Abstract
Introduction:
Cardiopulmonary exercise testing (CPET), the gold standard of cardiopulmonary evaluation, is used to determine VO2 levels at different aerobic exercise training intensities; however, it may not be feasible to conduct CPET in all clinical settings.
Aims:
To compare the heart rate reserve (HRR) and percent of 220-age methods for prescribing cycle ergometry exercise intensity using heart rate (HR) against the HRs obtained during a CPET in adults undergoing treatment for acute leukemia (AL).
Methods:
In this exploratory study, part of a larger randomized controlled trial, 14 adults with AL completed CPET on a cycle ergometer with indirect calorimetry within 96 hr of admission to a cancer hospital to determine VO2peak and HR corresponding to low (40% VO2peak), moderate (60% VO2peak), and high (75% VO2peak) exercise intensities. Analyses of variance were used to compare estimated HR for each intensity level using the HRR and percent of 220-age methods with HR determined via VO2peak.
Results:
HR corresponding to low-intensity exercise differed significantly across all three methods (p ≤ .05). No significant differences were observed between HR estimated via the percent of 220-age method and determined via VO2peak at moderate (100 ± 8 and 113 ± 24 bpm, p = .122) or high intensities (125 ± 10 and 123 ± 25 bpm, p = .994).
Conclusion:
In adults with AL, HR-based methods for defining aerobic exercise intensities should be used with caution. At low intensity, neither should be used, while at moderate and high intensities, the percent of 220-age equation might serve as an adequate substitute for CPET.
Keywords: acute leukemia, aerobic exercise intensity, chemotherapy, heart rate
Acute leukemia (AL) is a type of blood cancer that disrupts the production and/or maturation of hematopoietic cells in the bone marrow. In 2015, it accounted for more than 27,000 cancer cases in the United States (American Cancer Society [ACS], 2015). Acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL) are commonly treated via high-intensity induction chemotherapy. Treatment is successful in saving the lives of 65% of AML patients and 80% of ALL patients, but the 5-year survival rate is significantly lower in leukemia patients (only 30% for AML and 40% for ALL patients) when compared to other types of cancers such as breast and prostate (ACS, 2015).
Shortening the time between diagnosis and treatment completion is important for achieving remission and increases the rate of survival in AL patients (ALPs). However, severity of symptoms and side effects can decrease treatment adherence, for example, cancer-related fatigue that can lead to decreased fitness capacity and difficulties performing the activities of daily living and decreased health-related quality of life (Bryant, Walton, & Phillips, 2015; Bryant, Walton, Shaw-Kokot, Mayer, & Reeve, 2015). A few studies have examined potential benefits of exercise training in leukemia patients and have achieved successful outcomes in the alleviation of treatment-related symptoms, particularly fatigue (Alibhai et al., 2012; Battaglini et al., 2009; Chang et al., 2008; Klepin et al., 2011). There has been limited research, however, in determining the most effective exercise prescription paradigm (i.e., mode, duration, intensity, frequency, and timing within treatment) to maximize the benefits in patients with hematological cancers. Schmitz et al. (2010), authors of the “American College of Sports Medicine Roundtable on Exercise Guidelines for Cancer Survivors,” called for researchers to continue to explore different training regimens as well as the effects of exercise in other cancer populations.
At this time, the recommendations for exercise are generalized for the cancer population and are the same as the Physical Activity Guidelines for Americans, including the recommendation of 150 min of moderate intensity exercise per week (Schmitz et al., 2010). In the AL population, however, the prescription of exercise is challenging due not only to the intense treatment schedules and side effects but also to the inability of hospitals to perform accurate cardiorespiratory testing and use the results to prescribe exercise training intensity level. Cardiopulmonary exercise testing (CPET) to determine VO2peak is the gold standard for cardiopulmonary evaluation and is often used for determining exercise training intensities in different populations including cancer patients (Jones, Eves, Haykowsky, Joy, & Douglas, 2008). However, not every clinic or hospital has the equipment or personnel infrastructure to conduct and use the results of CPET to prescribe exercise intensity to their cancer patients. Thus, exercise intensity prescription varies based on availability of testing methods. In previous studies, exercise prescriptions for these patients have varied significantly, even though all represented attempts to be in the range of low- to moderate-intensity prescriptions: resting heart rate (RHR) + 30 bpm (Chang et al., 2008), 40–50% of heart rate reserve (HRR; Battaglini et al., 2009), and 60–75% of HRR (Alibhai et al., 2012).
