Abstract
Cluster of differentiation 59 (CD59) is a glycosylphosphatidylinositol-anchored protein. Cross-linking of CD59 with specific monoclonal antibodies can cause a series of intracellular signal transduction events. However, the underlying molecular mechanisms are poorly understood. Linker for activation of T-cells (LAT) is a crucial adaptor protein in T-cell signaling, and its phosphorylation and palmitoylation are essential for its localization and function. In a previous study by the present authors, it was demonstrated that CD59 may be responsible for LAT palmitoylation, thereby regulating T-cell signal transduction. The present study detected the co-localization of LAT and CD59 in lipid rafts by transfecting Jurkat cells with lentivirus vectors carrying the LAT-enhanced green fluorescent protein fusion protein. In addition, LAT and CD59 were shown to have a synergistic effect on the proliferation of Jurkat cells. The results also indicated that CD59 may transfer the palmitate group from phosphatidylinositol to LAT to form LAT palmitate, which then localizes to lipid rafts to regulate T-cell activation. The results of the present study provided novel insights into the role of CD59 in T-cell signal transduction.
Keywords: glycosylphosphatidylinositol, cluster of differentiation 59, linker for activation of T-cells, T-cell, signal transduction
Introduction
Glycosylphosphatidylinositols (GPIs) are complex glycolipids that anchor proteins to the outer leaflet of the cell membrane via their carboxyl terminal (1). The core structure of a GPI consists of a phosphatidylinositol (PI) moiety, a glucosamine (GlcN) moiety, three mannoses (Mans) and an ethanolamine-phosphate (EtNP) moiety (2). Previous studies have shown that GPIs are able to transduce activation signals into the cell (3–6). The molecular mechanisms underlying signal transduction are poorly understood. However, it is hypothesized that the PI moiety of GPI may participate in signal transduction via a palmitate group that is located at the second position of the PI (PI-2nd) (7).
Cluster of differentiation 59 (CD59) is an 18–21 kDa GPI-anchored glycoprotein (GPIAP), which belongs to the leukocyte antigen 6 (Ly6) family of proteins and has a high homology to the mouse protein, Ly6 (8). CD59 was initially shown to prevent C9 units from binding to the C5b-8 complex in order to inhibit the formation of membrane attack complex (9–11). Due to this crucial role, CD59 is widely expressed in the majority of tissues, including the heart, liver and kidneys, and circulating cells, such as leukocytes and red blood cells (12,13). The function of CD59 in complement regulation has been well documented (9–11); however, numerous studies have suggested that CD59 also has a role in T-cell activation and signal transduction (5–7). However, CD59 is a GPI-anchored protein and does not span the membrane; thus the mechanisms underlying the CD59-mediated transduction of signals into the cell remain unclear. It has been suggested that the function of CD59 in signal transduction is dependent on its localization to lipid rafts, which act as platforms for the associations of signaling molecules (14).
Linker for activation of T-cells (LAT) was first observed in 1990 and, until 1998, it was purified from activated Jurkat cells and named LAT based on its properties (15). LAT is one of the most important transmembrane adaptor proteins, and is expressed in mature T-cells, natural killer cells, mast cells, megakaryocytes and pre-B-cells (15–18). LAT has no intrinsic enzymatic activity, but it enables inducible recruitment of effector molecules to the plasma membrane (19). Human LAT has four extracellular amino acids, a single transmembrane domain and a long cytoplasmic tail that contains nine conserved tyrosine motifs. Examination of the amino acid sequence of LAT showed that the juxtamembrane region of LAT contains two cysteine (C) residues, C26 and C29 in humans, which are critical for LAT palmitoylation, raft localization, phosphorylation and function in T-cell receptor (TCR)-mediated signaling (20). LAT palmitoylation is undeniably essential for its function; however, the mechanism underlying the palmitoylation of LAT is unknown.
Based on the structural characteristics of CD59 and LAT, the authors of the present study hypothesized that CD59 may transfer a palmitate group to LAT, causing them to co-localize to lipid rafts in order to regulate T-cell signal transduction. Therefore, in the present study, Jurkat cells were transfected with lentivirus vectors carrying the LAT-enhanced green fluorescent protein (EGFP) fusion protein, in order to establish a cell line stably expressing the fusion protein. In addition, the present study aimed to investigate the biological roles of CD59 in the proliferation, activation and apoptosis of Jurkat cells via LAT, and to demonstrate that CD59 may be the candidate protein that transfers a palmitate group to LAT.
Materials and methods
Materials
Jurkat cells purchased from the cell bank of the Chinese Academy of Sciences (Beijing, China) were preserved in our lab. Negative (neg)-EGFP and the LAT-EGFP fusion protein were constructed by our lab. Lentiviral vectors were constructed by Shanghai GeneChem Co., Ltd. (Shanghai, China). RPMI-1640 medium was purchased from HyClone (GE Healthcare Life Sciences, Logan, UT, USA). The cell counting kit-8 (CCK-8) was purchased from Beijing Fanbo Biochemicals Co., Ltd. (Beijing, China). Guava Nexin Reagent was obtained from Merck Millipore (Darmstadt, Germany). Biotinylated rabbit anti-human CD59 (catalog no., 5516-2; dilution, 1:1,000; Abcam, Cambridge, UK) and phospholipase C-γ1 (PLCG1; dilution, 1:4,000; catalog no., 2112-1; Abcam) monoclonal antibodies, and zeta-chain-associated protein kinase 70 (ZAP70)/lymphocyte-specific protein tyrosine kinase (Lck) (C-term) polyclonal antibodies (dilution, 1,:1,000; catalog no., AB55208; Sigma-Aldrich; EMD Millipore, Billerica, MA, USA) were used. The β-actin antibody (dilution, 1:500; catalog no., D110007-0200) was obtained from Sangon Biotech Co., Ltd. (Shanghai, China). The goat anti-rabbit IgG (H+L) secondary antibody (catalog no., D111018; dilution 1:2,000) was purchased from Sangon Biotech Co., Ltd. TRITC-conjugated goat anti-rabbit IgG and the Mammalian Protein Extraction reagent were obtained from Beijing ComWin Biotech Co., Ltd. (Beijing, China). Enhanced chemiluminescence (ECL) reagent was purchased from GE Healthcare Life Sciences. The Transcriptor First Strand cDNA Synthesis kit and the FastStart Essential DNA Green Master were obtained from Roche Diagnostics (Basel, Switzerland).
