Abstract
The present study aimed to investigate the expression patterns of prothymosin-α (PTMA) and parathymosin (PTMS) in patients with squamous cell carcinoma (SCC), adenosquamous cell carcinoma (ASC) and adenocarcinoma (AC) of the gallbladder, and to assess their association with the clinicopathological characteristics and prognosis of the patients. A retrospective analysis of data pertaining to patients with SCC/ASC (n=46) and AC (n=80) of the gallbladder, who were treated with surgical resection, was conducted. Kaplan-Meier survival analysis was also performed to assess the correlation of the expression pattern with survival. The results revealed a higher percentage of patients with a large tumor diameter (>3 cm) in the SCC/ASC group as compared with those in the AC group (P<0.05). No significant differences were observed between patients with SCC/ASC and those with AC with respect to the patient sex, presence of gallstones, TNM stage, lymph node metastasis, invasive growth into anatomically contiguous structures, surgical methods used, survival rate, and the expression levels of PTMA and PTMA (P>0.05). However, positive expression of PTMA and PTMA was associated with tumor size, TNM stage, lymph node metastasis, locally invasive growth, and treatment with radical resection in patients with SCC/ASC and AC (P<0.05). In addition, positive expression of PTMA and PTMA was observed in a significantly lower number of patients with advanced AC as compared with those in early AC (P<0.05), while these expression levels were also associated with shorter survival in the SCC/ASC group and AC group (P<0.05). Cox multivariate analysis also demonstrated a negative correlation between PTMA and PTMA levels, and the postoperative survival rate in the two groups. In conclusion, the present study indicated that the expression levels of PTMA and PTMA were closely associated with the tumorigenesis and progression of SCC, ASC and AC of the gallbladder. Positive expression of PTMA and PTMA may serve as a valuable prognostic factor in these patients.
Keywords: squamous cell carcinoma, adenosquamous carcinoma, gallbladder, prothymosin-α, parathymosin, prognosis
Introduction
Gallbladder carcinoma (GBC) is a relatively rare, yet highly lethal neoplasm of the digestive tract. The most common histotype of GBC is adenocarcinoma (AC), while squamous cell carcinoma (SCC) and adenosquamous cell carcinoma (ASC) are relatively rare entities, accounting for 1.4–10.6% of all GBC cases (1). The biological and clinicopathological characteristics of SCC and ASC of the gallbladder are not well-characterized owing to their low incidence. Squamous metaplasia of the gallbladder mucosa or of the pluripotent basal cells in the gallbladder mucus membrane in response to chronic inflammation caused by gallstones has been suggested as the initial event in tumorigenesis (2–4). In addition, another study observed that SCC and ASC of the gallbladder may result from the exposure to certain carcinogens, including cholanthrene and methylanthracene (5). In addition, the histomorphology and biological characteristics of SCC and ASC differ from those of AC, in that the former two types of cancer exhibit a greater proliferation capacity and aggressive invasion of anatomically contiguous structures, while demonstrating a lower propensity for distant metastasis and involvement of regional lymph nodes (6). Notably, there are no detectable symptoms in the early stages of SCC and ASC of the gallbladder, with the exception of right upper abdominal pain and discomfort (7). The clinical outcomes of surgical resection in patients with advanced SCC or ASC of the gallbladder are typically poor (8). Therefore, early diagnosis and radical surgery may significantly improve the prognosis of these patients.
Prothymosin-α (PTMA), a member of the α-thymosin family, is a transcription factor that consists of 110 highly-conserved acidic amino acids in mammalian cells (9). Previous studies have indicated that PTMA may be associated with cell proliferation, apoptosis and the regulation of cell cycle progression in tumor cells (10,11). In addition, abnormal expression of PTMA has been reported in several malignant tumors, including gastric, colorectal and thyroid cancer, and upper urinary tract transitional cell carcinoma (12–15). Similarly, parathymosin (PTMS) is another homologue of PTMA (16,17), which was first isolated from the mouse thymus in 1985 (18) and was found to promote cell proliferation by downregulating the level of glucocorticoids (19). PTMA has been demonstrated to be associated with RNA synthesis and processing, while PTMS is involved in early DNA replication (20). Furthermore, the expression levels of PTMA and PTMS were reported to be closely correlated with malignant growth, metastasis and prognosis in the context of several tumors (19). Thus, it is hypothesized that high expression levels of PTMA and PTMS may be useful as potential prognostic biomarkers in patients with SCC and ASC of the gallbladder.
PTMA and PTMS are known to be involved in DNA transcription and replication, respectively. Previous studies have indicated the positive expression levels of PTMA/PTMS in gastric adenocarcinoma (14), colorectal cancer (15), and human upper urinary tract transitional cell carcinoma (12), and their expression levels have been demonstrated to be associated with tumor migration, tumor malignancy, and prognosis. However, the expression levels of PTMA/PTMS were still unknown in gallbaladder carcinoma. In this present study, the association between PTMA/PTMS expression and the prognosis in SCC/ASC and AC of the gallbladder was investigated.
Materials and methods
Patients
In the present study, patients with SCC and ASC were treated as a single experimental group since SCC and ASC are the minor types of GBC. A total of 46 patients with SCC or ASC of the gallbladder who were hospitalized between January 1995 and December 2009 were included into the present study. These 46 cases accounted for 4.34% of the total of 1,060 patients with GBC admitted to the Second Xiangya Hospital of Central South University (16/370; Changsha, China), the Xiangya Hospital of Central South University (14/325; Changsha, China), the Third Xiangya Hospital of Central South University (5/110; Changsha, China), the Hunan Provincial People's Hospital, The First Affiliated Hospital of Hunan Normal University (5/105; Changsha, China), The Hunan Cancer Hospital, The Affiliated Cancer Hospital of Xiangya School of Medicine, Central South University (4/100; Changsha, China), the Central Hospital of Changde (1/50; Changde, China) and the Central Hospital of Loudi (1/50; Loudi, China). The inclusion criterion was patients pathologically diagnosed with ACC, ASC, and SCC, whereas the exclusion criterion was patients pathologically diagnosed with neither GBC nor other types of ACC/ASC/SCC (21). All of the tumor samples were obtained by intraopherative biopsy, and none of the patients received any other treatment.
