Abstract
Purpose/Objectives
Systematically summarize findings from research conducted on adult acute leukemia survivors as they relate to symptoms and quality of life (QOL).
Data Sources
Systematic review of the literature from 1990–2013 found in the PubMed, PsycINFO®, EMBASE, and CINAHL® databases, as well as manual searches.
Data Synthesis
The review identified 16 quantitative studies and 1 qualitative study published from 1990–2013 that used a self-reported QOL or symptom questionnaire. Fatigue was the most commonly assessed and reported symptom, followed by depression.
Conclusions
Acute leukemia and its treatment have a significant impact in all QOL domains. Future studies should include longitudinal research, more than one recruitment site, increased minority representation, and home-based exercise interventions as ways to improve all domains of QOL.
Implications for Nursing
This review increases awareness of commonly reported symptoms faced by adults with acute leukemia. Oncology nurses are central in monitoring and reporting symptoms to the interdisciplinary team that may contribute to changes in function, with the overall goal of optimizing QOL over time.
Keywords: acute leukemia, acute leukemia survivors, quality of life, symptoms, patient-reported symptoms, systematic review
Leukemia has little age predilection; individuals have been diagnosed with the disease at various stages of life. Leukemia is a group of diseases that arise from the abnormal proliferation of mature myeloid and lymphocytic cells (National Cancer Institute [NCI], 2014c). Four types of leukemia exist: acute myelogenous leukemia (AML), acute lymphoblastic leukemia (ALL), chronic myelogenous leukemia, and chronic lymphoblastic leukemia (CLL). The term acute leukemia includes both AML and ALL. Acute leukemia is the most common hematologic cancer in the United States, with 21,800 new diagnoses and 11,900 deaths in 2014 (NCI, 2014a, 2014b). In 2011, an estimated 302,800 people were living with leukemia in the United States (NCI, 2014c). The five-year survival rate of a patient with leukemia is 57% (NCI, 2014c). AML and ALL are both commonly diagnosed in adults (NCI, 2014a, 2014b).
Acute leukemia presents aggressively, requiring intensive chemotherapy and prolonged hospital stays (Xuereb & Dunlop, 2003). Over time, gradual improvements have been made in remission and survival rates among adults with acute leukemia, but few treatment options exist, particularly for adults aged 60 years and older (Hiddemann et al., 1999; Stone, 2002). For adults with acute leukemia, induction chemotherapy is administered in the hospital during a three- to four-week stay. Expected treatment-related complications and symptoms include bone marrow suppression, neutropenic fever, and mucositis (Klepin et al., 2011; Stone, 2002). Symptoms are multidimensional, multiplicative in nature, and can occur solely or concurrently (Cleeland, 2007; Dodd et al., 2001). Symptoms are distressing and can disrupt activities of daily living, in addition to physical, social, emotional, and spiritual quality-of-life (QOL) domains. Albrecht (2014) conducted an integrative literature review of studies on adult acute leukemia survivors focused on physiologic and psychological symptoms and found variations in symptom severity and frequency. The current study builds on that review by summarizing findings of research conducted on adults with acute leukemia as they relate to symptoms and QOL, as reported directly by them.
Methods
Search Strategy
Following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses ([PRISMA], 2014) guidelines, the first and third authors of this article conducted a systematic literature search to identify studies published from 1990–2013. Hand searches of the references of selected articles also were conducted. The following words and combinations were used to conduct searches in PubMed, PsycINFO®, EMBASE, and CINAHL®: acute leukemia, leukemia acute, acute leukeamia, health related, health-related, symptom, symptoms, quality of life, function, functional, well being, well-being, and outcome assessment.
Inclusion and Exclusion Criteria
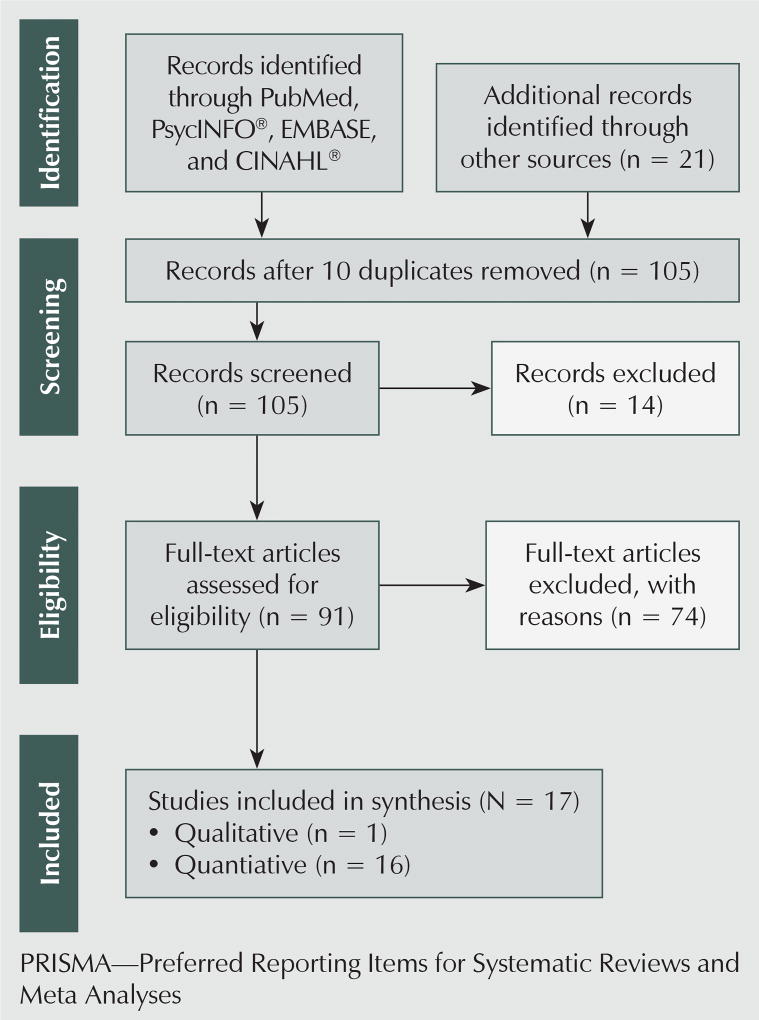
All retrospective, prospective, cross-sectional, exploratory, observational, qualitative, longitudinal, and randomized, controlled trial (RCT) studies using self-reported QOL or symptom instruments were eligible for inclusion. The search was limited to studies reported in English and with adults aged 18 years or older. The resulting group of studies looked at survivors undergoing treatment, survivors after induction treatment, and long-term survivors. Studies on survivors after hematopoietic stem cell transplantation were not included because of the complexity and toxicity of this treatment modality. Exclusions included case reviews, summary reports, clinical reviews, and literature and systematic reviews (see Figure 1).