Cell culture
Jurkat cells were maintained in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and antibiotics (100 U/ml penicillin and 100 µg/ml streptomycin). The cells were maintained at 37°C in a humidified atmosphere containing 5% CO2. Exponentially growing cells were used in all experiments.
Experimental groups
Jurkat cells were divided into four groups, as follows: i) Neg group, in which Jurkat cells were transfected with the neg-EGFP lentivirus; ii) neg + CD59 antibody (CD59Ab) group, in which the Jurkat cells transfected with the neg-EGFP lentivirus were treated with the CD59Ab; iii) LAT group, in which Jurkat cells were transfected with the LAT-EGFP lentivirus; and iv) LAT + CD59Ab group, in which the Jurkat cells transfected with the LAT-EGFP lentivirus were treated with the CD59Ab.
Fluorescence microscopy to assess the transfection efficiencies of neg-EGFP and LAT-EGFP into Jurkat cells
Jurkat cells were transfected with neg-EGFP or LAT-EGFP lentiviruses at a multiplicity of infection of 100 particles/cell in RPMI-1640 medium containing 5 µg/ml polybrene and Eni.S (infection enhancement solution), and then incubated at 37°C in 5% CO2 in 96-well plates for 12 h, after which the cells were incubated with fresh medium. After 2 weeks, the transfected cells expressing EGFP were observed under a fluorescence microscope.
Analysis of cell viability
Cell viability was measured using CCK-8 assays. Briefly, exponentially growing neg, neg + CD59Ab, LAT and LAT + CD59Ab cells (100 µl, 1×105 cells/ml) were seeded into 96-well plates and incubated for 48 h at 37°C in 5% CO2. Subsequently, 10 µl CCK-8 solution was added to each well followed by a 4-h incubation. The absorbance was determined at a wavelength of 490 nm using a microplate reader. All experiments were performed in quintuplicate.
Analysis of cell apoptosis
Guava Nexin Reagent contains Annexin V-phycoerythrin (PE) and 7-aminoactinomycin D (7-AAD). Annexin V-PE is used to detect phosphatidylserine, which is located on the external membrane of apoptotic cells, and its mean fluorescent intensity indicates early and late apoptotic cells. 7-AAD is an indicator of cell membrane structural integrity and binds to late apoptotic and dead cells. On the histogram, the lower-left quadrant indicates cells that are not undergoing apoptosis [Annexin V-PE(−), 7-AAD(−)], the lower-right quadrant indicates cells in the early stages of apoptosis [Annexin V-PE(+), 7-AAD(−)], the upper-right quadrant indicates cells in the late stages of apoptosis [Annexin V-PE(+), 7-AAD(+)] and the upper-left quadrant indicates necrotic cells [Annexin V-PE(−), 7-AAD(+)].
To analyze apoptosis, neg and LAT cells (106 cells/ml) were cultured in 24-well plates and treated with CD59Ab at 37°C in a humidified atmosphere containing 5% CO2 for 2 or 6 h. Subsequently, the cells were re-suspended in 100 µl RPMI-1640 medium containing 10% FBS and antibiotics, followed by addition of 80 µl Guava Nexin Reagent. After a 20-min incubation at room temperature in the dark, the cells were analyzed by flow cytometry. All assays were repeated three times.
Immunofluorescence staining
Cells (106 cells/ml) were plated onto glass coverslips, fixed in 4% paraformaldehyde for 20 min at room temperature, washed with PBS and then blocked with blocking buffer [10% goat serum (Beyotime Institute of Biotechnology, Shanghai, China) in PBS] for 30 min at room temperature. Fixed cells were incubated with rabbit anti-human CD59 (dilution, 1:50) primary antibody overnight at 4°C, washed with PBS, and incubated for 2 h with goat anti-rabbit IgG (TRITC) (dilution, 1:100) secondary antibody at room temperature in the dark, then washed again in PBS. Coverslips were mounted onto the glass slides using glycerine. Finally, images of the cells were captured using confocal laser scanning microscopy.
RNA extraction and reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated from the cell groups using Trizol reagent (Beijing ComWin Biotech Co., Ltd.), after which 1 µg RNA was reverse transcribed into cDNA in a 20 µl reaction volume using the Transcriptor First Strand cDNA Synthesis kit, according to the manufacturer's protocol. qPCR was performed using the FastStart Essential DNA Green Master and the following cycling conditions: Initial denaturation at 95°C for 5 min, denaturation at 94°C for 15 sec, annealing at 55°C for 30 sec and extension at 70°C for 30 sec. The primer sequences are shown in Table I. Each sample was analyzed three times and expression levels were normalized to GAPDH. Data were analyzed using the 2−ΔΔCq method (21).
Table I.
Sequences of primers used in this study.
| Gene | Sense primer (5′ to 3′) | Antisense primer (5′ to 3′) |
|---|---|---|
| PLCG1 | GCAGTGCCTTTGAAGAACAA | GGGACTGAGGTCACCATTCT |
| PI3K | TCTACCATGGAGGAGAACCC | AGCAAATGGAAAGGCAAAGT |
| LCK | GCATGGCATTCATTGAAGAG | CCTGGCTGTGTACTCGTTGT |
| LAT | CTACCCACCTGTCACCTCCT | CTGTTGGCACCATCAGAATC |
| GAPDH | GATGACCTTGCCCACAGCCT | ATCTCTGCCCCCTCTGCTGA |
PLCG1, phospholipase C gamma 1; PI3K, phosphoinositide 3-kinase; LCK, lymphocyte-specific protein tyrosine kinase; LAT, linker for activation of T-cells.