In addition, the pathological specimens of 80 patients with AC of the gallbladder, who received surgical treatment at the Second Xiangya Hospital of Central South University and the Central Hospital of Loudi between January 2000 and December 2009, were included in the present study. The clinical features of the selected patient samples, including the age, sex, histopathological subtype, TNM stage, lymph node invasion, distant metastasis, resection methods, tumor stages and survival rates, in the SCC/ASC and AC groups were embedded in paraffin and pathologically analyzed (Table I) (22,23). The present retrospective study was approved by the Medical Ethics Committee of Second Xiangya Hospital of Central South University. All the patients signed a written informed consent form prior to participation.
Table I.
Clinicopathological characteristics of patients with SCC/ASC and AC of the gallbladder.
| Characteristic | SCC/ASC (n=46), % | AC (n=80), % | χ2 | P-value |
|---|---|---|---|---|
| Sex | 0.986 | 0.352 | ||
| Male | 19 (41.3) | 26 (32.5) | ||
| Female | 27 (58.7) | 54 (67.5) | ||
| Age | 4.143 | 0.042 | ||
| ≤45 years | 3 (6.5) | 16 (20.0) | ||
| >45 years | 43 (93.5) | 64 (80.0) | ||
| Differentiation | 8.515 | 0.014 | ||
| Well-differentiated | 16 (34.8) | 27 (33.8) | ||
| Moderately differentiated | 24 (52.2) | 25 (31.3) | ||
| Poorly differentiated | 6 (13.0) | 28 (35.0) | ||
| Tumor diameter | 4.280 | 0.039 | ||
| ≤3 cm | 20 (43.5) | 50 (62.5) | ||
| >3 cm | 26 (56.5) | 30 (37.5) | ||
| Gallstones | 2.093 | 0.148 | ||
| Negative | 18 (39.1) | 42 (52.5) | ||
| Positive | 28 (60.9) | 38 (47.5) | ||
| TNM stages | 0.287 | 0.866 | ||
| I/II | 12 (26.1) | 21 (26.3) | ||
| III | 20 (33.5) | 38 (47.5) | ||
| IV | 14 (30.4) | 21 (26.3) | ||
| Lymph node invasion | 0.004 | 0.952 | ||
| Negative | 17 (37.0) | 30 (37.5) | ||
| Positive | 29 (63.0) | 50 (62.5) | ||
| Invasion of adjacent organs | 0.197 | 0.658 | ||
| Negative | 16 (34.8) | 31 (38.8) | ||
| Positive | 30 (62.5) | 49 (61.3) | ||
| Resection | 0.215 | 0.898 | ||
| Curative resection | 14 (30.4) | 26 (32.5) | ||
| Non-curative resection | 18 (39.1) | 28 (35.0) | ||
| No resection | 14 (30.4) | 26 (32.5) | ||
| Survival period, months | 10.07 (4–25) | 10.34 (3–27) | 0.014 | 0.906 |
| Prothymosin-α | 0.147 | 0.724 | ||
| − | 24 (52.2) | 33 (41.2) | ||
| + | 22 (47.8) | 47 (58.8) | ||
| Parathymosin | 1.100 | 0.321 | ||
| − | 24 (52.2) | 34 (42.5) | ||
| + | 22 (47.8) | 46 (57.5) |
SCC, squamous cell carcinoma; ASC, adenosquamous carcinoma; AC, adenocarcinoma.
Immunohistochemical analysis
Immunohistochemical examination to determine the PTMA and PTMS expression levels was performed with the standard protocol recommended for the EnVision™ Detection kit (Dako; Agilent Technologies, Inc., Glostrup, Denmark). Briefly, fresh pathological specimens were fixed in formalin, embedded in paraffin and sectioned at 3 µm thick. Next, the sections were incubated with anti-PTMA or anti-PTMA polyclonal antibody (1:200; ALS12216 and AT3490a; both purchased from Abgent, Inc., San Diego, CA, USA) for 2 hrs at 37°C, followed by visualization with DAB. Subsequent to dehydration and mounting of the specimens, the areas stained with PTMA or PTMA (appearing as brown spots) were reviewed and scored under a light microscope. The proportion of positively stained cells was counted at ×400 magnification. Specimens with >25% of positive cells were regarded as positive cases, whereas those with <25% positive cells were regarded as negative cases.
Statistical analysis
Statistical analysis was conducted with SPSS software (version 13 for Windows; SPSS Inc., Chicago, IL, USA). Data are presented as frequencies or as the mean ± standard error of the mean. Clinical variables were compared by χ2 test or Fisher's exact test. Survival curves were prepared with the Kaplan-Meier method and compared statistically by the log-rank test. Cox proportional hazards model was used for multivariate analysis. A value of P<0.05 was regarded to indicate a statistically significant difference. Data from patients who succumbed to the disease, or changed their home address or contact information was censored.
Results
Clinicopathological characteristics of patients with SCC/ASC and AC
The clinicopathological characteristics of 46 patients with ASC (n=20) or SCC (n=26) of the gallbladder, as well as 80 patients with AC of the gallbladder, are summarized in Table I. The SCC/ASC group included 19 men and 27 women, with a mean age of 55.8±9.6 years and age range of 35–82 years. Tumor staging revealed that 16 patients were at stage pT4 of the disease, 24 patients at pT3 and the remaining 6 patients at pT1/2. In the SCC/ASC group, 29 cases demonstrated positive lymph node invasion, and 28 cases were reported to have gallstones. TNM staging in the 46 patients with SCC/ASC indicated that 5 patients suffered from TNM stage I disease, 7 patients from TNM stage II, 20 patients from TNM stage III and 14 patients from TNM stage IV disease. In total, 14 patients underwent curative resection and 18 underwent non-curative resection, while surgical resection was not performed in 14 patients. All of the tumor samples were obtained by intraopherative biopsy, and none of the patients received any other treatment.