Figure 1.
Search and Selection Criteria in Accordance With PRISMA Guidelines
The search resulted in 94 articles from the databases and 21 articles identified by hand search. Of those, 10 were duplicate articles and were removed. An additional 14 of the 105 remaining studies were excluded based on an initial review of the title and abstracts that determined them to be irrelevant, leaving a total of 91 articles. Of the remaining articles, 74 were excluded because no QOL or symptom instruments were used and the age groups or publication types were outside the scope of this review. The final sample included 17 articles composed of 16 quantitative and 1 qualitative study.
Data Extraction
After the search for and selection of articles for review, two authors (ALB and AW) reviewed the identified articles and checked for inclusion criteria. They each examined the type of study, characteristics of the sample, and use of a QOL instrument to ensure inclusion. In case of a disagreement, the opinion of a third researcher (BBR) was accepted as the criterion for the articles’ inclusion or exclusion.
General Characteristics of Quality-of-Life Studies
Characteristics of the reviewed studies are summarized in Appendix A. One study used the term health-related quality of life (HRQOL), 13 studies used the term QOL, and 2 used neither terminology. For consistency, the term QOL is used throughout the current article. For the two studies that used neither terminology, they were included in the final sample based on their use of symptom instruments. The reviewed studies used eight measures to assess QOL or HRQOL (Functional Assessment of Cancer Therapy–General [FACT-G], Functional Assessment of Cancer Therapy–Spiritual [FACT-Sp], Functional Assessment of Cancer Therapy–Fatigue [FACT-F], SF-36®, Perceived QOL [PQOL], European Organisation for the Research and Treatment of Cancer Quality-of-Life Questionnaire–Core 30 [EORTC QLQC30], Schedule for the Evaluation of Individual QOL [SEIQOL], and the SEIQOL–Direct Weighting).
A survivor is defined as “from the time of diagnosis, through the balance of his or her life” (National Coalition for Cancer Survivorship, 2004, p. 1). Family members, friends, and caregivers also are impacted by the survivorship experience and are, therefore, included in this definition (National Coalition for Cancer Survivorship, 2004). The current article is a systematic review of existing literature and the terminology used in each individual article will be used. Some authors refer to those undergoing treatment as “patients,” “participants,” or “survivors.” The terminology used by the original author will be retained to best convey his or her meaning.
Eleven of the quantitative studies and the qualitative study were published more than five years ago. Eleven of the studies were conducted outside of the United States. One study used a sample from Portugal and the United States to present an international comparison of QOL (Forjaz & Guarnaccia, 2001). Sample sizes were fairly small, ranging from 10–479 adults, with a mean age of 59 years. Across all studies, more participants were male, which is consistent with prevalence rates for acute leukemia. All of the examined studies recruited convenience samples from multiple cancer center sites. Eight studies focused on mixed solid and non-solid tumors, and the remaining focused on acute leukemia only (n = 9). The current authors could not separate the data in articles that used a mixed population because of the small sample sizes of survivors with leukemia. The findings from eight mixed populations support the conclusion that symptoms and QOL are similar to those in the eight acute leukemia-only studies. A quantitative approach with a cross-sectional design was used in five studies, seven used a longitudinal design, two were of exercise interventions, and two had observational designs. One study used a mixed-methods design (Persson, Larsson, Ohlsson, & Hallberg, 2001).
Impact of Acute Leukemia on Physical, Psychosocial, and Spiritual Well-Being
The findings presented in this review address the impact of treatment on acute leukemia symptoms and physical well-being. In addition, the impact of treatment on psychological and spiritual well-being will be described.
