Abstract
DNA methylation is associated with tumorigenesis and may act as a potential biomarker for detecting cervical cancer. The aim of the present study was to explore the methylation status of the paired box gene 1 (PAX1) and the LIM homeobox transcription factor 1 α (LMX1A) gene in a spectrum of cervical lesions in an Eastern Chinese population. This single-center study involved 121 patients who were divided into normal cervix (NC; n=28), low-grade squamous intraepithelial lesion (LSIL; n=32), high-grade squamous intraepithelial lesion (HSIL; n=34) and cervical squamous cell carcinoma (CSCC; n=27) groups, according to biopsy results. Following extraction and modification of the DNA, quantitative assessment of the PAX1 and LMX1A genes in exfoliated cells was performed using pyrosequencing analysis. Receiver operating characteristic (ROC) curves were generated to calculate the sensitivity and specificity of each parameter and cut-off values of the percentage of methylation reference (PMR) for differentiation diagnosis. Analysis of variance was used to identify differences among groups. The PMR of the two genes was significantly higher in the HSIL and CSCC groups compared with that in the NC and LSIL groups (P<0.001). ROC curve analysis demonstrated that the sensitivity, specificity and accuracy for detection of CSCC were 0.790, 0.837 and 0.809, respectively, using PAX1; and 0.633, 0.357 and 0.893, respectively, using LMX1A. These results indicated that quantitative PAX1 methylation demonstrates potential for cervical cancer screening, while further investigation is required to determine the potential of LMX1A methylation.
Keywords: cervical cancer, paired box gene 1, LIM homeobox transcription factor 1 α, methylation, human papillomavirus
Introduction
Cervical cancer (CC) is one of the most common types of gynecological malignant tumor (1). The main histological type is cervical squamous cell carcinoma (CSCC), which accounts for 75–80% of cases; adenocarcinoma accounts for 10–15% and other histological subtypes represent 10–15% (2,3). Although there has been a declining trend over the past few decades, CC remains a major health problem for Chinese women, particularly for those living in rural areas (4). CC originating from cervical intraepithelial neoplasia (CIN) is often caused by high risk-human papilloma virus (HR-HPV) infection (5). CIN is a group of cervical lesions that are associated with CC and are divided into three grades (I, II and III) (6,7). The majority of low-grade CIN cases naturally subside, but high-grade lesions continue to develop and break through the sub-epithelial basement membrane, at which point they are referred to as cervical invasive carcinoma (7,8). Histology is currently the basis for the diagnosis and classification of CIN (6).
The early detection of lesions by screening remains the primary prevention method for CC. CIN often occurs in women aged 25–35 years (6) and the highest incidence of CC occurs at ~47 years of age (9), suggesting a slow evolution from precancerous lesions to CC. Additionally, HPV vaccination for the prevention of CC is offered in numerous regions around the world, but it is not widely applied in a number of countries, including mainland China (4,10). Finally, since the 1950s, due to the widespread use of cervical cytology screening, cervical precancerous and cancerous lesions may be identified and treated early, resulting in a significant decrease in the incidence, and mortality of CC (11,12). Therefore, early detection of lesions by screening is effective for CC prevention. In addition, screening and detection of high-grade CIN lesions and early CC, and providing timely treatments may represent effective measures for increasing the rate of cure in these diseases.
The Papanicolaou (Pap) test is currently the main screening method for cervical precancerous changes and CC (13,14). However, the sensitivity of the Pap test varies greatly, ranging between 30–87%, and sometimes being as low as 20% (14,15). In addition, the infrastructure used for Pap screening is expensive and is difficult to implement in developing countries (16). Although the thin-layer liquid-based cervical cytology technique has improved recently, the sensitivity of the test has led to an important number of equivocal cytological results that require confirmation (9,17). Due to the fact that persistent HR-HPV infection is a well-established cause of cervical neoplasia, HPV nucleotide detection is an attractive method for the detection of cervical lesions (18–20), but only a small proportion of the HR-HPV infected individuals exhibit cervical lesions, and the HPV test demonstrates limited specificity in diagnosing CC, particularly in young women (21,22).
DNA methylation involves intensive epigenetic modifications that serve important roles in gene expression or silencing in normal mammalian cells (10,23). DNA methylation-induced alteration of C-phosphate-G (CpG) islands in tumor suppressor gene promoter regions is often observed in human cancer (24–27). It is currently acknowledged that hyper- or hypo-methylation of tumor suppressor gene promoter regions may contribute to cell transformation and thus, that the DNA methylation status is a promising biomarker for the detection of cancer (28). For CC, HPV viral DNA methylation acts as a potential biomarker for early cancer detection. For example, methylation of multiple genes, including paired box gene 1 (PAX1) (29), LIM homeobox transcription factor 1α (LMX1A), NK6 transcription factor-related locus 1 (30), SRY-box 1, Wilms tumor 1 and one cut homeobox 1 (31), have demonstrated varying degrees of sensitivity, specificity, and accuracy for the detection of CIN grades III and above. Nevertheless, the diagnostic accuracy of these genes requires further evaluation.
The purpose of the present study was to explore the diagnostic accuracy of the quantitative methylation analysis of two genes, PAX1 and LMX1A, in a full spectrum of cervical lesions in an Eastern Chinese population.
Patients and methods
Study design
This single-center prospective clinical study was approved by the Ethics Committee of the Central Hospital of Minhang District (Shanghai, China). Between July 2013 and September 2014, 121 subjects with cervical cancer were recruited, and tested for PAX1 and LMX1A methylation genes prior to undergoing pathological examination at the gynecological department of the Central Hospital of Minhang District. The tissue histopathology of cervical tissue was used for diagnosis and the diagnostic accuracy of CIN and CC was assessed using the gene methylation test.
Patients
The study recruited adult women from a population undergoing routine health examination and those clinically diagnosed with CC (age range 21–57; mean age, 37.15±8.6). Potential participants were screened for eligibility using a structured questionnaire and detailed clinical assessment. The inclusion criteria were as follows: i) Patients and healthy women receiving cytological examination, using the thin-layer liquid-based technique (32), of the cervical exfoliated cells and quantitative detection of HR-HPV DNA; ii) women aged 21–57 years with a history of sexual activity; and iii) women who provided written informed consent to participate in the study. The exclusion criteria were: i) Refusal to undergo further colposcopy, cervical biopsy or cervical loop electrosurgical excision and hysterectomy; ii) history of other malignant tumors; iii) treatment for other cervical diseases during the study; or iv) histopathological diagnosis of cervical adenocarcinoma.
