Förster resonance energy transfer (FRET) is a non-radiative process that has been extensively applied for probing molecular events involved in a range of cellular and biological processes (1). FRET provides a means for measuring intermolecular spacings on the order ∼1–10 nm (2), an ability that has allowed measurement of cellular events such as protein folding, substrate interactions, enzyme kinetics, and intercellular signaling molecules. The advent of a wide range of fluorescent proteins from which to construct FRET reporters has enabled a variety of live-cell FRET studies to be readily conducted. In addition, newer techniques for gene manipulation have allowed generation of transgenic animal models expressing FRET reporters. The use of FRET-based reporters is now widespread, and in some cases FRET measurements have become the “gold-standard” The potential for developing new genetic approaches allowing localized intracellular biochemical assays that make use of FRET measurements is high.
Unfortunately, FRET approaches are associated with several limitations inherent in the physical process of FRET as well as the technologies used to measure FRET. For example, it is widely recognized that the fluorescence properties of many labels, especially fluorescent proteins, are sensitive to changes in local environment, such as changes in pH, ionic concentrations, oxidation, temperature, and refractive index. FRET measurements make use of two or more fluorescent labels, or proteins, each of which may present different sensitivities for changes in environmental factors. Hence, FRET measurements may be skewed by agonist-induced or unappreciated changes in local environment.
Arguably the largest limitation of FRET measurements is the low signal-to-noise ratio (SNR) associated with FRET imaging. While many technologies have been developed to measure FRET, in general, all of them suffer from poor SNR when compared to imaging of a single fluorescent label. The governing factors behind the reduced SNR include the loss of energy associated with the FRET process and the fact that, whether directly or indirectly, two fluorescent molecules contribute to the measured FRET signal. These factors carry over into many different technologies that have been developed to measure FRET: filter set-based (traditional fluorescence microscopy), acceptor photobleaching, spectral FRET, and fluorescence lifetime imaging microscopy (FLIM) FRET. While we, and others, have shown that current state-of-the-art techniques such as spectral imaging and FLIM can reduce variance in FRET measurements (3,4), these measurements still have low SNR when compared to imaging of a single label (Fig. 1). This low SNR, and the techniques of spectral and FLIM microscopy developed to overcome it, has often resulted in long exposure times (>1–2 sec) and uncertainty of the ability to differentiate between small differences in FRET efficiencies or interpret results based on small changes in FRET efficiency (7).
Figure 1.
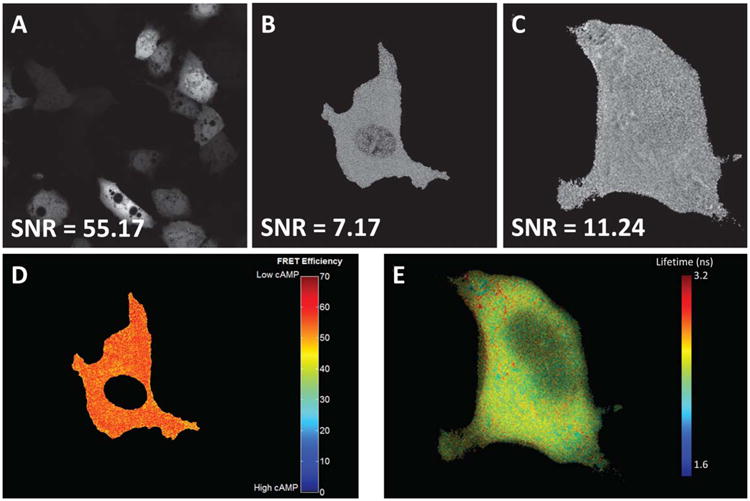
Current state-of-the-art FRET measurement technologies suffer from reduced signal-to-noise ratios (SNR) when compared to measurement of a single fluorescent label or protein. A: Image of single-label acceptor emission using acceptor excitation (Venus, excited at 488 nm, expressed in pulmonary microvascular endothelial cells); (B) image of calculated FRET efficiency using hyperspectral confocal imaging shows reduced SNR when compared to the single-label image in Panel (A)–image shows a single expressing cell (Turquoise-Epac-Venus cAMP reporter(5), excited at 405 nm, expressed in pulmonary microvascular endothelial cells); (C) image of fluorescence lifetime where greyscale intensity represents lifetime, masked to illustrate a single cell (Turqouoise-Epac-Venus cAMP reporter (5), expressed in HEK-293 cells); (D) the image from Panel (B), masked to remove nuclei, false-colored, and scaled to highlight changes in FRET efficiency; (E) the image from Panel (C), false-colored