For the purpose of practicality in these hospital settings, the HRR method and percent of 220-age equation have been used to prescribe exercise intensity without the benefit of CPET. In addition, in most exercise intervention research programs, for both supervised and unsupervised settings, heart rate (HR) has been used as a relatively easy and inexpensive way to monitor exercise intensity because of its practicality. No study, however, has examined the precision of using practical HR-based methods of exercise intensity determination such as the HRR or the percent of 220-age equation in ALPs initiating treatment, which leads to the simple questions of how well these methods of exercise intensity determination compare to the gold standard CPET in ALPs and whether the HR used for the determination of exercise intensity differs by cancer diagnosis or treatment type. Therefore, it is paramount that researchers conduct additional studies to allow for the development of more specific exercise plans for patients with cancer, including those with hematological malignancies (Battaglini, 2011).
In the present study, we compared HR training targets calculated using the HRR (often referred to as the Karvonen formula) and percent of 220-age equations against the HRs obtained directly from CPET for the determination of aerobic exercise intensity in newly diagnosed ALPs undergoing induction chemotherapy. The HR values from each of the three methods were compared at three different exercise intensity levels: low (40% of VO2peak), moderate (60% of VO2peak), and high (75% of VO2peak).
Materials and Methods
Participants
Study participants were 14 ALPs recruited from an ongoing larger trial at a North Carolina cancer hospital within 3 days of admission for treatment to the hematology oncology unit. A study coordinator informed patients about the study. If a patient expressed interest in participating, the coordinator asked the patient’s medical oncologist to evaluate her or his eligibility to participate based on review of the patient’s medical history and initial tests performed throughout the admission process and the following study inclusion/exclusion criteria: Participants were to be adults ≥21 years old, newly diagnosed with AML or ALL, admitted to begin induction chemotherapy with an expected hospital stay of 3–4 weeks, able to speak and understand English, and enrolled to participate in the parent study, Exercise and Quality of Life in Leukemia/Lymphoma Patients (EQUAL). They should not have cardiovascular disease; acute or chronic respiratory disease; acute or chronic bone, muscle, or joint abnormalities; altered mental state, dementia, or any other psychological condition that would prevent understanding of informed consent; another active malignancy; and active bleeding, acute thrombosis, ischemia, hemodynamic instability, or uncontrolled pain. After the medical oncologist cleared patients to participate in the study, we asked patients to sign an informed consent form approved by the University Biomedical Institutional Review Board prior to participating in any study activities.
All hematology patients admitted to the hospital undergo an electrocardiogram as well as an echocardiogram (ECHO) and multigated acquisition scan (MUGA) for the assessment of cardiovascular function prior to beginning treatment. We used the results of these tests as a screening tool for participation in the study as a mean to minimize the potential for an adverse event during the CPET.
Instrumentation
Participants performed all CPETs with indirect calorimetry on a mechanically braked cycle ergometer (Monark 874E, Goteborg, Sweden) within 96 hr of being admitted at an inpatient hematology oncology unit of the hospital. To determine oxygen uptake, we measured expired gases via a portable metabolic gas analysis system (Cosmed Portable K4b2, Rome, Italy). A hematology–oncology nurse assessed blood pressure (BP) using standard hospital equipment in the patient’s room prior to the CPET. We collected HR values using a Polar telemetry system (Polar Electro Inc., Lake Success, NY). At the end of each stage of CPET and at the end of the test, we used the Rate of Perceived Exertion Original Borg Scale to monitor rate of perceived exertion (RPE; Borg, 1982).
CPET
We used standard CPET procedures, adjusting the equipment to fit each patient, to determine VO2peak. Prior to beginning the cycling protocol, patients rested on the bike while we collected 2 min of resting metabolic data via expiratory gas analysis. Patients then cycled for 2 min at a resistance of 0 W as a warm-up, with the test starting immediately following the warm-up period.
The workload was initially 25 W and increased 5–20 W/min, depending on the physical state of the patient (Jones et al., 2008). Specifically, we determined workload increments for the remainder of the test based upon the patient’s medical history and metabolic response during the warm-up. Test termination criteria were volitional fatigue, achievement of a respiratory exchange ratio (RER) of greater than 1.10, or limitation by symptoms. Symptoms that would end a test immediately included chest pain, a BP response considered abnormal to exercise conditions, dizziness, or nausea.