The most common, standard treatment for de novo acute leukemia is intensive chemotherapy, which has short- and long-term physical and psychological effects on QOL (Mayer et al., 1994; Stone & Mayer, 1993). The short-term effects of intensive chemotherapy on QOL, fatigue, and physical function in survivors with AML are described as fairly stable over time and similar in younger (aged 18–59 years) and older (aged 60 years and older) adults (Mohamedali et al., 2012). In addition, physical function generally improved over time, but the improvement was somewhat greater in younger adults. Compared to younger adults, older adults tolerated intensive chemotherapy relatively well, as evidenced by little variation in QOL and physical function scores (Mohamedali et al., 2012).
In Stalfelt (1994), most patients were physically and psychologically affected during the induction period, particularly during week 3 of their hospitalization. One symptom that improved over time was nausea. Schumacher, Kessler, Buchner, Wewers, and van de Loo (1998) supported the finding that patients tended to suffer less from fatigue, nausea and emesis, loss of appetite, and sleep disturbance (p = 0.001) by the end of their treatment. Another study showed that 86% of survivors rated their health as good after discharge from the hospital following intensive care for life-threatening medical complications (Yau, Rohatiner, Lister, & Hinds, 1991). None of the survivors reported increased limitations in their daily activities, and 71% had returned to full-time employment.
One comparative study explored similarities and differences in physical QOL in 98 Portuguese and 109 American outpatient survivors (Forjaz & Guarnaccia, 2001). The article did not specify the type of hematologic malignancy. Overall, Portuguese survivors reported better physical functioning, less pain, more vitality, better social functioning, and better general QOL than the American survivors. Portuguese survivors tended to be older (X̅ age = 55.3 years, SD = 17.4, p = 0.0007) than American survivors (X̅ age = 48.9 years, SD = 16.3). Forjaz and Guarnaccia (2001) found that age and QOL vary among ethnic and racial groups and should be explored further.
Impact of Treatment and Acute Leukemia on Psychological and Spiritual Well-Being
Acute leukemia may have a negative psychological effect on survivors at the time of diagnosis and throughout their illness. Long-term survivors of acute leukemia (X̅ = 5.6 years) had higher distress scores on the Brief Symptom Inventory compared to Hodgkin lymphoma survivors (X̅ = 5.9 years), suggesting acute leukemia survivors experienced late symptom effects (Kornblith et al., 1998). Moderate-to-strong correlations were found between fatigue scores and depression scores in cross-sectional (Alibhai et al., 2012) and longitudinal studies (Alibhai, Leach, Kermalli, et al. 2007; Alibhai, Leach, Kowiger, et al., 2007; Battaglini et al., 2009).
Literature is lacking on long-term survivors’ symptoms and QOL in adults with acute leukemia. Overall, QOL improved over time for survivors with AML in all domains, including role functioning, emotional functioning (p = 0.001), and social functioning (p = 0.007) from the time of induction chemotherapy to the end of treatment. For patients who relapsed, QOL was lower with more symptoms experienced at the start of treatment compared to patients who had not relapsed (Persson et al., 2001).
Emotional functioning had the greatest improvement among all QOL domains in a study by Alibhai et al. (2012). Those who survived for more than two years experienced a change of attitude about what was important in their lives, but reverted to their earlier lifestyles within two years after treatment (Stalfelt, 1994). Anxiety and depressive symptoms are experienced by patients during treatment, and they also exhibit posttraumatic stress disorder symptoms, including high incidence of intrusive thoughts and avoidance. The findings were similar to those of Montgomery, Pocock, Titley, and Lloyd (2003), where 51% of patients with leukemia had distress and mild-to-moderate depression across their illness journey (from one month to eight years).
Patients at two years post-treatment felt that their QOL improved after entering remission, and their experiences were categorized into one of two themes: (a) believed in life, fought for it, and came through stronger, or (b) life went on, adapted, and found a balance in the new life (Persson & Hallberg, 2004). Most patients found benefits in this new value they placed on life; they readjusted priorities and enjoyed life more intensely, which outweighed the adverse effects of their treatments and remission status (Schumacher et al., 1998). A few patients reported poorer psychological QOL after entering remission, and their responses were categorized into the theme of “life was over, felt out of control, and lost belief in life” (Persson et al., 2001).
Only one study focused on spirituality as a QOL domain. Pearce, Coan, Herndon, Koenig, and Abernethy (2012) explored whether oncology inpatients (N = 150) in a malignant hematology and solid tumor oncology unit received spiritual care consistent with their needs, and, if they were inconsistent, whether the patients experienced deleterious effects on their outcomes. The majority of patients, 91%, expressed spiritual needs, and 67% received spiritual care from their healthcare providers. However, a subset (17%) received less spiritual care than desired from their healthcare providers. Those whose spiritual needs were not met reported more depressive symptoms, less meaning, and less peace about their lives. The study findings support that spirituality is an important QOL domain and spiritual needs should be addressed.
Discussion
Fatigue, depression, and anxiety are more prominent symptom concerns for survivors of acute leukemia because they interfere with survivors’ activities of daily living and ability to carry out social roles. In the current study, the authors assessed symptoms solely, not concurrently as symptom clusters (two or more symptoms occurring together) (Kim, McGuire, Tulman, & Barsevick, 2005). Assessing and understanding the symptom experience of the adult with acute leukemia is important to determine which symptoms are most troublesome during and after treatment and to appropriately intervene.
