Abstract
BACKGROUND
Rotator cuff tears affect millions of individuals each year, often requiring surgical intervention. However, repair failure remains common. We have previously shown that pulsed electromagnetic field (PEMF) therapy improved tendon-to-bone healing in a rat rotator cuff model. The purpose of this study was to determine the influence of both PEMF frequency and exposure time on rotator cuff healing.
METHODS
210 Sprague Dawley rats underwent acute bilateral supraspinatus injury and repair followed by either Physio-Stim® PEMF or High Frequency PEMF for 1, 3, or 6 hours daily. Control animals did not receive PEMF therapy. Mechanical and histological properties were assessed at 4, 8, and 16 weeks.
RESULTS
Improvements in different mechanical properties at various endpoints were identified for all treatment modalities when compared to non-treated animals, regardless of PEMF frequency or duration. Of note, one hour of Physio-Stim showed significant improvements in tendon mechanical properties across all time points, including increases in both modulus and stiffness as early as 4 weeks. Collagen organization improved for several of the treatment groups compared to controls. Additionally, improvements in collagen I and fibronectin expression were identified with PEMF treatment. Importantly, no adverse effects were identified in any mechanical or histological property.
CONCLUSIONS
Overall, results suggest that PEMF has a positive effect on rat rotator cuff healing for each electromagnetic fundamental pulse frequency and treatment duration tested in this study.
Keywords: PEMF, supraspinatus repair, rat, animal model, rotator cuff, tendon healing
LEVEL OF EVIDENCE: Basic Science Study, In Vivo Animal Model
Introduction
Rotator cuff tears affect millions of individuals each year, often requiring surgical intervention. Although recent advancements in surgical methods and rehabilitation protocols have improved clinical results, rotator cuff repair failure is still common.6 Contributing to repair failure is the nature of injured tissue, which tends to be disorganized and fibrotic. This scar tissue is unable to successfully recreate native tendon-to-bone properties after repair. In order to enhance native tissue healing and improve surgical outcomes, various non-invasive therapeutics have been utilized post-operatively, including ultrasound and shock wave therapy.16,21 Advantages of non-invasive therapeutic devices include their ease of use, relatively low cost, and ability to be brought into the patient’s home instead of requiring frequent visits to the clinic. Additionally, their use as a post-operative adjuvant therapy does not interfere with surgical techniques or standard rehabilitation protocols.
Pulsed electromagnetic field (PEMF) therapy has been approved by the FDA for treatment of fracture non-unions and for the enhancement of bone formation after lumbar and cervical spine fusion surgery. Because PEMF has been shown to decrease markers of inflammation, it is also of interest as a potential therapeutic in soft tissue healing environments. PEMF has been utilized in clinical studies to treat osteoarthritis, epicondylitis, and rotator cuff tears.9,18,19,26 At three months post-op, patients with small or medium rotator cuff tears showed improved range of motion after receiving PEMF therapy when compared to those receiving placebo. In order to investigate the structural and functional effects of PEMF on tendon-to-bone healing, we have previously utilized PEMF in a rat rotator cuff injury and transosseous repair model. Results demonstrated that PEMF therapy improved healing. These improvements included increased bone volume fraction, trabecular thickness, and bone mineral density at the repair site, similar to bone changes seen in other bone healing PEMF applications.2,10 Additionally, PEMF treated groups also showed improvements in tendon tissue properties including a 100% increase in tendon modulus after 4 weeks, and improved collagen alignment at later time points.24
However, PEMF signal characteristics typically vary between studies and applications in terms of waveform type, signal intensity, and treatment duration. Several studies have identified differences in cell and tissue response to PEMF of varying parameters, including frequency and treatment duration. For example, the formation of osteoclast-like cells in a culture of bone marrow cells, as well as concentrations of secreted inflammatory cytokines, can be either enhanced or suppressed by manipulating induced electric field intensity.3 Similarly, osteogenic differentiation of human mesenchymal stem cells differed following exposure to a variety of PEMF frequencies.17 Tendon cells isolated from human semitendinosus and gracilis tendons displayed altered regulation of gene expression dependent on field intensity, duration, and number of exposures.4 In a rat model of Achilles tendon injury and repair, alterations in PEMF frequency and signal amplitude affected tendon strength after repair.22 However, the effect of frequency and treatment duration has not yet been evaluated in our rat rotator cuff injury and repair animal model.
Therefore, the objective of this study was to determine the influence of both PEMF frequency and exposure time on rotator cuff healing. In this study, we utilized Physio-Stim® PEMF (PS, Orthofix, Inc., Lewisville, TX) as well as high frequency Physio-Stim PEMF (HF), which is similar in all aspects to Physio-Stim, with the exception of a higher fundamental frequency. Both treatments were tested using 1 hour, 3 hour, and 6 hour daily durations. We hypothesized that a PEMF signal with a higher fundamental frequency and longer daily treatment duration would 1) lead to further improvements in mechanical properties and 2) improve tissue morphology including cell shape, cellularity, and collagen fiber organization.