During the test, we recorded HR every 10 s and RPE at the end of every minute before increase to the next workload. We collected VO2 data every 4 s over 30 s intervals and used the average of the three highest values of VO2 during the last minute of the test to determine VO2peak. Following the test, patients could choose to rest or cool down by pedaling at 10 W until HR returned to under 120 bpm.
Determination of Exercise Intensity Level Using CPET, HRR, and the Percent of 220-Age Methods
Both the HRR method and the percent of 220-age equation are commonly used for the determination of aerobic exercise intensity in healthy and clinical populations due to their practicality and ease of use. Both methods rely on a calculation of the percentage of the predicted maximum HR that corresponds to each chosen intensity level (e.g., low intensity [40% of VO2peak] is determined by multiplying with .40 in these two equations). For these equations, HR is assumed to increase in a linear fashion, as intensity of exercise increases to the maximum (Robergs & Roberts, 1997).
For the present study, using the VO2peak determined from the CPET, we calculated VO2 values for low intensity (40% VO2peak), moderate intensity (60% VO2peak), and high intensity (75% VO2peak) exercise. By averaging the raw VO2 data collected every 4 s throughout the course of the test over 30-s intervals, we were able to match HR values collected every 10 s throughout the test to the VO2 corresponding to each exercise intensity. Figure 1 is a representation from one subject showing how we identified HR levels corresponding to each exercise intensity level using VO2peak obtained during the CPET. We then used the HRR and percent of 220-age methods to determine HR levels corresponding to the same three exercise intensities (40%, 60%, and 75% of VO2peak). The formula for the HRR method is (HRmax − RHR) × % intensity + RHR, where HRmax is maximum heart rate, calculated using the 220-age equation, RHR is resting heart rate, and % intensity is the percent of VO2peak that corresponds to each exercise intensity level. The formula for the percent of 220-age method is (220-age in years) × % intensity.
Figure 1.
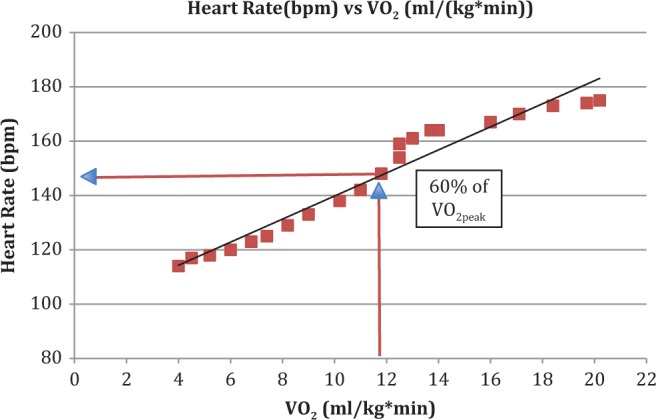
Heart rate (HR) versus VO2 from the cardiopulmonary exercise testing (CPET) used to determine VO2peak of one bone marrow transplant patient who completed the same testing procedure used in the present study. The VO2 values represent raw VO2 data recorded every 4 s during the CPET and averaged over 30-s intervals. The HR data were recorded every 10 s during the CPET.
Statistical Analysis
Using the G*power (version 3.1) analysis program, a sample size of 14 patients provided 70% power to detect an effect size of 0.60. A mean difference of 6 bpm and an SD of the difference of 10 bpm were used for the power calculation. Descriptive statistics of mean and SD are presented for age, height, weight, resting HR, and maximum HR achieved during the CPET. An α level set a priori at .05 was used for all analyses. All statistical analyses were performed using SPSS software version 22.0 for Mac OS (IBM Solutions, Durham, NC). Three models of aerobic exercise intensity determination calculated at the three different exercise intensities were compared using one-way within-subjects analysis of variance (ANOVA) models. If the ANOVA models were significant, post hoc analyses using the Sidak method to identify where significant differences in HR occurred between methods were employed.
Results
The purpose of this study was to compare the HR values computed using the HRR method and the percent 220-age equation to HR levels obtained from CPET for the determination of aerobic exercise intensity in ALPs undergoing induction treatment. We included 14 ALPs (12 with AML and 2 with ALL) in the analyses. Participant characteristics are presented in Table 1.