Symptoms can significantly affect QOL and activities of daily living, and intensity can be particularly severe during chemotherapy. Fatigue is a common, tenacious symptom that affects the majority of cancer survivors and has been identified as more difficult to treat than pain (Sekeres & Stone, 2002). The majority of all the reviewed studies measured fatigue with one or more fatigue scales at various points during and after treatment. Overall, fatigue improved over time from the start of treatment to the end of the study, but future studies that follow survivors for a longer period of time (more than two years) may help elucidate the late effects of treatment.
Understanding the experiences of an adult acute leukemia survivor from the start of treatment through the balance of his or her life provides trajectory data on how symptoms and QOL vary across the continuum. One limitation of the review was not including non-English articles and non-English instruments. Inclusion may have provided additional diverse, demographic, symptom, and QOL data on adults with acute leukemia.
The knowledge gaps in this area include underrepresentation of minorities, a lack of longitudinal studies or the use of theoretical or conceptual frameworks, and a lack of literature on long-term survivors’ needs. The studies include patients’ symptoms from start of treatment to long-term survivors, with varying symptoms across the illness journey.
One qualitative study was included in this review. Because qualitative studies allow survivors to express their experiences more thoroughly, using them would increase understanding of the subjective nature of the impact of acute leukemia and the impact that treatments has on patients’ lives. Using qualitative methods to complement standardized QOL-related instruments would more adequately capture the totality of a survivor’s experience than one measure alone. Additional studies could enhance the voice of the survivor through furthering the understanding of how symptoms affect all domains of QOL for this population. Although most of the reviewed studies focused on physical, psychological, and functional QOL and symptoms, only one study specifically measured spiritual QOL (Pearce et al., 2012), and only one addressed social QOL, specifically about returning to full-time employment (Yau et al., 1991).
Implications for Nursing Practice
This review increases awareness of commonly reported symptoms faced by adults with acute leukemia. Oncology nurses are central in reporting symptoms and changes in function to the provider, with the aim of ultimately improving QOL. In the 2013 Advanced Oncology Nursing Research national survey, the top priorities rated by oncology nurses were self-management interventions to improve symptom control, symptom management interventions, and interventions with technology to address symptoms (LoBiondo-Wood et al., 2014). The Oncology Nursing Society’s Putting Evidence Into Practice resources should be considered for symptoms such as depression and fatigue, the two most common symptoms presented in this review. Cognitive behavioral interventions or approach, mindfulness-based stress reduction, psychoeducational interventions, and exercise have been recommended for practice for survivors with depression and fatigue (Fulcher, Kim, Smith, & Sherner, 2014; Mitchell et al., 2014).
Conclusions
Future studies on QOL domains throughout the treatment course should include longitudinal designs, more than one recruitment site, and increased minority representation. Spiritual and social QOL are important aspects of QOL that often are the least reported and studied, as this current review supports. Studies with larger, diverse samples are needed to improve the understanding of the various QOL domains that impact overall health and survival. Future studies exploring the trajectories of symptoms and QOL in adult survivors will provide important data for interventional work.
Knowledge Translation.
Using qualitative methods to complement standard quality-of-life (QOL)–related instruments would more adequately capture the totality of a survivor’s experience than one measure alone.
Emphasis should be placed on evidence-based resources for common symptoms, such as fatigue and depression.
Future studies that explore trajectories of symptoms and QOL in adult survivors will provide important data for interventional work.
Acknowledgments
This study was supported, in part, by grants from the National Cancer Institute (No. 5R25CA116339) and the American Cancer Society (No. DSCNR-13-276-03). The study also was supported, in part, by the Jonas Nurse Leaders Scholar Program. Bryant can be reached at ashley_bryant@unc.edu, with copy to editor at ONFEditor@ons.org.
Appendix A
Reviewed Studies
| Study | Purpose | Domains | Sample | Design and Instruments | Findings |
|---|---|---|---|---|---|
| Alibhai et al., 2012 | To determine recruitment, retention, and ability of patients with acute leukemia undergoing induction chemotherapy to participate in an individualized exercise intervention during hospitalization, provide efficacy estimates on physical fitness outcome measures, and examine the safety of the exercise program |
|
35 patients with acute leukemia in Canada (18 were younger than age 60, 17 were age 60 and older); 46% were male and 74% were Caucasian. | Non-randomized, prospective, exercise intervention
|
Confirmed feasibility and safety of a carefully designed and controlled intervention in both older and younger patients with acute leukemia. Most commonly reported reason for not participating in daily exercise was fatigue. |
