Abstract
Tetramethylpyrazine (TMP), an effective component of the traditional Chinese medicine Chuanxiong Hort, has been proven to exhibit a beneficial effect in a number of types of malignant epithelial cancer. However, the mode of action of TMP on breast cancer cells remains unknown. The aim of the present study was to investigate the regulatory effect of TMP on breast cancer cells and its underlying molecular mechanism of action. Different concentrations of TMP were used to treat breast cancer cells, and subsequently, the effects on the viability, apoptosis, and migration and invasion abilities were determined. In addition, the expression and activity levels of the protein kinase B (Akt) signaling pathway and caspase-3 were explored via reverse transcription-quantitative polymerase chain reaction and western blot analysis. The results of the present study revealed that TMP significantly inhibited the viability, migration and invasion rates, and increased the apoptosis of MDA-MB-231 cells in a dose-dependent manner. The minimum effective dose was ~1,600 µM. Additional mechanistic studies demonstrated that 1,600 and 3,200 µM TMP significantly decreased the gene expression and activity of Akt and increased the activity of caspase-3. This mechanism may be responsible for the inhibition of viability, migration and invasion, and activation of apoptosis in breast cancer cells. The results of the present study suggested that TMP may be used in chemotherapy against breast cancer.
Keywords: tetramethylpyrazine, breast cancer, protein kinase B signaling pathway, caspase-3
Introduction
Worldwide, breast cancer occurs in the epithelial cells of the mammary gland and is the most common type of invasive malignancy in females (1). Every year, ~450,000 females succumb as a consequence of breast cancer, which is the second leading cause of cancer-associated mortality in females following lung cancer (1). The incidence rate of breast cancer is increasing around the world since the 1970s and has become a major public health problem (2). The breast is a non-vital organ so, theoretically, breast cancer should not be fatal; however, due to the loose connection between cells, breast cancer cells are released early from cancer nests via the blood or lymphatic vessels, leading to distant metastases and life-threatening disease for patients (3).
In recent years, comprehensive treatment models, focusing on local and systemic treatments, have become more popular in breast cancer therapy. Surgical intervention and radiation are the predominant local treatment options, whereas systemic treatments are on the basis of drug intervention (4). The classification of the type of cancer determines the treatment strategy and outcome. For example, hormone receptor positive breast cancer responds well to an endocrine therapy (5), whereas human epidermal growth factor (HER)2-targeting drugs, including trastuzumab (Herceptin) and pertuzumab (Perjeta), may result in an improved outcome for HER2+ breast cancer (6). Triple-negative breast cancer type has a poor prognosis, compared with the other types of breast cancer, due to the lack of targeted drug treatments (7).
The development of breast cancer cells is controlled by complex signaling networks. The major signaling pathways involved in mammary gland involution, signal transducer and activator of transcription (STAT3), nuclear factor-kappa B (NF-κB), transforming growth factor beta (TGF-β), and retinoid acid receptors (RARs)/retinoid X receptors (RXRs), are reviewed as part of the complex network of signaling pathways that crosstalk in a contextual-dependent manner. These factors, also involved in breast cancer development, and are important regulatory nodes for signaling amplification following weaning (8). A previous study demonstrated that the application of a single molecule is unlikely to suppress the cross-talk between cancerous cells (9). Therefore, multi-drug combination treatments have become the principal treatment strategy. At present, the most common types of therapeutics used for breast cancer treatment are chemotherapeutic agents, including Doxorubicin, Paclitaxel, Docetaxel, Thioridazine, Disulfiram and Camptothecin. Multi-drug combination treatments also include hormone blockers and monoclonal antibodies (10). Patient compliance is notably difficult with chemotherapeutic drugs, due to their severe toxicity for the human body (11). Therefore, exploring the development of novel cancer drugs with reduced side effects is important.
Tetramethylpyrazine (TMP), an effective component of the traditional Chinese medicine Chuanxiong, is the active ingredient of Umbelliferae plant root extracts and has primarily been used in the treatment of various neurovascular, including cerebral blood deficiency, cerebral thrombosis or cerebral infarction caused by cerebral embolism, and cardiovascular diseases, including angina and coronary heart disease (12,13). Furthermore, TMP has been demonstrated to exhibit beneficial effects in a number of types of epithelial malignant cancer including lung (14), ovarian (15) and hepatocellular cancer (16). Previous studies have validated that TMP exhibited the ability to reduce the resistance of breast cancer cells to chemotherapy (17,18). However, the detailed function and underlying molecular mechanism of TMP in breast cancer therapy remain unknown. Therefore, in the present study, the effect and mechanism of TMP on cell viability, apoptosis and migration was investigated.