Table 1.
Participant Characteristics.
| Subject | Age | Type of Leukemia | Weight (kg) | Height (cm) | Resting Heart Rate (bpm) |
|---|---|---|---|---|---|
| 1 | 34 | AML | 69.8 | 155 | 95 |
| 2 | 58 | AML | 90.3 | 187.9 | 59 |
| 3 | 64 | AML | 86.5 | 165 | 98 |
| 4 | 40 | AML | 128 | 198.1 | 81 |
| 5 | 57 | AML | 102.2 | 184.2 | 77 |
| 6 | 43 | AML | 95.5 | 182 | 86 |
| 7 | 28 | ALL | 116.6 | 164 | 93 |
| 8 | 67 | AML | 97.7 | 188 | 67 |
| 9 | 67 | AML | 47.5 | 153 | 69 |
| 10 | 57 | AML | 74.1 | 160 | 57 |
| 11 | 64 | AML | 74.1 | 163 | 57 |
| 12 | 69 | AML | 93 | 187 | 89 |
| 13 | 60 | AML | 94.8 | 182.5 | 60 |
| 14 | 39 | ALL | 60.6 | 188 | 75 |
| Mean ± SD | |||||
| All subjects | 53 ± 14 | 87.9 ± 21.4 | 175.6 ± 14.8 | 76 ± 15 | |
| Min–Max | |||||
| All subjects | 28–69 | 47.5–128.0 | 153.0–198.1 | 57–98 | |
Note. ALL = acute lymphoblastic leukemia; AML = acute myeloid leukemia; Min = minimum; Max = maximum; SD = standard deviation.
For low-intensity exercise, the HRR method significantly overestimated HR when compared to the HR obtained using the percentage of VO2peak method (HRR = 112 ± 12 bpm, %VO2 = 100 ± 22 bpm, p = .000), and the percent of 220-age equation significantly underestimated HR when compared to the percentage of VO2peak method (percent of 220-age = 67 ± 6, p = .026). The results of the analyses for all HRs calculated using all three methods for low intensity (40% of VO2peak) are presented in Figure 2.
Figure 2.
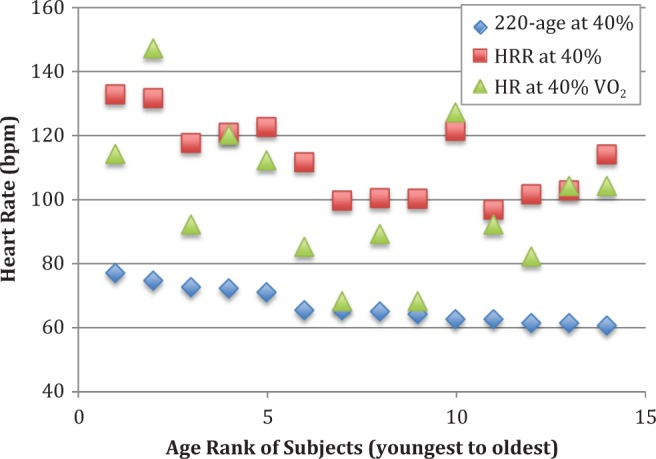
Heart rate (bpm) for each subject at low-intensity aerobic exercise (40% of VO2peak, observed during cardiopulmonary exercise testing) and calculated for this intensity using the heart rate reserve (HRR) and percent of 220-age methods. The 14 subjects are ranked along the x-axis by age, with the youngest ranked #1 and continuing to the oldest at #14.
At moderate-intensity exercise, there was a significant difference between the HR levels obtained using the HRR method and the percentage of VO2peak test (HRR = 130 ± 12 bpm, %VO2 = 113 ± 24 bpm, p = .004), but there was no significant difference between the HR estimated via the percent of 220-age method (100 ± 8 bpm) and HR measured at 60% of VO2peak (p = .122). The results of the analyses for all HRs calculated using all three methods for moderate intensity (60% of VO2peak) are presented in Figure 3.
Figure 3.