Patients in the AC group comprised 26 men and 54 women, with a mean age of 53.8±9.9 years and an age range of 33–80 years. In these cases, the tumor stage was classified as pT4 in 27 patients, pT3 in 25 patients and pT1/2 in 28 patients. Upon pathological analysis, 50 patients demonstrated positive lymph node invasion. Gallstones were detected in 38 patients. The TNM classification was stage I in 8 patients, stage II in 13 patients, stage III in 38 patients and stage IV in 21 patients. In total, 26 patients with AC underwent curative resection and 28 underwent non-curative resection, while surgical resection was not performed in 26 patients.
Patients were closely followed-up in the first 2 years after surgery. The mean overall survival in the SCC/ASC group was 10.07±0.78 months, while the <1-year and >1-year overall survival rates were 71.7 and 28.3%, respectively. Similarly, the mean overall survival in the AC group was 10.34±0.63 months, with a <1-year and >1-year overall survival rate in this group being 71.2 and 28.8%, respectively. No obvious difference was observed between lymph node invasion and survival period, however, a significant difference was identified in age, tumor differentiation, tumor diameter, TNM stage, and PTMA/PTMS expression between the SCC/ASC and AC groups (Table I).
Expression characteristics of PTMA and PTMS in SCC/ASC and AC groups
The expression levels of PTMA and PTMS were initially assessed in the SCC/ASC and AC groups. Upon immunohistochemical examination, the expression of PTMA and PTMS was observed to be localized in the cytoplasm and nucleus (Fig. 1). Positive expression of PTMA and PTMS in the SCC/ASC group was detected in 47.8% (22/46) and 47.8% (22/46) of patients, respectively. In the AC group, positive PTMA and PTMS expression was observed in 58.8% (47/80) and 57.5% (46/80) of patients, respectively (Table I). The between-group differences in this respect were not statistically significant (P>0.05).
Figure 1.
Expression of PTMA and PTMS in SCC, ASC and AC of the gallbladder. Positive expression of PTMA in (A) poorly differentiated ASC and (B) moderately differentiated SCC of the gallbladder. Positive expression of PTMS in (C) moderately and (D) poorly differentiated SCC of the gallbladder. Positive expression of PTMA in (E) poorly and (F) moderately differentiated AC of the gallbladder. Positive expression of PTMS in (G) moderately and (H) well-differentiated AC of the gallbladder. Magnification, ×200. PTMA, prothymosin-α; PTMS, parathymosin; SCC, squamous cell carcinoma; ASC, adenosquamous carcinoma; AC, adenocarcinoma.
Correlation of PTMA and PTMS expression levels with the clinicopathological characteristics
In the SCC/ASC group, positive expression of PTMA and PTMS was observed in a significantly reduced number of patients with smaller tumors (size, ≤3 cm), TNM stage I/II tumors, tumors not associated with lymph node invasion or distance metastasis, and tumors treated with curative resection (P<0.05 for all; Table II). A similar association of the PTMA and PTMS expression profiles with the aforementioned characteristics was observed in the AC group, while the tumor differentiation degree was also correlated with PTMA and PTMS in this group (Table III). Notably, no significant correlation of positively expressed PTMA and PTMS was observed with other clinicopathological characteristics, including the patient age, sex, pathologic subtype and presence of gallstones in the two groups (P>0.05; Tables II and III). These findings suggest that positive expression of PTMA and PTMS in tumor tissues may be associated with tumor metastasis and malignancy in patients with SCC, ASC and AC of the gallbladder.
Table II.
Correlation of PTMA and PTMS positive expression with the clinicopathological characteristics of patients with SCC and ASC of the gallbladder.
| PTMA | PTMS | ||||||
|---|---|---|---|---|---|---|---|
| Characteristic | Cases, n | Positive, n (%) | χ2 | P-value | Positive, n (%) | χ2 | P-value |
| Subtypes | 0.092 | 0.762 | 1.755 | 0.185 | |||
| SCC | 26 | 18 (69.2) | 18 (69.2) | ||||
| ASC | 20 | 13 (65.0) | 10 (50.0) | ||||
| Differentiation | 5.235 | 0.073 | 3.579 | 0.167 | |||
| Well-differentiated | 16 | 8 (50.0) | 7 (43.8) | ||||
| Moderately differentiated | 24 | 17 (70.8) | 16 (66.7) | ||||
| Poorly differentiated | 6 | 6 (100.0) | 5 (83.3) | ||||
| Tumor diameter | 12.081 | <0.001 | 9.942 | <0.001 | |||
| ≤3 cm | 20 | 8 (40.0) | 7 (35.0) | ||||
| >3 cm | 26 | 23 (88.5) | 21 (80.8) | ||||
| Gallstones | 1.885 | 0.178 | 0.001 | 0.955 | |||
| Negative | 18 | 10 (55.6) | 11 (61.1) | ||||
| Positive | 28 | 21 (75.0) | 17 (60.7) | ||||
| TNM stages | 7.797 | 0.020 | 5.805 | 0.059 | |||
| I/II | 12 | 5 (41.7) | 4 (33.3) | ||||
| III | 20 | 13 (65.0) | 13 (65.0) | ||||
| IV | 14 | 13 (92.9) | 11 (78.6) | ||||
| Lymph node invasion | 5.073 | 0.024 | 7.405 | 0.007 | |||
| Negative | 17 | 8 (47.1) | 6 (35.3) | ||||
| Positive | 29 | 23 (79.3) | 22 (75.9) | ||||
| Adjacent organs invasion | 6.240 | 0.012 | 5.625 | 0.019 | |||
| Negative | 16 | 7 (43.8) | 6 (37.5) | ||||
| Positive | 30 | 24 (80.0) | 22 (73.3) | ||||
| Resection | 6.165 | 0.046 | 7.346 | 0.025 | |||
| Curative resection | 14 | 6 (42.9) | 5 (35.7) | ||||
| Non-curative resection | 18 | 13 (72.2) | 11 (61.1) | ||||
| No resection | 14 | 12 (85.7) | 12 (85.7) | ||||
PTMA, prothymosin-α; PTMS, parathymosin; SCC, squamous cell carcinoma; ASC, adenosquamous carcinoma; AC, adenocarcinoma.
