Abstract
Unmethylated cytosine-phosphorothioate-guanine (CpG)-containing oligodeoxynucleotides (ODNs) are synthetic DNA sequences that mimic bacterial DNA, and are known to serve as ligands for Toll-like receptor 9 (TLR9). The interaction between a CpG ODNs with TLR9 activates the complex downstream cascade that contributes to exerting its function. In the present study, the results of clonogenic assays demonstrated that the activation of TLR9 by CpG ODNs significantly increased the radiosensitivity of A549 lung cancer cells, with a sensitivity enhancement ratio (SER) of 1.28. When the expression of TLR9 was effectively silenced, CpG ODNs used alone were identified to produce SERs as low as 1.01. Flow cytometry demonstrated that the interaction between TLR9 and CpG ODN 7909 alone did not significantly affect the rate of apoptosis, but may significantly enhance the radiation-induced apoptosis of A549 cells. Western blot analysis revealed that TLR9 activation by CpG ODN 7909 increased the levels of mitogen-activated protein kinase 14, cellular tumor antigen p53, B-cell lymphoma 2 associated X protein and genome polyprotein, and decreased Bcl-2 expression levels, whereas these effects were not observed in CpG ODN 7909-treated cells in which TLR9 was knocked down. These results suggest that CpG ODN 7909 may enhance radiosensitivity through TLR9 activation, and partially via the p53 pathway in A549 lung cancer cells.
Keywords: Toll-like receptors, CpG oligodeoxynucleotide 7909, apoptosis, irradiation, lung cancer, radiosensitizer, signal transduction
Introduction
Toll-like receptors (TLRs), a class of toll-homologous protein molecules expressed in mammals, are pattern-recognition receptors that are present in macrophages, mononuclear cells, dendritic cells, B cells and other immune cells (1). At present, 13 types of TLRs have been identified in mammals, namely TLR1 to TLR13, including TLR1-TLR11 in humans (2). Interactions between TLRs and their specific pathogen-associated molecular patterns mediate intracellular signaling pathways, increasing the levels of various chemical regulatory factors and cell surface molecules, thereby triggering innate, and adaptive immune responses. TLR9 specifically recognizes the unmethylated cytosine-phosphate-guanosine (CpG) motifs in bacteria, viruses and plasmids or synthetic double-, or single-stranded oligodeoxynucleotides (ODNs). CpG ODNs are synthetic DNA nucleotides containing unmethylated CpG sequences. As powerful immune modulators and immune adjuvants, the effectiveness of CpG ODNs has attracted increasing attention, particularly as potential therapies for malignant tumors that are difficult to treat (3). Wang et al (4) demonstrated that the immunomodulatory oligonucleotide had potent antitumor effects when used as monotherapy and in combination with conventional chemotherapeutic agents on non-small cell lung cancer (NSCLC) cells via TLR9.
Previous studies have demonstrated that CpG ODNs may contribute to the induction of apoptosis, inhibition of cancer cell growth and enhancement of radiotherapeutic, and chemotherapeutic sensitivity in various types of cancer (3,5–7). Several studies have also confirmed that TLR9 is highly expressed in numerous types of cancer cells, including lung, ovarian, pancreatic and breast cancer (8–11). However, there are disagreements regarding the associations between TLR9 expression, tumor development, and sensitivity to radiation and chemotherapy. According to a previous study, subsequent to recognizing CpG ODNs, TLR9 may activate the mitogen-activated protein kinase 14 (p38)/mitogen-activated protein kinase (MAPK) signal pathway (12); however, the downstream signal transduction pathway and subsequently target gene expressions have been inconclusive (13,14).
Our previous studies demonstrated that the combination of TLR9 and its agonist CpG ODNs increased the radiosensitivity of A549 lung cancer cells through reducing cell survival and colony formation ability, increasing the G2/M phase block and inducing cell apoptosis. CpG ODNs increased the radiosensitivity of radioresistant A549 cells, which may be mediated through the upregulation of TLR9 expression (15,16). Furthermore, our subsequent study demonstrated that the combination of TLR9 and its agonist CpG ODNs markedly increased the levels of cellular tumor antigen p53 (p53) protein phosphorylation induced by X-ray, which may be involved in the G2/M phase arrest and apoptosis induced by X-ray in human lung cancer A549 cells (17). Additional studies have indicated that p53 phosphorylation serves an important, and even a crucial, regulatory role in its own activation (18,19). Activation of p53 may result in cell growth retardation, induction of apoptosis or adaptation to DNA damage and survival, via regulating the expression of downstream target genes (20,21). Wild-type p53 has also been demonstrated to enhance the sensitivity of tumor cells to radiotherapy by inhibiting oncogene expression, arresting tumor cells in the G2/M phase, inhibiting tumor cell repair of radiation damage, and promoting apoptosis (14,15). Based on the aforementioned studies, we hypothesized that TLR9 may strengthen the effect of X-ray irradiation (IR) on NSCLC and consequently improve the radiosensitivity of lung cancer cells. Additionally, we also hypothesize that activation of the p53 pathway may be involved in this process; TLR9 expression may affect the proliferation and apoptosis of tumor cells through altering the expression of p53, and the associated downstream pathway via activating p38/MAPK signaling pathways. However, previous studies examining whether the TLR9-p53-p38/MAPK signaling pathways are involved in the process whereby TLR9 combines with CpG ODN to improve the radiosensitivity of lung cancer cells have not yet been sufficient.
In the present study, TLR9 gene expression was silenced using small interfering (si)RNA interference technology. Cells were then stimulated with CpG ODN 7909 and subjected to X-ray IR, and the radiation sensitivity and expression levels of p38, wild-type p53 and downstream target genes, including B-cell lymphoma 2 (Bcl-2), Bcl-2-associated X protein (Bax) and genome polyprotein (p21) were investigated. Therefore, the present study provides a preliminary investigation into the role of TLR9 in NSCLC radiotherapy, including the potential associated downstream pathway involved, which may assist in developing novel methods for predicting and improving the outcome of radiotherapy in NSCLC.
Materials and methods
Materials
Rabbit monoclonal antibodies against TLR9 (cat. no. 5845), p38 (cat. no. 8690), p53 (cat. no. 2527), Bax (cat. no. 2772), Bcl-2 (cat. no. 4223), p21 (cat. no. 2947) and β-actin (cat. no. 4970), were purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA). CpG ODN 7909, comprising a nucleotide sequence of 5′-TCGTCGTTTTGTCGTTTTGTCGTT-3′, was synthesized, purified and analyzed as previously described (11).