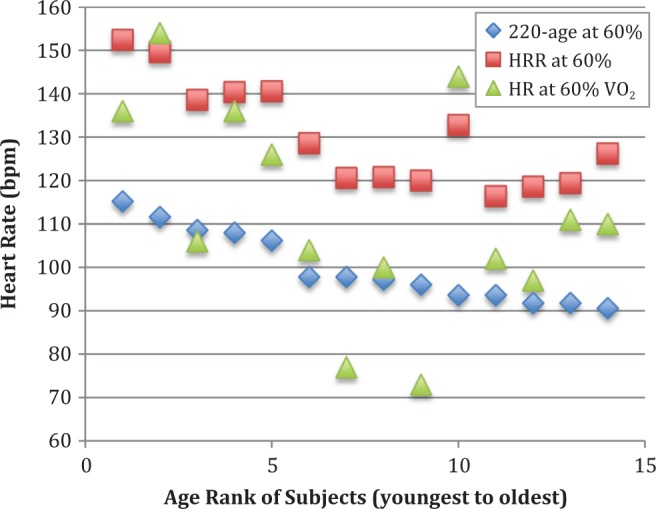
Heart rate (bpm) for each subject at moderate intensity aerobic exercise (60% of VO2peak, observed during cardiopulmonary exercise testing) and calculated for this intensity using the heart rate reserve (HRR) and percent of 220-age methods. The 14 subjects are ranked along the x-axis by age, with the youngest ranked #1 and continuing to the oldest at #14.
At high-intensity aerobic exercise, the HRR method once again significantly overestimated HR when compared to the percentage of VO2peak test (HRR = 144 ± 12 bpm, %VO2 = 124 ± 25 bpm, p = .003), while the HR estimated using the percent of 220-age equation (125 ± 10 bpm) again did not differ significantly from that determined using %VO2peak (p = .994). The results of the analyses for all HRs calculated using all three methods for high intensity (75% of VO2peak) are presented in Figure 4.
Figure 4.
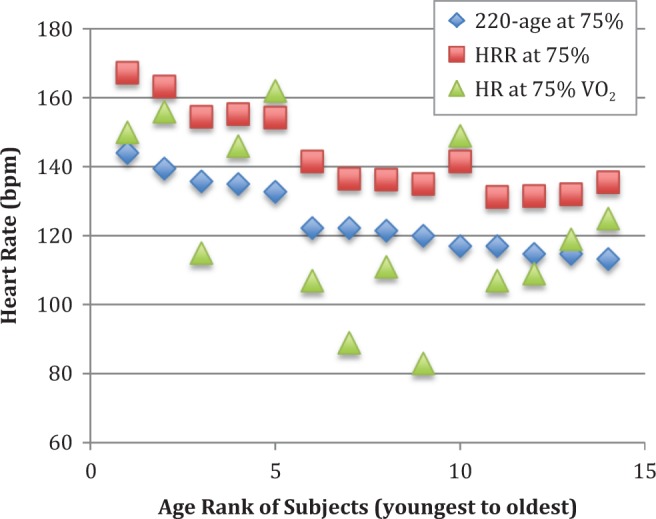
Heart rate (bpm) for each subject at high-intensity aerobic exercise (75% of VO2peak, observed during cardiopulmonary exercise testing) and calculated for this intensity using the heart rate reserve (HRR) and percent of 220-age methods. The 14 subjects are ranked along the x-axis by age, with the youngest ranked #1 and continuing to the oldest at #14.
Discussion
This was the first study to compare indirect HR training target estimation methods to an objective and direct measurement of HR at various training intensities. The comparison of all three methods across several intensity levels allowed us to examine the accuracy of such calculated methods when compared to the gold standard method of VO2 measurement via CPET.
Low Intensity
At the low-intensity exercise level of 40% of VO2peak, results indicated that both HR estimation methods differed from the VO2peak method, with the HRR overestimating and percent of 220-age underestimating HR. The overestimation of HR calculated with the HRR method may be due to an increase in fluids or other physiological alterations due to the disease or treatment that can affect HR. The underestimation from the percent of 220-age equation may be due to the fact that resting HR is not included in the calculation. Participants’ resting HRs were often above the value estimated for 40% of VO2peak intensity using the percent of 220-age equation. Based on these results, neither method is an acceptably accurate method for prescribing low-intensity aerobic exercise in this patient population.