Western blot analysis
Proteins were extracted from the neg cells, neg + CD59Ab cells, LAT cells and LAT + CD59Ab cells using Mammalian Protein Extraction Reagent, according to the manufacturers protocol. Protein concentrations were measured using the BCA Protein Assay kit (Beyotime Institute of Biotechnology), after which protein samples were resolved by 12 and 5% SDS-PAGE and transferred onto polyvinylidene fluoride membranes. The membranes were blocked with TBST (containing 5% non-fat milk) or bovine serum albumin (Solarbio, Beijing, China), then incubated with anti-PLCG1 (dilution, 1:1,000), anti-ZAP70 (dilution, 1:1,000), anti-Lck (dilution, 1:1,000) and anti-β-actin (dilution, 1:2,000) primary antibodies at 4°C overnight, followed by incubation with goat anti-rabbit IgG (H+L) secondary antibody (dilution, 1:5,000) for 2 h at room temperature. Immune complexes were detected using an ECL solution and visualized on the BioSpectrum 810 Imaging System (UVP, Inc., Upland, CA, USA).
Statistical analysis
The results are expressed as the mean ± standard deviation. The software used for statistical analysis was SPSS 17.0 for Windows (SPSS, Inc., Chicago, IL, USA). Multiple samples were compared with one-way analysis of variance combined with a Tukey's multiple comparison post-hoc test. P<0.05 was considered to indicate a significant difference.
Results
Fluorescence microscopy assessment of transfection efficiency
Jurkat cells were transfected with neg-EGFP or LAT-EGFP lentivirus vectors and the detection of green fluorescence was considered successful transfection. As shown in Fig. 1, green fluorescence was observed throughout the field of view, with the transfection efficiency reaching 80%. This suggested the successful establishment of stably transfected cell lines.
Figure 1.
Transfection efficiency of neg-EGFP and LAT-EGFP lentivirus vectors. Successfully transfected cells with enhanced green fluorescence protein. (A) Jurkat cells. (B) Jurkat cells transfected with neg-EGFP. (C) Jurkat cells. (D) Jurkat cells transfected with LAT-EGFP. EGFP, enhanced green fluorescence protein; LAT, linker for activation of T-cells; neg, negative.
Orientation and distribution of CD59 and LAT in different states of activation
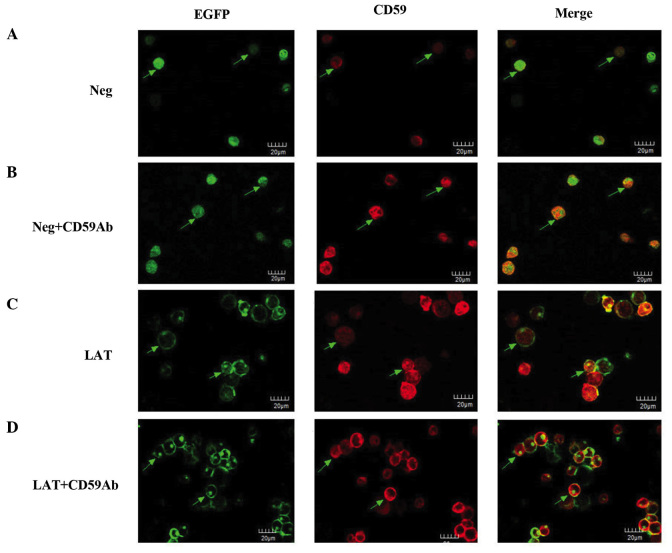
Immunofluorescence staining was used to observe the orientation and localization of CD59 and LAT. Both were distributed on the membrane (Fig. 2). The green fluorescence of the neg group corresponds to the expression of the EGFP reporter gene, which was diffusely distributed in the cytoplasm (Fig. 2A). The intensity of red and green fluorescence indicated the extent of expression of CD59 and LAT, respectively. As compared with the neg group, neg the expression of CD59 was enhanced in the neg + CD59Ab group (Fig. 2B). As shown in Fig. 2C and D, LAT was found predominantly in clusters in the cell membrane. After stimulation with the CD59Ab, the fluorescence signal of LAT became stronger (Fig. 2D). The red fluorescence of CD59 also showed clustering and was colocalized with the distribution of LAT (Fig. 2C and D). These results suggest that, when Jurkat cells are activated, CD59 is able to recruit a large number of LAT molecules to lipid rafts. Following ongoing activation, increasing numbers of CD59 and LAT are recruited to lipid rafts to regulate downstream signaling.
Figure 2.
Cellular localization of CD59 and LAT. (A) Neg group. Jurkat cells transfected with neg-EGFP lentiviruses showed green fluorescence corresponding to EGFP in the cytoplasm. CD59 molecules were stained red on the cytomembrane of the neg group cells. The merged images showed green and red fluorescence in neg cells corresponding to EGFP and CD59, respectively. (B) Neg + CD59Ab group. Neg cells were stimulated with CD59Ab. Green fluorescence corresponding to EGFP was expressed in the cytoplasm. CD59 red fluorescence was higher following stimulation with CD59Ab, as compared with the neg group. The merged images showed green and red fluorescence corresponding to EGFP and CD59, respectively, in neg cells following CD59Ab stimulation. (C) LAT group. Jurkat cells transfected with LAT-EGFP lentiviruses showed green fluorescence corresponding to LAT on the cell surface. CD59 was stained red on the cytomembrane of LAT group cells. The merged images showed colocalization of LAT and CD59 molecules to lipid rafts. (D) LAT + CD59Ab group. LAT group cells were stimulated with CD59Ab. Green fluorescence on the cell surface corresponding to LAT was higher in the LAT + CD59Ab group cells compared with the LAT group cells. Similarly, CD59 staining was higher in the LAT + CD59Ab group cells compared with the LAT group cells. The merged images showed that LAT and CD59 molecules were colocalized to lipid rafts in LAT cells stimulated with CD59Ab. CD59Ab, cluster of differentiation 59 antibody; LAT, linker for activation of T-cells; EGFP, enhanced green fluorescent protein.
Cross-linking CD59 in lipid rafts promotes LAT-mediated proliferation of Jurkat cells
CD59 and LAT are crucial molecules that participate in signal transduction in the membrane (14,22). As shown in Fig. 2, CD59 and LAT were colocalized to lipid rafts in Jurkat cells. To further investigate the relationship between CD59 and LAT, CCK8 assays were performed to examine the proliferation rate of the various groups. The results showed that the cell viability of the LAT group was significantly higher compared with the neg group (P<0.01; Fig. 3). Cross-linking with CD59 significantly increased the viabilities of both the LAT and neg group cells (both P<0.01; Fig. 3). These results suggested that cross-linking CD59 using a monoclonal antibody or overexpressing LAT were able to significantly increase the proliferation of Jurkat cells. Notably, the LAT group cells stimulated with the CD59Ab exhibited the greatest proliferation ability. These results suggest that GPI-anchored CD59 is able to recruit LAT into lipid rafts to induce the activation and proliferation of Jurkat cells.