Table III.
Correlation of prothymosin-α and parathymosin positive expression with the clinicopathological characteristics of patients with adenocarcinoma of the gallbladder.
| Prothymosin-α | Parathymosin | ||||||
|---|---|---|---|---|---|---|---|
| Characteristic | Cases | Positive (%) | χ2 | P-value | Positive (%) | χ2 | P-value |
| Differentiation | 9.274 | 0.010 | 5.599 | 0.061 | |||
| Well-differentiated | 27 | 12 (44.4) | 9 (33.3) | ||||
| Moderately differentiated | 25 | 18 (72.0) | 14 (56.0) | ||||
| Poorly differentiated | 28 | 23 (82.1) | 18 (64.3) | ||||
| Tumor diameter | 6.265 | 0.012 | 15.880 | <0.001 | |||
| ≤3 cm | 50 | 28 (56.0) | 17 (34.0) | ||||
| >3 cm | 30 | 25 (83.3) | 24 (80.0) | ||||
| Gallstones | 0.007 | 0.934 | 0.436 | 0.509 | |||
| Negative | 42 | 28 (66.7) | 23 (54.8) | ||||
| Positive | 38 | 25 (65.8) | 18 (47.4) | ||||
| TNM stages | 8.778 | 0.012 | 10.759 | 0.005 | |||
| I/II | 21 | 9 (42.9) | 7 (33.3) | ||||
| III | 38 | 26 (68.4) | 17 (44.7) | ||||
| IV | 21 | 18 (85.7) | 17 (81.0) | ||||
| Lymph node invasion | 5.669 | 0.017 | 4.086 | 0.047 | |||
| Negative | 30 | 15 (50.0) | 11 (36.7) | ||||
| Positive | 50 | 38 (76.0) | 30 (60.0) | ||||
| Invasion of adjacent organs | 10.067 | 0.002 | 7.307 | 0.007 | |||
| Negative | 31 | 14 (45.2) | 10 (32.3) | ||||
| Positive | 49 | 39 (79.6) | 31 (63.3) | ||||
| Resection | 12.435 | 0.002 | 13.032 | 0.001 | |||
| Curative resection | 26 | 11 (42.3) | 7 (26.9) | ||||
| Non-curative resection | 28 | 19 (67.9) | 14 (50.0) | ||||
| No resection | 26 | 23 (88.5) | 20 (76.9) | ||||
Prognostic value of PTMA and PTMS expression levels
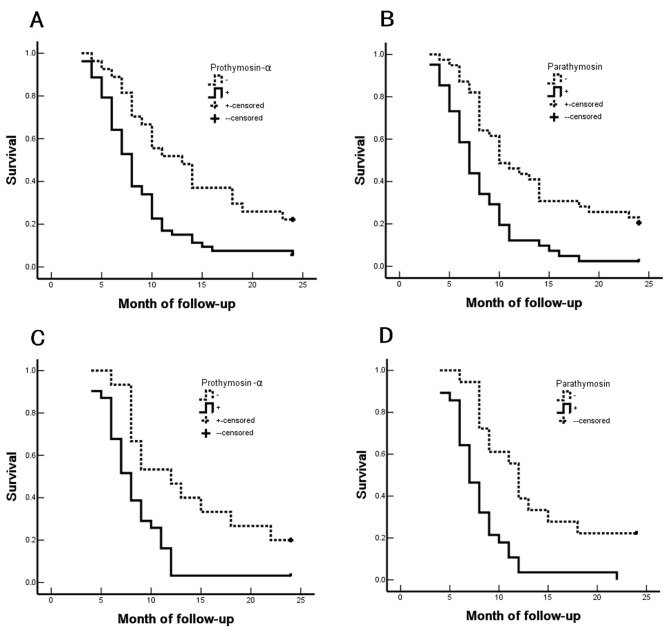
The study further investigated the prognostic value of the expression levels of PTMA and PTMS in patients in the SCC/ASC and AC groups. The median duration of follow-up in the SCC/ASC (n=46) and AC (n=80) groups was 10.07±0.78 and 10.34±0.63 months, respectively (Table I). Using Kaplan-Meier analysis, the tumor differentiation, tumor diameter, TNM stage, lymph node invasion, invasion of adjacent organs and resection treatment demonstrated a significant positive correlation with the survival of patients in the two groups (P<0.05; Tables IV–VII). Furthermore, in the SCC/ASC and AC groups, the survival of patients with positive expression of PTMA and PTMS was significantly shorter compared with that of patients with negative expression of PTMA and PTMS (Fig. 2). Furthermore, patients who underwent curative resection survived longer in comparison with those who underwent non-curative resection in the SCC/ASC and AC groups, which suggests that curative resection efficiently improved the prognosis of patients (P<0.05 for all; Tables IV–VII). Taken together, the aforementioned results indicated that the clinicopathological characteristics of patients, including tumor differentiation, tumor diameter, TNM stage, lymph node invasion, invasion of adjacent organs and resection treatment, as well as positive expression of PTMA and PTMS, were closely associated with the postoperative survival. These findings suggest the diagnostic and prognostic relevance of PTMA and PTMS expression in the management of patients with GBC.
Table IV.