Moderate Intensity
At the moderate-intensity exercise level of 60% of VO2peak, there was no significant difference between the HR estimated using the percent of 220-age equation and the HR derived from the VO2peak test. However, the difference of 13 bpm between the two methods is clinically significant; therefore, these results should be interpreted with caution. This difference in bpm is large enough to significantly compromise the utility of the exercise prescription, as it represents underprescription of an intensity that is targeted to promoting desirable changes in certain physiological parameters, such as cardiorespiratory fitness.
During moderate-intensity exercise, the cardiorespiratory system is working harder to supply the muscles with oxygen more quickly in order to maintain a higher level of force production. The increased cardiac output required at this intensity may cause HRs to become more similar to those predicted by the HRR and percent of 220-age equations (Brooks, Fahey, & Baldwin, 2005).
The HRR method again overestimated HR when compared to the HR determined via VO2peak, just as it did at low intensity. The reasons for this overestimation are likely the same as they were for low intensity: a decreased ability to carry oxygen as a result of the disease or the myelosuppression from chemotherapy.
High Intensity
At the high-intensity exercise level of 75% of VO2peak, we found results similar to those at moderate intensity, in that there was no significant difference between HR derived using the percent of 220-age method and HR determined from the VO2peak test. At high intensity, the difference between the mean HR derived using the percent 220-age method and mean HR determined from the VO2peak test was only 2 bpm, a significant heterogeneity reduction in HR responses between subjects, possibly due to the higher HR needed for subjects to be able to maintain a higher level of power output. As seen at the other intensities, the HRR method overestimated HR when compared to the VO2peak method of measuring HR.
Implications for Research and Practice
Although the statistical outcomes at the moderate- and high-intensity levels were more favorable than at the low intensity level, with HRs derived by the percent of 220-age equation not differing significantly from those measured using the gold standard CPET method, the overall picture when looking at individual participants is much different. Not only is there wide variation between HRs derived using each of the two prediction methods and the HRs from the VO2peak test, but this variation is not systematic or consistent between or even within participants. These variable differences across intensities suggest that another method or scale might need to be included in the determination of exercise intensity in this specific population.
As patients go through treatment, their exercise tolerance changes daily due to infections, fluids shifts, treatment side effects, and the disease process itself. For the purposes of practicality and scalability, without compromising prescription accuracy, a subjective parameter based on the daily physical well-being of the patient might be a more accurate tool for prescribing intensity, for example, the RPE. The physiological changes due to the treatment and its side effects may change the daily ability of these cancer patients to exercise, and a subjective measure used to ascertain how they feel and how able they are to perform on any given day may assist trainers and help care providers to quantify and monitor their exercise intensity until a better method of exercise quantification is determined.
In terms of direct application in a hospital setting, based on these results, neither the HRR method nor the percent of 220-age equation appears to be a reliable method for the determination of exercise intensity due to the large variation in HR between patients at each level of intensity. The average difference of 13 bpm between the HR predicted by the percent of 220-age equation and the actual HR from the VO2 method is clinically relevant, especially for ALPs who experience changes in exercise tolerance depending on their cancer and treatments. Based on the results of this study, we recommend that, for this patient population, HR-based equations and HR in general should not be used exclusively to determine exercise intensity. As discussed earlier, including other parameters along with HR such as self-reported outcomes (e.g., levels of fatigue, pain) and perhaps even other physiological parameters that are monitored daily by nurses and could be checked prior to training, including hematocrit, hemoglobin, absolute neutropenic count, fevers, and so on, as well as information on the patient’s perception of levels of physical exertion during exercise may help guide exercise specialist on the adjustment of training intensity. To this time, researchers have used only HR and RPE in previous studies to prescribe exercise intensity, with this study being the first to look at the accuracy of using HR-based methods for the determination of exercise intensity in leukemia patients. Therefore, we can make no definitive statement as to the recommended method for prescribing exercise intensity in the ALP population at this time.
A variety of physiological factors can affect HR, including fluid levels, stress, medications, and others. Maintenance of overall physical function during the various phases of treatment for AL is very important for assisting patients to better tolerate treatment and potentially attain more favorable long-term prognoses (Wood et al., 2013). The attainment of a training response is not always possible; however, for patients experiencing fewer treatment-related side effects, a training progression aimed at producing more prominent positive response should be explored. Future studies should examine the most appropriate and precise method for prescribing the training dose to promote a more specific physiological response. It may be that, for this cancer population, functional training involving more of a resistance training focus would be more appropriate. The cardiovascular component of the overall fitness and health of the patient could be maintained with light doses of aerobic training for which the intensity determination could be based on each patient’s capacity as determined on a daily basis. Future studies should, therefore, examine different training protocols, with different exercise intensities, along with the examination of more appropriate ways of quantifying exercise intensity, especially aerobic training.