Cell culture and small interfering (si)RNA transfection
The human NSCLC A549 cell line was obtained from Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences (Shanghai, China) and grown in RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.). The cell lines were cultured at 37°C in a humidified air containing 5% CO2. When the cells reached 70% confluence, they were transfected with 100 nM siRNA targeting: Human TLR9 mRNA forward, 5′-CUAGACCUGUCCCACAAUATT-3′ and reverse, 5′-UAUUGUGGGACAGGUCUAGTT-3′; wild-type p53 mRNA forward, 5′-CCACUGGAUGGAGAAUAUUTT-3′ and reverse, 5′-AAUAUUCUCCAUCCAGUGGTT-3′; or scramble (control) siRNA 1 forward, 5′-UUCUCCGAACGUGUCACGUTT-3′ and reverse, 5′-ACGUGACACGUUCGGAGAATT-3′; scramble (control) siRNA 2 forward, 5′-UUCUCCGAACGUGUCACGUTT-3′ and reverse, 5′-ACGUGACACGUUCGAGAATT-3′), using Lipofectamine® 2000 reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. All siRNAs were fluorescein amidite (FAM)-labeled, and were obtained from Shanghai GenePharma Co., Ltd. (Shanghai, China). Subsequent to incubation for 6 h at 37°C in a humidified incubator containing 5% CO2, the medium was replaced with RPMI-1640 medium supplemented with 10% fetal bovine serum. Following another incubation for 18 h at 37°C, the cells were used for subsequent experimentation. Then, 24 h after transfection, the expression of green fluorescent protein was observed under an Olympus IX73 fluorescence microscope (Olympus Corporation, Tokyo, Japan) to evaluate the transfection efficiency. A total of 500 cells were selected randomly, and the percentage of cells expressing green fluorescent protein was calculated.
Radiation treatment
A549 cells and other transfected cells were exposed to various doses of 6 MV X-ray IR, which was performed with the use of a linear accelerator (Varian Medical Systems Inc., Palo Alto, CA, USA), and the dose rate at an IR distance of 100 cm was 2 Gy/min, as determined by Fricke's chemical dosimeter (15). Various doses of radiation (2, 4, 6, 8 and 10 Gy) were applied in the colony formation assay, while 10 Gy radiation was used for the remaining experiments.
Western blot analysis
Proteins were extracted from the cells with 10% SDS cell lysis solution (including 1:100 phenylmethylsulfonyl fluoride; Beyotime Institute of Biotechnology, Haimen, China) and protein concentration was measured using a BCA assay kit (Beyotime Institute of Biotechnology). Then, 6% SDS-PAGE (Beyotime Institute of Biotechnology) was used to separate the protein extracts (~50 µg/lane), which was then transferred to polyvinylidene fluoride membranes (EMD Millipore, Billerica, MA, USA). The membranes were then blocked with tris-buffered saline with 1% Tween-20 (TBST) containing 5% skimmed milk for 2 h at room temperature, and subsequently incubated with the primary antibodies (TLR9, p38, p53, Bax, Bcl-2, p21 or β-actin; dilution, 1:800) at room temperature. The membranes were then washed with TBST three times, followed by incubation with the horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (cat no. 4970; 1:5,000; Cell Signaling Technology, Inc.) for 1 h at room temperature. The membranes were finally washed with TBST and protein expression was determined with enhanced chemiluminescence kit (ECL; EMD Millipore). β-actin was applied as an endogenous reference for quantification. The images were analyzed using Adobe Photoshop CS3 software (Adobe Systems, Inc., San Jose, CA, USA).
Colony formation assay
Following X-ray IR, cells were collected and used to prepare a single-cell suspension. According to the radiation dose (0, 2, 4, 6, 8 and 10 Gy), cells were seeded in triplicate in 60-mm petri dishes at densities of 500, 500, 1,000, 500, 1,000 and 8,000 cells/dish, respectively. Following incubation at 37°C in an incubator for 2 weeks, the cells were washed twice with PBS, fixed with 4% paraformaldehyde for 30 min and stained with 0.5% crystal violet for 15 min at room temperature. Subsequently, cell colonies containing >50 cells were counted. The formed colonies were observed using light microscope (×100). The colony formation rate was calculated as the number of colonies divided by the number of seeded cells. According to single-hit, multi-target model, D0, Dq, N and SF2 were obtained (22). D0 represents the slope rate of survival curve, which indicates the radiosensitivity of cells; Dq represents the initial shoulder of survival curve, which is associated with the efficacy of the DNA repair system of the cell; and N is an extrapolation value of D0, which is an associated parameter reflecting primary radiation sensitivity of cells. SF2 was the survival fraction following 2 Gy irradiation and used as an index of intrinsic radiosensitivity. The sensitivity enhancement ratio (SER) was calculated as the D0 (lethal dose in one-hit multi-target model) value of cells receiving IR treatment alone in the control group divided by the D0 value of other cells receiving IR treatment alone, or CpG ODN 7909 plus IR treatment.
Apoptosis assays
Apoptosis was evaluated with the Annexin V-fluorescein isothiocyanate (FITC) Apoptosis Detection kit (BD Biosciences, San Jose, CA, USA) according to the manufacturer's protocol. All cells, including adherent and non-adherent cells, were collected at 48 h subsequent to IR, and washed twice with cold PBS. Following centrifugation at 800 × g for 5 min at 4°C, the deposits were resuspended in binding buffer, and incubated with FITC-conjugated Annexin-V and propidium iodide for 15 min at room temperature in the dark. Immediately, the cells were analyzed using a Cytomics™ FC500 flow cytometer (Beckman Coulter, Inc., Fullerton, CA, USA). Data were analyzed using Summit version 5.2 software (Beckman Coulter, Inc.).
Statistical analysis
All experiments were repeated three times. Statistical analysis was performed using SPSS 20.0 (IBM Corp., Armonk, NY, USA) and GraphPad Prism 5.0 (fitting the survival curve in a single-hit, multi-target model) (GraphPad Software, Inc., La Jolla, CA, USA). Values are presented as the mean ± standard deviation of triplicate experiments. One-way analysis of variance followed by Bonferroni post-hoc test was used for data with ≥3 groups or Student's t-test for data with two groups. P<0.05 was considered to indicate a statistically significant difference.
Results
Silencing of TLR9 expression in A549 cell lines by siRNA
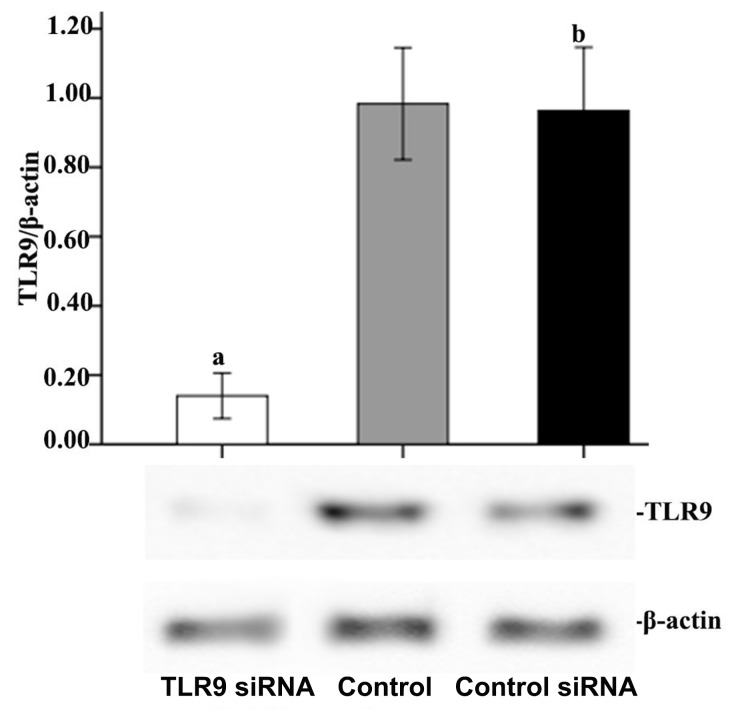
To knock down the expression of TLR9, siRNA targeting TLR9 were applied to the cells. A total of 24 h after transfection, the transfection efficiency was observed under an Olympus IX73 fluorescence microscope (Olympus Corporation, Tokyo, Japan). Scramble siRNA (control siRNA) was applied to exclude the effect of the liposome transfection on the measured effects. All siRNAs were FAM-labeled. Following transfection, >70% of cells in each transfected group were FAM-positive, indicating high transfection efficiency. Western blot analyses revealed decreased TLR9 expression levels in TLR9 siRNA-transfected cells compared with untransfected cells. As presented in Fig. 1, 24 h subsequent to transfection, TLR9 protein expression was significantly reduced in A549 cells transfected with TLR9 siRNA compared with that in control cells, demonstrating 85.76% inhibition based on the densitometric analysis (P<0.05). However, there was no significant difference between the control siRNA and control groups with regard to TLR9 protein expression, which suggested that transfection using Lipofectamine had very little or no effect on A549 cells.