Materials and methods
Cell culture
The breast cancer cell line MDA-MB-231 was purchased from the Institute of Biochemistry and Cell Biology at the Chinese Academy of Sciences (Shanghai, China). MDA-MB-231 cells were maintained in Dulbecco's modified Eagle's medium (DMEM; Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum (Thermo Fisher Scientific, Inc.), 100 U/ml penicillin and 100 µg/ml streptomycin (HyClone; GE Healthcare, Logan, UT, USA). Cells were cultured at 37°C in a humidified incubator containing 5% CO2. The culture medium was changed every 3 days. Only cells in the exponential growth phase were included in the present study.
Cell viability analysis
A Cell Counting kit-8 (CCK-8; Dojindo Molecular Technologies, Inc., Kumamoto, Japan) was used to analyze the viability of breast cancer cells following TMP (Dalian Meilun Biotech Co., Ltd, Dalian, China) treatment for 24, 48 and 72 h at 37°C. A total of 5×103 MDA-MB-231 cells/well were seeded (5,000) on each film and transferred to 96-well plates. DMEM was removed after 8 h, and DMEM containing 0, 800, 1,600 and 3,200 µM TMP was used to treat the cells for 24, 48 and 72 h. Furthermore, the 0 µM TMP group was used as the control. The conditioned culture medium was removed prior to CCK-8 examination. Subsequently, 100 µl DMEM and 10 µl CCK-8 solution were added to each well, followed by CCK-8 incubation at 37°C for 2.5 h. The optical density at 450 nm was determined using a microplate reader (BioTek Instruments, Inc., Winooski, VT, USA).
Cell apoptosis analysis
A total of 2×105 MDA-MB-231 cells/well were seeded onto each film and placed in 6-well plates. Cells used in this experiment were sub-confluent. Cells were collected after 72 h TMP treatment at 37°C. The concentrations of TMP were 0, 800, 1,600 and 3,200 µM, the 0 µM TMP group was used as the control. Then, cells were treated in accordance with the protocol of the Vybrant® Apoptosis Assay kit (Thermo Fisher Scientific, Inc.). In detail, ice-cold PBS was used to wash the cells three times. Subsequently, cells were centrifuged at 300 × g for 5 min at room temperature and re-suspended in 1X Annexin-Binding Buffer. The apoptosis rate was determined by staining with Annexin V-allophycocyanin and propidium iodide. All cells were analyzed by FACScan flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA). Data were acquired using CellQuest™ software (version 5.1; BD Biosciences) to reveal the impact of TMP on cell apoptosis.
Cell migration and invasion analysis
A total of 2×105 MDA-MB-231 cells/well were seeded into the upper chamber of an 8.0-µm pore Transwell apparatus (EMD Millipore, Billerica, MA, USA) and maintained in DMEM containing 0.2% bovine serum albumin (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). Conditioned DMEM with different concentrations (0, 800, 1,600 and 3,200 µM) of TMP was added to the lower chamber. Furthermore, the 0 µM TMP group was used as the control. After 1 h incubation at 37°C in an atmosphere containing 5% CO2, the upper chamber was washed with PBS and cells on the top surface of the insert were removed with a cotton swab. The invasion assay procedure was similar to that of the cell migration assay, except that the Transwell membrane was coated with Matrigel diluted 1:3 (BD Biosciences) and the cells were incubated for 12 h at 37°C. Cells that migrated to the bottom surface of the insert were fixed with 4% paraformaldehyde for 30 min at room temperature and stained with 0.1% crystal violet for 12 h at room temperature for subsequent observations by using a light microscope (cat. no. IX71; Olympus Corporation, Tokyo, Japan). Images were taken randomly at magnification, ×200 and the total cell count was calculated by counting the number of cells in five randomly-selected observation fields.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from MDA-MB-231 cells using the AxyPrep™ Multisource Total RNA Miniprep kit (Axygen Scientific, Inc., Union City, CA, USA) according to the manufacturer's protocol in an environment of 4°C. cDNA was synthesized using the PrimeScript™ RT reagent kit (Takara Bio, Inc., Otsu, Japan). qPCR was performed using an ABI 7500 Sequencing Detection System and SYBR® Premix Ex Taq™ (Takara Bio, Inc.). Cycling conditions were as follows: 40 cycles of 95°C for 5 sec; and 60°C for 34 sec. The comparative 2−∆∆Cq method (19) was used to calculate the relative expression level of each target gene with β-actin as the control gene. All primers used to amplify target genes are listed in Table I.
Table I.
Sequences of the primers used in the quantitative polymerase chain reaction.
| Gene | Sequence (5′-3′) |
|---|---|
| Akt1 | |
| Forward | ATGAGCGACGTGGCTATTGTGAAG |
| Reverse | GAGGCCGTCAGCCACAGTCTGGATG |
| Akt2 | |
| Forward | ATGAATGAGGTGTCTGTCATCAAAGAA |
| Reverse | GGCTGCTTGAGGCTGTTGGCGACC |
| Akt3 | |
| Forward | CAGTCTGTCTGCTACAGCCTGGATA |
| Reverse | ATGAGCGATGTTACCATTGT |
| β-actin | |
| Forward | CCAACCGCGAGAAGATGA |
| Reverse | CCAGAGGCGTACAGGGATAG |
Akt, protein kinase B.