and scaled to highlight changes in fluorescence lifetime. Images (A, B, and D) were taken using identical objectives and acquisition parameters using a Nikon A1R spectral confocal microscope, with the exception of using a 488 nm laser for direct excitation of Venus in Panel (A). Images were then linearly unmixed using a non-negatively constrained unmixing algorithm implemented in MATLAB software, and converted to 8-bit and scaled linearly for display. Images (C, E) were provided by courtesy of Dr. Kees Jalink, van Leeuwenhoek Centre of Advanced Microscopy, Amsterdam, The Netherlands. SNR was calculated as described by Bernas and colleagues (6)–in brief, pixels in regions of similar (but not oversaturated) intensity were identified using an 8-way high-pass filter and the mean intensity (signal) was calculated from the most homogeneous regions, while noise was calculated as the standard deviation of these regions and the SNR calculated as the ratio of mean intensity to standard deviation. [Color figure can be viewed in the online issue which is available at wileyonlinelibrary.com]
A key next step in enabling effective FRET-based studies is the development of new approaches for FRET microscopy that allow accurate and low-variance FRET measurements. The field is actively pursuing several areas of research. First, biological innovations such as the development of improved fluorescent labels (5) are leading to FRET reporters with improved quantum efficiencies and FRET-transfer efficiencies. Second, technological innovations such as the development of new imaging modalities, more sensitive imaging detectors, and more efficient optical transmission pathways are providing improved SNR for a range of microscopic applications, including FRET imaging (3,8). Third, the development of more accurate mathematical and statistical analysis approaches is providing the ability to understand, remove bias, and potentially improve the accuracy of FRET efficiency calculations, making better use of the data that can be acquired using current microscope systems (9).
Literature on FRET analysis techniques and calculation of FRET indices and efficiencies is vast, ranging from theoretical physics (10) to developmental biology (11). In addition, FRET measurements and calculations can be relatively complex. The wide range of potential analysis techniques and the relative complexity of coding new image analysis algorithms have, in part, limited wide-spread adoption of new mathematical analysis techniques. The manuscript by Nagy and colleagues, in this edition of Cytometry, Part A, helps to alleviate this burden by implementing a series of improved FRET parameter and FRET efficiency calculations within an easy-to-use MATLAB graphic user interface (this issue, page 376). Key advantages of this program include its ability to: 1) operate on the most widely-used FRET microscopy approach (traditional filter set-based fluorescence microscopy); 2) provide a sequential workflow to guide users through FRET efficiency calculations; 3) perform sophisticated operations, such as visualizing and calculating spectral overspill (a.k.a. cross-talk) parameters; 4) gate, or subselect, FRET data to improve the reliability (reduce the variance) of FRET measurements; 5) perform pixel-by-pixel or region-based FRET measurements, in addition to whole-image measurements; and 6) use statistical approaches based on maximum likelihood estimation (MLE) algorithms, which the authors have previously shown to reduce the variance of FRET measurements through FRET outlier rejection (9). Hence, this software program should enable investigators to improve the reliability of their current FRET measurements by providing an easy-to-use interface that allows improved estimation of spectral overspill parameters and improved calculation of FRET efficiencies. Furthermore, statistical approaches such as MLE could potentially be applied to image data acquired from alternative microscope modalities, such as spectral or fluorescence lifetime FRET.