Examination and grouping
All the participants received a histological examination and underwent a colposcopic cervical biopsy by a trained medical doctor. Cervical exfoliated cell specimens were taken by a nurse using a Cervex-Brush (Rovers Medical Devices, Oss, The Netherlands) and smeared onto a slide for cytological examination. The hospital team comprised 10 pathologists, each with >20 years of experience. Biopsied tissues were fixed in 10% neutral-buffered formalin for 24 h at 37°C. Paraffin-embedded sections (4-µm thick) were cut for hematoxylin and eosin staining (5–15 min at 37°C) and immunohistochemistry. To generate frozen (−20°C) sections, fresh tissues were embedded in Tissue-Tek O.C.T compound (Sakura Finetek Europe B.V., Flemingweg, The Netherlands) immediately after removal. Frozen sections (7 µm) were used for immunofloresence.
The participants were then grouped according to the tissue biopsy results as NC (normal cervix), LSIL (low-grade squamous intraepithelial lesion), HSIL (high-grade squamous intraepithelial lesion) or CSCC (cervical squamous cell carcinoma). Since 60% of the grade I CIN lesions naturally fade and require no treatment (only follow-up if the disease does not progress within 2 years), CIN1 lesions were classified as LSIL. Approximately 20% of CIN2 lesions progress to CIN3 and 5% of these lesions eventually become invasive cancer; therefore, CIN2 and CIN3 lesions were classified as HSIL (33).
Immunohistochemistry
For immunohistochemistry, all antibodies and reagents were purchased from Fuzhou Maixin Biotechnology Development Co., Ltd. (Fuzhou, China) and immunohistochemistry was performed according to the UltraSensitive™ SP (Mouse/Rabbit) IHC kit (cat no. KIT-9710; Fuzhou Maixin Biotechnology Development Co., Ltd.) manufacturer's protocol. The first step was to bake the paraffin section at 60°C for 2 h, followed by xylene dewaxing and alcohol hydration for 3 h at 60°C, following these procedures: xylene I for 60 min, xylene II for 30 min, 100% alcohol for 30 min, 95% alcohol for 15 min, 75% alcohol for 15 min, 50% alcohol for 15 min and washing with distilled water for 15 min. In order to block inactivated endogenous peroxidase, cells were incubated with 3% H2O2 at 37°C for 10 min, followed by washing with phosphate-buffered saline (PBS) three times for 5 min. Then, for antigen repair, 0.01 M citric acid tissue antigen repair solution (pH 6.0) was used for boiling (at 95°C, for 15 to 20 min), and then the cells were allowed to cool naturally for 20 min. Then, the cylinder was rinsed with cold water and cooling was accelerated to room temperature, followed by washing with PBS three times for 5 min. Normal sheep serum was enclosed with the cells and incubated at 37°C for 20 min, followed by removing the normal sheep serum without washing. The primary antibodies p16 (cat no. MAB-0673; Fuzhou Maixin Biotechnology Development Co., Ltd.) at 1:100 dilution and ki67 (cat no. MAB-0672; Fuzhou Maixin Biotechnology Development Co., Ltd) at 1:200 dilution were added respectively and refrigerated at 4°C overnight, and washed with PBS three times for 5 min (with PBS buffer used as a negative control). A total of 50 µl biotinylated goat anti-mouse/rabbit IgG [Buffer C from the UltraSensitive SP (Mouse/Rabbit) IHC kit; cat no. KIT-9710] was performed at 37°C for a 30-min incubation, followed by washing with PBS three times for 5 min. A total of 50 µl horseradish peroxidase-labeled streptomycin avidin working liquid [Buffer D from the UltraSensitive SP (Mouse/Rabbit) IHC kit, product number: KIT-9710], for the specific recognition of the biotin-labeled secondary antibody, was then added for a 30-min incubation at 37°C, followed by washing with PBS three times for 5 min. This method ensures a higher sensitivity (34). Then 3,3′-Diaminobenzidine/H2O2 reaction staining at 37°C for 3–10 min was performed. After washing with the tap water for 5 times within 15 min, the haematin was used for re-dying at 37°C for 1 min, followed by normal alcohol dehydration at 37°C for 5–10 min, treated with xylene for 4–6 min at 37°C to increase the transmittance of the specimen, and the specimen was covered with a square coverslip by adding the neutral gum to seal, followed by drying naturally at room temperature for later observation.
Immunofluorescence
Frozen sections (7 µm thick) were fixed with 2% paraformaldehyde in PBS at room temperature for 10 min followed by extraction using 0.5% Triton X-100 in PBS for 5 min, at room temperature. Blocking was then performed using 0.01 M PBS (pH 7.4) containing 10% normal goat serum and 0.3% Trixton X-100 for 1 h at room temperature, followed by the addition of the primary antibodies as follows: Human Anti-CDKN2A mouse monoclonal antibody (cat no. D199930; Sangon Biotech Co., Ltd., Shanghai, China) at a 1:100 dilution, diluted with with 0.01 M PBS (pH 7.4) containing 1% BSA and 0.3% Triton X-100 and human Ki-67 (D3B5) Rabbit mAb (Alexa Fluor® 647 Conjugate; cat no. 12075; Cell Signaling Technology) at a 1:50 dilution, diluted with 0.0 1 M PBS (pH 7.4) containing 1% BSA and 0.3% Triton X-100, and incubated with these antibodies at 4°C overnight. This was followed by washing with PBS three times for 10 min in the dark. Then, incubation with the secondary antibody FITC-conjugated Donkey Anti-Mouse IgG (cat no. D110081; Sangon Biotech Co., Ltd.) was performed at a 1:100 dilution, diluted with 0.01 M PBS (pH 7.4) containing 1% BSA and 0.3% Triton X-100 and incubated for 1 h at room temperature, followed by washing with PBS three times for 15 min. Fluorescence microscopy was performed following sealing. DNA was visualized using ~1.5 g/ml Hoechst 33342 (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). Fluorescence microscopy was performed using a 60× Plan Apo (NA 1.40) oil immersion objective lens and a Nikon TE2000-U inverted microscope equipped with a SPOT-RT CCD system.