Figure 1.
Effect of TLR9 siRNA on TLR9 expression in A549 cells. A549 cells were transfected with TLR9 siRNA or scrambled siRNA (control siRNA), or remained untransfected (control). At 24 h following transfection, western blot analyses were performed to examine the inhibition efficiency. The graph depicts mean ± standard deviation from three independent experiments of optical density of the TLR9 western blot bands. aP<0.05 vs. control group, bP>0.05 vs. control group. TLR9, toll-like receptor 9; si, small interfering.
Clonogenic assays for determination of radiosensitivity of A549 cells in response to CpG ODN 7909 combined with TLR9
Colony formation assays were performed to evaluate the radiation-enhancing effects of CpG ODN 7909 combined with TLR9. As demonstrated in Fig. 2 and Table I, the cell survival curve, fitted using a single-hit, multi-target model, indicated that A549 cells treated with CpG ODN 7909 plus IR exhibited decreased colony formation ability and lower values of D0, Dq, N and SF2 compared with cells treated with IR alone; the sensitivity enhancement ratios (SER) was 1.28. However, following the knockdown of TLR9 expression by siRNA, CpG ODN 7909 was unable to decrease the colony forming capacity of lung cancer cells and the SER was 1.01. Control-siRNA transfection had no significant effect on the colony formation ability of A549 cells.
Figure 2.
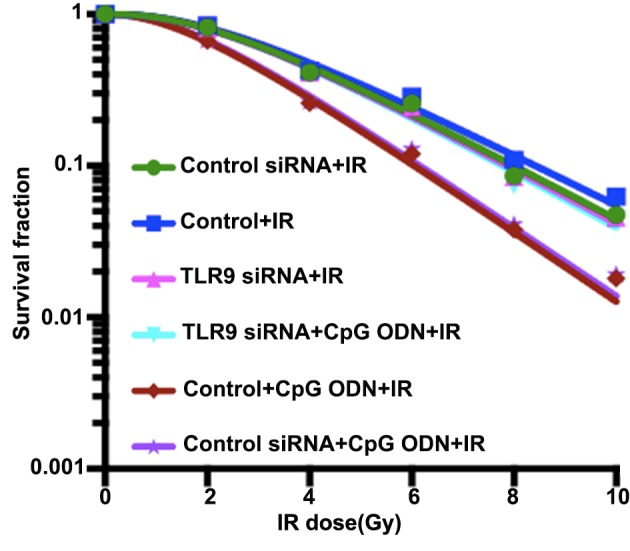
Activation of TLR9 by CpG ODN 7909 enhances the radiosensitivity of A549 cells. Compared with that of untransfected control cells, the dose-survival curve of TLR9 siRNA-transfected cells exhibited a broader initial shoulder (indicating increase in Dq) and a smaller slope rate (indicating increase in D0). TLR9 siRNA-transfected cells were more resistant to cell death post-IR. These results implied that CpG ODN 7909 plus IR resulted in decreased colony formation ability in untransfected control cells compared with TLR9 siRNA-transfected cells, with sensitivity enhancement ratios of 1.28 and 1.01, respectively. TLR9, toll-like receptor 9; CpG ODNs, cytosine-phosphorothioate-guanine-containing oligodeoxynucleotides; IR, irradiation; TLR9, toll-like receptor 9; si, small interfering; D0, slope rate of survival curve; Dq, initial shoulder of survival curve.
Table I.
Comparison of radiosensitivity of untransfected, control siRNA-transfected and TLR9 siRNA-transfected A549 cells treated with IR or CpG ODN 7909.
| Group | D0 | Dq | N | SF2 | SER |
|---|---|---|---|---|---|
| TLR9 siRNA + IR | 2.38 | 4.39 | 2.85 | 0.82 | 1.03 |
| Control + IR | 2.46 | 4.56 | 2.86 | 0.84 | – |
| Control siRNA + IR | 2.42 | 4.40 | 2.82 | 0.82 | 1.02 |
| TLR9 siRNA + CpG ODN + IR | 2.41 | 4.40 | 2.82 | 0.81 | 1.01 |
| Control + CpG ODN + IR | 1.92 | 2.90 | 2.52 | 0.67 | 1.28 |
| Control siRNA + CpG ODN + IR | 1.91 | 3.00 | 2.57 | 0.68 | 1.29 |
A549 cells treated with CpG ODN 7909 plus IR exhibited lower values of D0, Dq, N and SF2 compared with those treated with IR alone, and the SER was 1.28. No significant difference between the D0, Dq, N and SF2 values in the TLR9 siRNA-transfected A549 cells treated with CpG ODN 7909 plus IR compared with those treated with IR alone was observed, and the SER was 1.01. Compared with those of TLR9 siRNA-transfected A549 cells, the D0, Dq, N and SF2 values of control siRNA transfection cells demonstrated no significant difference. D0, slope rate of survival curve; Dq, initial shoulder of survival curve; N, extrapolation value of D0, which is an associated parameter reflecting primary radiation sensitivity of cells; SF2, the survival fraction after 2 Gy irradiation; TLR9, toll-like receptor 9; CpG ODN, cytosine-phosphorothioate-guanine-containing oligodeoxynucleotides; IR, irradiation; SER, sensitivity enhancement ratio; si, small interfering.