Correlation of the average survival with the clinicopathological characteristics of patients with squamous cell and adenosquamous carcinomas of the gallbladder.
| Groups | Cases, n | The mean survival period (months) | χ2-value | P-value |
|---|---|---|---|---|
| Sex | 0.767 | 0.381 | ||
| Male | 19 | 10.74 (6–24) | ||
| Female | 27 | 9.85 (4–24) | ||
| Age | 2.023 | 0.155 | ||
| ≤45 years | 3 | 15.67 (8–24) | ||
| >45 years | 43 | 9.84 (4–25) | ||
| Subtypes | 0.223 | 0.637 | ||
| Squamous cell carcinoma | 26 | 10.19 (4–24) | ||
| Adenosquamous carcinoma | 20 | 10.25 (4–24) | ||
| Differentiation | 19.125 | <0.001 | ||
| Well-differentiated | 16 | 13.81 (5–24) | ||
| Moderately differentiated | 24 | 8.92 (4–18) | ||
| Poorly differentiated | 6 | 5.83 (4–9) | ||
| Tumor diameter | 31.337 | <0.001 | ||
| ≤3 cm | 20 | 14.35 (7–24) | ||
| >3 cm | 26 | 7.04 (4–11) | ||
| Gallstones | 3.730 | 0.053 | ||
| Negative | 18 | 8.22 (4–12) | ||
| Positive | 28 | 11.50 (4–24) | ||
| TNM stages | 51.139 | <0.001 | ||
| I/II | 12 | 17.00 (9–24) | ||
| III | 20 | 9.20 (7–15) | ||
| IV | 14 | 5.86 (4–8) | ||
| Lymph node invasion | 16.219 | <0.001 | ||
| Negative | 17 | 14.24 (4–24) | ||
| Positive | 29 | 7.86 (4–15) | ||
| Adjacent organs invasion | 32.271 | <0.001 | ||
| Negative | 16 | 15.75 (9–24) | ||
| Positive | 30 | 7.27 (4–12) | ||
| Resection | 50.165 | <0.001 | ||
| Curative resection | 14 | 16.64 (10–24) | ||
| Non-curative resection | 18 | 8.50 (6–12) | ||
| Non-resection | 14 | 6.00 (4–8) | ||
| Prothymosin-α | 8.478 | 0.004 | ||
| − | 15 | 13.87 (6–24) | ||
| + | 31 | 8.45 (4–24) | ||
| Parathymosin | 13.824 | <0.001 | ||
| − | 18 | 13.61 (6–24) | ||
| + | 28 | 8.04 (4–22) |
Table VII.
Cox multivariate analysis of survival rate for patients with adenocarcinoma of the gallbladder.
| 95% CI | ||||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | Subgroups | B-value | SE | Wald | P-value | RR | Low | High |
| Differentiation | Well-/moderately/poorly | 1.264 | 0.494 | 6.547 | 0.011 | 3.540 | 1.344 | 9.321 |
| Tumor diameter | ≤3/>3 cm | 1.307 | 0.484 | 7.292 | 0.007 | 3.695 | 1.431 | 9.541 |
| Gallstones | Negative/positive | 0.251 | 0.239 | 1.103 | 0.294 | 1.285 | 0.805 | 2.053 |
| TNM stages | I/II/III/IV | 1.344 | 0.469 | 8.212 | 0.004 | 3.834 | 1.529 | 9.614 |
| Lymph node invasion | Negative/positive | 1.409 | 0.563 | 6.263 | 0.012 | 4.092 | 1.357 | 12.335 |
| Adjacent organs invasion | Negative/positive | 1.586 | 0.516 | 9.447 | 0.002 | 4.884 | 1.776 | 13.428 |
| Resection | Curative/non-curative/non-resection | 1.526 | 0.617 | 6.117 | 0.013 | 4.600 | 1.373 | 15.415 |
| Prothymosin-α | −/+ | 1.743 | 0.563 | 9.585 | 0.002 | 5.714 | 1.896 | 17.227 |
| Parathymosin | −/+ | 1.352 | 0.474 | 8.136 | 0.004 | 3.865 | 1.526 | 9.787 |
SE, standard error; RR, relative risk; CI, confidence interval.
Figure 2.
Correlation of survival rates with PTMA and PTMS positive expression in SCC/ASC or AC of the gallbladder. The overall survival rate of patients with SCC/ASC of the gallbladder with positive expression of (A) PTMA and (B) PTMS. The overall survival rate of patients with AC of the gallbladder with positive expression of (C) PTMA and (D) PTMS. PTMA, prothymosin-α; PTMS, parathymosin; SCC, squamous cell carcinoma; ASC, adenosquamous carcinoma; AC, adenocarcinoma. Data from patients who succumbed to the disease, or changed their home address or contact information was censored.
Discussion
GBC is a relatively rare tumor of the digestive system, with AC of the gallbladder accounting for >85% of all gallbladder tumors. The majority of cases with AC of the gallbladder involve highly or moderately differentiated tumors. However, as rare histotypes of GBC, the clinicopathological features of SCC and ASC are not well-characterized. Histologically, SCC and ASC of the gallbladder have a squamous cell component that differs from that observed in AC. Furthermore, SCC/ASC and AC of the gallbladder exhibit certain differences with respect to their clinical characteristics. SCC and ASC of the gallbladder present a greater proliferation capacity (4), which suggests that patients with SCC or ASC may have poorer prognosis in comparison with those with AC. Therefore, the present study attempted to compare patients with these different histopathological subtypes to assess the correlation between their clinicopathological characteristics and the PTMA/PTMS expression in SC/ASC and AC of the gallbladder.