Figure 3.
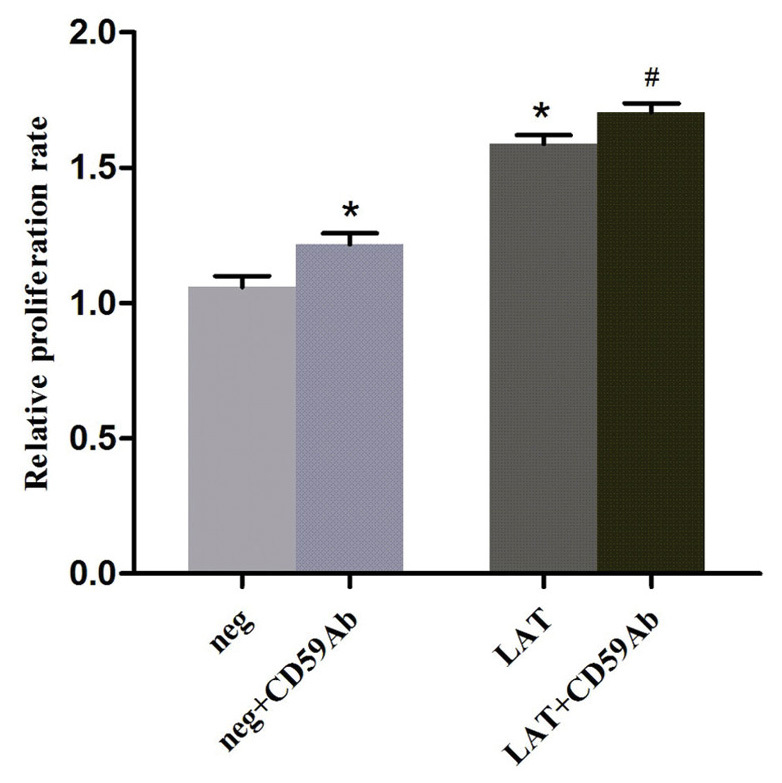
Cell proliferation was assessed using the CCK-8 assay. The neg, neg + CD59Ab, LAT and LAT + CD59Ab cells were treated with CCK-8 for 4 h. Data are expressed as the mean ± standard deviation. *P<0.01 vs. the neg group; #P<0.01 vs. the LAT group. CCK-8, cell counting kit-8; neg, negative; CD59Ab, cluster of differentiation 59 antibody; LAT, linker for activation of T-cells.
Cross-linking CD59 and overexpressing LAT reduces apoptosis in Jurkat cells
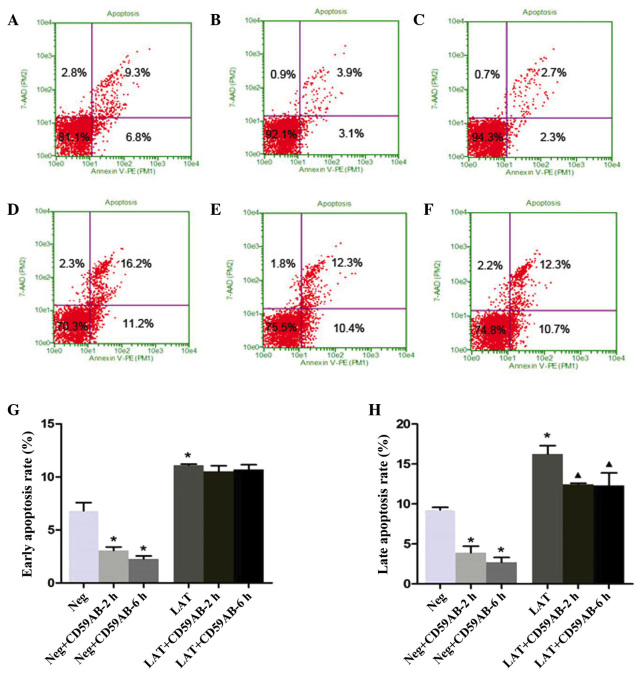
To observe the apoptotic effects induced by CD59, the neg and LAT cells treated with CD59Ab were stained with Annexin V-PE and 7-AAD and analyzed by flow cytometry. As shown in Fig. 4A, the percentages of early apoptotic (right lower quadrant) and late apoptotic neg cells (right upper quadrant) were 6.8 and 9.3%, respectively. The apoptosis rates gradually decreased with a longer incubation with CD59Ab (Fig. 4B and C). The LAT cells showed a higher percentage of late apoptotic cells compared with the neg group (16.2 vs. 9.3%; Fig. 4D). However, the percentage of late apoptotic LAT cells was decreased following treatment with CD59Ab for 2 or 6 h (12.3 and 12.3%, respectively; Fig. 4E and F), although the ratio indicated no significant difference (Fig. 4G). These results, as well as the results of a previous study (23), suggest that GPI-anchored proteins, such as CD59, are able to assist SRC family kinase-mediated signal transduction in lipid rafts, and promote the proliferation and suppress the apoptosis of Jurkat cells through cross-linking via its corresponding antibody.
Figure 4.
Effect of CD59 on the apoptosis of neg and LAT cells. The apoptosis of (A) Jurkat cells transfected with neg-EGFP lentivirus, (B) neg cells treated with the CD59 monoclonal antibody for 2 h, (C) neg cells treated with the CD59 monoclonal antibody for 6 h, (D) Jurkat cells transfected with LAT-EGFP lentivirus, (E) LAT cells treated with the CD59 monoclonal antibody for 2 h and (F) LAT cells treated with the CD59 monoclonal antibody for 6 h was assessed by flow cytometry. (G) Statistical analysis of the percentages of early apoptotic cells in the different groups. *P<0.05 vs. the neg group. (H) Statistical analysis of the percentages of late apoptotic cells in the different groups. *P<0.05 vs. the neg group. ▲P<0.05 vs. the LAT group. CD59Ab, cluster of differentiation 59 antibody; LAT, linker for activation of T-cells; EGFP, enhanced green fluorescent protein; 7-AAD, 7-aminoactinomycin D; PE, phycoerythrin.