The outlook for future development of FRET approaches is high. Trimolecular and higher molecular FRET have provided a means for probing multi-step molecular interactions. Spectral imaging has provided a means for imaging FRET in the presence of additional fluorescent labels, allowing calculation of FRET efficiencies within specific subcellular regions. Confocal and multiphoton imaging techniques have provided the ability to potentially perform FRET in 3 dimensions. However, each of these techniques relies on an underlying assumption that sufficient SNR is available to make a reliable measurement of FRET efficiency. SNR is especially important if techniques for thin optical sectioning or rapid time-lapse imaging are applied, which by nature limit the number of molecules examined and photons detected. Continued optimization of the biological, technological, and theoretical aspects of FRET measurements will enable 3- dimensional spatially- and temporally resolved biochemical studies to be conducted on single cells and localized subcellular compartments and within living tissues.
Acknowledgments
Authors would like to thank Dr. Kees Jalink, at the van Leeuwenhoek Centre of Advanced Microscopy in Amsterdam, for contributing the fluorescence lifetime image data.
Grant sponsor: NIH, Grant numbers: P01 HL066299; S10 RR027535; Grant sponsor: Abraham Mitchell Cancer Research Fund.
Literature Cited
- 1.Förster T. Energy migration and fluorescence. J Biomed Opt. 2012;17:0110021–01100210. doi: 10.1117/1.JBO.17.1.011002. [DOI] [PubMed] [Google Scholar]
- 2.Stryer L, Haugland RP. Energy transfer: A spectroscopic ruler. Proc Natl Acad Sci USA. 1967;58:719. doi: 10.1073/pnas.58.2.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leavesley SJ, Britain A, Cichon LK, Nikolaev VO, Rich TC. Assessing FRET using spectral techniques. Cytometry A. 2013;83A:898–912. doi: 10.1002/cyto.a.22340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leavesley SJ, Nakhmani A, Gao Y, Rich TC. Automated image analysis of FRET signals for subcellular cAMP quantification. In: Zaccolo M, editor. cAMP Signaling: Methods and Protocols. New York: Springer; 2015. pp. 59–70. [DOI] [PubMed] [Google Scholar]
- 5.Klarenbeek J, Goedhart J, van Batenburg A, Groenewald D, Jalink K. Fourth-generation Epac-based FRET sensors for cAMP feature exceptional brightness, photostability and dynamic range: Characterization of dedicated sensors for FLIM, for ratiometry and with high affinity. PLoS One. 2015:e0122513. doi: 10.1371/journal.pone.0122513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bernas T, Robinson JP, Asem EK, Rajwa B. Loss of image quality in photobleaching during microscopic imaging of fluorescent probes bound to chromatin. J Biomed Opt. 2005;10:064015–064019. doi: 10.1117/1.2136313. [DOI] [PubMed] [Google Scholar]
- 7.Rich TC, Webb KJ, Leavesley SJ. Can we decipher the information content contained within cyclic nucleotide signals? J Gen Physiol. 2014;143:17–27. doi: 10.1085/jgp.201311095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gadella TW, Jovin TM, Clegg RM. Fluorescence lifetime imaging microscopy (FLIM): Spatial resolution of microstructures on the nanosecond time scale. Biophys Chem. 1993;48:221–239. [Google Scholar]
- 9.Nagy P, Szabó Á, Váradi T, Kovács T, Batta G, Szöllősi J. Maximum likelihood estimation of FRET efficiency and its implications for distortions in pixelwise calculation of FRET in microscopy. Cytometry A. 2014;85A:942–952. doi: 10.1002/cyto.a.22518. [DOI] [PubMed] [Google Scholar]
- 10.Scholes GD. Long-range resonance energy transfer in molecular systems. Annu Rev Phys Chem. 2003;54:57–87. doi: 10.1146/annurev.physchem.54.011002.103746. [DOI] [PubMed] [Google Scholar]
- 11.Ponsioen B, Zhao J, Riedl J, Zwartkruis F, Van Der Krogt G, Zaccolo M, Moolenaar WH, Bos JL, Jalink K. Detecting cAMP-induced Epac activation by fluorescence resonance energy transfer: Epac as a novel cAMP indicator. EMBO Rep. 2004;5:1176–1180. doi: 10.1038/sj.embor.7400290. [DOI] [PMC free article] [PubMed] [Google Scholar]


