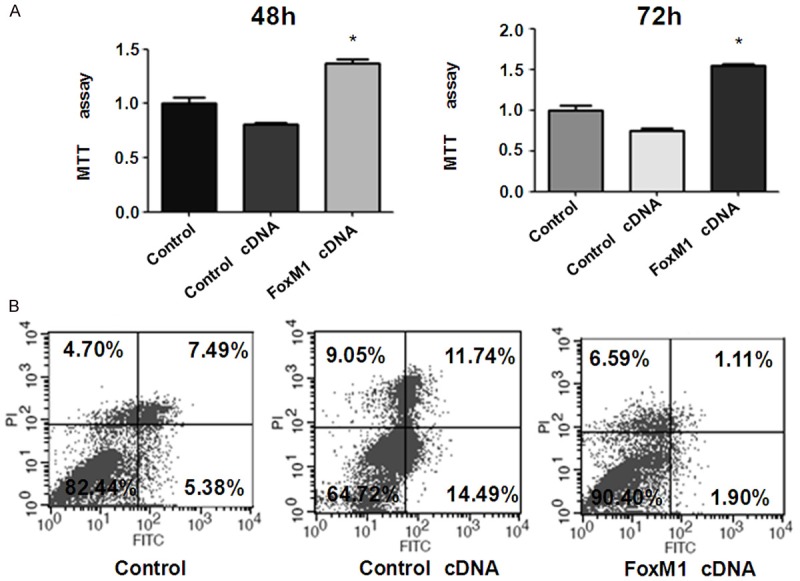
Overexpression of FoxM1 enhanced cell proliferation and inhibited apoptosis. A. MTT assay was used to measure cell proliferation in RT4 cells after FoxM1 cDNA transfection. The transfected cells (5 × 103) were seeded in a 96-well culture plate. After 48 h and 72 h, cells were then incubated with MTT reagent (0.5 mg/ml) for 2 h at 37°C. The absorbance was measured at 560 nm. All values were normalized to those of the controls. *P < 0.05 vs Control cDNA. B. Flow cytometry was used to detect cell apoptosis in bladder cancer cells transfected with FoxM1 cDNA. The transfected cells were cultured in 6-well plate for 48 h. Then, the cells were collected and resuspended in binding buffer with 5 μl propidium iodide and 5 μl FITC-conjugated anti-Annexin V antibody. Apoptosis was analyzed by a FACScalibur flow cytometer.
Over-expression of FoxM1 promoted cell migration and invasion
To further determine the role of FoxM1 in cell migration and invasion, we used Transwell chamber assay to measure the cell invasion in RT4 cells after FoxM1 cDNA transfection. We found that FoxM1 cDNA transfected cells showed significant promotion of cell invasion compared to empty vector-transfected control cells (Figure 6A and 6B). Wound healing assay was performed to measure the migratory activity in bladder cancer cells after overexpression of FoxM1. We found that FoxM1 cDNA transfected RT4 cells showed a remarkably increase in cell migration (Figure 6C). Altogether, over-expression of FoxM1 enhanced cell motility in bladder cancer cells. Mechanistically, overexpression of FoxM1 decreased p27 level and increased the expression of VEGF in bladder cancer cells (Figure 7 and Supplementary Figure 1).
Figure 6.