DNA methylation: Cervical DNA extraction, bisulfite treatment and modification
DNA was extracted from the obtained exfoliated cells using an Omniscript RT kit (Qiagen, Inc., Valencia, CA, USA), according to the manufacturer's protocols. The extracted DNA was subjected to bisulfite treatment, DNA was tested for purity and concentration using Nanodrop™ 2000 (Thermo Fisher Scientific, Inc., Waltham, MA, USA). 500 ng DNA was placed into a polymerase chain reaction (PCR) tube, followed by denaturation at 95°C for 5 min, and refolding at 60°C for three rounds (for 25, 85 and then 175 min) of reciprocation on a PCR instrument using an EpiTect Bisulfite kit (Qiagen GmbH, Hilden, Germany). The reaction solution was transferred to a 1.5 ml Eppendorf tube and was then treated with Buffer BL, Buffer BW and Buffer BD (part of the EpiTect Bisulfite kit), respectively. Subsequently, the Eppendorf tube was centrifuged at 95°C for 1 min at a speed of 14,100 × g in the EpiTect spin columns (Qiagen GmbH), and finally the sulfite-treated product was eluted with 20 µl Buffer EB, according to the manufacturer's protocols. Polymerase chain reaction (PCR) was used to amplify the target fragments. The primer sequences used were as follows: PCR primer forward, 5′-TATTTTGGGTTTGGGGTCGC-3′ and reverse, 5′-CCCGAAAACCGAAAACCG-3′; sequencing primer, 5′-TTTTTGTTTTAGAGAGGTTAGTAAT-3′. The primers were all synthesized by Sangon Biotech Co., Ltd. (Shanghai, China). PCR was performed as follows: i) Initial denaturation at 95°C for 5 min; ii) denaturation at 94°C for 30 sec, annealing at 64°C for 30 sec and elongation at 72°C for 40 sec, for a total of 40 cycles; and iii) final elongation at 72°C for 10 min. The products were separated using a 2% agarose gel.
Quantitative bisulfite pyrosequencing analysis
Quantitative detection of methylation was performed for the suppressor genes in the cervical tissues. According to the methylation sequencing kit (Qiagen GmbH, Hilden, Germany), a reaction solution was prepared, containing 0.1 mol/l Tris Ac buffer (pH 7.7), 2 mmol/l EDTA, 10 mmol/l Mg(Ac) 2, 0.1% bovine serum albumin, 1 mmol/l dithiothreitol, 3 µmol/l 5′-phosphorylated adenosine sulfate, 0.4 µg/l polyvidone, 0.4 mmol/l D luciferin, 2×10-4 U/l ATP sulfurylase, 2×10−3 U/l dual phosphatase ATP and 18×10−3 U/l Klenow DNA polymerase (without exonuclease activity and containing 14.6 mg/l luciferase; New England Biolabs, Ipswich, MA, USA). Next, the methylation of the samples was measured and the average value was calculated based on the degree of methylation of the nine loci (according to the criteria (35) of methylation grouping that represents the degree of methylation for each sample).
Statistical analysis
One-way analysis of variance with Tukey's test used for post hoc analysis was used to evaluate differences in the percentage of methylation reference (PMR) among the groups. Continuous data are presented as the mean ± standard deviation. Categorical data were analyzed using the χ2 test. Receiver operating characteristic (ROC) curves were generated to confirm the accuracy of diagnosis for each gene, and the sensitivity and specificity were calculated. P<0.05 was considered to indicate a statistically significant difference. Statistical analysis was performed using SPSS 20.0 (IBM Corp., Armonk, NY, USA).
Results
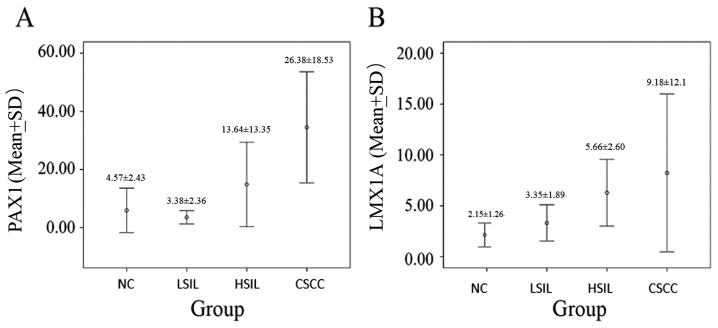
The patient distribution was n=28 for the NC group, n=32 for the LSIL, n=34 for the HSIL and n=27 for the CSCC group (Table I). The mean patient age increased with disease severity (P<0.006). The proportion of HPV-negative samples was significantly lower in the NC group compared with in the CIN and CC groups (P<0.05). The proportions of positive PAX1 and LMX1A methylated genes were higher in cervical tissues and exfoliated cells in the HSIL and CSCC groups compared with those in the LSIL and NC groups, but no significant difference was observed between the NC and LSIL groups (Table I; Fig. 1).
Table I.
Characteristics of participants and gene methylation.