CD59 and LAT induce the upregulation and activation of crucial protein tyrosine kinases (PTKs)
The present study demonstrated that CD59 and LAT induced the proliferation of Jurkat cells. To determine the enzymes or signal transducers involved in the regulatory mechanism, the mRNA expression levels of PLCG1, phosphoinositide 3-kinase (PI3K), Lck and LAT, and the protein expression levels of Lck, PI3K, FYN, ZAP70 and PLCG1, were measured. The mRNA expression levels of PLCG1, PI3K, Lck and LAT in LAT cells were significantly higher compared with those in neg cells (Table II). The expression of PI3K in LAT cells was ~16-fold greater compared with neg cells. Furthermore, the expression of PI3K was increased in the neg + CD59Ab group compared with the neg group, although it was less than the mRNA expression of PI3K in the LAT group. These results suggest that LAT overexpression exerts stronger activating effects in Jurkat cells than cross-linking the GPIAP CD59. Compared with the LAT group, there was no significant increase in the mRNA expression levels of any of the PTKs in the LAT + CD59Ab group (Table II). Therefore, the dual intervention did not enhance the activation of Jurkat cells compared with overexpresson of LAT alone. Notably, the mRNA expression levels of LAT in both the neg and LAT cells were decreased following treatment with CD59Ab (Table II). One possible explanation for this is that numerous positive regulators set the activation threshold in T-cells, and SRC kinase-dependent hyperphosphorylation of inhibitory receptors such as csk binding protein and cytotoxic T-lymphocyte-associated protein 4, may result in their recruitment to lipid rafts to displace LAT.
Table II.
Reverse transcription-quantitative polymerase chain reaction assessment of mRNA expression levels in the different groups.
| ΔCq value | ||||
|---|---|---|---|---|
| Gene | Neg | neg+CD59Ab | LAT | LAT+CD59Ab |
| PLCG1 | 9.74 | 9.09 | 7.95b | 8.63 |
| PI3K | 16.32 | 15.15a | 12.47b | 12.36 |
| LCK | 5.09 | 5.26 | 4.23b | 4.38 |
| LAT | 8.38 | 9.45 | 6.35b | 7.42 |
mRNA expression of PI3K in neg+CD59Ab group compared with in neg group
mRNA expression levels of PLCG1, PI3K, Lck and LAT in LAT group compared with those in neg group. PLCG1, phospholipase C-γ1; PI3K, phosphoinositide 3-kinase; LCK, lymphocyte-specific protein tyrosine kinase; LAT, linker for activation of T-cells; CD59Ab, cluster of differentiation 59 antibody; LAT, linker for activation of T-cells.
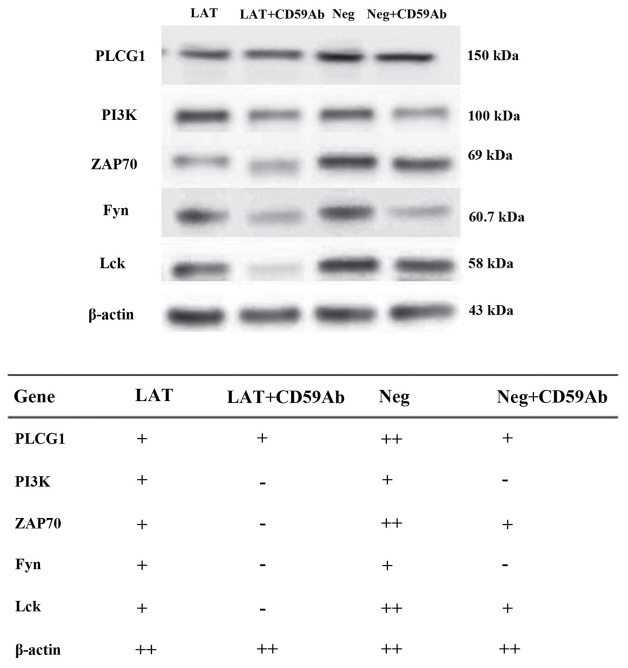
The expression of various molecules that interact with LAT in the T-cell signaling pathway were detected. The results indicated that cross-linking CD59 led to a significant reduction in the concentrations of upstream PTKs, including ZAP-70, Lck and FYN. PLCG1 and PI3K are two downstream signaling molecules modulated by LAT (24). As shown in Fig. 5, there was no change in the concentration of PLCG1 after cross-linking CD59. However, the band of PI3K was too light to quantify, which was likely because the majority of PI3K was phosphorylated to participate in the PI3K/Akt signaling pathway. These results suggested that the signal transduction induced by CD59 mainly affected the PI3K/Akt pathway via LAT, a crucial adaptor (some results are not shown).
Figure 5.
Western blotting was performed to detect the expression of PLCG1, PI3K, ZAP70, Fyn and Lck in the neg, neg + CD59Ab, LAT and LAT + CD59Ab groups. CD59Ab, cluster of differentiation 59 antibody; LAT, linker for activation of T-cells; PLCG1, phospholipase C-γ1; PI3K, phosphoinositide 3-kinase; ZAP70, zeta-chain-associated protein kinase 70; Lck, lymphocyte-specific protein tyrosine kinase.
Discussion
CD59 is a GPI-anchored glycoprotein that protects cells from attack by the complement system (8–11). Previous studies have shown that cross-linking of CD59 can lead to a series of intracellular signal transduction events (25–28). However, the molecular mechanisms underlying CD59-mediated T-cell signal transduction are unclear. A mechanism has been suggested based on the structure of the GPI anchor and the positioning of the CD59 molecule in lipid rafts (14). Lipid rafts, which mainly contain cholesterol and sphingolipids, are considered signaling platforms to mediate signal transduction (29–32). Numerous raft-associated proteins, including LAT and SRC family kinases, have been shown to have important roles in signal transduction in lymphocytes (20,33).