Western blot analysis
A total of 2×105 MDA-MB-231 cells/well were seeded onto each film and placed in 6-well plates. After 48 h incubation with 0, 800, 1,600 and 3,200 µM TMP, cells were washed with PBS, detached from the well using 0.25% trypsin and centrifuged at 1,000 × g for 5 min at room temperature. Furthermore, the 0 µM TMP group was used as the control. Cytoplasmic proteins were extracted using NE-PER® Nuclear and Cytoplasmic Extraction Reagents (Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol. Protein concentration was detected in accordance with the instructions of the BCA protein assay kit (Thermo Fisher Scientific, Inc.). SDS-PAGE (10% gel) was used to separate total-protein kinase B (t-Akt; cat. no. 4685), phosphorylated-Akt (p-Akt; cat. no. 4060), total-caspase-3 (t-casp3; cat. no. 9662) and β-actin (dilution of all antibodies, 1:1,000; cat. no. 4970; Cell Signaling Technology, Inc., Danvers, MA, USA), and SDS-PAGE (15% gel) was used to separate cleaved-caspase-3 (cleaved-casp3; dilution 1:1,000; cat. no. 9662; Cell Signaling Technology, Inc.). Cell homogenates containing equal amounts of protein (30 µg) were subjected to SDS-PAGE and transferred to 0.22 µm polyvinylidene difluoride membranes, which were subsequently blocked with 5% fat free milk at room temperature for 1 h. All primary antibodies were purchased from Cell Signaling Technology, Inc. and the membranes were incubated with these antibodies overnight at 4°C. The following day, the membranes were washed three times with Tris-buffered saline containing Tween-20 (TBST) and the secondary horseradish peroxidase-conjugated anti-rabbit immunoglobulin G antibody (dilution, 1:5,000; cat. no. 7074; Cell Signaling Technology, Inc.) was applied to the membranes for 1 h at room temperature. Following three washes in TBST, the membranes were incubated in enhanced chemiluminescence (ECL) solution according to the protocol of the ECL detection kit (GE Healthcare) at room temperature. Positive immunoreactive bands were quantified and normalized to β-actin.
Statistical analysis
Each sample was analyzed in triplicate and the experiments were repeated three times. Data from all experiments are expressed as the mean ± standard error of the mean. The differences between experimental groups and controls were assessed using Student's t-test or one-way analysis of variance with post hoc differences via the Student-Newman-Keuls test. P<0.05 was considered to indicate a statistically significant difference.
Results
TMP inhibits the viability of MDA-MB-231 cells
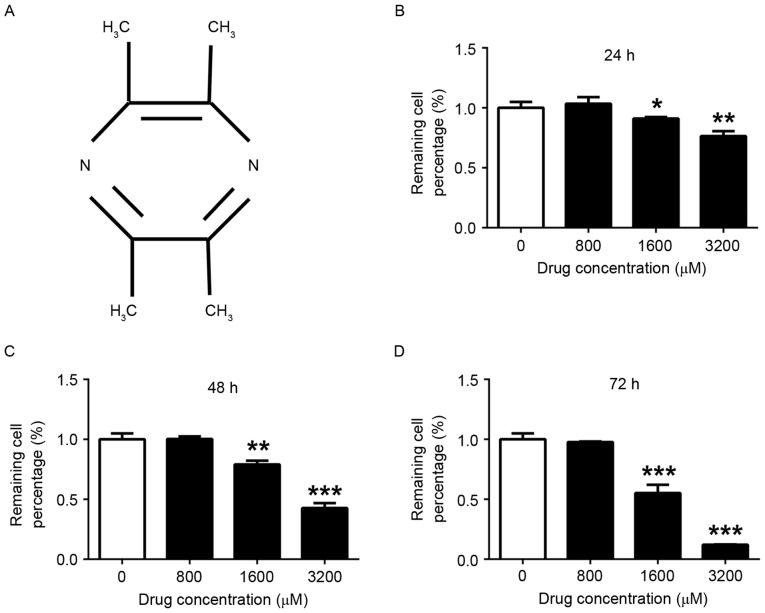
The chemical structure of TMP was depicted in Fig. 1A. Following treatment with TMP (0, 800, 1,600 and 3,200 µM) for 24, 48 and 72 h, the viability of MDA-MB-231 cells was determined using the CCK8 assay. Fig. 1B-D demonstrates that TMP at 1,600 and 3,200 µM significantly inhibited the viability of MDA-MB-231 cells after 24 (1,600 µM group, P<0.05; 3,200 µM group, P<0.01), 48 (1,600 µM group, P<0.01; 3,200 µM group, P<0.001) and 72 h (1,600 µM group, P<0.001; 3,200 µM group, P<0.001), compared with the viability of control cells. Furthermore, the suppression of viability occurred in a dose-depended manner. However, there was no significant effect on cell viability when the concentration of TMP was <800 µM.