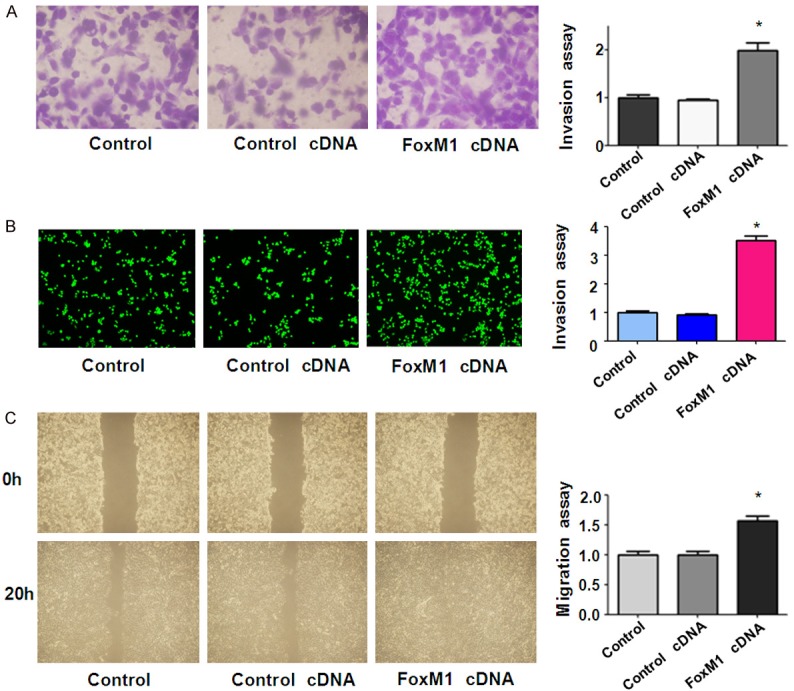
Overexpression of FoxM1 enhanced motility activity in bladder cancer cells. (A, B) Invasion assays were used to measure the cell invasion in RT4 cells after FoxM1 cDNA transfection. The tranfected cells were seeded in DMEM without serum in the upper chamber. The bottom chamber was added with complete medium. After 20 h of incubation, the invaded cells through Matrigel matrix membrane were stained with Wright’s-Giemsa (A) or 4 μg/ml Calcein AM (B) at 37°C for 1 h. The labeled invasive cells were photographed under a microscope. (C) Wound healing assays was used to detect the cell migration in RT4 after FoxM1 cDNA transfection. Cells were seeded in 6-well plates and grown to almost confluency. Then, monolayers of cells were scratched with 200 μL small yellow pipette tips and washed twice with PBS. The scratched area was photographed with a microscope at 0 h and 20 h, respectively.
Figure 7.
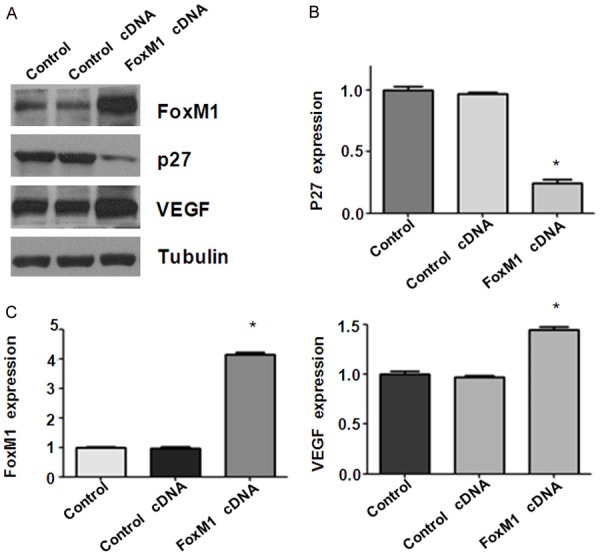
Overexpression of FoxM1 inhibited p27 and upregulated VEGF levels. (A) Western blot analysis was used to detect the expression of FoxM1, p27, and VEGF in bladder cancer cells after FoxM1cDNA transfection for 48 h. (B, C) Quantitative results for Western blotting in (A). *P < 0.01, vs control siRNA.
Discussion
FoxM1 plays a pivotal role in regulation of cell proliferation, differentiation, and apoptosis. Abnormal expression of FoxM1 has been reported in various human malignancies including bladder cancer [22-24]. For example, FoxM1 was found to be overexpressed in bladder tumor samples as compared with normal bladder tissues [22]. Similar study showed that overexpressed FoxM1 is observed in bladder cancer clinical specimens [24]. Another study also supported that FoxM1 expression was up-regulated in the majority of the bladder cancer tissue specimens at both mRNA and protein levels [23]. Moreover, FoxM1 expression was significantly correlated with TNM (tumor, node, and metastasis) stage and histological grade and metastasis, suggesting that FoxM1 up-regulation was associated with poor prognosis in bladder cancer [23]. Interestingly, recent study identified that FoxM1 was differentially expressed between muscle-invasive bladder cancer subtypes [25]. Although FoxM1 was associated the poor prognosis in bladder cancer, the mechanism of FoxM1-mediated tumor progression has not been fully elucidated. In the present study, we found that FoxM1 promoted cell growth and migration and invasion partly through downregulation of p27 and upregulation of VEGF in bladder cancer cells.