Materials and Methods
Methods used in this study, including animal care, surgical techniques, tendon mechanics, and tendon histology, are identical to previously published work.24
Study design
210 (including 60 from our previous study24) adult male Sprague-Dawley rats (400–450 g) were used in this University of Pennsylvania Institutional Animal Care and Use Committee approved study. Animals were housed in a conventional facility in 12-hour light/dark cycles and were fed standard rat chow ad libitum. Animals underwent bilateral acute supraspinatus injury and repair1 followed by randomization into treatment groups, receiving either Physio-Stim® PEMF (PS, Orthofix, Inc., Lewisville, TX) or High Frequency PEMF (HF, similar to PS but with a higher fundamental frequency) for 1, 3, or 6 hours daily. Control animals did not receive PEMF therapy (non-PEMF). The control and 3 hour PS groups used in this study were taken from our previously published work.
Animals were sacrificed at 4, 8, or 16 weeks (n=10 per group per time point). At the time of sacrifice, right shoulders (n=7 per group per time point) were immediately dissected, fixed in formalin, and processed for histological analysis. Left shoulders (n=10 per group per time point) were left intact and animals were frozen at −20°C and thawed for dissection at the time of mechanical testing.
Detachment and Repair Surgery
Animals were subjected to bilateral supraspinatus detachment and repair as described. For analgesia, buprenorphine (0.05 mg/kg) was administered subcutaneously 30 minutes prior to surgery, 6–8 hours post-operatively, and then every 8–12 hours for the next 48 hours. Briefly, with the arm held in external rotation and adduction and the supraspinatus tendon was exposed. The tendon was grasped with a simple grasping stitch using 5–0 polypropylene suture (Surgipro II, Covidien, Mansfield, MA) and was sharply transected from its bony insertion. For repair, a 5 mm diameter high speed bur (Multipro 395, Dremel, Mt. Prospect, IL) was used to remove the remaining fibrocartilage from the footprint of the tendon insertion site. A 0.5 mm bone tunnel was drilled from anterior to posterior through the greater tuberosity. Suture was then passed through the bone tunnel and tied, securing the supraspinatus to the footprint. The wound was flushed with saline, and the deltoid and skin sutured closed with 4-0 Vicryl (Ethicon, Bridgewater, NJ).
PEMF Exposure
Excluding non-PEMF controls, animals received daily PEMF exposure until the time of sacrifice using a commercial PEMF signal (PS, Physio-Stim®, Orthofix, Inc., Lewisville, TX) or a high-frequency PEMF signal (HF). The maximum amplitude and fundamental frequency of the Physio-Stim® PEMF signal are 1.19 mT and 3.85 kHz, respectively. HF PEMF maintains similar parameters to PS, but with a fundamental frequency of 40.85 kHz. Physio-Stim® was chosen partly due to it being FDA approved for treatment of long-bone fracture non-unions.7,25 As detailed in a previous study24, 24 hours after surgery, animals were placed on custom built PEMF racks (Orthofix, Inc., Lewisville, TX) with an electromagnetic coil for each module, sized to hold a standard rat cage. Tendon mechanical testing: Supraspinatus and humerus tendon-bone units were dissected from the shoulder and cleaned of muscle and other connective tissue under a stereomicroscope. Stain lines were placed along the length of the tendon using Verhoeff’s stain for optical strain measurements. Cross sectional area was measured using a custom laser device.5 Samples were immersed in a 37°C phosphate buffered saline (PBS) bath and subjected to a mechanical testing protocol consisting of a preload to 0.08 N, ten cycles of preconditioning (0.1–0.5N at 1% strain/s), stress relaxation to 5% strain (at 5%/s) followed by a 600s hold, and finally a ramp to failure at 0.3%/s. Maximum stress and maximum load were calculated only from samples that experienced physiological failure. This included failure at the site of injury or in the midsubstance of the tendon, but excluded samples that failed at the grip fixture. Exclusion varied by group, but averaged around 1–2 for 4 week groups, and 3–4 for 8 and 16 week groups.