| Variable | NC (n=28) | LSIL (n=32) | HSIL (n=34) | CSCC (n=27) | P-value |
|---|---|---|---|---|---|
| Age, years | 36.2±7.7 | 28.3±7.6 | 39.7±10.5 | 44.4±8.8 | 0.006 |
| HPV-negative | 18 | 6 | 3 | 2 | <0.001 |
| High-risk HPV | 10 | 26 | 31 | 25 | |
| HPV DNA | 89.85±95.77 | 480.23±702.79 | 630.28±623.75 | 1650.80±4595.88 | 0.23 |
| PAX1 in tissue | 4.92±4.45 | 5.55±5.05 | 10.21±14.39 | 41.97±23.02 | <0.001 |
| PAX1 in exfoliated cell | 4.57±2.43 | 3.38±2.36 | 13.64±13.35 | 26.38±18.53 | <0.001 |
| LMX1A in tissue | 4.53±3.76 | 5.05±3.06 | 4.70±5.12 | 14.36±18.31 | <0.001 |
| LMX1A in exfoliated cell | 2.15±1.26 | 3.35±1.89 | 5.66±2.60 | 9.18±12.1 | <0.001 |
| TCT, n | <0.001 | ||||
| Normal (NC) | 20 | 7 | 4 | 5 | |
| Low (CIN1) | 1 | 6 | 2 | 1 | |
| High (CIN2-3) | 1 | 1 | 17 | 18 | |
| ASC-US | 6 | 18 | 11 | 1 | |
| CSCC | 0 | 0 | 0 | 2 |
Values are in n, unless otherwise stated. NC, normal cervix; LSIL, low-grade squamous intraepithelial lesion; HSIL, high-grade squamous intraepithelial lesion; CSCC, cervical squamous-cell carcinoma; HPV, human papilloma virus; PAX1, paired box gene 1; LMX1A, LIM homeobox transcription factor 1 α; LMX2A, LIM homeobox transcription factor 2 α; TCT, thin-layer liquid-based cervical cytology; CIN, cervical intraepithelial neoplasia; ASC-US, atypical squamous cells of undetermined significance.
Figure 1.
Quantitative comparison of methylation of (A) PAX1 and (B) LMX1A in the cervical exfoliated cells among different groups. PAX1, paired box gene 1; LMX1A, LIM homeobox transcription factor 1 α; SD, standard deviation. NC, negative control; LSIL, low-grade squamous intraepithelial lesion; HSIL, high-grade squamous intraepithelial lesion; CSCC, cervical squamous-cell carcinoma.
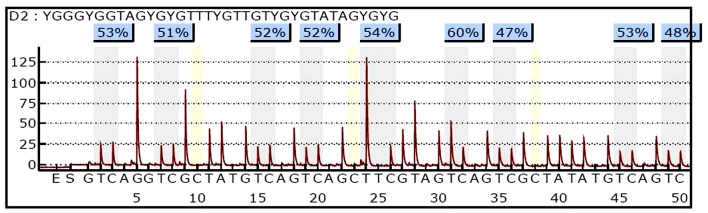
The methylation levels of PAX1 and LMX1A were quantitatively detected by pyrosequencing. An example of the PAX1 pyrosequencing results of a single specimen was demonstrated in Fig. 2.
Figure 2.
An example of the PAX1, paired box gene 1 pyrosequencing results of a single specimen. The gray areas are the 9 CpG sites, and the methylation percentage of each CpG site was automatically displayed at the top of the CpG site.
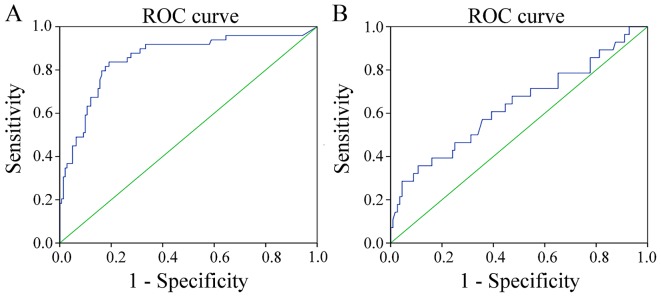
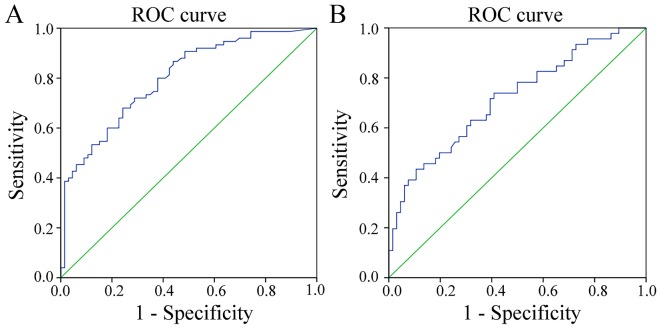
To evaluate the diagnostic potential of the two genes, respective ROC curves were produced (Figs. 3 and 4). Methylated PAX1 demonstrated a greater ability to detect cancer compared with LMX1A (the sensitivities at the cut-off points were 0.837 and 0.357, respectively; P<0.001), although the specificity values for the two genes were similar and high (0.809 and 0.893, respectively; Table II). In addition, when comparing the ability to distinguish HSIL lesions from normal tissues and LSIL, the sensitivities at the cut-off values were similar, but not high (0.680 and 0.739, respectively), while the specificities of the two genes differed significantly (0.758 vs. 0.591; P<0.001; Table III).
Figure 3.
ROC curves of the diagnostic threshold of quantitative methylation of (A) paired box gene 1 and (B) LIM homeobox transcription factor 1 α in the exfoliated cells. Cervical squamous-cell carcinoma vs. high-grade squamous intraepithelial lesion+low-grade squamous intraepithelial lesion+negative control. ROC, receiver operating characteristic; CSCC, cervical squamous-cell carcinoma.
Figure 4.
ROC curves of the diagnostic threshold of quantitative methylation of (A) paired box gene 1 and (B) LIM homeobox transcription factor 1 α in the exfoliated cells. high-grade squamous intraepithelial lesion vs. low-grade squamous intraepithelial lesion+negative control. ROC, receiver operating characteristic.
Table II.
Sensitivity and specificity of PAX1 and LMX1A for distinguishing CSCC from HSIL, LSIL and NC (CSCC vs. HSIL+LSIL+NC).
| Gene | Cut-off | AUC | Sensitivity, % | Specificity, % | P-value | OR (95% CI) |
|---|---|---|---|---|---|---|
| PAX1 | 11.78 | 0.790 | 0.837 | 0.809 | <0.001 | 0.788–0.923 |
| LMX1A | 7.185 | 0.633 | 0.357 | 0.893 | 0.029 | 0.508–0.758 |
CSCC, cervical squamous-cell carcinoma; HSIL, high-grade squamous intraepithelial lesion; NC, normal cervix; LSIL, low grade squamous intraepithelial lesion; AUC, area under the curve; OR, odds ratio; CI, confidence interval; PAX1, paired box gene 1; LMX1A, LIM homeobox transcription factor 1 α.
Table III.