Emerging evidence has suggested that FoxM1 functions as an oncoproetin in multiple human cancers [26,27]. We determined the biological function of FoxM1 in bladder cancer cells via downregulation or overexpression of FoxM1. Consistent with one study [23], we found that down-regulation of FoxM1 inhibited cell proliferation, migration, and invasion in bladder cancer cells. Keeping with one report [24], down-regulation of FoxM1 induced cell apoptosis in bladder cancer. Mechanistically, depletion of FoxM1 inhibited cell growth and triggered apoptosis partly via upregulation of p27 in bladder cancer cells. VEGF is an important molecule that involved in tumor cell invasion and metastasis [28,29]. It has been reported that FoxM1 regulated VEGF expression in various cell types [30-32]. In this study, we found that FoxM1 down-regulation led to a significant reduction of VEGF expression in bladder cancer cells. Consistently, over-expression of FoxM1 resulted in a remarkably increased in the expression of VEGF in bladder cancer cells. Our findings indicated that downregulation of FoxM1 could potentiate the anti-motility activity in part through inhibition of VEGF in bladder cancer cells.
Since FoxM1 plays an oncogenic role in human cancers, down-regulation of FoxM1 could be a useful for the treatment of cancer. Multiple natural compounds have been discovered to inhibit the expression of FoxM1. For instance, plumbagin induced growth inhibition of human glioma cells via downregulation of the expression and activity of FoxM1 [33]. Genistein inhibited cell growth accompanied by induction of apoptosis through attenuation of FoxM1 in pancreatic cancer cells [34]. Diarylheptanoids suppressed cell growth via modulation of FoxM1 in pancreatic cancer cells [35]. Sorafenib inhibited cell proliferation and invasion via suppressing FoxM1 in human hepatocellular carcinoma cells [36]. Therefore, inhibition of FoxM1 by these natural agents could be effective therapeutic approach for human cancer treatment.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Antoni S, Ferlay J, Soerjomataram I, Znaor A, Jemal A, Bray F. Bladder cancer incidence and mortality: a global overview and recent trends. Eur Urol. 2017;71:96–108. doi: 10.1016/j.eururo.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 3.Kamat AM, Bagcioglu M, Huri E. What is new in non-muscle-invasive bladder cancer in 2016? Turk J Urol. 2017;43:9–13. doi: 10.5152/tud.2017.60376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cookson MS, Herr HW, Zhang ZF, Soloway S, Sogani PC, Fair WR. The treated natural history of high risk superficial bladder cancer: 15-year outcome. J Urol. 1997;158:62–67. doi: 10.1097/00005392-199707000-00017. [DOI] [PubMed] [Google Scholar]
- 5.Li F, Hong X, Hou L, Lin F, Chen P, Pang S, Du Y, Huang H, Tan W. A greater number of dissected lymph nodes is associated with more favorable outcomes in bladder cancer treated by radical cystectomy: a meta-analysis. Oncotarget. 2016;7:61284–61294. doi: 10.18632/oncotarget.11343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kang W, Cui Z, Chen Q, Zhang D, Zhang H, Jin X. Narrow band imaging-assisted transurethral resection reduces the recurrence risk of non-muscle invasive bladder cancer: a systematic review and meta-analysis. Oncotarget. 2017;8:23880–23890. doi: 10.18632/oncotarget.13054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gartel AL. FOXM1 in cancer: interactions and vulnerabilities. Cancer Res. 2017;77:3135–3139. doi: 10.1158/0008-5472.CAN-16-3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bella L, Zona S, Nestal de Moraes G, Lam EW. FOXM1: a key oncofoetal transcription factor in health and disease. Semin Cancer Biol. 2014;29:32–39. doi: 10.1016/j.semcancer.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 9.Laoukili J, Stahl M, Medema RH. FoxM1: at the crossroads of ageing and cancer. Biochim Biophys Acta. 2007;1775:92–102. doi: 10.1016/j.bbcan.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 10.Wierstra I, Alves J. FOXM1, a typical proliferation-associated transcription factor. Biol Chem. 2007;388:1257–1274. doi: 10.1515/BC.2007.159. [DOI] [PubMed] [Google Scholar]