GPI-anchored molecules lack transmembrane regions and are especially enriched in lipid rafts (34). These molecules can induce intracellular signal transduction events (35). Therefore, there must be a mechanism connecting the exterior and interior of the plasma membrane. Subczynski and Kusumi (36) speculated that this mechanism may occur via a type of non-specific transmembrane protein. The present study focused on the role of CD59 in Jurkat cells in order to determine how CD59 may interact with LAT to transduce signals.
LAT is a 36–38 kDa type III transmembrane adaptor protein (37), which functions as an important integration node at the plasma membrane for various signaling complexes (38). Microclusters of LAT are composed of signaling complexes that include adaptors and effectors for T-cell activation (39). Phosphorylation and palmitoylation are crucial for the localization and function of transmembrane adaptor proteins (40). LAT can be phosphorylated by the ZAP70/Syk PTK, which promotes the recruitment of various signaling molecules to the plasma membrane (41). Protein palmitoylation is an important form of post-translational covalent modification characterized by lipid acylation (42). Following palmitoylation, LAT is recruited to lipid rafts, where it is phosphorylated to serve as a linker for activated T-cells. In the GPI structure, a palmitate group is attached to the second carbon of the inositol ring (43), and the present study assumed that LAT palmitoylation is associated with the function of the palmitate group. Lentivirus vectors carrying neg-EGFP and LAT-EGFP were constructed and transfected into Jurkat cells. The results of confocal microscopy indicated that CD59 and LAT colocalized to lipid rafts.
Activation of Jurkat cells through specific GPI-anchored molecules, such as CD55 and CD59 in humans, results in the phosphorylation of PTKs, elevation of Ca2+ concentrations and an increase in proliferation (6) and cytokine release (44). Too much calcium can cause damage to mitochondria and the release of cytochrome c, which activates caspases to induce apoptosis (45). In order to observe the effect of CD59 on LAT cells and the molecular mechanisms underlying apoptosis, the effects of CD59 on the proliferation and apoptosis of neg and LAT cells were examined. The present study demonstrated that CD59 and LAT molecules were able to promote the growth of Jurkat cells. LAT cells showed enhanced proliferation and had a relatively high late-apoptosis rate, which may have been caused by activation-induced cell death.
TCR engagement triggers signaling cascades that result in enhanced gene transcription, cell proliferation and differentiation, and even apoptosis (46,47). The earliest events in this pathway are the activation of Lck and Fyn, which are PTKs from the Src family. These enzymes were found to phosphorylate tyrosine residues within the immunoreceptor tyrosine-based activation motifs (ITAMs) of the cytosolic domains of TCR-CD3ξ chains. Phosphorylated ITAMs serve as binding sites for the Src homology 2 (SH2) domains of ZAP70, which is then phosphorylated by Lck or Fyn to further activate downstream substrates (15). LAT is a substrate of activated ZAP70 and is one of the most important tyrosine-phosphorylated proteins following TCR activation; LAT is able to bind to the SH2 domains of growth factor receptor bound protein 2 and PLCG1 and the p85 subunit of PI3K (48–50). In the present study, results from immunoblotting and RT-qPCR indicated that the expression of non-phosphorylated proteins in LAT and neg cells was decreased following cross-linking of CD59. For neg cells, the mRNA expression levels of PLCG1 and PI3K were increased after CD59 cross-linking, while there was a decrease in the mRNA expression level of LAT following cross-linking. For LAT cells, there was no significant difference in the mRNA expression levels of PTKs following cross-linking of CD59, with the exception of the mRNA expression level of LAT, which decreased markedly following cross-linking of CD59. These results suggested that CD59 in lipid rafts in combination with palmitoylated LAT were able to promote T-cell signal transduction, facilitate the phosphorylation of PTKs and enhance gene transcription. However, the over-activation of T-cells can trigger and recruit inhibitory receptors into lipid raft to displace and suppress activators, in order to maintain T-cell homeostasis (51).
In conclusion, the present study demonstrated the that GPI-anchored protein CD59 was able to promote TCR signal transduction, of which the mechanism may involve the CD59-mediated transfer of a palmitate group to LAT and subsequent regulation of T-cell signaling. These findings suggest that other GPI-anchored proteins may be involved in signal transduction in T-cells. Furthermore, the results of the present study suggested that CD59 and LAT were able to synergistically promote Jurkat cell proliferation. Future studies should further investigate how GPI-anchored proteins are able to modulate T-cell signal transduction.
Acknowledgements
We would like to thank Dr Peng Zhao (Department of Cancer, Biotherapy Center of the Central Hospital of Qingdao) for his assistance with recovery of the Jurkat cells. This study was supported by the National Natural Science Foundation of China (grant no. 81273206).