Tendon Histology
Histological analysis was performed to assess cell shape, cellularity, and collagen fiber organization at the injury site and midsubstance of the repaired supraspinatus tendon. The injury site was defined as the central portion of the fibrous scar tissue adjacent to the bony insertion, and the midsubstance as the region proximal to the myotendinous junction. Supraspinatus-humerus units were processed using standard paraffin techniques. 7 μm sections were stained with hematoxylin and eosin (H&E). Regions of interest were imaged at magnification x200. Cell shape and cellularity were evaluated by three blinded graders using a semi-quantitative method. A custom grading scale was created as previously described.24 Briefly, images were blinded and arranged in order for each histological property (for example, for cell density, images were arranged from least to most cellular). A representative image was selected for each tertile to create grades of 1, 2, and 3 for cell shape, and from each quartile to create grades of 1, 2, 3, and 4 for cellularity. These representative images are pre-selected for grade determination instead of using descriptive terminology to select grades. Images were then randomly organized and blinded for grading. Each grader compared each histological image to the representative images and selected the appropriate grade. This analytical method has been validated and is commonly utilized in our lab.23, 24 Circular standard deviation of collagen fibers was determined by images taken with a polarizing microscope and analyzed with custom software as described previously.8,23
Tendon Immunohistochemistry
Immunohistochemistry analysis was performed by incubating sections with specific antibodies for COLI, COLIII, FN, DCN, IL1-β, TNF-α, and MMP13 and developed using a standard avidin biotin complex (ABC)/3,3′-diaminobenzidine (DAB) method. After deparaffinization and rehydration, sections underwent digestion, were blocked with 3% hydrogen peroxide to inhibit endogenous peroxidase, washed in phosphate buffered saline, blocked to prevent non-specific binding, and incubated with primary antibody. Detection of the antibody was accomplished using either the Dako EnVision+ System HRP kit (used for FN staining only, Agilent Technologies, Santa Clara, CA) or HRP-tagged secondary antibody followed by Vectastain Elite ABC HRP kit (universal, for all other targets, Vector Laboratories, Burlingame, CA). Details regarding each target are listed in Table 1. Slides were incubated in DAB for 3 to 5 minutes. A total of 5 histologic sections per antibody per time point per group were evaluated. The injury region was imaged at magnification ×200 and qualitatively assessed in a blinded fashion. No statistics were planned or performed. The methods used were qualitative and were used to descriptively evaluate local changes.
Table 1.
List of antibodies
| Protein Target | Digestion | Primary Antibody | Secondary Antibody |
|---|---|---|---|
| Collagen Type 1 | Hyaluronidase (0.5 mg/ml) Acetic Acid (0.5N) |
EMD Millipore1 AB755P, 1:200 | goat anti-rabbit, BD Biosciences6 550338, 1:200 |
| Collagen Type III | Protease K (0.4 mg/ml) Hyaluronidase (0.5 mg/ml) Acetic Acid (0.5 N) |
Sigma-Aldrich2 C7805, 1:500 | rat anti-mouse, BD Biosciences 550331, 1:100 |
| Decorin | Chondroitinase-ABC(0.2U/ml) Acetic Acid (0.5 N) |
Kerafast3 LF-114, 1:300 | goat anti-rabbit, BD Biosciences 550338, 1:200 |
| Fibronectin | Hyaluronidase (0.5 mg/ml) | EMD Millipore AB2040, 1:200 | EnVision+HRP anti-rabbit, Agilent Tech.7, undiluted |
| IL-1β | Pepsin (0.5%) | EMD Millipore AB1832, 1:400 | goat anti-rabbit, JacksonImmuno8 111-035-003, 1:100 |
| TNFα | Pepsin (0.5%) | Novus Biologicals4 19532, 1:750 | goat anti-rabbit, JacksonImmuno 111-035-003, 1:100 |
| MMP13 | Hyaluronidase (0.5 mg/ml) | Santa Cruz Biotech.5, 30073, 1:200 | goat anti-rabbit, BD Biosciences 500338, 1:200 |
IL-1β, interleukin-1-beta; TNFα, tumor necrosis factor alpha; MMP13, matrix metalloproteinase 13
Billerica, MA;
St. Louis, MO;
Boston, MA;
Littleton, CO;
Dallas, TX;
San Jose, CA;
Santa Clara, CA;
West Grove, PA
Statistical Analysis
Sample sizes were determined using a priori power analyses using data from a previous study.8 Statistical comparisons were made between control (non-PEMF) group and each treatment group at each time point. Mechanical testing and collagen fiber organization comparisons were made using one-way analysis of variance tests (ANOVAs) at each time point with post-hoc tests correcting for multiple comparisons. Non-parametric histological comparisons (cellularity and cell shape) were made using Mann-Whitney U tests. Significance was set at p<0.05 (p<0.0083 for follow up t-tests).
Funding provided by Orthofix, Inc.
Results
Mechanical properties
Improvements in different mechanical properties for various end points were identified for all treatment modalities when compared to non-treated animals, regardless of PEMF frequency or duration (Fig 1). Specifically, one hour of PS treatment led to increased tendon stiffness at all time points (Fig 1C, p=0.008 at 4 weeks, p=0.04 at 8 weeks, p=0.03 at 16 weeks), as well as an increase in modulus at 4 weeks (Fig 1E, p=0.008). Both stiffness and modulus increased by over 100% at 4 weeks for this treatment group. One hour of HF PEMF treatment increased stiffness (Fig 1C, p<0.001) and modulus (Fig 1E, p=0.05) at 8 weeks. Animals treated with three hours of PS had a decreased cross sectional area (Fig 1A, p=0.05), increased max stress (Fig 1B, p=0.04), and increased stiffness (Fig 1C, p=0.001) at 4 weeks, as well as increased modulus at 4 (Fig 1E, p=0.01) and 8 weeks (p=0.05). Three hours of HF PEMF led to increased max load at 4 weeks (Fig 1D, p=0.03), and increased stiffness at 4 (Fig 1C, p=0.001) and 8 weeks (p<0.001). Treatment with six hours of PS increased modulus substantially at 4 weeks (Fig 1E, p=0.01), and also increased stiffness (Fig 1C, p=0.01) and modulus (Fig 1E, p=0.05) at 8 weeks. Cross-sectional area was also reduced at 4 weeks (Fig 1A, p=0.05), but increased at 16 weeks (p=0.006). The final treatment regimen, 6 hours of HF PEMF, also resulted in significant mechanical improvements, including increased stiffness at all time points (Fig 1C, p=0.004 at 4 weeks, p=0.01 at 8 weeks, p=0.03 at 16 weeks) and a three-fold increase in tendon modulus 8 weeks after repair (Fig 1E, p<0.001). Tendon cross sectional area was increased at 16 weeks (Fig 1A, p=0.04). No differences were noted in percent relaxation in any group (data not shown). Importantly, no adverse effects were seen in any mechanical property.