| To provide estimates of the effects of exercise on QOL and fatigue, and to understand the impact of exercise on acute leukemia treatment tolerability, including length of stay in the hospital, development of sepsis, ICU admission, and delay in subsequent chemotherapy | |||||
|
| |||||
| Alibhai, Leach, Kermalli, et al., 2007 | To examine QOL and self-reported functional status over a six-month period in older adults with newly diagnosed acute leukemia who were being man-aged with either intensive chemotherapy or less-aggressive approaches |
|
65 patients in Canada with acute leukemia with a mean age of 72.1 years; 71% were male and ethnicity was not reported. | Prospective, longitudinal
|
At baseline, functional status was high but QOL was negatively affected in global health and most QOL domains. Over time, QOL remained stable or improved in most patients and was generally similar between intensive chemotherapy and non-intensive chemotherapy groups. Basic ADL scores did not change over time, whereas instrumental ADL scores declined slightly regardless of treatment. Receiving intensive chemotherapy does not appear to lead to worse QOL or functional status than more palliative approaches. |
|
| |||||
| Alibhai, Leach, Kowiger, et al., 2007 | To characterize the prevalence and severity of fatigue, correlations between fatigue and QOL and functional status, and examine correlations between fatigue and potential sociodemographic and clinical variables |
|
65 patients in Canada with acute leukemia with a mean age of 72.1 years; 71% were male and ethnicity was not reported. | Prospective, longitudinal
|
Fatigue scores had moderate-to-strong correlations with global health and all five QOL domains; strongest correlations were with global health and physical function. Moderate-to-strong correlations between fatigue scores and depression scores were noted. Fatigue improved slightly over time in intensive chemotherapy and non-intensive chemotherapy groups. |
|
| |||||
| Battaglini et al., 2009 | To examine the feasibility of administering an in-hospital exercise program to patients with acute leukemia undergoing chemotherapy |
|
10 patients with acute leukemia in the United States ranging in age from 18–55 years; 70% were male and ethnicity was not reported. | Non-randomized, controlled trial exercise intervention
|
Significant improvements were noted in cardiorespiratory endurance (p = 0.009; baseline, 8.9 ± 8.8 minutes; postexercise intervention, 17 ± 14.3 minutes). Significant reductions in total fatigue scores also were noted (p = 0.009; baseline, 4.6 ± 1.7; postexercise intervention, 1.8 ± 1.6). Depression scores were reduced as well (p = 0.023; baseline, 19 ± 11.5; postexercise intervention, 12 ± 8.2). Marginally significant decrease was seen in interleukin-6 (p = 0.059), with no significant changes in interleukin-10 (p = 0.223) or interferon-γ (p = 0.882). |
|
| |||||
| Efficace et al., 2012 | To investigate whether baseline patient-reported symptom severity independently predicted overall survival in a heterogeneous hematologic population, mainly with advanced disease, followed up in a prospective study |
|
119 patients in Italy with various cancers and a mean age of 70 years; 36% were male and ethnicity was not reported. | Prospective, observational
|
The median survival of the entire cohort was 4.8 months (range = 0–28 months). The MDASI was completed at baseline by 91% of patients. The final multivariate model retained two parameters as independent prognostic factors for survival: clinical prognostic group and patient’s self-reported severity of drowsiness. HRs were found for curable versus terminal, 0.055 (95% CI [0.022, 0.136], p < 0.001), and for advanced versus terminal, 0.193 (95% CI [0.103, 0.362], p < 0.001). Patient’s self-reported severity of drowsiness independently predicted survival with an HR of 1.801 (95% CI [1.044, 3.107], p = 0.033). Additional sensitivity analysis confirmed the independent prognostic value of variables identified in this study. |
|
| |||||
| Forjaz et al., 2001 | To investigate health-related QOL differences between 98 Portuguese and 109 American outpatients with hematologic malignancies |
|
98 Portuguese (51% male with a mean age of 55 years) and 108 American (53% male with a mean age of 49 years) patients with various cancer types. | Prospective, cross-sectional
|
Portuguese patients reported better physical functioning, less pain, more vitality, better social functioning, and better general QOL than American patients. |
|
| |||||
| Klepin et al., 2011 | To test feasibility and use of a bedside GA to detect impairment in multiple geriatric domains in older adults initiating chemotherapy for acute leukemia |
|
54 patients with acute leukemia in the United States, with a mean age of 70.8 (52% aged 60–69 and 35% aged 70–79); 59% were male and 96% were Caucasian. | Prospective, observational cohort study
|
93% completed the entire GA; the mean time was 44 minutes (SD = 14). Impairments detected included cognitive (32%), depression (39%), distress (54%), impairments in ADLs (48%), impaired physical performance (54%), and comorbidities (46%). |
|
| |||||
| Kornblith et al., 1998 | To compare long-term psychosocial adaptation of Hodgkin disease and acute leukemia survivors |
|
273 patients with Hodgkin disease (mean age of 29.6 years [SD = 28]) and 206 with acute leukemia (mean age of 36.4 years [SD = 34]) in the United States. | Prospective, cross-sectional, telephone interviews