Sensitivity and specificity of PAX1 and LMX1A for distinguishing HSIL from LSIL+NC (HSIL vs. LSIL+NC).
| Gene | Cut-off | AUC | Sensitivity, % | Specificity, % | P-value | OR (95% CI) |
|---|---|---|---|---|---|---|
| PAX1 | 5.405 | 0.799 | 0.680 | 0.758 | <0.001 | 0.727–0.871 |
| LMX1A | 4.730 | 0.716 | 0.739 | 0.591 | <0.001 | 0.619–0.813 |
PAX1, paired box gene 1; LMX1A, LIM homeobox transcription factor 1 α; HSIL, high-grade squamous intraepithelial lesion; LSIL, low grade squamous intraepithelial lesion; NC, normal cervix; AUC, area under the curve; OR, odds ratio; CI, confidence interval.
Discussion
DNA methylation serves an important role in the regulation of gene expression or silencing in normal mammalian cells and has been proposed as a potential biomarker for the detection of cervical cancer (10,23). DNA methylation-induced alteration of CpG islands in the tumor suppressor gene promoter regions are often observed in human cancer. The results of the present study revealed that the PMR of the two genes were significantly higher in the HSIL and CSCC groups compared with those in the NC, and LSIL groups. ROC curve analysis demonstrated that the sensitivity, specificity and accuracy for detecting CSCC of 0.790, 0.837 and 0.809, respectively using LMX1A and 0.633, 0.357 and 0.893, respectively using PAX1. Previous studies focused on detecting cervical cancer were hindered by inconsistent results of quantitative DNA methylation analysis, and moderate sensitivities and specificities using the available genes (10,23).
Genes of the paired box (PAX) family serve important roles in embryonic development and organogenesis, and may be expressed persistently in stem cells and mature cells (36,37). Specific PAX proteins are able to maintain stem cell properties, and are involved in the development and progression of solid tumors and hematologic cancer (38). As a downstream product of the gene, the PAX protein may be expressed in tissue-specific stem cells (39). Previous studies have suggested that the anti-apoptotic function of PAX proteins may encourage tumor cells to continuously grow without undergoing apoptosis (38,40). In breast cancer cells, PAX2 and estrogen receptor complexes regulate the expression of human epidermal growth factor 2, which determines the response of tumor cells to tamoxifen (41). PAX3 and PAX7, as well as forkhead box protein O1, participate in rhabdomyosarcoma formation through chromosomal rearrangement (42,43). In hepatocellular carcinoma, PAX5 inhibits tumor formation by mediating P53-associated signaling pathways (44). However, to the best of our knowledge, there is no current literature regarding the mechanisms of the PAX1 protein in malignant tumor development.
PAX1, as a member of the PAX family, serves an important regulatory role in the early development of an embryo, and is involved in the formation of bone, thymus and parathyroid glands (38,45,46). Inactivation of PAX1 has been observed in patients with CC and is considered to be associated with the methylation of the promoter region (47,48). In a hospital-based study on CC detection, Huang et al (49) observed that the quantitative measurement of PAX1 hypermethylation in cervical samples was highly sensitive and more specific compared with the Hybrid Capture 2 HPV test (0 vs. 5.9% in normal tissue). In the past, the study of PAX1 gene methylation in the cervix was found to be used for the differential diagnosis of invasive carcinoma (50). In the present study, the pyrosequencing quantitative methylation method confirmed the methylation analysis levels of PAX1, which identified CSCC or HSIL to a certain degree, as the AUCs were 0.790 (95% CI, 0.788–0.923) and 0.799 (95% CI, 0.727–0.871), respectively. When PAX1 methylation was detected for differentiating CSCC, the sensitivity and specificity were 0.837 and 0.809, respectively. However, these indices decreased to 0.680 and 0.758 when HSIL was screened for. These data revealed that the detection of PAX1 methylation has clinical diagnostic value in differentiating invasive CC, but may not be sufficient alone in screening for HSIL. A number of studies have indicated the potential value of PAX1 for the screening and detection of CC (49,51,52), in line with the findings of the present study, but the association between PAX1 and tumors requires further investigation. The present study used methylation-specific PCR to demonstrate that the PAX1 gene is abnormally methylated in cervical cancer specimens, with methylation rates as high as 87.5%, which is significantly different to those in normal cervical tissues and cervical precancerous lesions (49). Furthermore, the diagnostic sensitivity was twice that of the HPV-HC2 assay (53).
LMX1A is an important homeobox transcription factor in the process of cell development; it binds to AT-rich sequences in the insulin promoter and stimulates the transcription of insulin (54). In a previous study, LMX1A methylation testing demonstrated great potential for cervical lesion screening with a sensitivity, specificity and accuracy of 0.77, 0.88 and 0.90, respectively (30). In the present study, the pyrosequencing method was used to confirm the methylation of the LMX1A gene in cervical epithelial malignant transformation, but it was hardly methylated in LSIL. Therefore, LMX1A methylation in cervical tissue detection may provide valuable information regarding the differentiation of invasive cancer, HSIL and LSIL. ROC analysis revealed a sensitivity and specificity of 0.357 and 0.893, respectively, for CSCC; while the specificity for HSIL was 0.591. These unsatisfactory data indicated that it may be necessary to combine other detection methods to improve accuracy. LMX1A methylation is dysregulated in gastric, bladder, breast, ovarian and pancreatic cancer (55–58). A study on various types of cancer may provide useful insights into the involvement of LMX1A methylation in tumorigenesis.
The present study has certain limitations. In addition to the small sample size, there was no combined analysis of the two genes or combined analysis of either gene using another test, as the preliminary results suggested that combined examination of multiple indices may be a feasible approach to improving the diagnostic accuracy of differentiating cervical lesions. In addition, the association between the two gene methylation statuses and disease prognosis was not analyzed due to problems at follow-up. Further multicenter studies, with larger sample sizes and strictly designed diagnostic criteria are required to obtain definitive conclusions.
In conclusion, quantitative detection of PAX1 methylation exhibited good diagnostic value in differentiating HSIL from CSCC in cervical tissues, while the efficiency of LMX1A methylation as a diagnostic tool requires further investigation.