Figure 1.
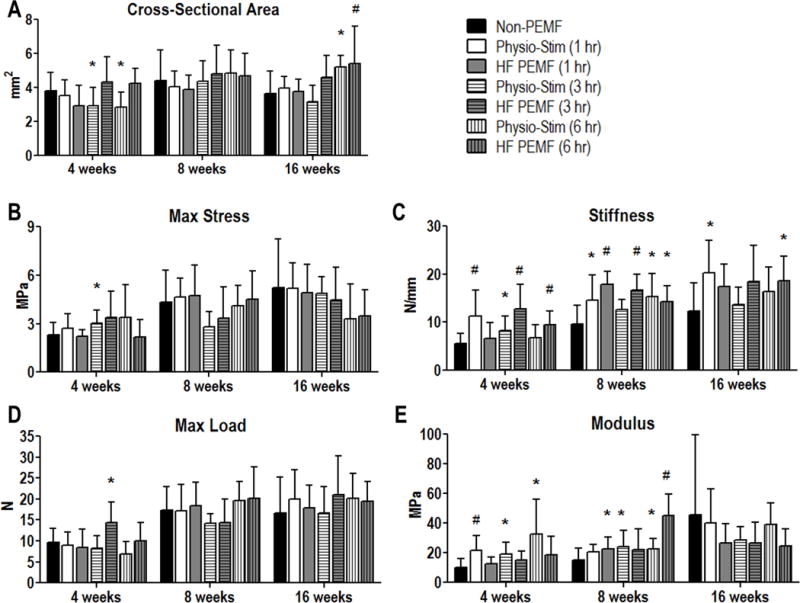
Tendon mechanical properties. A: Cross-sectional area was significantly reduced at 4 weeks after treatment with either 3 or 6 hours of Physio-Stim. However, at 16 weeks, this was reversed for the Physio-Stim (6 hr) group. HF PEMF (6 hr) trended toward increased area at this time point as well. B: Max stress was significantly greater in the Physio-Stim (3 hr) group at 4 weeks when compared to the non-PEMF group. C: Tendon stiffness was increased in all treatment groups for at least one evaluated time point. D: Maximum failure load was significantly greater in the Physio-Stim (3 hr) group at 4 weeks when compared to the non-PEMF group. E: Tendon modulus was improved for each Physio-Stim treatment duration at 4 weeks. At 8 weeks, it was increased for HF PEMF (1hr) and (6 hr) as well as for Physio-Stim (3 hr) and (6 hr). Data represented as mean+SD. * denotes p<0.05. # denotes p<0.0083 which corrects for multiple comparisons.
Tendon histology
No differences were measured in tendon cell shape or cell density (cellularity) in any treatment group when compared to non-PEMF controls (Fig 2). Overall cell density decreased in both the insertion (Fig 2A, p<0.001) and midsubstance (Fig 2B, p<0.001) with time, but this was not specific to any treatment group. Collagen organization showed improvements at the tendon insertion at 16 weeks in animals treated with 3 hours of HF PEMF (Fig 3A, p=0.005). This group also showed improved alignment at 8 weeks in the tendon midsubstance (Fig 3B, p=0.005). Additional improvements were identified at 16 weeks for 1 hour HF PEMF (p=0.006) and 6 hours of PS (p=0.03) in the midsubstance (Fig 3B). No adverse effects were identified in any histological property.
Figure 2.
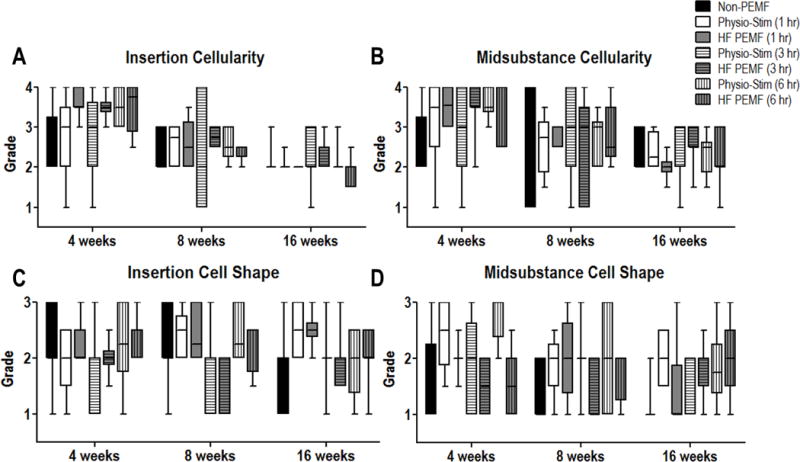
Histological analysis. No significant differences were noted between non-PEMF and treatment groups at any time point for any of the tested parameters.