Effect of TLR9 expression on apoptosis in A549 cells treated with CpG ODN 7909 plus IR
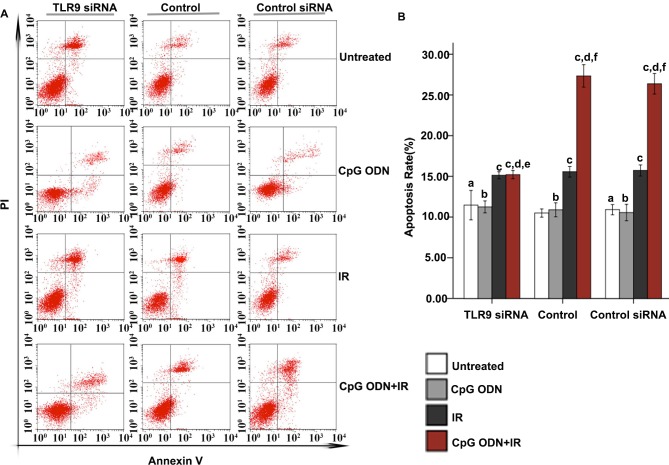
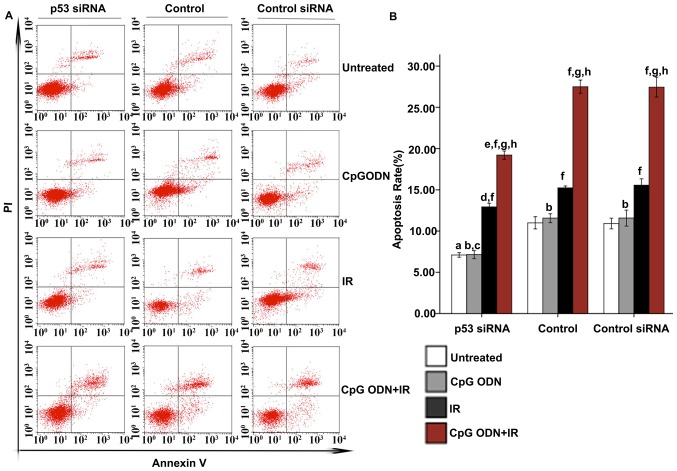
Flow cytometry was applied to analyze apoptosis. As indicated in Fig. 3, in the untreated control, TLR9 siRNA-transfected and control siRNA-transfected groups, there was no significant difference in apoptosis rates between untreated cells and cells treated with CpG ODN 7909 alone. Furthermore, in all three groups, compared with the untreated cells, there was a significantly increased apoptosis rate in cells treated with IR alone and those treated with CpG ODN 7909 plus IR (P<0.05). In the untransfected control and control siRNA groups, increased apoptosis was observed in cells treated with CpG ODN 7909 plus IR compared with that in cells treated with IR alone; however, this was not observed in the TLR9 siRNA group. Compared with the corresponding cells in the control group, cells in the TLR9 siRNA group that were untreated or treated with CpG ODN 7909 or IR alone did not exhibit any significant difference in the rate of apoptosis; however, cells in the TLR9 siRNA group treated with CpG ODN 7909 combined with IR exhibited a significantly decreased apoptosis rate compared with those in the control group. When receiving the same treatment, there was no significant difference between the untransfected control and control siRNA groups with regard to the apoptosis rate.
Figure 3.
Effect of TLR9 siRNA on apoptosis in A549 cells treated with CpG ODN plus IR. At 48 h following IR, apoptosis was evaluated using Annexin V-fluorescein isothiocyanate/PI staining and flow cytometric analysis. (A) Representative flow cytometry results: Bottom right quadrant, cells stained primarily by Annexin V (early apoptotic cells); top right quadrant, cells stained by PI and Annexin V (late apoptotic cells); top left quadrant, cells stained primarily by PI (necrotic cells); bottom left quadrant, cells negative for Annexin V and PI. (B) The rates of apoptosis (cells in bottom right and top right quadrants) in each transfection and treatment group were quantified and presented as the mean ± standard deviation. aNot significantly different (P>0.05) compared with untreated cells in the control group. bNot significantly different (P>0.05) compared with untreated cells in the same group. cP<0.05 compared with untreated cells in the same group. dP<0.05 compared with CpG ODN-treated cells in the same group. eNot significantly different (P>0.05) compared with IR-treated cells in the same group. fP<0.05 compared with IR-treated cells in the same group. TLR9, toll-like receptor 9; CpG ODNs, cytosine-phosphorothioate-guanine-containing oligodeoxynucleotides; IR, irradiation; PI, propidium iodide; si, small interfering; CITC, fluorescein isothiocyanate.
Effects of TLR9 activation by CpG ODN 7909 on the p53-mediated pathway in A549 lung cancer cells
Effect of TLR9 activation by CpG ODN 7909 on the expression of p38
The expression of p38 was detected by western blot analysis to explore whether the interaction between CpG ODN and TLR9 activated the p38/MAPK signaling pathway. As presented in Fig. 4, in the untransfected control and control siRNA groups, the cells treated with CpG ODN 7909 alone and those treated with CpG ODN 7909 plus IR exhibited increased p38 expression levels compared with untreated cells. By contrast, the treatments had no significant effect on p38 levels in the TLR9 siRNA group. In all the three groups, compared with untreated cells, there was no significant difference in p38 levels in cells treated with IR alone.
Figure 4.
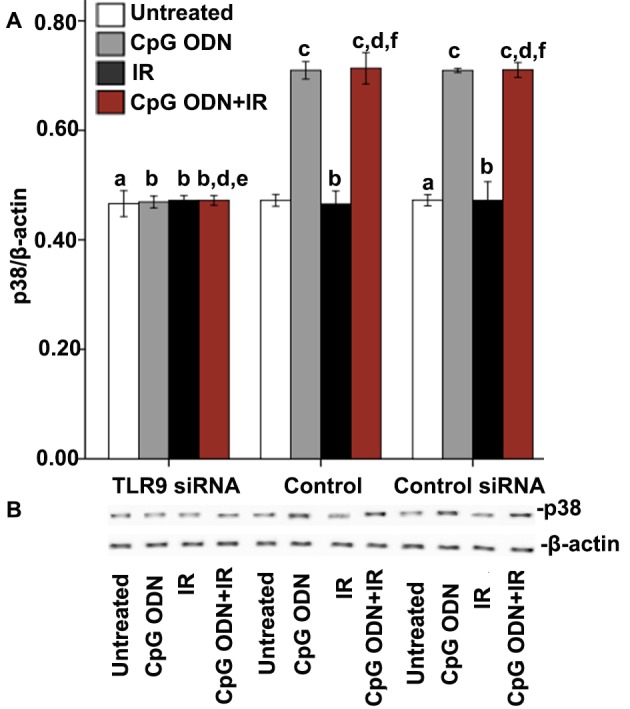
Effect of TLR9 activation by CpG ODN 7909 on the expression of p38 in A549 cells. (A) Quantification and (B) representative image of western blot analyses performed to detect the expression of p38. The graph presents data as the mean ± standard deviation from three independent determinations of optical density of the p38 western blot bands. aNot significantly different (P>0.05) compared with untreated cells in the control group. bNot significantly different (P>0.05) compared with untreated cells in the same group. cP<0.05 compared with untreated cells in the same group. dNot significantly different (P>0.05) compared with CpG ODN-treated cells in the same group. eNot significantly different (P>0.05) compared with IR-treated cells in the same group. fP<0.05 compared with IR-treated cells in the same group. TLR9, toll-like receptor 9; CpG ODNs, cytosine-phosphorothioate-guanine-containing oligodeoxynucleotides; IR, irradiation; p38, mitogen-activated protein kinase 14.