Acknowledgements
The present study was supported by the National Wu Jieping Foundation for Clinical Scientific Research (grant no. 320.6750.13152) and the Project of Minhang Central Hospital (grant no. 2016MHJC06).
References
- 1.Divine LM, Huh WK. Tertiary prevention of cervical cancer. Clin Obstet Gynecol. 2014;57:316–324. doi: 10.1097/GRF.0000000000000020. [DOI] [PubMed] [Google Scholar]
- 2.Vizcaino AP, Moreno V, Bosch FX, Muñoz N, Barros-Dios XM, Borras J, Parkin DM. International trends in incidence of cervical cancer: II. Squamous-cell carcinoma. Int J Cancer. 2000;86:429–435. doi: 10.1002/(SICI)1097-0215(20000501)86:3<429::AID-IJC20>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 3.Vizcaino AP, Moreno V, Bosch FX, Muñoz N, Barros-Dios XM, Parkin DM. International trends in the incidence of cervical cancer: I. Adenocarcinoma and adenosquamous cell carcinomas. Int J Cancer. 1998;75:536–545. doi: 10.1002/(SICI)1097-0215(19980209)75:4<536::AID-IJC8>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 4.Li J, Kang LN, Qiao YL. Review of the cervical cancer disease burden in mainland China. Asian Pac J Cancer Prev. 2011;12:1149–1153. [PubMed] [Google Scholar]
- 5.Burd EM. Human papillomavirus and cervical cancer. Clin Microbiol Rev. 2003;16:1–17. doi: 10.1128/CMR.16.1.1-17.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar V, Abbas AK, Fausto N, Mitchell RN. Robbins Basic Pathology. Saunders Elsevier; Dublin: 2007. [Google Scholar]
- 7.Waxman AG, Chelmow D, Darragh TM, Lawson H, Moscicki AB. Revised terminology for cervical histopathology and its implications for management of high-grade squamous intraepithelial lesions of the cervix. Obstet Gynecol. 2012;120:1465–1471. doi: 10.1097/AOG.0b013e31827001d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agorastos T, Miliaras D, Lambropoulos AF, Chrisafi S, Kotsis A, Manthos A, Bontis J. Detection and typing of human papillomavirus DNA in uterine cervices with coexistent grade I and grade III intraepithelial neoplasia: Biologic progression or independent lesions? Eur J Obstet Gynecol Reprod Biol. 2005;121:99–103. doi: 10.1016/j.ejogrb.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 9.Leda G, Akila NV, Carlos AP, William PT, Sharmila M. http://www.cancernetwork.com/cancer-management/cervical. [Nov 01;2015 ];Cervical Cancer. [Google Scholar]
- 10.Jin B, Li Y, Robertson KD. DNA methylation: Superior or subordinate in the epigenetic hierarchy? Genes Cancer. 2011;2:607–617. doi: 10.1177/1947601910393957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee JY, Soong SJ. Cancer mortality in the South, 1950 to 1980. South Med J. 1990;83:185–190. doi: 10.1097/00007611-199002000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Joshi S, Kulkarni V, Darak T, Mahajan U, Srivastava Y, Gupta S, Krishnan S, Mandolkar M, Bharti AC. Cervical cancer screening and treatment of cervical intraepithelial neoplasia in female sex workers using ‘screen and treat’ approach. Int J Womens Health. 2015;7:477–483. doi: 10.2147/IJWH.S80624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pak SC, Martens M, Bekkers R, Crandon AJ, Land R, Nicklin JL, Perrin LC, Obermair A. Pap smear screening history of women with squamous cell carcinoma and adenocarcinoma of the cervix. Aust N Z J Obstet Gynaecol. 2007;47:504–507. doi: 10.1111/j.1479-828X.2007.00788.x. [DOI] [PubMed] [Google Scholar]
- 14.Geldenhuys L, Murray ML. Sensitivity and specificity of the Pap smear for glandular lesions of the cervix and endometrium. Acta Cytol. 2007;51:47–50. doi: 10.1159/000325682. [DOI] [PubMed] [Google Scholar]
- 15.Nanda K, McCrory DC, Myers ER, Bastian LA, Hasselblad V, Hickey JD, Matchar DB. Accuracy of the Papanicolaou test in screening for and follow-up of cervical cytologic abnormalities: A systematic review. Ann Intern Med. 2000;132:810–819. doi: 10.7326/0003-4819-132-10-200005160-00009. [DOI] [PubMed] [Google Scholar]
- 16.Lazcano-Ponce E, Alonso P, Ruiz-Moreno JA, Hernández-Avila M. Recommendations for cervical cancer screening programs in developing countries. The need for equity and technological development. Salud Publica Mex. 2003;45(Suppl 3):S449–S462. doi: 10.1590/S0036-36342003000900020. [DOI] [PubMed] [Google Scholar]
- 17.Nuovo J, Melnikow J, Howell LP. New tests for cervical cancer screening. Am Fam Physician. 2001;64:780–786. [PubMed] [Google Scholar]
- 18.Flores-Miramontes MG, Torres-Reyes LA, Alvarado-Ruíz L, Romero-Martínez SA, Ramírez-Rodríguez V, Balderas-Peña LM, Vallejo-Ruíz V, Piña-Sánchez P, Cortés-Gutiérrez EI, Jave-Suárez LF, Aguilar-Lemarroy A. Human papillomavirus genotyping by linear array and next-generation Sequencing in cervical samples from Western Mexico. Virol J. 2015;12:161. doi: 10.1186/s12985-015-0391-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Othman N, Othman NH. Detection of human papillomavirus DNA in routine cervical scraping samples: Use for a national cervical cancer screening program in a developing nation. Asian Pac J Cancer Prev. 2014;15:2245–2249. doi: 10.7314/APJCP.2014.15.5.2245. [DOI] [PubMed] [Google Scholar]