In the present study, patients with SCC/ASC of the gallbladder accounted for 4.34% (46/1,060) of the total patients with carcinoma of the gallbladder treated at the participating hospitals, which is consistent with the incidence reported in earlier studies (2,6,20). Similar to the clinical presentation of AC, patients with early-stage SCC and ASC presented with no specific symptoms other than those typically associated with chronic cholecystitis, due to which the majority of patients were not diagnosed at an early stage. Clinical signs and symptoms, such as persistent abdominal pain, palpable mass and jaundice, typically occur in the advanced stages of the disease (5). The population of the present study mainly included patients with advanced stages of SCC, ASC and AC.
Several previous studies have described that SCC and ASC of the gallbladder with a squamous cell component exhibit a greater proliferation capability, lower propensity for metastasis, greater tendency for involvement of adjacent organs, and worse overall prognosis, as compared with that associated with AC of the gallbladder (2–6). In the present study, no significant difference was observed with respect to the incidence of lymph node metastasis, and invasion of the surrounding tissues and organs between the SCC/ASC and AC groups. However, the number of patients having tumors with a size of >3 cm in the SCC/ASC group was markedly higher compared with that in the AC group.
The present study also identified that the patients with advanced stages in the SCC/ASC and AC groups had an extremely poor prognosis, as all patients with TNM stages III/IV succumbed to the disease within 25–27 months. Patients with TNM stage I/II tumors had a markedly higher survival rate, which underlines the importance of early diagnosis in these patients (Tables IV and VI). Furthermore, the average postoperative survival was comparable in the SCC/ASC and AC patients, which suggested that all these histotypes were associated with a poor prognosis (Table I). The percentage of patients who underwent curative resection was not significantly difference between the two study groups. However, the survival rate of patients who underwent curative resection group was significantly increased as compared with that of patients who underwent non-curative resection or did not undergo resection in the SCC/ASC and AC groups (Tables IV–VII). This indicates that curative resection may improve the prognosis of patients with SCC, ASC and AC of the gallbladder.
Table VI.
Correlation of the average survival with the clinicopathological characteristics of patients with adenocarcinoma of the gallbladder.
| Groups | Cases, n | The mean survival period (months) | χ2 | P-value |
|---|---|---|---|---|
| Sex | 2.567 | 0.109 | ||
| Male | 26 | 9.58 (3–24) | ||
| Female | 54 | 11.30 (3–24) | ||
| Age | 0.003 | 0.956 | ||
| ≤45 years | 16 | 10.81 (4–24) | ||
| >45 years | 64 | 10.72 (3–24) | ||
| Differentiation | 32.501 | <0.001 | ||
| Well-differentiated | 27 | 15.07 (5–24) | ||
| Moderately differentiated | 25 | 10.60 (4–24) | ||
| Poorly differentiated | 28 | 6.68 (3–14) | ||
| Tumor diameter | 68.283 | <0.001 | ||
| ≤3 cm | 50 | 13.70 (6–24) | ||
| >3 cm | 30 | 5.80 (3–10) | ||
| Gallstones | 0.246 | 0.620 | ||
| Negative | 42 | 10.19 (3–24) | ||
| Positive | 38 | 11.34 (4–24) | ||
| TNM stages | 105.825 | <0.001 | ||
| I/II | 21 | 18.96 (5–24) | ||
| III | 38 | 9.29 (6–15) | ||
| IV | 21 | 5.14 (3–7) | ||
| Lymph node invasion | 42.372 | <0.001 | ||
| Negative | 30 | 16.27 (4–24) | ||
| Positive | 50 | 7.42 (3–14) | ||
| Adjacent organs invasion | 55.535 | <0.001 | ||
| Negative | 31 | 16.68 (7–24) | ||
| Positive | 49 | 6.98 (3–11) | ||
| Resection | 113.141 | <0.001 | ||
| Curative resection | 26 | 18.31 (10–24) | ||
| Non-curative resection | 28 | 8.64 (6–11) | ||
| No resection | 26 | 5.42 (3–9) | ||
| Prothymosin-α | 10.079 | 0.001 | ||
| − | 27 | 14.07 (4–24) | ||
| + | 53 | 9.04 (3–24) | ||
| Parathymosin | 15.255 | <0.001 | ||
| − | 39 | 13.44 (4–24) | ||
| + | 41 | 8.17 (3–24) |
PTMA and PTMS, which were originally obtained by bovine thymus extraction, are members of the α-thymosin family (24). Previous studies have demonstrated that PTMA and PTMS activate the immune response to curb the viral infection in patients with human immunodeficiency virus, hepatitis B virus or hepatitis C virus (25,26). Elevated expression levels of PTMA and PTMS have been reported in numerous human malignant tumor tissues (12,14,15). However, the correlation between the expression levels of PTMA and PTMS in SCC, ASC and AC of the gallbladder have not been addressed in previous studies. In the present study, the percentage of patients with positive expression of PTMA and PTMS was not significantly between the SCC/ASC and AC of the gallbladder groups (Tables II and III). In addition, it was observed that positive expression of PTMA and PTMS was not associated with the differentiation of SCC/ASC or AC of the gallbladder (Tables II and III). In the SCC/ASC and AC groups, negative expression of PTMA and PTMS was detected in a significantly reduced number of patients with highly-differentiated tumors, maximal tumor diameter of ≤3 cm, TNM stage I/II tumors, lack of lymph node metastasis, absence of invasion into the surrounding tissues and organs, and in patients who underwent radical resection. Similarly, positive expression of PTMA and PTMS was reported in an increased number of patients with a maximal tumor diameter of >3 cm, TNM stage III/IV disease, lymph node metastasis and invasion into the surrounding tissues and organs, as well as patients who did not undergo resection (Tables II and III).