Study Limitations
The sample size in the present study was small. A larger sample size might allow for the drawing of more definite conclusions about the prescription of aerobic exercise intensity using HR. There was a large variation in participant age, 28–69 years old, and there were large differences in RHR values, from 57 to 98 bpm. RHR values may have differed due to factors such as medications and initiation of treatment prior to completion of the VO2peak test. Of the 14 participants, 1 was on medication a week prior to enrollment which could have altered HR and BP responses during testing. The participant had been prescribed this medication due to the levels of stress and anxiety the patient exhibited after diagnosis rather than to address any cardiovascular comorbidity. The medical oncologist had cleared this patient for participation, and this patient experienced no adverse effects during testing. Nevertheless, this patient’s HR response to exercise could have been affected by the medication even though we observed no abnormal response during testing. It is also important to note that this evaluation used only one single bout of exercise performed to maximal effort. A familiarization testing session where participants learn and experience a CPET prior to the day of testing is always a desirable procedure to maximize the chance for a subject to produce their maximal effort on the test day. When participants are familiarized with the testing procedures ahead of time, they usually become less anxious, are used to the testing equipment, and are more prone to perform at their best. Due to the nature and logistics of leukemia treatments, where treatments may need to be started immediately, and the likelihood of patients’ physical states changing significantly from day to day, a familiarization session prior to testing is usually not feasible in this population.
Since we developed this study as a preliminary examination using data from a larger trial designed to evaluate other outcomes, a future study could focus specifically on HR and potential alterations HR may exhibit during treatment. The results of the present study could be used to inform the calculation of power for future trials aimed at confirming or refuting these preliminary findings.
Conclusion
The HRR method and the percent of 220-age equation are commonly used methods for calculating HR values corresponding to varying levels of aerobic exercise intensity in different populations (da Cunha, Farinatti, & Midgley, 2011; Engels, Zhu, & Moffatt, 1998). Although the use of HR is a fairly easy and practical method for quantifying exercise intensity, it is important to keep in mind that HR is sensitive to daily changes. In cancer patients, whose physical status can change day to day due to treatment and its side effects, HR responses can vary even more significantly than in apparently healthy individuals.
Based on the results of this study, in adults with AL, HR-based methods for the determination of aerobic exercise intensities should be used with caution. For low-intensity exercise, neither percent of 220-age nor HRR was accurate in comparison to direct determination of HR from VO2, while at moderate and high intensities, the percent of 220-age equation produced HR values similar to those directly determined using VO2peak in some, but not all, patients. However, though the differences between HR values derived from the percent of 220-age equation and those directly determined from VO2 at moderate and high intensities were not statistically significant, at moderate intensity this difference did appear to be clinically significant.
Our results lead us to the conclusion that neither the HRR method nor the percent of 220-age equation should be the sole method used to determine exercise intensity in this population. We recommend that future studies explore other parameters and methods to be used alone or in conjunction with HR to better determine physical exertion (exercise intensity) prescriptions for patients undergoing induction chemotherapy.
Footnotes
Author Contribution: C. Story, A. Bryant, and C. Battaglini contributed to conception, design, data acquisition, data analysis, and interpretation; drafted the manuscript; critically revised the manuscript; gave final approval; and agreed to be held accountable for all aspects of work, ensuring integrity and accuracy. Phillips, B. contributed to data acquisition, data analysis, and interpretation; drafted the manuscript; critically revised the manuscript; gave final approval; and agreed to be held accountable for all aspects of work, ensuring integrity and accuracy. C. Bailey contributed to data acquisition and interpretation; drafted the manuscript; critically revised the manuscript; gave final approval; and agreed to be held accountable for all aspects of work, ensuring integrity and accuracy. E. Shield contributed to design, data acquisition, data analysis, and interpretation; drafted the manuscript; critically revised the manuscript; gave final approval; and agrees to be held accountable for all aspects of work, ensuring integrity and accuracy.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Alvin R. Tarlov and John E. Ware Post-Doctoral Research Award in Patient Reported Outcomes, R25CA116339 Cancer Care Quality Training Post-Doctoral Fellowship, and UNC University Cancer Research Funds.