References
- 1.Lisanti MP, Sargiacomo M, Graeve L, Saltiel AR, Rodriguez-Boulan E. Polarized apical distribution of glycosyl-phosphatidylinositol-anchored proteins in a renal epithelial cell line; Proc Natl Acad Sci USA; 1988; pp. 9557–9561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fujita M, Kinoshita T. GPI-anchor remodeling: Potential functions of GPI-anchors in intracellular trafficking and membrane dynamics. Biochim Biophys Acta. 2012;1821:1050–1058. doi: 10.1016/j.bbalip.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 3.Davis LS, Patel SS, Atkinson JP, Lipsky PE. Decay-accelerating factor functions as a signal transducing molecule for human T cells. J Immunol. 1988;141:2246–2252. [PubMed] [Google Scholar]
- 4.Thompson LF, Ruedi JM, Glass A, Low MG, Lucas AH. Antibodies to 5′-nucleotidase (CD73), a glycosyl-phosphatidylinositol-anchored protein, cause human peripheral blood T cells to proliferate. J Immunol. 1989;143:1815–1821. [PubMed] [Google Scholar]
- 5.Presky DH, Low MG, Shevach EM. Role of phosphatidylinositol-anchored proteins in T cell activation. J Immunol. 1990;144:860–868. [PubMed] [Google Scholar]
- 6.Korty PE, Brando C, Shevach EM. CD59 functions as a signal-transducing molecule for human T cell activation. J Immunol. 1991;146:4092–4098. [PubMed] [Google Scholar]
- 7.Corda D, Zizza P, Varone A, Bruzik KS, Mariggiò S. The glycerophosphoinositols and their cellular functions. Biochem Soc Trans. 2012;40:101–107. doi: 10.1042/BST20110679. [DOI] [PubMed] [Google Scholar]
- 8.Davies A, Simmons DL, Hale G, Harrison RA, Tighe H, Lachmann PJ, Waldmann H. CD59, an LY-6-like protein expressed in human lymphoid cells, regulates the action of the complement membrane attack complex on homologous cells. J Exp Med. 1989;170:637–654. doi: 10.1084/jem.170.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farkas I, Baranyi L, Ishikawa Y, Okada N, Bohata C, Budai D, Fukuda A, Imai M, Okada H. CD59 blocks not only the insertion of C9 into MAC but inhibits ion channel formation by homologous C5b-8 as well as C5b-9. J Physiol. 2002;539:537–545. doi: 10.1113/jphysiol.2001.013381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meri S, Morqan BP, Davies A, Daniels RH, Olavesen MG, Waldmann H, Lachmann PJ. Human protectin (CD59), an 18,000–20,000 MW complement lysis restricting factor, inhibits C5b-8 catalysed insertion of C9 into lipid bilayers. Immunology. 1990;71:1–9. [PMC free article] [PubMed] [Google Scholar]
- 11.Rollins SA, Sims PJ. The complement-inhibitory activity of CD59 resides in its capacity to block incorporation of C9 into membrane C5b-9. J Immunol. 1990;144:3478–3483. [PubMed] [Google Scholar]
- 12.Hideshima T, Okada N, Okada H. Expression of HEF20, a regulatory molecule of complement activation, on peripheral blood mononuclear cells. Immunology. 1990;69:396–401. [PMC free article] [PubMed] [Google Scholar]
- 13.Meri S, Waldmann H, Lachmann PJ. Distribution of protectin (CD59), a complement membrane attack inhibitor, in normal human tissues. Lab Invest. 1991;65:532–537. [PubMed] [Google Scholar]
- 14.Kimberley FC, Sivasankar B, Paul Morgan B. Alternative roles for CD59. Mol Immunol. 2007;44:73–81. doi: 10.1016/j.molimm.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 15.Zhang W, Sloan-Lancaster J, Kitchen J, Trible RP, Samelson LE. LAT: The ZAP-70 tyrosine kinase substrate that links T cell receptor to cellular activation. Cell. 1998;92:83–92. doi: 10.1016/S0092-8674(00)80901-0. [DOI] [PubMed] [Google Scholar]
- 16.Su YW, Jumaa H. LAT links the pre-BCR to calcium signaling. Immunity. 2003;19:295–305. doi: 10.1016/S1074-7613(03)00202-4. [DOI] [PubMed] [Google Scholar]
- 17.Facchetti F, Chan JK, Zhang W, Tironi A, Chilosi M, Parolini S, Notarangelo LD, Samelson LE. Linker for activation of T cells (LAT), a novel immunohistochemical marker for T cells, NK cells, mast, cells and megakaryocytes: Evaluation in normal and pathological conditions. Am J Pathol. 1999;154:1037–1046. doi: 10.1016/S0002-9440(10)65356-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weber JR, Orstavik S, Torqersen KM, Danbolt NC, Berg SF, Ryan JC, Taskén K, Imboden JB, Vaage JT. Molecular cloning of the cDNA encoding pp36, a tyrosine-phosphorylated adaptor protein selectively expressed by T cells and natural killer cells. J Exp Med. 1998;187:1157–1161. doi: 10.1084/jem.187.7.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li CY, Peng J, Ren LP, Gan LX, Lu XJ, Liu Q, Gu W, Guo XJ. Roles of histone hypoacetylation in LAT expression on T cells and Th2 polarization in allergic asthma. J Transl Med. 2013;11:26. doi: 10.1186/1479-5876-11-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang W, Trible RP, Samelson LE. LAT palmitoylation: Its essential role in membrane microdomain targeting and tyrosine phosphorylation during T cell activation. Immunity. 1998;9:239–246. doi: 10.1016/S1074-7613(00)80606-8. [DOI] [PubMed] [Google Scholar]
- 21.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 22.Dustin ML, Depoil D. New insights into the T cell synapse from single molecule techniques. Nat Rev Immunol. 2011;11:672–684. doi: 10.1038/nri3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wange RL. LAT, the linker for activation of T cells: a bridge between T cell-specific and general signaling pathways. Sci STKE. 2000;2000:re1. doi: 10.1126/stke.2000.63.re1. [DOI] [PubMed] [Google Scholar]
- 24.Chen Y, Thelin WR, Yang B, Milgram SL, Jacobson K. Transient anchorage of cross-linked glycosyl-phosphatidylinositol-anchored proteins depends on cholesterol, Src family kinases, caveolin and phosphoinositides. J Cell Biol. 2006;175:169–178. doi: 10.1083/jcb.200512116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okada H, Nagami Y, Takahashi K, Okada N, Hideshima T, Takizawa H, Kondo J. 20 KDa homologous restriction factor of complement resembles T cell activating protein. Biochem Biophys Res Commun. 1989;162:1553–1559. doi: 10.1016/0006-291X(89)90852-8. [DOI] [PubMed] [Google Scholar]