Effect of CpG ODN 7909-mediated TLR9 activation on the expression of p53 pathway-associated proteins
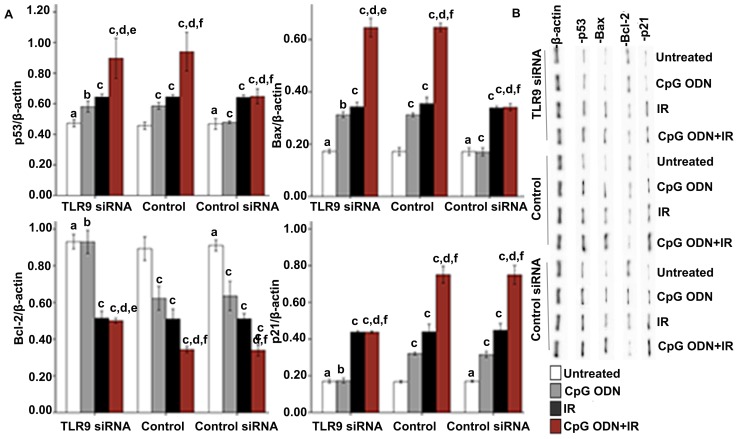
X-ray IR may lead to apoptosis via activation of the p53 signal pathway (16). In order to investigate whether TLR9 activation by CpG ODN 7909 enhanced the radiosensitivity of A549 lung cancer cells of via the p53 pathway, various p53 pathway-associated proteins, including p53, Bax, Bcl-2 and p21, were detected by western blotting. As indicated in Fig. 5, in the control and control siRNA groups, significantly increased expression levels of p53, Bax and p21, and unchanged expression levels of Bcl-2 were observed in cells treated with CpG ODN 7909 alone compared with those in the untreated cells. By contrast, no significant difference was observed in the TLR9 siRNA group. In all the three groups, compared with the untreated cells, increased expression levels of p53, Bax and p21, and decreased Bcl-2 expression were observed in cells treated with IR alone and in cells treated with CpG ODN 7909 plus IR. In the untransfected control and control siRNA groups, the levels of p53, Bax and p21 expression were increased, and the Bcl-2 levels were decreased significantly in the cells treated with CpG ODN7909 plus IR compared with the cells treated with IR alone; however, the combined treatment did not lead to the same effects in the TLR9 siRNA group. When receiving the same treatment, no significant difference between control and control siRNA groups for all detected p53 pathway associated proteins was observed.
Figure 5.
Effect of TLR9 activation by CpG ODN 7909 on p53 pathway-associated proteins in A549 cells. (A) Quantification and (B) representative image of western blot analyses applied to detect the expression of p53 pathway-associated proteins, including p53, Bax, Bcl-2 and p21. The graph presents data as the mean ± standard deviation from three independent determinations of optical density of the protein western blot bands. aNot significantly different (P>0.05) compared with untreated cells in the control group. bNot significantly different (P>0.05) compared with untreated cells in the same group. cP<0.05 compared with untreated cells in the same group. dP<0.05 compared with CpG ODN-treated cells in the same group. eNot significantly different (P>0.05) compared with IR-treated cells in the same group. fP<0.05 compared with IR-treated cells in the same group. TLR9, toll-like receptor 9; CpG ODNs, cytosine-phosphorothioate-guanine-containing oligodeoxynucleotides; IR, irradiation; p53, cellular tumor antigen p53; Bcl-2, B-cell lymphoma 2; Bax, Bcl-2-associated X protein; p21, genome polyprotein.
Effect of blocking the p53 signal transduction pathway on apoptosis in A549 cells treated with CpG ODN 7909 plus IR
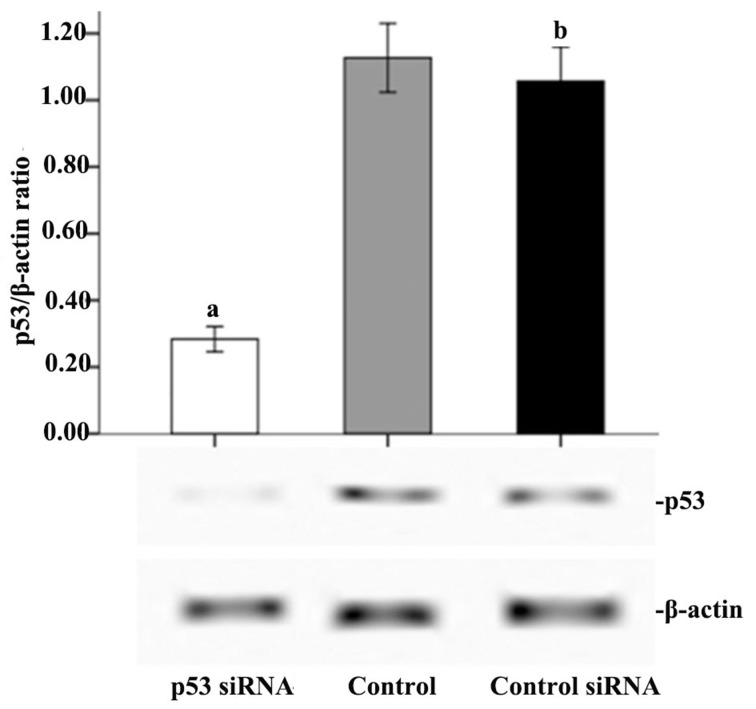
As demonstrated in Fig. 6, p53 was successfully knocked down by transfection of the cells with p53 siRNA. Therefore, the p53-mediated signal transduction pathway was blocked to verify its effect on the apoptosis of cells A549 cells treated with CpG ODN 7909 plus IR. As indicated in Fig. 7, the rate of apoptosis of the untreated cells, cells treated with CpG ODN7909 or IR respectively, and CpG ODN 7909 plus IR in the p53 siRNA group all decreased notably compared with those in the control group. In p53 siRNA, control or control siRNA groups, the apoptosis rate of cells treated with CpG ODN7909 alone did not vary significantly compared with those of cells without any treatment; but there was a significantly increased apoptosis rate in cells treated with IR alone or CpG ODN7909 combined with IR compared with that in cells without any treatment (P<0.05); in addition, compared with cells treated with IR or CpG ODN7909 alone, increased apoptosis was observed in the cells treated with CpG ODN7909 plus IR. No significant difference between control and control siRNA groups for the apoptosis rate was observed in any treatment group.
Figure 6.
Effect of p53 siRNA on p53 expression in A549 cells. A549 cells were transfected with p53 siRNA, scrambled siRNA (control siRNA), or left untransfected (control). At 24 h following transfection, western blot analyses were performed to examine the inhibition efficiency. The graph presents data as the mean ± standard deviation from three independent determinations of optical density of the p53 western blot bands. aP<0.05 compared with control. bNot statistically different (P>0.05) compared with control. p53, cellular tumor antigen p53; si, small interfering.
Figure 7.
Effect of blocking the p53 transduction pathway on apoptosis. At 48 h following IR, the apoptosis rates of all cells were evaluated using Annexin V-FITC/PI staining and flow cytometric analysis. (A) Representative flow cytometry results: Bottom right quadrant, cells stained primarily by Annexin V (early apoptotic cells); top right quadrant, cells stained by both PI and Annexin V (late apoptotic cells); top left quadrant, cells stained primarily by PI (necrotic cells); bottom left quadrant, cells negative for both Annexin V and PI. (B) The rates of apoptosis (cells in both bottom right and top right quadrants) in each transfection and treatment group were quantified and presented as the mean ± standard deviation. aP<0.05 compared with untreated cells of the control group. bNot significantly different (P>0.05) compared with untreated cells in the same group. cP<0.05 compared with CpG ODN-treated cells of the control group. dP<0.05 compared with IR-treated cells of the control group. eP<0.05 compared with CpG ODN plus IR-treated cells of the control group. fP<0.05 compared with untreated cells in the same group. gP<0.05 compared with CpG ODN-treated cells in the same group. hP<0.05 compared with IR-treated cells in the same group. CpG ODNs, cytosine-phosphorothioate-guanine-containing oligodeoxynucleotides; IR, irradiation; FITC, fluorescein isothiocyanate; PI, propidium iodide; p53, cellular tumor antigen p53; si, small interfering.