- 11.Huang C, Du J, Xie K. FOXM1 and its oncogenic signaling in pancreatic cancer pathogenesis. Biochim Biophys Acta. 2014;1845:104–116. doi: 10.1016/j.bbcan.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li L, Wu D, Yu Q, Wu P. Prognostic value of FOXM1 in solid tumors: a systematic review and meta-analysis. Oncotarget. 2017;8:32298–32308. doi: 10.18632/oncotarget.15764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quan M, Wang P, Cui J, Gao Y, Xie K. The roles of FOXM1 in pancreatic stem cells and carcinogenesis. Mol Cancer. 2013;12:159. doi: 10.1186/1476-4598-12-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wierstra I. FOXM1 (Forkhead box M1) in tumorigenesis: overexpression in human cancer, implication in tumorigenesis, oncogenic functions, tumor-suppressive properties, and target of anticancer therapy. Adv Cancer Res. 2013;119:191–419. doi: 10.1016/B978-0-12-407190-2.00016-2. [DOI] [PubMed] [Google Scholar]
- 15.Shi M, Cui J, Xie K. Signaling of miRNAs-FOXM1 in cancer and potential targeted therapy. Curr Drug Targets. 2013;14:1192–1202. doi: 10.2174/13894501113149990192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wierstra I. The transcription factor FOXM1 (Forkhead box M1): proliferation-specific expression, transcription factor function, target genes, mouse models, and normal biological roles. Adv Cancer Res. 2013;118:97–398. doi: 10.1016/B978-0-12-407173-5.00004-2. [DOI] [PubMed] [Google Scholar]
- 17.Halasi M, Gartel AL. Targeting FOXM1 in cancer. Biochem Pharmacol. 2013;85:644–652. doi: 10.1016/j.bcp.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 18.Su J, Zhou X, Yin X, Wang L, Zhao Z, Hou Y, Zheng N, Xia J, Wang Z. The effects of curcumin on proliferation, apoptosis, invasion, and NEDD4 expression in pancreatic cancer. Biochem Pharmacol. 2017;140:28–40. doi: 10.1016/j.bcp.2017.05.014. [DOI] [PubMed] [Google Scholar]
- 19.Ma J, Zeng F, Ma C, Pang H, Fang B, Lian C, Yin B, Zhang X, Wang Z, Xia J. Synergistic reversal effect of epithelial-to-mesenchymal transition by miR-223 inhibitor and genistein in gemcitabine-resistant pancreatic cancer cells. Am J Cancer Res. 2016;6:1384–1395. [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou X, Su J, Feng S, Wang L, Yin X, Yan J, Wang Z. Antitumor activity of curcumin is involved in down-regulation of YAP/TAZ expression in pancreatic cancer cells. Oncotarget. 2016;7:79076–79088. doi: 10.18632/oncotarget.12596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Su J, Zhou X, Wang L, Yin X, Wang Z. Curcumin inhibits cell growth and invasion and induces apoptosis through down-regulation of Skp2 in pancreatic cancer cells. Am J Cancer Res. 2016;6:1949–1962. [PMC free article] [PubMed] [Google Scholar]
- 22.Pignot G, Vieillefond A, Vacher S, Zerbib M, Debre B, Lidereau R, Amsellem-Ouazana D, Bieche I. Hedgehog pathway activation in human transitional cell carcinoma of the bladder. Br J Cancer. 2012;106:1177–1186. doi: 10.1038/bjc.2012.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu D, Zhang Z, Kong CZ. High FOXM1 expression was associated with bladder carcinogenesis. Tumour Biol. 2013;34:1131–1138. doi: 10.1007/s13277-013-0654-x. [DOI] [PubMed] [Google Scholar]