Figure 1.
TMP inhibits the viability of MDA-MB-231 cells. (A) Chemical structure of TMP. MDA-MB-231 cells were treated with 0, 800, 1,600 and 3,200 µM TMP for (B) 24 h, (C) 48 h and (D) 72 h. There was no significant inhibition of MDA-MB-231 cell viability <1,600 µM. *P<0.05, **P<0.01, ***P<0.001 vs. control. TMP, tetramethylpyrazine.
TMP enhances the apoptosis of MDA-MB-231 cells
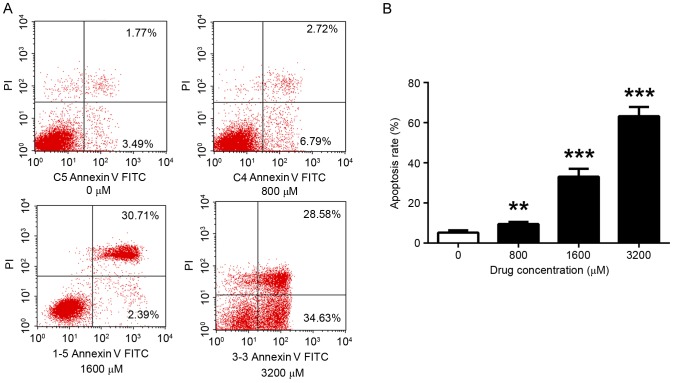
The effect of TMP on the apoptosis of MDA-MB-231 cells was examined using flow cytometry. Consistent with effect observed in the viability assay, apoptosis was altered in a dose-dependent manner. The level of apoptosis significantly increased with increasing TMP concentrations. After 72 h incubation, the results indicated that 800, 1,600 and 3,200 µM TMP significantly increased the apoptosis rate (9.51, 33.10 and 63.21%, respectively), compared with the control (5.26%; P<0.01, P<0.001 and P<0.001, respectively) (Fig. 2).
Figure 2.
TMP enhances the apoptosis of MDA-MB-231 cells. (A) Apoptosis following 48 h of treatment with TMP (0, 800, 1,600 and 3,200 µM) was measured, using PI and Annexin V-allophycocyanin labeling and flow cytometry analysis. (B) The apoptosis rate was calculated which revealed that TMP induced the apoptosis of breast cancer cells at a concentration of 800 µM and above. **P<0.01, ***P<0.001 vs. control. TMP, tetramethylpyrazine; PI, propidium iodide; FITC, fluorescein isothiocyanate.
TMP impairs the migration and invasion of MDA-MB-231 cells
Migration and invasion of MDA-MB-231 cells was significantly inhibited by 800, 1,600 and 3,200 µM TMP, compared with the control (P<0.05, P<0.001 and P<0.001, respectively). The inhibition efficiency was positively associated with drug concentration. Furthermore, the migratory and invasive capabilities of the cells was decreased by >50%, compared with the control, when TMP concentration >,1600 µM (Fig. 3). The results of the present study revealed that TMP may prevent the migration and invasion of breast cancer cells.
Figure 3.

TMP inhibits the migratory and invasive capabilities of MDA-MB-231 cells. (A) The ability of MDA-MB-231 cells to migrate and invade other tissues was determined after 12 h incubation with 0, 800, 1,600 and 3,200 µM TMP by staining with 0.1% crystal violet. (B) The staining indicated that 800, 1,600 and 3,200 µM TMP decreased the number of migrated and invasive cells. TMP exhibits its effects in a dose-dependent manner. *P<0.05, ***P<0.001 vs. control. TMP, tetramethylpyrazine.
TMP may regulate the viability, migration, invasion and apoptosis of breast cancer cells by decreasing the expression or activity of Akt and caspase-3
To additionally explore the results of the present study, the alterations in the expression and activity of Akt and caspase-3 were investigated. The results demonstrated that 1,600 and 3,200 µM TMP significantly inhibited the gene expression of Akt1 (P<0.01 and P<0.001, respectively), Akt2 (P<0.01 and P<0.001, respectively) and Akt3 (P<0.05 and P<0.01, respectively; Fig. 4A), compared with the control. Furthermore, TMP downregulated the activity of Akt and caspase-3, the relative expression of p-Akt to t-Akt significantly decreased (1,600 µM group: P<0.01; 3,200 µM group: P<0.01), and the relative expression of cleaved-casp3 to t-casp3 significantly increased (800 µM group: P<0.05; 1,600 µM group: P<0.001; 3,200 µM group: P<0.001) (Fig. 4B and C). These results indicate that Akt and caspase-3 may serve important roles in cell viability, migration, invasion, and apoptosis.
Figure 4.