- 20.Natter C, Polterauer S, Rahhal-Schupp J, Cacsire Castillo-Tong D, Pils S, Speiser P, Zeillinger R, Heinze G, Grimm C. Association of TAP gene polymorphisms and risk of cervical intraepithelial neoplasia. Dis Markers. 2013;35:79–84. doi: 10.1155/2013/368732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cuzick J, Clavel C, Petry KU, Meijer CJ, Hoyer H, Ratnam S, Szarewski A, Birembaut P, Kulasingam S, Sasieni P, Iftner T. Overview of the European and North American studies on HPV testing in primary cervical cancer screening. Int J Cancer. 2006;119:1095–1101. doi: 10.1002/ijc.21955. [DOI] [PubMed] [Google Scholar]
- 22.Whitlock EP, Vesco KK, Eder M, Lin JS, Senger CA, Burda BU. Liquid-based cytology and human papillomavirus testing to screen for cervical cancer: A systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2011;155:687–697, W214-W215. doi: 10.7326/0003-4819-155-10-201111150-00376. [DOI] [PubMed] [Google Scholar]
- 23.Loscalzo J, Handy DE. Epigenetic modifications: Basic mechanisms and role in cardiovascular disease (2013 Grover Conference series) Pulm Circ. 2014;4:169–174. doi: 10.1086/675979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sakamoto A, Akiyama Y, Shimada S, Zhu WG, Yuasa Y, Tanaka S. DNA Methylation in the Exon 1 region and complex regulation of Twist1 expression in gastric cancer cells. PLoS One. 2015;10:e0145630. doi: 10.1371/journal.pone.0145630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fan H, Zhang H, Pascuzzi PE, Andrisani O. Hepatitis B virus X protein induces EpCAM expression via active DNA demethylation directed by RelA in complex with EZH2 and TET2. Oncogene. 2016;35:715–726. doi: 10.1038/onc.2015.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmid CA, Robinson MD, Scheifinger NA, Müller S, Cogliatti S, Tzankov A, Müller A. DUSP4 deficiency caused by promoter hypermethylation drives JNK signaling and tumor cell survival in diffuse large B cell lymphoma. J Exp Med. 2015;212:775–792. doi: 10.1084/jem.20141957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sood S, Srinivasan R. Alterations in gene promoter methylation and transcript expression induced by cisplatin in comparison to 5-Azacytidine in HeLa and SiHa cervical cancer cell lines. Mol Cell Biochem. 2015;404:181–191. doi: 10.1007/s11010-015-2377-3. [DOI] [PubMed] [Google Scholar]
- 28.Kulis M, Esteller M. DNA methylation and cancer. Adv Genet. 2010;70:27–56. doi: 10.1016/B978-0-12-380866-0.60002-2. [DOI] [PubMed] [Google Scholar]
- 29.Chao TK, Ke FY, Liao YP, Wang HC, Yu CP, Lai HC. Triage of cervical cytological diagnoses of atypical squamous cells by DNA methylation of paired boxed gene 1 (PAX1) Diagn Cytopathol. 2013;41:41–46. doi: 10.1002/dc.21758. [DOI] [PubMed] [Google Scholar]
- 30.Lai HC, Lin YW, Huang RL, Chung MT, Wang HC, Liao YP, Su PH, Liu YL, Yu MH. Quantitative DNA methylation analysis detects cervical intraepithelial neoplasms type 3 and worse. Cancer. 2010;116:4266–4274. doi: 10.1002/cncr.25252. [DOI] [PubMed] [Google Scholar]
- 31.Lai HC, Lin YW, Huang TH, Yan P, Huang RL, Wang HC, Liu J, Chan MW, Chu TY, Sun CA, et al. Identification of novel DNA methylation markers in cervical cancer. Int J Cancer. 2008;123:161–167. doi: 10.1002/ijc.23519. [DOI] [PubMed] [Google Scholar]
- 32.Hammou JC, Bertino B, Blancheri A, Kon Man P, Patoz L. Pap test: Liquid-based-thin-layer. A new method: Results. Gynecol Obstet Fertil. 2003;31:833–840. doi: 10.1016/j.gyobfe.2003.01.001. (In French) [DOI] [PubMed] [Google Scholar]
- 33.WHO, corp-author. http://screening.iarc.fr/colpochap.php?chap=2. [Aug 15;2015 ];An introduction to cervical intraepithelial neoplasia (CIN) [Google Scholar]
- 34.Elias JM, Margiotta M, Gaborc D. Sensitivity and detection efficiency of the peroxidase antiperoxidase (PAP), avidin-biotin peroxidase complex (ABC), and peroxidase-labeled avidin-biotin (LAB) methods. Am J Clin Pathol. 1989;92:62–67. doi: 10.1093/ajcp/92.1.62. [DOI] [PubMed] [Google Scholar]
- 35.Xu J, Xu L, Yang BH, Wang LF, Lin X, Tu H. Assessing methylation status of PAX1 in cervical scrapings, as a novel diagnostic and predictive biomarker, was closely related to screen cervical cancer. Int J Clin Exp Pathol. 2015;8:1674–1681. [PMC free article] [PubMed] [Google Scholar]
- 36.Wehr R, Gruss P. Pax and vertebrate development. Int J Dev Biol. 1996;40:369–377. [PubMed] [Google Scholar]
- 37.Feiner N, Meyer A, Kuraku S. Evolution of the vertebrate Pax4/6 class of genes with focus on its novel member, the Pax10 gene. Genome Biol Evol. 2014;6:1635–1651. doi: 10.1093/gbe/evu135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lang D, Powell SK, Plummer RS, Young KP, Ruggeri BA. PAX genes: Roles in development, pathophysiology, and cancer. Biochem Pharmacol. 2007;73:1–14. doi: 10.1016/j.bcp.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 39.Lang D, Lu MM, Huang L, Engleka KA, Zhang M, Chu EY, Lipner S, Skoultchi A, Millar SE, Epstein JA. Pax3 functions at a nodal point in melanocyte stem cell differentiation. Nature. 2005;433:884–887. doi: 10.1038/nature03292. [DOI] [PubMed] [Google Scholar]