Discussion
As a monotherapy and combined with other treatment methods, CpG ODNs have exhibited vast potential for the treatment of cancer (5,23). Using in vitro experiments and in vivo animal models, previous studies have identified that CpG ODNs combined with chemotherapy or radiation may increase the therapeutic effects of these conventional therapies in treating cancer (16,24,25). CpG ODNs may have broad application prospects for the prevention and treatment of malignant tumors (26). It has been demonstrated previously that CpG ODNs exert their effects by interacting with TLR9 and activating the subsequent pathways (27).
In the present study, the effect of CpG ODNs treatment and TLR9 expression on the radiosensitivity of A549 lung cancer cells was investigated, and whether the p53 signaling pathway was the downstream pathway involved in exerting these effects was explored.
The results of colony forming assays revealed that the activation of TLR9 by CpG ODN 7909 significantly increased the radiosensitivity of A549 lung cancer cells, whereas CpG ODNs used alone did not significantly affect radiosensitivity when the expression of TLR9 was knocked down. This indicated that the interaction of CpG ODNs with TLR9 was responsible for increasing sensitivity of the cancer cells to X-ray IR.
Cell apoptosis is an important factor affecting tumor development; the tumorigenesis and progression of malignant tumors depends on the inhibition of the cell death processes, and unlimited malignant hyperplasia of tumor cells. Therefore, interventions that may cause tumor cell apoptosis represent potential tumor treatment strategies. In the present study, X-ray IR alone induced marked increases in apoptosis in the three groups of cells (untransfected, TLR9 siRNA-transfected and control siRNA-transfected A549 cells) compared with the respective untreated cells, whereas CpG ODN 7909 alone had no significant effect on apoptosis in any group. Furthermore, the combined apoptosis-inducing effect of TLR9 and CpG ODNs following IR treatment was significantly increased compared with that of X-ray IR alone, which was indicated by the lack of increase in apoptosis following the application of the combined treatment in cells with TLR9 gene silencing. This evidence suggests that the interaction of CpG ODNs with TLR9 is associated with the significant enhancement of radiation-induced apoptosis in NSCLC cells to a certain extent.
The interaction between CpG ODNs and TLR9 activates the p38/MAPK signaling pathway, and subsequently the p53 signal pathway (28,29). The p38/MAPK pathway is involved in various biological effects, including the stress response, cell proliferation and apoptosis (30). A previous study identified that the overexpression of p38 MAPK proteins by the transfection of exogenous p38 MAPK into cells improved the curative effect of various pro-apoptotic genes dependent on the p38/MAPK signal pathway, and p38 MAPK may regulate apoptosis by activating p53 (13,31). This suggests that TLR9 may affect the proliferation and apoptosis of tumor cells through altering the expression of p53 via activation of the p38/MAPK pathway. It is well-known that the p53-mediated apoptotic pathway is important in the apoptotic cell death of tumors; the tumor suppressor p53 regulates cell cycle arrest, apoptosis and DNA repair processes by controlling various target genes that contain p53 sequence-specific DNA binding sites (32–34).
X-ray IR may result in apoptosis in various types of human cancer and consequently kill cancer cells; increased apoptosis rates usually indicate increased radiosensitivity (35). A previous study has demonstrated that X-ray IR may increase wild-type p53 protein levels and subsequently induce p53-dependent apoptotic cell death (36).
In order to explore whether the p53-mediated apoptotic pathway was involved in signal transduction downstream of TLR9, the levels of the p53 pathway-associated proteins p53, Bax, Bcl-2 and p21 were examined. Bax and Bcl-2 are pro-apoptotic and anti-apoptotic genes, respectively, in the Bcl-2 family. Bax is the primary regulator of Bcl-2 and serves an important role in modulating tumor cells (37,38). X-ray IR may increase the expression of Bax in cancer cells and result in apoptosis (39). A previous study indicated that p21, a member of the cyclin-dependent kinase inhibitor family, may cause G2/M phase arrest by inhibiting the function of cyclin-dependent kinase 1 (40). As a target gene of the p53 signal transduction pathway and upstream gene of certain pro-apoptotic genes, p21 may be involved in the IR-induced apoptosis of cancer cells (41).
The results of the present study indicated that CpG ODN 7909 treatment increased the expression of p38, p53, Bax and p21, and decreased the expression of Bcl-2, whereas TLR9 knockdown attenuated the effect of CpG ODN 7909 treatment alone. This suggested that CpG ODN 7909 may activate the p38 MAPK-p53 pathway, but only through TLR9. X-ray IR alone significantly increased the expression of p53, Bax and p21, and decreased the expression of Bcl-2, but did not significantly alter the expression of p38. Furthermore, the effect of X-ray IR on the p53 pathway was not affected by the expression of TLR9. The combined treatment of CpG ODN 7909 and X-ray IR did not result in an increased expression of p38 compared with that in cells treated with CpG ODN 7909 alone, but did induce increased expression of p53, Bax and p21 and decreased expression of Bcl-2 in cells treated with either CpG ODN 7909 or X-ray IR single treatment. The results regarding the p38 MAPK-p53 pathway-associated expression combined with effect of TLR9 expression on apoptosis indicate that TLR9 activation by CpG ODN 7909 may activate the p38 MAPK-p53 pathway, but not induce apoptosis. A potential reason may be that TLR9 activation by CpG ODN 7909 may activate other pathways that counteract the effect of the p53 pathway on inducing apoptosis directly. However, the activation of the p38 MAPK-p53-mediated pathway may significantly enhance the IR-induced apoptosis of A549 cells, thus increasing the radiosensitivity of A549 cells. Additional studies are required to establish the underlying reason and mechanism for this.
In order to verify whether the p53 pathway affects the radiosensitivity of A549 lung cancer cells, the expression of p53 was knocked down. The results indicated that, although the apoptosis rate was decreased compared with that in control cells, X-ray IR may induce apoptosis in p53 siRNA-transfected cells. In addition, combined treatment of CpG ODN 7909 and IR also produced this effect, which indicated that the activation of the p53 pathway was partially, but not fully, responsible for IR-induced apoptosis, and therefore contributed to enhancing the radiosensitivity of A549 lung cancer cells.
In conclusion, the results of the present study demonstrated that TLR9 activation by CpG ODN 7909 may produce a therapeutic effect by enhancing the radiotherapeutic sensitivity of A549 cells. This mechanism is at least partially associated with the activation of the TLR9-p53-mediated pathway. The results of the present study may be useful in improving the effectiveness of radiotherapy for lung cancer and in developing a TLR9-targeted treatment with CpG ODNs as a radiosensitizer. However, a number of factors remain unclear, including the following: The specific and exact mechanisms of the interaction of CpG ODNs with TLR9; the process by which identification of CpG ODNs by immune or tumor cells leads to the eventual effects; whether CpG ODNs may be developed as a radiosensitizer, and applied to patients safely and effectively in a clinical setting; and what the optimal dose and administration route would be. Therefore, additional studies are required to address to these issues.