Figure 3.
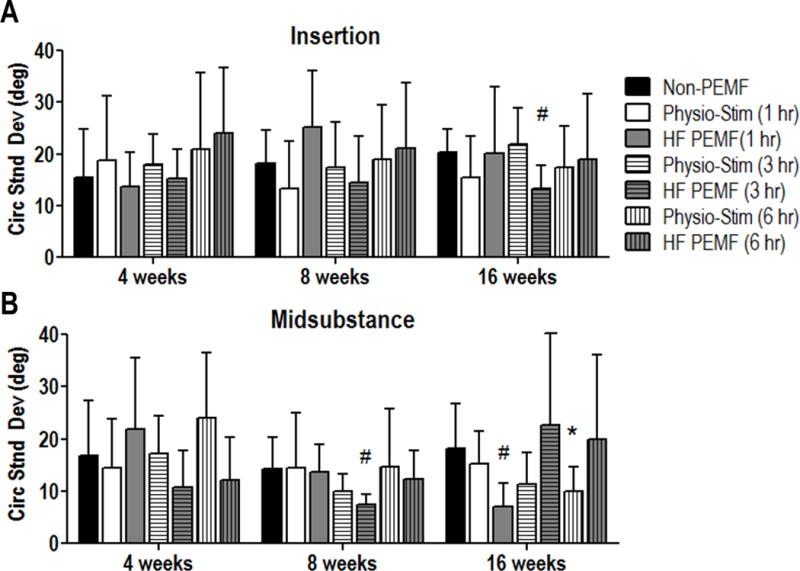
Collagen fiber alignment. A: By 16 weeks, collagen alignment was increased at the site of injury (insertion), as depicted by a decreased circular standard deviation, for tendons treated with HF PEMF (3 hr). B: HF PEMF (3 hr) also demonstrated improved collagen alignment in the tendon midsubstance at 8 weeks. HF PEMF (1 hr) and Physio-Stim (6 hr) also improved collagen alignment in this region at 16 weeks. No adverse effects were seen. Data represented as mean+SD. * denotes p<0.05. # denotes p<0.0083 which corrects for multiple comparisons.
Tendon immunohistochemistry
Based on significant improvements in early healing demonstrated by mechanical testing, immunohistochemical analysis was performed on non-PEMF controls and the 1 hour PS group. Our results were consistent across specimens, providing confidence in our results and conclusions. Tendon protein composition was altered in the PEMF treated groups (Figure 4). Collagen I was notably increased at the injury site of the supraspinatus at 4 weeks after PEMF treatment compared to controls (Figure 4, upper panel), but these differences were no longer seen after 8 weeks post-operatively. Similarly, fibronectin expression was increased and was more diffuse at the injury site at 4 weeks after PEMF treatment compared to controls (Figure 4, lower panel), but again, these differences were no longer apparent at 8 weeks. No differences were seen in Collagen III, IL1β, TNFα, or decorin (Supplementary Figure 1). MMP13 staining failed to develop above the minimum level of detection (data not shown).
Figure 4.