- 26.Lipp AM, Juhasz K, Paar C, Ogris C, Eckerstorfer P, Thuenauer R, Hesse J, Nimmervoll B, Stockinger H, Schütz GJ, et al. Lck mediates signal transmission from CD59 to the TCR/CD3 pathway in Jurkat T cells. PLoS One. 2014;9:e85934. doi: 10.1371/journal.pone.0085934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suzuki KG. Single-Molecule Imaging of Signal Transduction via GPI-Anchored Receptors. Methods Mol Biol. 2016;1376:229–238. doi: 10.1007/978-1-4939-3170-5_19. [DOI] [PubMed] [Google Scholar]
- 28.Stefanová I, Horejsí V, Ansotegui IJ, Knapp W, Stockinger H. GPI-anchored cell-surface molecules complexed to protein tyrosine kinases. Science. 1991;254:1016–1019. doi: 10.1126/science.1719635. [DOI] [PubMed] [Google Scholar]
- 29.Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 30.Jacobson K, Mouritsen OG, Anderson RG. Lipid rafts: At a crossroad between cell biology and physics. Nat Cell Biol. 2007;9:7–14. doi: 10.1038/ncb0107-7. [DOI] [PubMed] [Google Scholar]
- 31.Goswami D, Gowrishankar K, Bilgrami S, Ghosh S, Raghupathy R, Chadda R, Vishwakarma R, Rao M, Mayor S. Nanoclusters of GPI-anchored proteins are formed by cortical actin-driven activity. Cell. 2008;135:1085–1097. doi: 10.1016/j.cell.2008.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee MY, Ryu JM, Lee SH, Park JH, Han HJ. Lipid rafts play an important role for maintenance of embryonic stem cell self-renewal. J Lipid Res. 2010;51:2082–2089. doi: 10.1194/jlr.M001545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kabouridis PS, Magee AI, Ley SC. S-acylation of LCK protein tyrosine kinase is essential for its signaling function in T lymphocytes. EMBO J. 1997;16:4983–4998. doi: 10.1093/emboj/16.16.4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cinek T, Horejsí V. The nature of large noncovalent complexes containing glycosyl-phosphatidylinositol-anchored membrane glycoproteins and protein tyrosine kinases. J Immunol. 1992;149:2262–2270. [PubMed] [Google Scholar]
- 35.Suzuki KG. Lipid rafts generate digital-like signal transduction in cell plasma membranes. Biotechnol J. 2012;7:753–761. doi: 10.1002/biot.201100360. [DOI] [PubMed] [Google Scholar]
- 36.Subczynski WK, Kusumi A. Dynamics of raft molecules in the cell and artificial membranes: Approaches by pulse EPR spin labeling and single molecule optical microscopy. Biochim Biophys Acta. 2003;1610:231–243. doi: 10.1016/S0005-2736(03)00021-X. [DOI] [PubMed] [Google Scholar]
- 37.Fuller DM, Zhang W. Regulation of lymphocyte development and activation by the LAT family of adapter proteins. Immunol Rev. 2009;232:72–83. doi: 10.1111/j.1600-065X.2009.00828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bartelt RR, Houtman JC. The adaptor protein LAT serves as an integration node for signaling pathways that drive T cell activation. Wiley Interdiscip Rev Syst Biol Med. 2013;5:101–110. doi: 10.1002/wsbm.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Balagopalan L, Coussens NP, Sherman E, Samelson LE, Sommers CL. The LAT story: A tale of cooperativity, coordination, and choreography. Cold Spring Harb Perspect Biol. 2010;2:a005512. doi: 10.1101/cshperspect.a005512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin J, Weiss A, Finco TS. Localization of LAT in glycolipid-enriched microdomains is required for T cell activation. J Biol Chem. 1999;274:28861–28864. doi: 10.1074/jbc.274.41.28861. [DOI] [PubMed] [Google Scholar]
- 41.Jiang Y, Cheng H. Evidence of LAT as a dual substrate for Lck and Syk in T lymphocytes. Leuk Res. 2007;31:541–545. doi: 10.1016/j.leukres.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 42.Tanimura N, Saitoh S, Kawano S, Kosugi A, Miyake K. Palmitoylation of LAT contributes to its subcellular localization and stability. Biochem Biophys Res Commun. 2006;341:1177–1183. doi: 10.1016/j.bbrc.2006.01.076. [DOI] [PubMed] [Google Scholar]
- 43.Ferguson MAJ, Kinoshita T, Hart GW. Glycosylphosphatidylinositol anchors. In: Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME, editors. Essentials of Glycobiology. 2nd. Cold Spring Harbor; New York, NY: 2009. pp. 3–6. [Google Scholar]
- 44.Deckert M, Ticchioni M, Mari B, Mary D, Bernard A. The glycosylphosphatidylinositol-anchored CD59 protein stimulates both T cell receptor zeta/ZAP-70-dependent and -independent signaling pathways in T cells. Eur J Immunol. 1995;25:1815–1822. doi: 10.1002/eji.1830250704. [DOI] [PubMed] [Google Scholar]
- 45.Li B, Chu X, Gao M, Xu Y. The effects of CD59 gene as a target gene on breast cancer cells. Cell Immunol. 2011;272:61–70. doi: 10.1016/j.cellimm.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 46.Weiss A, Littman DR. Signal transduction by lymphocyte antigen receptors. Cell. 1994;76:263–274. doi: 10.1016/0092-8674(94)90334-4. [DOI] [PubMed] [Google Scholar]
- 47.Wange RL, Samelson LE. Complex complexes: Signaling at the TCR. Immunity. 1996;5:197–205. doi: 10.1016/S1074-7613(00)80315-5. [DOI] [PubMed] [Google Scholar]
- 48.Trüb T, Frantz JD, Miyazaki M, Band H, Shoelson SE. The role of a lymphoid-restricted, Grb2-like SH3-SH2-SH3 protein in T cell receptor signaling. J Biol Chem. 1997;272:894–902. doi: 10.1074/jbc.272.2.894. [DOI] [PubMed] [Google Scholar]
- 49.Fukazawa T, Reedquist KA, Panchamoorthy G, Soltoff S, Trub T, Druker B, Cantley L, Shoelson SE, Band H. T cell activation-dependent association between the p85 subunit of the phosphatidylinositol 3-kinase and Grb2/phospholipase C-gamma 1-binding phosphotyrosyl protein pp36/38. J Biol Chem. 1995;270:20177–20182. doi: 10.1074/jbc.270.34.20177. [DOI] [PubMed] [Google Scholar]
- 50.Sieh M, Batzer A, Schlessinger J, Weiss A. GRB2 and phospholipase C-gamma 1 associate with a 36- to 38-kilodalton phosphotyrosine protein after T-cell receptor stimulation. Mol Cell Biol. 1994;14:4435–4442. doi: 10.1128/MCB.14.7.4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kalland ME, Solheim SA, Skånland SS, Taskén K, Berge T. Modulation of proximal signaling in normal and transformed B cells by transmembrane adapter Cbp/PAG. Exp Cell Res. 2012;318:1611–1619. doi: 10.1016/j.yexcr.2012.05.014. [DOI] [PubMed] [Google Scholar]