The present study further demonstrated that the degree of tumor differentiation, maximal tumor diameter, TNM stage, lymph node metastasis, invasion of the surrounding tissues and history of surgical resection were closely associated with the average survival of patients with SCC/ASC (Table IV) and AC (Table VI) of the gallbladder. In addition, the survival of patients with a positive expression of PTMA and PTMS was significantly shorter when compared with that of patients with negative expression of PTMA and PTMS (Fig. 2). Cox multivariate analysis also revealed that poor differentiation, a maximal tumor diameter of ≥3 cm, TNM stages III or IV disease, occurrence of lymph node metastasis, invasion of the surrounding tissues and organs, and non-resection were negatively correlated with the postoperative survival of patients with SCC/ASC and AC of the gallbladder (Tables V and VII). These findings indicate that PTMA and PTMS expression levels may be independent prognostic predictors. Furthermore, the results suggest that PTMA and PTMS may serve an important role in the occurrence, progression, biological behavior and prognosis of gallbladder SCC, ASC and AC, which is consistent with the observations of previous studies on PTMA and PTMS expression in other malignant tumors (19,20). The present study also identified that positive expression of PTMA and PTMS in patients with SCC/ASC and AC of the gallbladder (Tables II and III) was associated with rapid progression, and these patients were prone to regional lymph node metastasis with strong invasiveness. Therefore, it is suggested that these two factors may be important biological markers for early diagnosis of GBC. However, the molecular mechanism underlying the effects of PTMA and PTMS in GBC requires further exploration.
Table V.
Cox multivariate analysis of survival rate of patients with SCC and ASC of the gallbladder.
| 95% CI | ||||||||
|---|---|---|---|---|---|---|---|---|
| Characteristics | Subgroups | B-value | SE | Wald | P-value | RR | Low | High |
| Subtypes | SCC/ASC | 0.308 | 0.460 | 0.448 | 0.503 | 1.361 | 0.522 | 3.352 |
| Differentiation | Well-/moderately/poorly | 1.317 | 0.582 | 5.121 | 0.024 | 3.732 | 1.193 | 11.678 |
| Tumor diameter | ≤3/>3 cm | 1.951 | 0.691 | 7.972 | 0.005 | 7.036 | 1.816 | 27.258 |
| Gallstones | Negative/positive | 0.859 | 0.483 | 3.163 | 0.075 | 2.361 | 0.916 | 6.084 |
| TNM stages | I/II/III/IV | 1.352 | 0.548 | 6.087 | 0.014 | 3.865 | 1.320 | 11.314 |
| Lymph node invasion | Negative/positive | 1.580 | 0.673 | 5.512 | 0.019 | 4.855 | 1.298 | 18.157 |
| Invasion of adjacent organs | Negative/positive | 2.625 | 0.819 | 10.273 | 0.001 | 13.805 | 2.773 | 68.734 |
| Resection | Curative/non-curative/non-resection | 1.080 | 0.467 | 5.348 | 0.021 | 2.945 | 1.179 | 7.355 |
| Prothymosin-α | −/+ | 1.736 | 0.688 | 6.367 | 0.012 | 5.675 | 1.473 | 21.856 |
| Parathymosin | −/+ | 1.563 | 0.770 | 4.120 | 0.042 | 4.773 | 1.055 | 21.589 |
SCC, squamous cell carcinoma; ASC, adenosquamous carcinoma; SE, standard error; RR, relative risk; CI, confidence interval.
In conclusion, PTMA and PTMS are two important biological markers that reflect tumorigenesis, tumor progression, clinical biological behavior, and the prognosis of patients with SCC, ASC and AC of the gallbladder. It was identified that the positive expression of PTMA/PTMS may be associated with poor prognosis in both SCC/ASC and AC of the gallbladder. Therefore, the detection of the expression levels of PTMA and/or PTMS in gallbladder tissues may have important clinicopathological significance in the prevention and early diagnosis of GBC.
References
- 1.Roa JC, Tapia O, Cakir A, Basturk O, Dursun N, Akdemir D, Saka B, Losada H, Bagci P, Adsay NV. Squamous cell and adenosquamous carcinomas of the gallbladder: Clinicopathological analysis of 34 cases identified in 606 carcinomas. Mod Pathol. 2011;24:1069–1078. doi: 10.1038/modpathol.2011.68. [DOI] [PubMed] [Google Scholar]
- 2.Kim WS, Jang KT, Choi DW, Choi SH, Heo JS, You DD, Lee HG. Clinicopathologic analysis of adenosquamous/squamous cell carcinoma of the gallbladder. J Surg Oncol. 2011;103:239–242. doi: 10.1002/jso.21813. [DOI] [PubMed] [Google Scholar]
- 3.Kondo M, Dono K, Sakon M, Shimizu J, Nagano H, Nakamori S, Umeshita K, Wakasa K, Monden M. Adenosquamous carcinoma of the gallbladder. Hepatogastroenterology. 2002;49:1230–1234. [PubMed] [Google Scholar]
- 4.Nishihara K, Nagai E, Izumi Y, Yamaguchi K, Tsuneyoshi M. Adenosquamous carcinoma of the gallbladder: A clinicopathological, immunohistochemical and flow-cytometric study of twenty cases. Jpn J Cancer Res. 1994;85:389–399. doi: 10.1111/j.1349-7006.1994.tb02372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mingoli A, Brachini G, Petroni R, Antoniozzi A, Cavaliere F, Simonelli L, Chirletti P, Modini C. Squamous and adenosquamous cell carcinomas of the gallbladder. J Exp Clin Cancer Res. 2005;24:143–150. [PubMed] [Google Scholar]
- 6.Chan KM, Yu MC, Lee WC, Jan YY, Chen MF. Adenosquamous/squamous cell carcinoma of the gallbladder. J Surg Oncol. 2007;95:129–134. doi: 10.1002/jso.20576. [DOI] [PubMed] [Google Scholar]