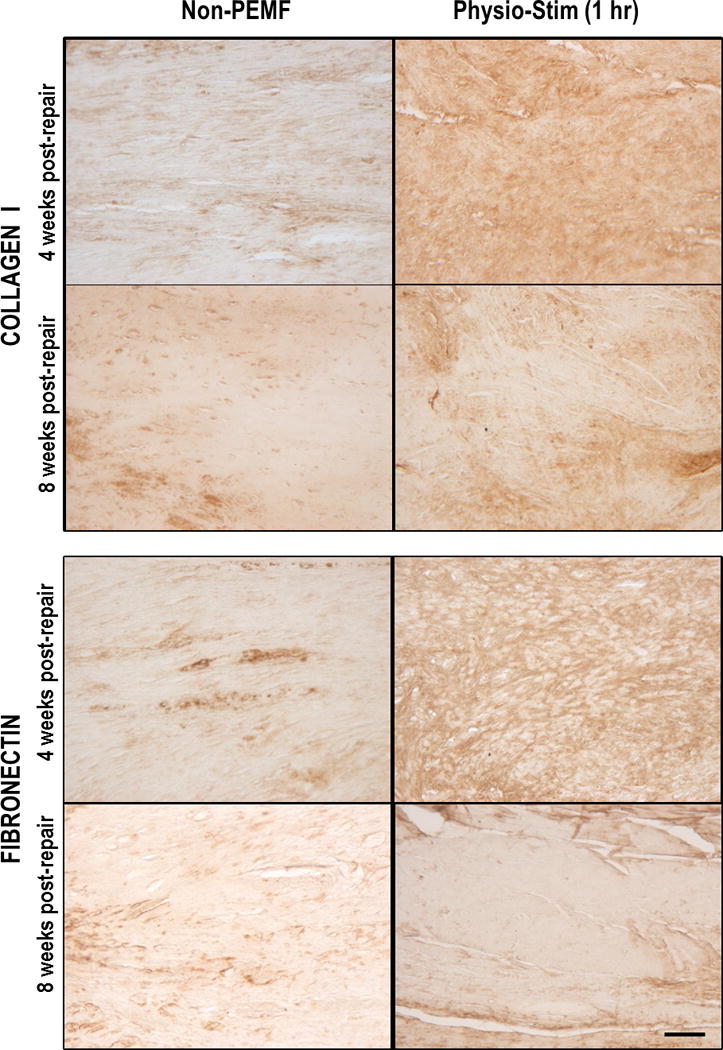
Immunohistochemical analysis. Representative images for Collagen I staining show increased, diffuse staining for ColI protein for Physio-Stim (1hr) after 4 weeks of treatment (top panel). Protein expression was similar to non-PEMF group by 8 weeks. Representative images for fibronectin staining also show increased, diffuse staining for this ECM protein after 4 weeks of treatment (bottom panel), but expression levels were similar across groups by 8 weeks. Scale bar: 250 μm.
Discussion
Previous work demonstrated that Physio-Stim PEMF treatment improved tendon and bone properties when applied after supraspinatus injury and repair. However, the effects of pulse frequency and daily treatment duration were unknown. This study examined the effect of systemic exposure of an FDA approved, commercially available PEMF waveform, as well as a nearly identical waveform with a higher fundamental frequency. The primary focus was to identify differences in healing when both frequency and exposure time were varied. Overall, results suggest that PEMF has a positive effect on rat rotator cuff healing for each electromagnetic fundamental pulse frequency and treatment duration tested in this study. Contrary to our hypotheses, neither higher fundamental frequency nor longer duration lead to consistently better tendon mechanical or histological properties when compared to other PEMF treatment regimens investigated in this study. The improvements in tendon properties seen for both treatments (PS, HF) and all exposure durations (1, 3, and 6 hours) supports the potential for PEMF as a clinical adjunctive treatment to rotator cuff repair. Specifically, the earlier improvements in tendon modulus by the PS 1hr PEMF treatment makes it ideal as it utilizes an FDA approved signal and minimizes patient treatment time, which may increase the likelihood of patient compliance. Perhaps most importantly, this study also confirmed that there were no detrimental effects of PEMF across tested parameters. In addition, our previous study, which also quantified rat ambulation and passive joint range of motion, found no differences with PEMF treatment, suggesting that animal limb function was not impaired.24
Tendon mechanical properties, including tendon stiffness and modulus, were significantly improved with all six treatment modalities compared with control at some point in the healing process (either at 4, 8 or 16 weeks). Of note, one hour of Physio-Stim led to two-fold increases in stiffness and modulus four weeks post-operatively, indicating improvements in early healing processes. Tissue stiffness continued to be significantly greater with this treatment type and exposure time compared to controls throughout the study duration. Repair failure often occurs early post-operatively, and can be due to inadequate strength of the initial repair construct.12 Improved tissue properties at early time points could increase suture retention strength, in turn allowing superior tendon-to-bone healing and preventing retear. Although the tendon enthesis is a critical component of tendon function, our goal was to assess the tendon-bone construct as a whole during mechanical testing, as the samples are tested with the enthesis intact. Indeed, the majority of our samples failed at the insertion site 4 weeks after surgery, supporting the idea that tendon-to-bone healing is pertinent to prevent early retear. However, at 16 weeks, many samples failed at the midsubstance or at the tendon grip, suggesting that it is important to assess the full tendon to understand healing properties over time of this whole complex, including the enthesis.
Additionally, collagen fiber organization was improved for several treatments, suggesting a greater degree of organization in PEMF-treated tissues. Collagen alignment in the direction of mechanical load imparts a significant anisotropic characteristic to tendons, increasing their ability to transfer load from muscle to bone.13 This suggests that improvements in collagen alignment with PEMF may be due to a more intact enthesis to allow such loading. Scar tissue after injury is typically disorganized and fibrotic; therefore, improved matrix remodeling and collagen fiber organization may partially explain the improved mechanical strength seen in treated groups. These findings suggest PEMF treatment increases cell metabolism, matrix production, and collagen remodeling.