|
Hodgkin disease survivors’ risk of having a high distress score on the BSI was almost twice that of acute leukemia survivors (OR = 1.9), with 21% of Hodgkin versus 14% of acute leukemia survivors (p < 0.05) having scores that were 1.5 SDs above the norm, suggestive of a possible psychiatric disorder. Hodgkin survivors reported greater fatigue (POMS fatigue, p = 0.01, and vigor, p = 0.001, subscales), greater conditioned CINV (p < 0.05), greater impact of cancer on their family life (p = 0.004), and poorer sexual functioning (p = 0.0001) than acute leukemia survivors. |
|
| |||||
| Mohamedali et al., 2012 | To investigate the short-term effects of intensive chemotherapy on QOL, fatigue, and physical function in patients with acute leukemia; and to compare changes in physical function, QOL, and fatigue in older (aged 60 years and older) and younger (aged 18–59 years) patients |
|
103 patients with acute leukemia in Canada; 65 patients were younger than age 60 (68% male) and 38 were age 60 and older (34% male); 68% in the younger group and 82% in the older group were Caucasian, respectively. | Prospective, longitudinal cohort
|
Both QOL and physical function were worse than normative data. QOL was fairly stable over time and similar in both age groups, whereas physical function generally improved over time, but the improvement was somewhat greater in younger compared to older adults. Compared to younger adults, older adults tolerate intensive chemotherapy quite well from QOL and physical function perspectives. |
|
| |||||
| Montgomery et al., 2002 | To evaluate the clinical usefulness of a novel QOL measure, the SEIQOL–Direct Weighting, in a sample of patients with either leukemia or lymphoma |
|
51 patients with various cancers in the United Kingdom with a mean age of 54 years; 71% were male and ethnicity was not reported. | Prospective, cross-sectional
|
The inverse relationship between QOL (SEIQOL score) and psychological distress (depression and anxiety) as measured by total HADS score was confirmed. The domain of “family” was nominated most frequently (82%) as an important area from which QOL is derived; this domain was also weighted by 58% of patients as first or second in importance. The fact that “health,” as a domain, was not, in this sample, the most important determinant of QOL was an interesting finding which reinforces the value of using an instrument which does not impose an external, clinician-derived set of values. The association between total mean HADS scores and SEIQOL scores suggests that depression and anxiety is an important risk factor for a diminished QOL experience, but a causal relationship cannot be established from the data. |
|
| |||||
| Montgomery et al., 2003 | To examine relationships between coping style, QOL, and psychological distress in a sample of patients with leukemia and lymphoma |
|
51 patients with various cancer in the United Kingdom with a mean age of 54 years; 71% were male and ethnicity was not reported. | Prospective, cross-sectional
|
51% with moderate distress; 14% with severe distress; 27% identified as having adjusted poorly to their diagnosis and having low scores on the Fighting Spirit subscale of the MAC and high scores on the Hopeless/Helpless subscale. Those with a worse coping style were most likely to suffer from severe psychological distress. |
|
| |||||
| Pearce et al., 2012 | To determine if inpatients receive spiritual care consistent with their needs. When inconsistent, are there deleterious effects on patient outcomes? |
|
150 patients with various cancers from the United States with a mean age of 59 years; 54% were male and ethnicity was not reported. | Prospective, cross-sectional
|
91% had spiritual needs, and the majority desired and received spiritual care from their healthcare providers. Those who received less spiritual care than desired reported more depressive symptoms [adjusted β (SE) 0–1.2 (0.47), p = 0.013] and less meaning and peace [adjusted β (SE) 0–2.37 (1.15), p = 0.042]. |
|
| |||||
| Persson et al., 2001 | To investigate QOL and sense of coherence for patients with acute leukemia and malignant lymphoma at the start of treatment and during two years. The secondary aim was to compare questionnaires responses with patients’ statements in open-ended interviews. |
|
16 patients with various cancers from Sweden with a mean age of 57 years. | Prospective, longitudinal
|
QOL at start of treatment was reduced mostly with role and social functioning and by presence of fatigue, dyspnea, and sleep disturbances; patients who relapsed at the start of treatment had a significantly more reduced QOL in most functional aspects and a higher level for most symptoms compared to those who did not relapse. Patients with acute leukemia were more affected with social functioning and global QOL and had a significantly more positive development in physical functioning and global QOL. |
|
| |||||
| Persson et al., 2004 | To narrate the lived experience of falling ill, being in treatment, and life following this event | – | 18 patients with various cancers from Sweden with a mean age of 58 years; 44% were male and ethnicity was not reported. |
|
(a) Believed in life, fought for it, and came through stronger; (b) life went on, adapted and found a balance in the new life; and (c) life was over, felt out of control, and lost belief in life |
|
| |||||