- 24.Inoguchi S, Seki N, Chiyomaru T, Ishihara T, Matsushita R, Mataki H, Itesako T, Tatarano S, Yoshino H, Goto Y, Nishikawa R, Nakagawa M, Enokida H. Tumour-suppressive microRNA-24-1 inhibits cancer cell proliferation through targeting FOXM1 in bladder cancer. FEBS Lett. 2014;588:3170–3179. doi: 10.1016/j.febslet.2014.06.058. [DOI] [PubMed] [Google Scholar]
- 25.Rinaldetti S, Wirtz RM, Worst TS, Eckstein M, Weiss CA, Breyer J, Otto W, Bolenz C, Hartmann A, Erben P. FOXM1 predicts overall and disease specific survival in muscle-invasive urothelial carcinoma and presents a differential expression between bladder cancer subtypes. Oncotarget. 2017;8:47595–47606. doi: 10.18632/oncotarget.17394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koo CY, Muir KW, Lam EW. FOXM1: from cancer initiation to progression and treatment. Biochim Biophys Acta. 2012;1819:28–37. doi: 10.1016/j.bbagrm.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 27.Raychaudhuri P, Park HJ. FoxM1: a master regulator of tumor metastasis. Cancer Res. 2011;71:4329–4333. doi: 10.1158/0008-5472.CAN-11-0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simons M, Gordon E, Claesson-Welsh L. Mechanisms and regulation of endothelial VEGF receptor signalling. Nature reviews. Nat Rev Mol Cell Biol. 2016;17:611–625. doi: 10.1038/nrm.2016.87. [DOI] [PubMed] [Google Scholar]
- 29.Mazzola CR, Chin J. Targeting the VEGF pathway in metastatic bladder cancer. Expert Opin Investig Drugs. 2015;24:913–927. doi: 10.1517/13543784.2015.1041588. [DOI] [PubMed] [Google Scholar]
- 30.Chen H, Zou Y, Yang H, Wang J, Pan H. Downregulation of FoxM1 inhibits proliferation, invasion and angiogenesis of HeLa cells in vitro and in vivo. Int J Oncol. 2014;45:2355–2364. doi: 10.3892/ijo.2014.2645. [DOI] [PubMed] [Google Scholar]
- 31.Jiang L, Wang P, Chen L, Chen H. Down-regulation of FoxM1 by thiostrepton or small interfering RNA inhibits proliferation, transformation ability and angiogenesis, and induces apoptosis of nasopharyngeal carcinoma cells. Int J Clin Exp Pathol. 2014;7:5450–5460. [PMC free article] [PubMed] [Google Scholar]
- 32.Wen N, Wang Y, Wen L, Zhao SH, Ai ZH, Wu B, Lu HX, Yang H, Liu WC, Li Y. Overexpression of FOXM1 predicts poor prognosis and promotes cancer cell proliferation, migration and invasion in epithelial ovarian cancer. J Transl Med. 2014;12:134. doi: 10.1186/1479-5876-12-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu X, Cai W, Niu M, Chong Y, Liu H, Hu W, Wang D, Gao S, Shi Q, Hu J, Zhou X, Yu R. Plumbagin induces growth inhibition of human glioma cells by downregulating the expression and activity of FOXM1. J Neurooncol. 2015;121:469–477. doi: 10.1007/s11060-014-1664-2. [DOI] [PubMed] [Google Scholar]
- 34.Wang Z, Ahmad A, Banerjee S, Azmi A, Kong D, Li Y, Sarkar FH. FoxM1 is a novel target of a natural agent in pancreatic cancer. Pharm Res. 2010;27:1159–1168. doi: 10.1007/s11095-010-0106-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dong GZ, Jeong JH, Lee YI, Lee SY, Zhao HY, Jeon R, Lee HJ, Ryu JH. Diarylheptanoids suppress proliferation of pancreatic cancer PANC-1 cells through modulating shh-Gli-FoxM1 pathway. Arch Pharm Res. 2017;40:509–517. doi: 10.1007/s12272-017-0905-2. [DOI] [PubMed] [Google Scholar]
- 36.Wei JC, Meng FD, Qu K, Wang ZX, Wu QF, Zhang LQ, Pang Q, Liu C. Sorafenib inhibits proliferation and invasion of human hepatocellular carcinoma cells via up-regulation of p53 and suppressing FoxM1. Acta Pharmacol Sin. 2015;36:241–251. doi: 10.1038/aps.2014.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