Acknowledgements
Not applicable
Funding
The present study was supported by a grant from the Shanghai Municipal Health Bureau Commission of Health and Family Planning (grant no. 20134y156).
Availability of data and materials
All data generated or analyzed during the present study are included in this published article.
Authors' contributions
TKQ was the guarantor of integrity of entire study, study concepts/study design and definition of intellectual content. SJY, XL and XBZ performed data analysis/data acquisition, SJY, WC, XC and QZ performed the literature research and SJY and TKQ performed manuscript editing. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of interest.
References
- 1.Mutwiri G. TLR9 agonists: Immune mechanisms and therapeutic potential in domestic animals. Vet Immunol Immunopathol. 2012;148:85–89. doi: 10.1016/j.vetimm.2011.05.032. [DOI] [PubMed] [Google Scholar]
- 2.Yoneda K, Sugimoto K, Shiraki K, Tanaka J, Beppu T, Fuke H, Yamamoto N, Masuya M, Horie R, Uchida K, Takei Y. Dual topology of functional Toll-like receptor 3 expression in human hepatocellular carcinoma: Differential signaling mechanisms of TLR3-induced NF-kappaB activation and apoptosis. Int J Oncol. 2008;33:929–936. [PubMed] [Google Scholar]
- 3.He H, Genovese KJ, Swaggerty CL, Nisbet DJ, Kogut MH. Differential induction of nitric oxide, degranulation, and oxidative burst activities in response to microbial agonist stimulations in monocytes and heterophils from young commercial turkeys. Vet Immunol Immunopathol. 2008;123:177–185. doi: 10.1016/j.vetimm.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 4.Wang H, Rayburn ER, Wang W, Kandimalla ER, Agrawal S, Zhang R. Chemotherapy and chemosensitization of non-small cell lung cancer with a novel immunomodulatory oligonucleotide targeting Toll-like receptor 9. Mol Cancer Ther. 2006;5:1585–1592. doi: 10.1158/1535-7163.MCT-06-0094. [DOI] [PubMed] [Google Scholar]
- 5.Holtick U, Scheulen ME, von Bergwelt-Baildon MS, Weihrauch MR. Toll-like receptor 9 agonists as cancer therapeutics. Expert Opin Investig Drugs. 2011;20:361–372. doi: 10.1517/13543784.2011.553187. [DOI] [PubMed] [Google Scholar]
- 6.Petrangolini G, Tortoreto M, Perego P, Carenini N, De Cesare M, Balsari A, Zunino F, Pratesi G. Combination of metronomic gimatecan and CpG oligodeoxynucleotides against an orthotopic pancreatic cancer xenograft. Cancer Biol Ther. 2008;7:596–601. doi: 10.4161/cbt.7.4.5548. [DOI] [PubMed] [Google Scholar]
- 7.Brignole C, Marimpietri D, Di Paolo D, Perri P, Morandi F, Pastorino F, Zorzoli A, Pagnan G, Loi M, Caffa I, et al. Therapeutic targeting of TLR9 inhibits cell growth and induces apoptosis in neuroblastoma. Cancer Res. 2010;70:9816–9826. doi: 10.1158/0008-5472.CAN-10-1251. [DOI] [PubMed] [Google Scholar]
- 8.Droemann D, Albrecht D, Gerdes J, Ulmer AJ, Branscheid D, Vollmer E, Dalhoff K, Zabel P, Goldmann T. Human lung cancer cells express functionally active Toll-like receptor 9. Respir Res. 2005;6:1. doi: 10.1186/1465-9921-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu HQ, Wang B, Zhu SK, Tian Y, Zhang JH, Wu HS. Effects of CPG ODN on biological behavior of PANC-1 and expression of TLR9 in pancreatic cancer. World J Gastroenterol. 2011;17:996–1003. doi: 10.3748/wjg.v17.i8.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tuomela J, Sandholm J, Karihtala P, Ilvesaro J, Vuopala KS, Kauppila JH, Kauppila S, Chen D, Pressey C, Härkönen P, et al. Low TLR9 expression defines an aggressive subtype of triple-negative breast cancer. Breast Cancer Res Treat. 2012;135:481–493. doi: 10.1007/s10549-012-2181-7. [DOI] [PubMed] [Google Scholar]
- 11.Kundu SD, Lee C, Billips BK, Habermacher GM, Zhang Q, Liu V, Wong LY, Klumpp DJ, Thumbikat P. The toll-like receptor pathway: A novel mechanism of infection induced carcinogenesis of prostate epithelial cells. Prostate. 2008;68:223–229. doi: 10.1002/pros.20710. [DOI] [PubMed] [Google Scholar]
- 12.Chang JH, Park JY, Kim SK. Dependence on p38 MAPK signalling in the up-regulation of TLR2, TLR4 and TLR9 gene expression in Trichomonas vaginalis-treated HeLa cells. Immunology. 2006;118:164–170. doi: 10.1111/j.1365-2567.2006.02347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Amicis F, Giordano F, Vivacqua A, Pellegrino M, Panno ML, Tramontano D, Fuqua SA, Andò S. Resveratrol, through NF-Y/p53/Sin3/HDAC1 complex phosphorylation, inhibits estrogen receptor gene expression via p38MAPK/CK2 signaling in human breast cancer cells. FASEB J. 2011;25:3695–3707. doi: 10.1096/fj.10-178871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riazantseva NV, Novitskiĭ VV, Kaĭgorodova EV, Chasovskikh NIu, Starikova EG. Mitogenactivated protein kinases JNK and p38 as redox-dependent molecular targets correction of programmed cell death disturbances in oxidative stress condition. Usp Fiziol Nauk. 2009;40:3–11. (In Russian) [PubMed] [Google Scholar]
- 15.Zha L, Qiao T, Yuan S, Lei L. Enhancement of radiosensitivity by CpG-oligodeoxyribonucleotide-7909 in human non-small cell lung cancer A549 cells. Cancer Biother Radiopharm. 2010;25:165–170. doi: 10.1089/cbr.2009.0686. [DOI] [PubMed] [Google Scholar]
- 16.Yan L, Xu G, Qiao T, Chen W, Yuan S, Li X. CpG-ODN 7909 increases radiation sensitivity of radiation-resistant human lung adenocarcinoma cell line by overexpression of Toll-like receptor 9. Cancer Biother Radiopharm. 2013;28:559–564. doi: 10.1089/cbr.2012.1450. [DOI] [PubMed] [Google Scholar]
- 17.Liu XQ, Qiao TK, Chen W, Yuan SJ. Role of ATM kinase in the effect of CpG-oligodeoxynucleotide-7909 on X-ray-induced G2/M phase arrest and apoptosis in A549 cells. Chin J Radiol Med Prot. 2012;3:270–273. (In Chinese) [Google Scholar]
- 18.Viadiu H, Fronza G, Inga A. Structural studies on mechanisms to activate mutant p53. Subcell Biochem. 2014;85:119–32. doi: 10.1007/978-94-017-9211-0_7. [DOI] [PubMed] [Google Scholar]
- 19.Merino D, Malkin D. p53 and hereditary cancer. Subcell Biochem. 2014;85:1–16. doi: 10.1007/978-94-017-9211-0_1. [DOI] [PubMed] [Google Scholar]