- 40.Sharma R, Sanchez-Ferras O, Bouchard M. Pax genes in renal development, disease and regeneration. Semin Cell Dev Biol. 2015;44:97–106. doi: 10.1016/j.semcdb.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 41.Hurtado A, Holmes KA, Geistlinger TR, Hutcheson IR, Nicholson RI, Brown M, Jiang J, Howat WJ, Ali S, Carroll JS. Regulation of ERBB2 by oestrogen receptor-PAX2 determines response to tamoxifen. Nature. 2008;456:663–666. doi: 10.1038/nature07483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Galili N, Davis RJ, Fredericks WJ, Mukhopadhyay S, Rauscher FJ, III, Emanuel BS, Rovera G, Barr FG. Fusion of a fork head domain gene to PAX3 in the solid tumour alveolar rhabdomyosarcoma. Nat Genet. 1993;5:230–235. doi: 10.1038/ng1193-230. [DOI] [PubMed] [Google Scholar]
- 43.Bennicelli JL, Advani S, Schäfer BW, Barr FG. PAX3 and PAX7 exhibit conserved cis-acting transcription repression domains and utilize a common gain of function mechanism in alveolar rhabdomyosarcoma. Oncogene. 1999;18:4348–4356. doi: 10.1038/sj.onc.1202812. [DOI] [PubMed] [Google Scholar]
- 44.Liu W, Li X, Chu ES, Go MY, Xu L, Zhao G, Li L, Dai N, Si J, Tao Q, et al. Paired box gene 5 is a novel tumor suppressor in hepatocellular carcinoma through interaction with p53 signaling pathway. Hepatology. 2011;53:843–853. doi: 10.1002/hep.24124. [DOI] [PubMed] [Google Scholar]
- 45.McGaughran JM, Oates A, Donnai D, Read AP, Tassabehji M. Mutations in PAX1 may be associated with Klippel-Feil syndrome. Eur J Hum Genet. 2003;11:468–474. doi: 10.1038/sj.ejhg.5200987. [DOI] [PubMed] [Google Scholar]
- 46.Bannykh SI, Emery SC, Gerber JK, Jones KL, Benirschke K, Masliah E. Aberrant Pax1 and Pax9 expression in Jarcho-Levin syndrome: Report of two Caucasian siblings and literature review. Am J Med Genet A. 2003;120A:1–246. doi: 10.1002/ajmg.a.20192. [DOI] [PubMed] [Google Scholar]
- 47.Schnittger S, Rao VV, Deutsch U, Gruss P, Balling R, Hansmann I. Pax1, a member of the paired box-containing class of developmental control genes, is mapped to human chromosome 20p11.2 by in situ hybridization (ISH and FISH) Genomics. 1992;14:740–744. doi: 10.1016/S0888-7543(05)80177-6. [DOI] [PubMed] [Google Scholar]
- 48.Zhang Y, Chen FQ, Sun YH, Zhou SY, Li TY, Chen R. Effects of DNMT1 silencing on malignant phenotype and methylated gene expression in cervical cancer cells. J Exp Clin Cancer Res. 2011;30:98. doi: 10.1186/1756-9966-30-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang TH, Lai HC, Liu HW, Lin CJ, Wang KH, Ding DC, Chu TY. Quantitative analysis of methylation status of the PAX1 gene for detection of cervical cancer. Int J Gynecol Cancer. 2010;20:513–519. doi: 10.1111/IGC.0b013e3181c7fe6e. [DOI] [PubMed] [Google Scholar]
- 50.Chen W, Yang HJ, Xu J, Zhu HP. Quantitative analysis of LMX1A and PAX1 gene methylation in cervical cancer and cervical intraepithelial neoplasia. China Oncol. 2015;25:19–24. [Google Scholar]
- 51.Kan YY, Liou YL, Wang HJ, Chen CY, Sung LC, Chang CF, Liao CI. PAX1 methylation as a potential biomarker for cervical cancer screening. Int J Gynecol Cancer. 2014;24:928–934. doi: 10.1097/IGC.0000000000000155. [DOI] [PubMed] [Google Scholar]
- 52.Lai HC, Ou YC, Chen TC, Huang HJ, Cheng YM, Chen CH, Chu TY, Hsu ST, Liu CB, Hung YC, et al. PAX1/SOX1 DNA methylation and cervical neoplasia detection: A Taiwanese Gynecologic Oncology Group (TGOG) study. Cancer Med. 2014;3:1062–1074. doi: 10.1002/cam4.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lim EH, Ng SL, Li JL, Chang AR, Ng J, Ilancheran A, Low J, Quek SC, Tay EH. Cervical dysplasia: Assessing methylation status (Methylight) of CCNA1, DAPK1, HS3ST2, PAX1 and TFPI2 to improve diagnostic accuracy. Gynecol Oncol. 2010;119:225–231. doi: 10.1016/j.ygyno.2010.07.028. [DOI] [PubMed] [Google Scholar]
- 54.German MS, Wang J, Fernald AA, Espinosa R, III, Le Beau MM, Bell GI. Localization of the genes encoding two transcription factors, LMX1 and CDX3, regulating insulin gene expression to human chromosomes 1 and 13. Genomics. 1994;24:403–404. doi: 10.1006/geno.1994.1639. [DOI] [PubMed] [Google Scholar]
- 55.Dong W, Feng L, Xie Y, Zhang H, Wu Y. Hypermethylation-mediated reduction of LMX1A expression in gastric cancer. Cancer Sci. 2011;102:361–366. doi: 10.1111/j.1349-7006.2010.01804.x. [DOI] [PubMed] [Google Scholar]
- 56.Zhao Y, Guo S, Sun J, Huang Z, Zhu T, Zhang H, Gu J, He Y, Wang W, Ma K, et al. Methylcap-seq reveals novel DNA methylation markers for the diagnosis and recurrence prediction of bladder cancer in a Chinese population. PLoS One. 2012;7:e35175. doi: 10.1371/journal.pone.0035175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Su HY, Lai HC, Lin YW, Chou YC, Liu CY, Yu MH. An epigenetic marker panel for screening and prognostic prediction of ovarian cancer. Int J Cancer. 2009;124:387–393. doi: 10.1002/ijc.23957. [DOI] [PubMed] [Google Scholar]
- 58.Hagihara A, Miyamoto K, Furuta J, Hiraoka N, Wakazono K, Seki S, Fukushima S, Tsao MS, Sugimura T, Ushijima T. Identification of 27 5′ CpG islands aberrantly methylated and 13 genes silenced in human pancreatic cancers. Oncogene. 2004;23:8705–8710. doi: 10.1038/sj.onc.1207783. [DOI] [PubMed] [Google Scholar]