| Schumacher et al., 1998 | To evaluate QOL in patients with acute leukemia treated according to the protocol of the German AML Cooperative Group |
|
28 patients with acute leukemia in Germany. The mean age was 46 years; 43% were male and ethnicity was not reported. | Prospective, longitudinal
|
Although most patients with acute leukemia eventually relapse, the evaluation of QOL in patients undergoing treatment shows that subjective benefit outweighs the adverse effects of antileukemic therapy. |
|
| |||||
| Stalfelt, 1994 | To explore QOL in patients with acute leukemia during the course of their disease |
|
27 patients with acute leukemia from Sweden; mean age was 46.9 years (range = 18–74) and 51% were male. Ethnicity was not reported. | Prospective, longitudinal
|
Induction treatment entailed physical and psychological distress with decreased QOL but with continued ability to enjoy various leisure activities. Patients who survived experienced a change of attitude with regard to what was important in their lives. They reverted to their previous lifestyle within two years. |
|
| |||||
| Yau et al., 1991 | To evaluate long-term outcomes and QOL in patients discharged from the hospital following intensive care for life-threatening medical complications of hematologic malignancy, identify factors that might influence long-term prognosis in such cases |
|
92 patients with various cancers in London, England; 63% were male and age and ethnicity were not reported. | Retrospective, longitudinal
|
QOL of six of the seven long-term survivors is good, while that of the other is acceptable. None of the patients reported any increased limitation of their daily activities, five had returned to full-time employment, and all seven stated that they would be willing to undergo intensive care again under the same circumstances. |
ADL—activities of daily living; BSI—Beck Symptom Inventory; CES-D—Center for Epidemiologic Studies Depression; CI—confidence interval; CINV—chemotherapy-induced nausea and vomiting; DT—Distress Thermometer; ESAS—Edmonton Symptom Assessment Scale; EORTC QLQ-C30—European Organisation for the Research and Treatment of Cancer Quality of Life Questionnaire–Core 30; FACT-F—Functional Assessment of Cancer Therapy–Fatigue; FACT-G—Functional Assessment of Cancer Therapy–General; FACT-Sp—Functional Assessment of Cancer Therapy–Spiritual; FLIC—Functional Living Index-Cancer; GA—geriatric assessment; GDS—Geriatric Depression Scale; HADS—Hospital Anxiety and Depression Scale; HR—hazard ratio; ICU—intensive care unit; LIP—Life Ingredient Profile; LGC—Lund Gerontological Centre Generic Global QOL; MAC—Mental Adjustment to Cancer; MDASI—MD Anderson Symptom Inventory; PAIS-SR—Psychosocial Adjustment to Illness Scale–Self Report; PQOL—Perceived Quality of Life; QOL—quality of life; RPFS—Revised Piper Fatigue Scale; SEIQOL—Schedule for the Evaluation of Individual Quality of Life; VAS—Visual Analog Scale
References
- Albrecht T. Physiologic and psychological symptoms experienced by adults with acute leukemia: An integrative literature review. Oncology Nursing Forum. 2014;41:286–295. doi: 10.1188/14.ONF.286-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alibhai S, Leach M, Kermalli H, Gupta V, Kowiger M, Tomlinson G, Minden MD. The impact of acute myeloid leukemia (AML) and its treatment on quality of life and functional status in older adults. Critical Reviews in Oncology/Hematology. 2007;64:19–30. doi: 10.1016/j.critrevonc.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Alibhai S, Leach M, Kowiger ME, Tomlinson GA, Brandwein JM, Minden MD. Fatigue in older adults with acute myeloid leukemia: Predictors and associations with quality of life and functional status. Leukemia. 2007;21:845–848. doi: 10.1038/sj.leu.2404576. [DOI] [PubMed] [Google Scholar]
- Alibhai S, O’Neill S, Fisher-Schlombs K, Breunis H, Brandwein J, Timilishain N, Culos-Reed S. A clinical trial of supervised exercise for adult inpatients with acute myeloid leukemia (AML) undergoing induction chemotherapy. Leukemia Research. 2012;36:1255–1261. doi: 10.1016/j.leukres.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglini CB, Hackney AC, Garcia R, Groff D, Evans E, Shea T. The effects of an exercise program in leukemia patients. Integrative Cancer Therapies. 2009;8:130–138. doi: 10.1177/1534735409334266. [DOI] [PubMed] [Google Scholar]
- Cleeland CS. Symptom burden: Multiple symptoms and their impact as patient-reported outcomes. Journal of the National Cancer Institute. Monographs. 2007;37:16–21. doi: 10.1093/jncimonographs/lgm005. [DOI] [PubMed] [Google Scholar]
- Dodd M, Janson S, Facione N, Faucett J, Froelicher ES, Humphries J, Taylor D. Advancing the science of symptom management. Journal of Advanced Nursing. 2001;33:668–676. doi: 10.1046/j.1365-2648.2001.01697.x. [DOI] [PubMed] [Google Scholar]
- Efficace F, Cartoni C, Niscola P, Tendas A, Meloni E, Scarmucci L, Mandelli F. Predicting survival in advanced hematologic malignancies: Do patient-reported symptoms matter? European Journal of Haematology. 2012;89:410–416. doi: 10.1111/ejh.12004. [DOI] [PubMed] [Google Scholar]
- Forjaz M, Guarnaccia C. A comparison of Portuguese and American patients with hematological malignancies: A cross-cultural survey of health-related quality of life. Psycho-Oncology. 2001;10:251–258. doi: 10.1002/pon.522. [DOI] [PubMed] [Google Scholar]
- Fulcher CD, Kim HJ, Smith PR, Sherner TL. Putting Evidence Into Practice: Evidence-based interventions for depression. Clinical Journal of Oncology Nursing. 2014;18(Suppl. 3):S26–S37. doi: 10.1188/14.CJON.S3.26-37. [DOI] [PubMed] [Google Scholar]