In order to further elucidate these improvements, we chose to evaluate protein expression for the control group and the group receiving one hour of Physio-Stim, as this group showed significant improvements in tendon mechanical properties across all time points, including important increases in both modulus and stiffness as early as 4 weeks. Additionally, a one hour treatment time per day would be ideal for our patient population. Based on qualitative assessment of these analyses, PEMF treatment (1hr PS) resulted in increased collagen I at the site of injury (insertion) four weeks after injury and repair. Collagen I is the primary component of tendon matrix, and the increase in protein expression during early healing supports our mechanical findings of improved stiffness and modulus.20 Additionally, at this time point, no differences were seen in collagen III. The ratio of collagen I to collagen III deposition is important in tendon healing, and a higher proportion of collagen I is typically seen during later healing stages.15 This suggests that healing mechanisms may be occurring more quickly with PEMF treatment. Another indicator of healing and tissue remodeling, fibronectin, was also increased at four weeks with PEMF treatment. This hydrophilic glycoprotein is produced during healing to form fibrous scar tissue. Fibronectin supports cell proliferation and migration at the injury site, in turn promoting additional metabolic activities and matrix production.14 Additionally, fibronectin plays an important role in the assembly of other matrix proteins, including collagen I.11 Therefore, increased fibronectin in PEMF treated animals could affect newly deposited collagen organization during healing.
This study is not without limitations. An acute model of supraspinatus injury with immediate repair does not recapitulate the typical chronic conditions seen clinically. Additionally, FDA approved PEMF treatments are applied locally with anatomically specific devices. In this study, PEMF was delivered systemically; future studies could assess the effects of localized PEMF exposure. Protein expression studies were qualitative in nature, limiting their interpretation.
Although we were unable to identify an optimal PEMF frequency or duration, improvements in healing properties across treatment regimens for tendon-to-bone healing suggests potential for this therapy in other tendon applications. Evaluating the effects of PEMF on soft tissue tendon healing in commonly injured tendons such as the Achilles may cause similar improvements as an augment to surgical repair. Additionally, this treatment could be applied for tendinopathy or partial tears, potentially avoiding costly surgical procedures and improving recovery time. The non-invasive nature of PEMF, along with a history of being safe and well-tolerated in a wide patient population, makes it attractive as an adjunctive treatment to current tendon repair practices, both surgical and non-surgical.
Conclusions
Based on the results of this study, we speculate that PEMF treatment may increase tendon cell metabolism, which in turn increases both collagen production and matrix remodeling. These proposed changes are supported by our findings of improved mechanical properties and increased collagen alignment. Our previous work indicates that PEMF treatment does not alter joint function24; in conjunction with these current findings, these animal studies promote the evaluation of PEMF to improve rotator cuff healing in the clinic.
Supplementary Material
Supplemental Figure S1 Representative images for Collagen III, IL1β, TNFα, and decorin for non-PEMF and PEMF groups at 4 and 8 weeks post-repair. No differences were seen between groups at either time point. Scale bar represents 250 μm.
References
- 1.Beason DP, Connizzo BK, Dourte LM, Mauck RL, Soslowsky LJ, Steinberg DR, et al. Fiber-aligned polymer scaffolds for rotator cuff repair in a rat model. J Shoulder Elbow Surg. 2012;21:245–50. doi: 10.1016/j.jse.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 2.Chang K, Chang WH. Pulsed electromagnetic fields prevent osteoporosis in an ovariectomized female rat model: a prostaglandin E2-associated process. Bioelectromagnetics. 2003;24:189–98. doi: 10.1002/bem.10078. [DOI] [PubMed] [Google Scholar]
- 3.Chang K, Chang WH, Wu ML, Shih C. Effects of different intensities of extremely low frequency pulsed electromagnetic fields on formation of osteoclast-like cells. Bioelectromagnetics. 2003;24:431–9. doi: 10.1002/bem.10118. [DOI] [PubMed] [Google Scholar]
- 4.de Girolamo L, Vigano M, Galliera E, Stanco D, Setti S, Marazzi MG, et al. In vitro functional response of human tendon cells to different dosages of low-frequency pulsed electromagnetic field. Knee Surg Sports Traumatol Arthrosc. 2015;23:3443–53. doi: 10.1007/s00167-014-3143-x. [DOI] [PubMed] [Google Scholar]
- 5.Favata M. PhD Thesis. University of Pennsylvania; 2006. Scarless healing in the fetus: implications and strategies for postnatal tendon repair. [Google Scholar]
- 6.Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am et al J Bone Joint Surg Am. 2004;86-A:219–24. doi: 10.2106/00004623-200402000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Garland DE, Moses B, Salyer W. Long-term follow-up of fracture nonunions treated with PEMFs. Contemp Orthop. 1991;22:295–302. [PubMed] [Google Scholar]