References
- Alibhai S. M., O’Neill S., Fisher-Schlombs K., Breunis H., Brandwein J. M., Timilshina N.…Culos-Reed S. N. (2012). A clinical trial of supervised exercise for adult inpatients with acute myeloid leukemia (AML) undergoing induction chemotherapy. Leukemia Research, 36, 1255–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Cancer Society. (2015). Cancer facts and figures 2015. Retrieved April 8, 2015, from http://www.cancer.org/acs/groups/content/@editorial/documents/document/acspc-044552.pdf
- Battaglini C. L. (2011). Physical activity and hematological cancer survivorship. Recent Results in Cancer Research, 186, 275–304. [DOI] [PubMed] [Google Scholar]
- Battaglini C. L., Hackney A. C., Garcia R., Groff D., Evans E., Shea T. (2009). The effects of an exercise program in leukemia patients. Integrative Cancer Therapies, 8, 130–138. [DOI] [PubMed] [Google Scholar]
- Borg G. (1982). Psychophysical bases of perceived exertion. Medicine & Science in Sports & Exercise, 14, 377–381. [PubMed] [Google Scholar]
- Brooks G. A., Fahey T. D., Baldwin K. M. (2005). Exercise physiology: Human bioenergetics and its applications. Boston, MA: McGraw Hill. [Google Scholar]
- Bryant A. L., Walton A. M., Phillips B. (2015). Cancer-related fatigue: Scientific progress has been made in 40 years. Clinical Journal of Oncology Nursing, 19, 137–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant A. L., Walton A. M., Shaw-Kokot J., Mayer D. K., Reeve B. B. (2015). A systematic review of psychometric properties of health-related quality-of-life and symptom instruments in adult leukemia survivors. Cancer Nursing. Advance online publication; doi:10.1097/NCC.0000000000000327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang P. H., Lai Y. H., Shun S. C., Lin L. Y., Chen M. L., Yang Y.…Cheng S. Y. (2008). Effects of a walking intervention on fatigue-related experiences of hospitalized acute myelogenous leukemia patients undergoing chemotherapy: A randomized controlled trial. Journal of Pain and Symptom Management, 35, 524–534. [DOI] [PubMed] [Google Scholar]
- da Cunha F. A., Farinatti P. T., Midgley A. W. (2011). Methodological and practical application issues in exercise prescription using the heart rate reserve and oxygen uptake reserve methods. Journal of Science and Medicine in Sport/Sports Medicine Australia, 14, 46–57. [DOI] [PubMed] [Google Scholar]
- Engels H. J., Zhu W., Moffatt R. J. (1998). An empirical evaluation of the prediction of maximal heart rate. Research Quarterly for Exercise and Sport, 69, 94–98. [DOI] [PubMed] [Google Scholar]
- Jones L. W., Eves N. D., Haykowsky M., Joy A. A., Douglas P. S. (2008). Cardiorespiratory exercise testing in clinical oncology research: Systematic review and practice recommendations. Lancet Oncology, 9, 757–765. [DOI] [PubMed] [Google Scholar]
- Klepin H. D., Danhauer S. C., Tooze J. A., Stott K., Daley K., Vishnevsky T.…Mihalko S. L. (2011). Exercise for older adult inpatients with acute myelogenous leukemia: A pilot study. Journal of Geriatric Oncology, 2, 11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robergs R. A., Roberts S. O. (1997). Exercise physiology: Exercise, performance and clinical applications. St. Louis, MO: Mosby. [Google Scholar]
- Schmitz K. H., Courneya K. S., Matthews C., Demark-Wahnefried W., Galvao D. A., Pinto B. M.…Schwartz A. L. (2010). American college of sports medicine roundtable on exercise guidelines for cancer survivors. Medicine and Science in Sports and Exercise, 42, 1409–1426. [DOI] [PubMed] [Google Scholar]
- Wood W. A., Deal A. M., Reeve B. B., Abernethy A. P., Basch E., Mitchell S. A.…Battaglini C. (2013). Cardiopulmonary fitness in patients undergoing hematopoietic SCT: A pilot study. Bone Marrow Transplantation, 48, 1342–1349. [DOI] [PubMed] [Google Scholar]