TMP decreases the gene expression and protein activity of the Akt signaling pathway and increases caspase-3 activity. (A) The reverse transcription-quantitative polymerase chain reaction revealed that TMP significantly downregulated the gene expression of Akt1, Akt2 and Akt3 at 1,600 and 3,200 µM. (B) Western blot analysis indicated that TMP decreased the activity of the Akt signaling pathway and increased the activity of caspase-3. β-actin was the loading control. (C) Quantification of western blot analysis results which validated that 1,600 and 3,200 µM significantly decreased the relative expression of p-Akt to t-Akt and increased the relative expression of cleaved-casp3 to t-casp3. *P<0.05, **P<0.01, ***P<0.001 vs. control. TMP, tetramethylpyrazine; Akt, protein kinase B; p-, phosphorylated; t-, total; casp3, caspase-3.
Discussion
The majority of types of malignant tumor, including breast cancer, are characterized by continuous cell division and viability, suppression of the initiation of apoptosis, the ability to metastasize, and potential recurrence. It is difficult to remove all cancer cells using surgical techniques due to their ability to spread to other tissues via the blood stream or lymphatic system. Therefore, drug interventions to clear cancer cells from the blood or lymphatic systems are important. Due to the occurrence of severe side effects and the possibility of drug resistance against common chemotherapeutic therapies, there is a requirement to identify less toxic and more efficacious treatment alternatives. Consequently, natural alternative products have received growing attention.
TMP was extracted, isolated and purified from the traditional Chinese medicine Chuanxiong Hort. Although the content of TMP in Chuanxiong Hort is abundant, the extraction process is time- and energy-consuming (12). Therefore, the majority of TMP is artificially synthesized (20). Previous studies have focused on identifying the underlying molecular mechanisms of TMP activity due to its well established antitumor effect and its ability to reverse resistance to chemotherapy treatments, while causing less adverse reactions (16,17). For example, TMP was able to inhibit the growth and migration of glioma by regulating calcium influxes (21) and additionally, TMP was revealed to decrease the metastases of melanoma by suppressing vascular endothelial growth factor activity (22). Furthermore, TMP has been identified to serve a function in the reversal of multidrug resistance in a number of types of malignant tumor, including reversing the multi-drug resistance in hepatocellular carcinoma via inhibiting P-gp, MRP2, MRP3 and MRP5 (16); TMP could effectively reverse multi-drug resistance of bladder cancer cells and its mechanisms may be associated with the alteration of MRP1, GST, BCL-2 and TOPO-II (23); TMP as a salvage agent for patients with relapsed or refractory non-Hodgkin's lymphoma may also be associated with its effect on the expression of P-gp (24). However, the effect of TMP was different in various types of tumors. Previous studies have demonstrated that 200 µM TMP significantly inhibited hepatocellular carcinoma cell proliferation (25) and that 300 µg/ml TMP significantly inhibited the viability of acute lymphocytic leukemia cell lines (26). These results were similar to the results of the present study where it was observed that breast cancer cells were inhibited by 1,600 µM TMP. However, only a limited number of studies have investigated the effect and mode of action of TMP on breast cancer cells. A recent study revealed that the combination treatment of the tetramethylpyrazine piperazine derivative DLJ14 and adriamycin inhibited the progression of resistant breast cancer (27); however, the function of DLJ14 alone remains unknown. In fact, DLJ14 is a tetramethylpyrazine piperazine derivative; therefore, tetramethylpyrazine and DLJ14 are different drugs (27). It has been demonstrated that TMP causes apoptotic death and tumor regression in human breast cancer cells in in vitro, and in vivo models (28). However, there were no further studies evaluating the effect of TMP on the migration and invasion abilities of breast cancer, and the underlying molecular mechanisms following TMP treatment remain unknown. The results of the present study identified that TMP may regulate breast cancer cell migration, invasion and apoptosis by affecting the activity of Akt, and caspase-3, which is distinct from the results of the aforementioned studies (18,28). Our study not only confirmed the effect of TMP on the migration and invasion of breast cancer in addition to its role of apoptosis, but further found it possible to directly targets in breast cancer cells.
Excessive viability and apoptosis disorders are the two primary reasons for the genesis and development of malignant tumors. The Akt signaling pathway has been identified to serve functions in a number of types of disease, particularly in malignancies (29). The Akt signaling pathway principally regulates the activity of cancer cells (30,31). Previous studies have indicated that the Akt signaling pathway is a key factor in the viability and migration of a number of types of tumor including colorectal (32), and prostate cancer (33), and glioblastoma (34) and osteosarcoma (35). Additionally, the Akt signaling pathway is an important regulator of breast cancer (36–38). Apoptosis disorders are an additional cause for the occurrence of breast cancer. The initiation of apoptosis is typically triggered via caspase-3 (39), which is being used to treat cancer; for example, a number of chemotherapeutic drugs, including melatonin, doxorubicin and cisplatin, induce cancer cell apoptosis by upregulating the activity of caspase-3 (40,41). Therefore, novel drugs, which repress Akt signaling or increase caspase-3 activity, may be effective tools to improve breast cancer prognosis.