- 20.Khan I, Garikapati KR, Shaik AB, Makani VKK, Rahim A, Shareef MA, Reddy VG, Pal-Bhadra M, Kamal A, Kumar CG. Design, synthesis and biological evaluation of 1, 4-dihydro indeno[1,2-c] pyrazole linked oxindole analogues as potential anticancer agents targeting tubulin and inducing p53 dependent apoptosis. Eur J Med Chem. 2017;144:104–115. doi: 10.1016/j.ejmech.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 21.Parvathaneni S, Lu X, Chaudhary R, Lal A, Madhusudan S, Sharma S. RECQ1 expression is upregulated in response to DNA damage and in a p53-dependent manner. Oncotarget. 2017;8:75924–75942. doi: 10.18632/oncotarget.18237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spring E, Holmberg P. Evaluation of experimental irradiation fractionation with the single-hit, multi-target model. Acta Radiol Ther Phys Biol. 1968;7:297–306. doi: 10.3109/02841866809133203. [DOI] [PubMed] [Google Scholar]
- 23.Yang L, Wu X, Wan M, Yu Y, Yu Y, Wang L. CpG oligodeoxynucleotides with double stem-loops show strong immunostimulatory activity. Int Immunopharmacol. 2013;15:89–96. doi: 10.1016/j.intimp.2012.10.020. [DOI] [PubMed] [Google Scholar]
- 24.Sommariva M, de Cesare M, Meini A, Cataldo A, Zaffaroni N, Tagliabue E, Balsari A. High efficacy of CpG-ODN, cetuximab and cisplatin combination for very advanced ovarian xenograft tumors. J Transl Med. 2013;11:25. doi: 10.1186/1479-5876-11-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang S, Liu X, Qiao T, Zhang Q. Radiosensitization by CpG ODN7909 in an epidermoid laryngeal carcinoma Hep-2 cell line. J Int Med Res. 2017;45:2009–2022. doi: 10.1177/0300060517728634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sfondrini L, Sommariva M, Tortoreto M, Meini A, Piconese S, Calvaruso M, Van Rooijen N, Bonecchi R, Zaffaroni N, Colombo MP, et al. Anti-tumor activity of CpG-ODN aerosol in mouse lung metastases. Int J Cancer. 2013;133:383–393. doi: 10.1002/ijc.28028. [DOI] [PubMed] [Google Scholar]
- 27.Xing N, Qiao T, Zhuang X, Yuan S, Zhang Q, Xu G. CpG oligodeoxyribonucleotide 7909 enhances radiosensitivity via downregulating Oct-4 expression in radioresistant lung cancer cells. Onco Targets Ther. 2015;8:1443–1449. doi: 10.2147/OTT.S84467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Honda K, Yanai H, Mizutani T, Negishi H, Shimada N, Suzuki N, Ohba Y, Takaoka A, Yeh WC, Taniguchi T. Role of a transductional-transcriptional processor complex involving MyD88 and IRF-7 in Toll-like receptor signaling; Proc Natl Acad Sci USA; 2004; pp. 15416–15421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hong MY, Gao JL, Cui JZ, Wang KJ, Tian YX, Li R, Wang HT, Wang H. Effect of c-Jun NH2-terminal kinase-mediated p53 expression on neuron autophagy following traumatic brain injury in rats. Chin Med J (Engl) 2012;125:2019–2024. [PubMed] [Google Scholar]
- 30.Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298:1911–1912. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- 31.Strittmatter F, Gratzke C, Walther S, Göttinger J, Beckmann C, Roosen A, Schlenker B, Reich O, Stief CG, Hennenberg M. Alpha1-adrenoceptor signaling in the human prostate involves regulation of p38 mitogen-activated protein kinase. Urology. 2011;78:969.e7–e13. doi: 10.1016/j.urology.2011.03.036. [DOI] [PubMed] [Google Scholar]
- 32.Aloni-Grinstein R, Schwartz D, Rotter V. Accumulation of wild-type p53 protein upon gamma-irradiation induces a G2 arrest-dependent immunoglobulin kappa light chain gene expression. EMBO J. 1995;14:1392–1401. doi: 10.1002/j.1460-2075.1995.tb07125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu R, Ji P, Liu B, Qiao H, Wang X, Zhou L, Deng T, Ba Y. Apigenin enhances the cisplatin cytotoxic effect through p53-modulated apoptosis. Oncol Lett. 2017;13:1024–1030. doi: 10.3892/ol.2016.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang P, Cui J, Wen J, Guo Y, Zhang L, Chen X. Cisplatin induces HepG2 cell cycle arrest through targeting specific long noncoding RNAs and the p53 signaling pathway. Oncol Lett. 2016;12:4605–4612. doi: 10.3892/ol.2016.5288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vellanki SH, Grabrucker A, Liebau S, Proepper C, Eramo A, Braun V, Boeckers T, Debatin KM, Fulda S. Small-molecule XIAP inhibitors enhance gamma-irradiation-induced apoptosis in glioblastoma. Neoplasia. 2009;11:743–752. doi: 10.1593/neo.09436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Borges HL, Chao C, Xu Y, Linden R, Wang JY. Radiation-induced apoptosis in developing mouse retina exhibits dose-dependent requirement for ATM phosphorylation of p53. Cell Death Differ. 2004;11:494–502. doi: 10.1038/sj.cdd.4401366. [DOI] [PubMed] [Google Scholar]
- 37.Rossé T, Olivier R, Monney L, Rager M, Conus S, Fellay I, Jansen B, Borner C. Bcl-2 prolongs cell survival after Bax-induced release of cytochrome c. Nature. 1998;391:496–499. doi: 10.1038/35160. [DOI] [PubMed] [Google Scholar]
- 38.Guo B, Zhai D, Cabezas E, Welsh K, Nouraini S, Satterthwait AC, Reed JC. Humanin peptide suppresses apoptosis by interfering with Bax activation. Nature. 2003;423:456–461. doi: 10.1038/nature01627. [DOI] [PubMed] [Google Scholar]
- 39.Arafat W, Zhou T, Naoum GE, Buchsbaum DJ. Targeted radiotherapy potentiates the cytotoxicity of a novel anti-human DR5 monoclonal antibody and the adenovirus encoding soluble TRAIL in prostate cancer. J Egypt Natl Canc Inst. 2015;27:205–215. doi: 10.1016/j.jnci.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 40.Torricelli C, Salvadori S, Valacchi G, Souček K, Slabáková E, Muscettola M, Volpi N, Maioli E. Alternative pathways of cancer cell death by rottlerin: Apoptosis versus autophagy. Evid Based Complement Alternat Med. 2012;2012:980658. doi: 10.1155/2012/980658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murphy M, Mabruk MJ, Lenane P, Liew A, McCann P, Buckley A, Billet P, Leader M, Kay E, Murphy GM. The expression of p53, p21, Bax and induction of apoptosis in normal volunteers in response to different doses of ultraviolet radiation. Br J Dermatol. 2002;147:110–117. doi: 10.1046/j.1365-2133.2002.04749.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during the present study are included in this published article.