- 7.Rekik W, Ben Fadhel C, Boufaroua AL, Mestiri H, Khalfallah MT, Bouraoui S, Mzabi-Rgaya S. Case report: Primary pure squamous cell carcinoma of the gallbladder. J Visc Surg. 2011;148:e149–e151. doi: 10.1016/j.jviscsurg.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 8.Oohashi Y, Shirai Y, Wakai T, Nagakura S, Watanabe H, Hatakeyama K. Adenosquamous carcinoma of the gallbladder warrants resection only if curative resection is feasible. Cancer. 2002;94:3000–3005. doi: 10.1002/cncr.10578. [DOI] [PubMed] [Google Scholar]
- 9.Haritos AA, Goodall GJ, Horecker BL. Prothymosin alpha: Isolation and properties of the major immunoreactive form of thymosin alpha 1 in rat thymus; Proc Natl Acad Sci USA; 1984; pp. 1008–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Letsas KP, Frangou-Lazaridis M. Surfing on prothymosin alpha proliferation and anti-apoptotic properties. Neoplasma. 2006;53:92–96. [PubMed] [Google Scholar]
- 11.Jiang X, Kim HE, Shu H, Zhao Y, Zhang H, Kofron J, Donnelly J, Burns D, Ng SC, Rosenberg S, Wang X. Distinctive roles of PHAP proteins and prothymosin-alpha in a death regulatory pathway. Science. 2003;299:223–226. doi: 10.1126/science.1076807. [DOI] [PubMed] [Google Scholar]
- 12.Jou YC, Tung CL, Tsai YS, Shen CH, Syue-Yi C, Shiau AL, Tsai HT, Wu CL, Tzai TS. Prognostic relevance of prothymosin-alpha expression in human upper urinary tract transitional cell carcinoma. Urology. 2009;74:951–957. doi: 10.1016/j.urology.2008.11.060. [DOI] [PubMed] [Google Scholar]
- 13.Letsas KP, Vartholomatos G, Tsepi C, Tsatsoulis A, Frangou-Lazaridis M. Fine-needle aspiration biopsy-RT-PCR expression analysis of prothymosin alpha and parathymosin in thyroid: Novel proliferation markers? Neoplasma. 2007;54:57–62. [PubMed] [Google Scholar]
- 14.Wang M, Pan JY, Song GR, Chen HB, An LJ, Qu SX. Altered expression of estrogen receptor alpha and beta in advanced gastric adenocarcinoma: Correlation with prothymosin alpha and clinicopathological parameters. Eur J Surg Oncol. 2007;33:195–201. doi: 10.1016/j.ejso.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 15.Zhang M, Cui F, Lu S, Lu H, Jiang T, Chen J, Zhang X, Jin Y, Peng Z, Tang H. Increased expression of prothymosin-α, independently or combined with TP53, correlates with poor prognosis in colorectal cancer. Int J Clin Exp Pathol. 2014;7:4867–4876. [PMC free article] [PubMed] [Google Scholar]
- 16.Frangou-Lazaridis M, Clinton M, Goodall GJ, Horecker BL. Prothymosin alpha and parathymosin: Amino acid sequences deduced from the cloned rat spleen cDNAs. Arch Biochem Biophys. 1988;263:305–310. doi: 10.1016/0003-9861(88)90640-6. [DOI] [PubMed] [Google Scholar]
- 17.Clinton M, Frangou-Lazaridis M, Panneerselvam C, Horecker BL. The sequence of human parathymosin deduced from a cloned human kidney cDNA. Biochem Biophys Res Commun. 1989;158:855–862. doi: 10.1016/0006-291X(89)92801-5. [DOI] [PubMed] [Google Scholar]
- 18.Haritos AA, Salvin SB, Blacher R, Stein S, Horecker BL. Parathymosin alpha: A peptide from rat tissues with structural homology to prothymosin alpha; Proc Natl Acad Sci USA; 1985; pp. 1050–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hannappel E, Huff T. The thymosins. Prothymosin alpha, parathymosin, and beta-thymosins: Structure and function. Vitam Horm. 2003;66:257–296. doi: 10.1016/S0083-6729(03)01007-0. [DOI] [PubMed] [Google Scholar]
- 20.Vareli K, Frangou-Lazaridis M, van der Kraan I, Tsolas O, van Driel R. Nuclear distribution of prothymosin alpha and parathymosin: Evidence that prothymosin alpha is associated with RNA synthesis processing and parathymosin with early DNA replication. Exp Cell Res. 2000;257:152–161. doi: 10.1006/excr.2000.4857. [DOI] [PubMed] [Google Scholar]
- 21.Lazcano-Ponce EC, Miquel JF, Muñoz N, Herrero R, Ferrecio C, Wistuba II, Alonso de Ruiz P, Aristi Urista G, Nervi F. Epidemiology and molecular pathology of gallbladder cancer. CA Cancer J Clin. 2001;51:349–364. doi: 10.3322/canjclin.51.6.349. [DOI] [PubMed] [Google Scholar]
- 22.Edge SB, Compton CC. The American Joint Committee on Cancer: The 7th edition of the AJCC cancer staging Manual and the Future of TNM. Ann Surg Oncol. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 23.Hamilton SR, Aaltonen LA. Patholigy and Genetics of Tumours of the Digestive System. IARC Press; Lyon: 2000. [Google Scholar]
- 24.Okamoto K, Isohashi F. Purification and primary structure of a macromolecular-translocation inhibitor II of glucocorticoid-receptor binding to nuclei from rat liver. Inhibitor II is the 11.5-kDa Zn2+-binding protein (parathymosin) Eur J Biochem. 2000;267:155–162. doi: 10.1046/j.1432-1327.2000.00987.x. [DOI] [PubMed] [Google Scholar]
- 25.Hoch K, Volk DE. Structures of thymosin proteins. Vitam Horm. 2016;102:1–24. doi: 10.1016/bs.vh.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 26.Mosoian A, Teixeira A, High AA, Christian RE, Hunt DF, Shabanowitz J, Liu X, Klotman M. Novel function of prothymosin alpha as a potent inhibitor of human immunodeficiency virus type 1 gene expression in primary macrophages. J Virol. 2006;80:9200–9206. doi: 10.1128/JVI.00589-06. [DOI] [PMC free article] [PubMed] [Google Scholar]