The present study focused on understanding the effect and mode of action of TMP on the viability, migration, invasion and apoptosis of breast cancer cells. The results of the present study demonstrated that TMP is effective against a number of cancer cell characteristics. In addition, TMP was able to modulate the activity of the Akt signaling pathway and caspase-3, up to a concentration of 1,600 µM. However, the viability, migration, invasion and apoptosis of breast cancer cells were not significantly inhibited following treatment with 800 µM TMP. Additionally, the influence of TMP on the activity of Akt and caspase-3 was more significant than the effect on their expression. In order to understand the mode of action of TMP on Akt and caspase-3 signaling pathways, and the effect of TMP in vivo, additional studies are required.
The results of the present study revealed that TMP was effective against the viability, migration, invasion and apoptosis of breast cancer cells. It is hypothesized that the molecular mechanisms underlying these actions involve the Akt signaling pathway and caspase-3. The results of the present study suggest that TMP is a novel drug candidate for the regulation of breast cancer genesis and development; however, additional in vivo studies are required.
Acknowledgements
The present study was supported by the Health System ‘Outstanding Young Talent’ Cultivation Plan of Shanghai Jinshan District (grant no. JSYQ201620), the Science and Technology Innovation Fund Projects of Shanghai Jinshan District (grant no. 2015-3-24) and the Medical Subject Construction Fund Project of Shanghai Jinshan District (grant no. JSZK2015B06).
References
- 1.Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality and prevalence across five continents: Defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24:2137–2150. doi: 10.1200/JCO.2005.05.2308. [DOI] [PubMed] [Google Scholar]
- 2.Sim X, Ali RA, Wedren S, Goh DL, Tan CS, Reilly M, Hall P, Chia KS. Ethnic differences in the time trend of female breast cancer incidence: Singapore, 1968–2002. BMC Cancer. 2006;6:261. doi: 10.1186/1471-2407-6-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gujam FJ, Going JJ, Edwards J, Mohammed ZM, McMillan DC. The role of lymphatic and blood vessel invasion in predicting survival and methods of detection in patients with primary operable breast cancer. Crit Rev Oncol Hematol. 2014;89:231–241. doi: 10.1016/j.critrevonc.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 4.Hack CC, Voiß P, Lange S, Paul AE, Conrad S, Dobos GJ, Beckmann MW, Kümmel S. Local and systemic therapies for breast cancer patients: Reducing Short-term symptoms with the methods of integrative medicine. Geburtshilfe Frauenheilkd. 2015;75:675–682. doi: 10.1055/s-0035-1557748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nagaraj G, Ellis MJ, Ma CX. The natural history of hormone receptor-positive breast cancer: Attempting to decipher an intriguing concept. Oncology (Williston Park) 2012;26:696–700. [PubMed] [Google Scholar]
- 6.Tang Y, Wang Y, Kiani MF, Wang B. Classification, treatment strategy and associated drug resistance in breast cancer. Clin Breast Cancer. 2016;16:335–343. doi: 10.1016/j.clbc.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 7.Rybárová S, Hodorová I, Hajduková M, Schmidtová K, Mojzis J, Kajo K, Kviatkovská Z, Plank L, Benický M, Mirossay A, et al. Expression of MDR proteins in breast cancer and its correlation with some clinical and pathological parameters. Neoplasma. 2006;53:128–135. [PubMed] [Google Scholar]
- 8.Zaragozá R, García-Trevijano ER, Lluch A, Ribas G, Viña JR. Involvement of different networks in mammary gland involution after the pregnancy/lactation cycle: Implications in breast cancer. IUBMB Life. 2015;67:227–238. doi: 10.1002/iub.1365. [DOI] [PubMed] [Google Scholar]
- 9.Espinoza-Fonseca LM. Targeting MDM2 by the small molecule RITA: Towards the development of new multi-target drugs against cancer. Theor Biol Med Model. 2005;2:38. doi: 10.1186/1742-4682-2-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Núñez C, Capelo JL, Igrejas G, Alfonso A, Botana LM, Lodeiro C. An overview of the effective combination therapies for the treatment of breast cancer. Biomaterials. 2016;97:34–50. doi: 10.1016/j.biomaterials.2016.04.027. [DOI] [PubMed] [Google Scholar]