- 8.Gimbel JA, Van Kleunen JP, Mehta S, Perry SM, Williams GR, Soslowsky LJ. Supraspinatus tendon organizational and mechanical properties in a chronic rotator cuff tear animal model. J Biomech. 2004;37:739–749. doi: 10.1016/j.jbiomech.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 9.Iannitti T, Fistetto G, Esposito A, Rottigni V, Palmieri B. Pulsed electromagnetic field therapy for management of osteoarthritis-related pain, stiffness and physical function: clinical experience in the elderly. Clin Interv Aging. 2013;8:1289–1293. doi: 10.2147/CIA.S35926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jing D, Shen G, Huang J, Xie K, Cai J, Xu Q, Wu X, Luo E. Circadian rhythm affects the preventive role of pulsed electromagnetic fields on ovariectomy-induced osteoporosis in rats. Bone. 2010;46:487–95. doi: 10.1016/j.bone.2009.09.021. [DOI] [PubMed] [Google Scholar]
- 11.Kadler KE, Hill A, Canty-Laird EG. Collagen fibrillogenesis: fibronectin, integrins, and minor collagens as organizers and nucleators. Curr Opin Cell Biol. 2008;20:495–501. doi: 10.1016/j.ceb.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ladermann A, Denard PJ, Burkhart SS. Management of failed rotator cuff repair: a systematic review. J ISAKOS. 2016;1:32–37. doi: 10.1136/jisakos-2015-000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lake SP, Miller KS, Elliott DM, Soslowsky LJ. Effect of fiber distribution and realignment of the nonlinear and inhomegeneous mechanical properties of human supraspinatus tendon under longitudinal tensile loading. J Orthop Res. 2009;27:1596–602. doi: 10.1002/jor.20938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lenselink EA. Role of fibronectin in normal wound healing. Int Wound J. 2015;12:313–316. doi: 10.1111/iwj.12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu SH, Yang RS, al-Shaikh R, Lane JM. Collagen in tendon, ligament, and bone healing. A current review. Clin Orthop Relat Res. 1995;318:265–78. [PubMed] [Google Scholar]
- 16.Lovric V, Ledger M, Goldberg J, Harper W, Bertollo N, Pelletier MH, et al. The effects of low-intensity pulsed ultrasound on tendon-bone healing in a transosseous-equivalent sheep rotator cuff model. Knee Surg Traumatol Arthrosc. 2013;21:466–75. doi: 10.1007/s00167-012-1972-z. [DOI] [PubMed] [Google Scholar]
- 17.Luo F, Hou T, Zhang Z, Xie Z, Wu X, Xu J. Effects of pulsed electromagnetic field frequencies on the osteogenic differentiation of human mesenchymal stem cells. Orthopedics. 2012;35:e526–31. doi: 10.3928/01477447-20120327-11. [DOI] [PubMed] [Google Scholar]
- 18.Miller SL, Bradshaw R, Coughlin DG, Waldorff EI, Ryaby JT, Lotz JC. Pulsed electromagnetic field (PEMF) reduces expression of genes associated with disc degeneration in human intervertebral disc cells. Spine J. 20116;16:770–776. doi: 10.1016/j.spinee.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 19.Osti L, Biono AD, Maffulli N. Pulsed electromagnetic fields after rotator cuff repair: a randomized, controlled study. Orthopedics. 2015;38:e223–8. doi: 10.3928/01477447-20150305-61. [DOI] [PubMed] [Google Scholar]
- 20.Provenzano PP, Vanderby R., Jr Collagen fibril morphology and organization: implications for force transmission in ligament and tendon. Matrix Biol. 2006;25:71–84. doi: 10.1016/j.matbio.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 21.Springer J, Badgett RG. ACP Journal Club: Optimized extracorporeal shock-wave therapy improved pain and functioning in chronic plantar fasciitis. Ann Intern Med. 2015;163:JC8. doi: 10.7326/ACPJC-2015-163-10-008. [DOI] [PubMed] [Google Scholar]
- 22.Strauch B, Patel MK, Rosen DJ, Mahadevia S, Brindzei N, Pilla AA. Pulsed magnetic field therapy increases tensile strength in a rat Achilles’ tendon repair model. J Hand Surg. 2006;31A:1131–1135. doi: 10.1016/j.jhsa.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 23.Thomopoulos S, Williams GR, Gimbel JA, Favata M, Soslowsky LJ. Variation of biomechanical, structural, and compositional properties along the tendon to bone insertion site. J Orthop Res. 2003;21:413–419. doi: 10.1016/S0736-0266(03)00057-3. [DOI] [PubMed] [Google Scholar]
- 24.Tucker JJ, Cirone JM, Morris TR, Nuss CA, Huegel J, Waldorff EI, et al. Pulsed electromagnetic field therapy improves tendon-to-bone healing in a rat rotator cuff repair model. J Orthop Res. 2016 doi: 10.1002/jor.23333. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.United States Food & Drug Administration. Premarket Approval (PMA) P850007 [Internet] Silver Spring (MD): United States Department of Health & Human Services; 1986. [cited 12 April 2017]. Available from: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P850007. [Google Scholar]
- 26.Uzunca K, Birtane M, Tastekin N. Effectiveness of pulsed electromagnetic field therapy in lateral epicondylitis. Clin Rheumatol. 2007;26:69–74. doi: 10.1007/s10067-006-0247-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure S1 Representative images for Collagen III, IL1β, TNFα, and decorin for non-PEMF and PEMF groups at 4 and 8 weeks post-repair. No differences were seen between groups at either time point. Scale bar represents 250 μm.