- 11.Ma P, Mumper RJ. Paclitaxel nano-delivery systems: A comprehensive review. J Nanomed Nanotechnol. 2013;4:1000164. doi: 10.4172/2157-7439.1000164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y, Fu Q, Zhao W. Tetramethylpyrazine inhibits osteosarcoma cell proliferation via downregulation of NF-κB in vitro and in vivo. Mol Med Rep. 2013;8:984–988. doi: 10.3892/mmr.2013.1611. [DOI] [PubMed] [Google Scholar]
- 13.Li WM, Liu HT, Li XY, Wu JY, Xu G, Teng YZ, Ding ST, Yu C. The effect of tetramethylpyrazine on hydrogen peroxide-induced oxidative damage in human umbilical vein endothelial cells. Basic Clin Pharmacol Toxicol. 2010;106:45–52. doi: 10.1111/j.1742-7843.2009.00470.x. [DOI] [PubMed] [Google Scholar]
- 14.Zheng CY, Xiao W, Zhu MX, Pan XJ, Yang ZH, Zhou SY. Inhibition of cyclooxygenase-2 by tetramethylpyrazine and its effects on A549 cell invasion and metastasis. Int J Oncol. 2012;40:2029–2037. doi: 10.3892/ijo.2012.1375. [DOI] [PubMed] [Google Scholar]
- 15.Yin J, Yu C, Yang Z, He JL, Chen WJ, Liu HZ, Li WM, Liu HT, Wang YX. Tetramethylpyrazine inhibits migration of SKOV3 human ovarian carcinoma cells and decreases the expression of interleukin-8 via the ERK1/2, p38 and AP-1 signaling pathways. Oncol Rep. 2011;26:671–679. doi: 10.3892/or.2011.1334. [DOI] [PubMed] [Google Scholar]
- 16.Wang XB, Wang SS, Zhang QF, Liu M, Li HL, Liu Y, Wang JN, Zheng F, Guo LY, Xiang JZ. Inhibition of tetramethylpyrazine on P-gp, MRP2, MRP3 and MRP5 in multidrug resistant human hepatocellular carcinoma cells. Oncol Rep. 2010;23:211–215. [PubMed] [Google Scholar]
- 17.Zhang Y, Liu X, Zuo T, Liu Y, Zhang JH. Tetramethylpyrazine reverses multidrug resistance in breast cancer cells through regulating the expression and function of P-glycoprotein. Med Oncol. 2012;29:534–538. doi: 10.1007/s12032-011-9950-8. [DOI] [PubMed] [Google Scholar]
- 18.Zhang P, Zheng BB, Wang HY, Chen JH, Liu XY, Guo XL. DLJ14, a novel chemo-sensitization agent, enhances therapeutic effects of adriamycin against MCF-7/A cells both in vitro and in vivo. J Pharm Pharmacol. 2014;66:398–407. doi: 10.1111/jphp.12168. [DOI] [PubMed] [Google Scholar]
- 19.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 20.Tang ZY, Wang SL, Lin Y. Progress in the pharmacokinetics and clinical pharmacodynamics of tetramethylpyrazine in nervous system. Chin J Clin Pharmacol. 2010;26:535–539. [Google Scholar]
- 21.Fu YS, Lin YY, Chou SC, Tsai TH, Kao LS, Hsu SY, Cheng FC, Shih YH, Cheng H, Fu YY, Wang JY. Tetramethylpyrazine inhibits activities of glioma cells and glutamate neuro-excitotoxicity: Potential therapeutic application for treatment of gliomas. Neuro Oncol. 2008;10:139–152. doi: 10.1215/15228517-2007-051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen L, Lu Y, Wu JM, Xu B, Zhang LJ, Gao M, Zheng SZ, Wang AY, Zhang CB, Zhang WW, Lei N. Ligustrazine inhibits B16F10 melanoma metastasis and suppresses angiogenesis induced by vascular endothelial growth factor. Biochem Biophys Res Commun. 2009;386:374–379. doi: 10.1016/j.bbrc.2009.06.042. [DOI] [PubMed] [Google Scholar]
- 23.Wang S, Lei T, Zhang M. The reversal effect and its mechanisms of tetramethylpyrazine on multidrug resistance in human bladder cancer. PLoS One. 2016;11:e0157759. doi: 10.1371/journal.pone.0157759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang XG, Jiang C. Ligustrazine as a salvage agent for patients with relapsed or refractory non-Hodgkin's lymphoma. Chin Med J (Engl) 2010;123:3206–3211. [PubMed] [Google Scholar]
- 25.Cao J, Miao Q, Miao S, Bi L, Zhang S, Yang Q, Zhou X, Zhang M, Xie Y, Zhang J, Wang S. Tetramethylpyrazine (TMP) exerts antitumor effects by inducing apoptosis and autophagy in hepatocellular carcinoma. Int Immunopharmacol. 2015;26:212–220. doi: 10.1016/j.intimp.2015.03.028. [DOI] [PubMed] [Google Scholar]
- 26.Wang XJ, Xu YH, Yang GC, Chen HX, Zhang P. Tetramethylpyrazine inhibits the proliferation of acute lymphocytic leukemia cell lines via decrease in GSK-3β. Oncol Rep. 2015;33:2368–2374. doi: 10.3892/or.2015.3860. [DOI] [PubMed] [Google Scholar]
- 27.Chen J, Wang W, Wang H, Liu X, Guo X. Combination treatment of ligustrazine piperazine derivate DLJ14 and adriamycin inhibits progression of resistant breast cancer through inhibition of the EGFR/PI3K/Akt survival pathway and induction of apoptosis. Drug Discov Ther. 2014;8:33–41. doi: 10.5582/ddt.8.33. [DOI] [PubMed] [Google Scholar]
- 28.Pan J, Shang JF, Jiang GQ, Yang ZX. Ligustrazine induces apoptosis of breast cancer cells in vitro and in vivo. J Cancer Res Ther. 2015;11:454–458. doi: 10.4103/0973-1482.147378. [DOI] [PubMed] [Google Scholar]
- 29.Mundi PS, Sachdev J, McCourt C, Kalinsky K. AKT in cancer: New molecular insights and advances in drug development. Br J Clin Pharmacol. 2016;82:943–956. doi: 10.1111/bcp.13021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matson DR, Hardin H, Buehler D, Lloyd RV. AKT activity is elevated in aggressive thyroid neoplasms where it promotes proliferation and invasion. Exp Mol Pathol. 2017;103:288–293. doi: 10.1016/j.yexmp.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 31.Robert M, Frenel JS, Bourbouloux E, Berton Rigaud D, Patsouris A, Augereau P, Gourmelon C, Campone M. Efficacy of buparlisib in treating breast cancer. Expert Opin Pharmacother. 2017;18:2007–2016. doi: 10.1080/14656566.2017.1410139. [DOI] [PubMed] [Google Scholar]
- 32.Zhu L, Tian G, Yang Q, De G, Zhang Z, Wang Y, Nie H, Zhang Y, Yang X, Li J. Thyroid hormone receptor β1 suppresses proliferation and migration by inhibiting PI3K/Akt signaling in human colorectal cancer cells. Oncol Rep. 2016;36:1419–1426. doi: 10.3892/or.2016.4931. [DOI] [PubMed] [Google Scholar]
- 33.Lim W, Jeong M, Bazer FW, Song G. Coumestrol inhibits proliferation and migration of prostate cancer cells by regulating AKT, ERK1/2 and JNK MAPK cell signaling cascades. J Cell Physiol. 2016;232:862–871. doi: 10.1002/jcp.25494. [DOI] [PubMed] [Google Scholar]
- 34.Clark PA, Bhattacharya S, Elmayan A, Darjatmoko SR, Thuro BA, Yan MB, van Ginkel PR, Polans AS, Kuo JS. Resveratrol targeting of AKT and p53 in glioblastoma and glioblastoma stem-like cells to suppress growth and infiltration. J Neurosurg. 2016;126:1448–1460. doi: 10.3171/2016.1.JNS152077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Han XG, Du L, Qiao H, Tu B, Wang YG, Qin A, Dai KR, Fan QM, Tang TT. CXCR1 knockdown improves the sensitivity of osteosarcoma to cisplatin. Cancer Lett. 2015;369:405–415. doi: 10.1016/j.canlet.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 36.Yamaguchi M, Osuka S, Weitzmann MN, Shoji M, Murata T. Increased regucalcin gene expression extends survival in breast cancer patients: Overexpression of regucalcin suppresses the proliferation and metastatic bone activity in MDA-MB-231 human breast cancer cells in vitro. Int J Oncol. 2016;49:812–822. doi: 10.3892/ijo.2016.3669. [DOI] [PubMed] [Google Scholar]
- 37.Jiang Q, Pan Y, Cheng Y, Li H, Liu D, Li H. Lunasin suppresses the migration and invasion of breast cancer cells by inhibiting matrix metalloproteinase-2/-9 via the FAK/Akt/ERK and NF-κB signaling pathways. Oncol Rep. 2016;36:253–262. doi: 10.3892/or.2016.4798. [DOI] [PubMed] [Google Scholar]
- 38.Yang SX, Polley E, Lipkowitz S. New insights on PI3K/AKT pathway alterations and clinical outcomes in breast cancer. Cancer Treat Rev. 2016;45:87–96. doi: 10.1016/j.ctrv.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ried SJ, Shi Y. Molecular mechanisms of caspase regulation during apoptosis. Nat Rev Mol Cell Biol. 2004;5:897–907. doi: 10.1038/nrm1496. [DOI] [PubMed] [Google Scholar]
- 40.Fan LL, Sun GP, Wei W, Wang ZG, Ge L, Fu WZ, Wang H. Melatonin and doxorubicin synergistically induce cell apoptosis in human hepatoma cell lines. World J Gastroenterol. 2010;16:1473–1481. doi: 10.3748/wjg.v16.i12.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang L, Jin F, Qin A, Hao Y, Dong Y, Ge S, Dai K. Targeting Notch1 signaling pathway positively affects the sensitivity of osteosarcoma to cisplatin by regulating the expression and/or activity of Caspase family. Mol Cancer. 2014;13:139. doi: 10.1186/1476-4598-13-139. [DOI] [PMC free article] [PubMed] [Google Scholar]