- Hiddemann W, Kern W, Schoch C, Fonatsch C, Heinecke A, Wormann B, Buchner T. Management of acute leukemia in elderly patients. Journal of Clinical Oncology. 1999;17:3569–3579. doi: 10.1200/JCO.1999.17.11.3569. [DOI] [PubMed] [Google Scholar]
- Kim HJ, McGuire DB, Tulman L, Barsevick AM. Symptom clusters: Concept analysis and clinical implications for cancer nursing. Cancer Nursing. 2005;28:270–282. doi: 10.1097/00002820-200507000-00005. [DOI] [PubMed] [Google Scholar]
- Klepin HD, Geiger A, Tooze J, Kritchevsky SB, Williamson J, Pardee TS, Powell B. The feasibility of inpatient geriatric assessment for older adults receiving induction chemotherapy for acute myelogenous leukemia. Journal of American Geriatrics Society. 2011;59:1837–1846. doi: 10.1111/j.1532-5415.2011.03614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornblith A, Herndon J, Zuckerman E, Cella D, Cherin E, Wolchok S, Holland J. Comparison of psychosocial adaptation of advanced stage Hodgkin’s disease and acute leukemia survivors. Annals of Oncology. 1998;9:297–306. doi: 10.1023/A:1008297130258. [DOI] [PubMed] [Google Scholar]
- LoBiondo-Wood G, Brown CG, Knobf MT, Lyon D, Mallory G, Mitchell SA, Fellman B. Priorities for oncology nursing research: The 2013 national survey. Oncology Nursing Forum. 2014;41:67–76. doi: 10.1188/14.ONF.67-76. [DOI] [PubMed] [Google Scholar]
- Mayer R, Davis RB, Schiffer C, Berg D, Powell B, Frei E. Intensive postremission chemotherapy in adults with acute myeloid leukemia. New England Journal of Medicine. 1994;331:896–903. doi: 10.1056/NEJM199410063311402. [DOI] [PubMed] [Google Scholar]
- Mitchell SA, Hoffman AJ, Clark JC, DeGennaro RM, Poirier P, Robinson CB, Weisbrod BL. Putting Evidence Into Practice: An update of evidence-based interventions for cancer-related fatigue during and following treatment. Clinical Journal of Oncology Nursing. 2014;18(Suppl. 3):S38–S58. doi: 10.1188/14.CJON.S3.38-58. [DOI] [PubMed] [Google Scholar]
- Mohamedali H, Breunis H, Timilshina N, Brandwein J, Gupta V, Li M, Alibhai S. Older age is associated with similar quality of life and physical function compared to younger age during intensive chemotherapy for acute myeloid leukemia. Leukemia Research. 2012;36:1241–1248. doi: 10.1016/j.leukres.2012.05.020. [DOI] [PubMed] [Google Scholar]
- Montgomery C, Pocock M, Titley K, Lloyd K. Individual quality of life in patients with leukemia and lymphoma. Psycho-Oncology. 2002;11:239–243. doi: 10.1002/pon.557. [DOI] [PubMed] [Google Scholar]
- Montgomery C, Pocock M, Titley K, Lloyd K. Predicting psychological distress in patients with leukemia and lymphoma. Journal of Psychosomatic Research. 2003;54:289–292. doi: 10.1016/S0022-3999(02)00396-3. [DOI] [PubMed] [Google Scholar]
- National Cancer Institute. General information about adult acute lymphoblastic leukemia. 2014a Retrieved from http://www.cancer.gov/cancertopics/pdq/treatment/adultALL/Patient/page1.
- National Cancer Institute. General information about adult acute myeloid leukemia. 2014b Retrieved from http://www.cancer.gov/cancertopics/pdq/treatment/adultAML/Patient/page1.
- National Cancer Institute. SEER stat fact sheets: Leukemia. 2014c Retrieved from http://seer.cancer.gov/statfacts/html/leuks.html.
- National Coalition for Cancer Survivorship. Survivorship definitions. 2004 Retrieved from http://cancercontrol.cancer.gov/ocs/statistics/definitions.html.
- Persson L, Hallberg I. Lived experience of survivors of leukemia or malignant lymphoma. Cancer Nursing. 2004;27:303–313. doi: 10.1097/00002820-200407000-00007. [DOI] [PubMed] [Google Scholar]
- Persson L, Larsson G, Ohlsson O, Hallberg I. Acute leukaemia or highly malignant lymphoma patients’ quality of life over two years: A pilot study. European Journal of Cancer Care. 2001;10:36–47. doi: 10.1046/j.1365-2354.2001.00236.x. [DOI] [PubMed] [Google Scholar]
- Pearce M, Coan A, Herndon J, Koenig H, Abernethy A. Unmet spiritual care needs impact emotional and spiritual wellbeing in advanced cancer patients. Supportive Care in Cancer. 2012;20:2269–2276. doi: 10.1007/s00520-011-1335-1. [DOI] [PubMed] [Google Scholar]
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses. The PRISMA statement. 2014 Retrieved from http://prisma-statement.org/statement.htm.
- Sekeres MA, Stone RM. The challenge of acute myeloid leukemia in older patients. Current Opinions in Oncology. 2002;14:24–30. doi: 10.1097/00001622-200201000-00005. [DOI] [PubMed] [Google Scholar]
- Schumacher A, Kessler T, Buchner T, Wewers D, van de Loo J. Quality of life in adult patients with acute myeloid leukemia receiving intensive and prolonged chemotherapy—A longitudinal study. Leukemia. 1998;12:586–592. doi: 10.1038/sj.leu.2400977. [DOI] [PubMed] [Google Scholar]
- Stalfelt A. Quality of life of patients with acute myeloid leukemia. Leukemia Research. 1994;18:257–267. doi: 10.1016/0145-2126(94)90028-0. [DOI] [PubMed] [Google Scholar]
- Stone R. The difficult problem of acute myeloid leukemia in the older adult. CA: A Cancer Journal for Clinicians. 2002;52:363–371. doi: 10.3322/canjclin.52.6.363. [DOI] [PubMed] [Google Scholar]
- Stone RM, Mayer RJ. Treatment of the newly diagnosed adult with de novo acute myeloid leukemia. Hematology/Oncology Clinics of North America. 1993;7:47–64. [PubMed] [Google Scholar]
- Xuereb MC, Dunlop R. The experience of leukaemia and bone marrow transplant: Searching for meaning and agency. Psycho-Oncology. 2003;12:397–409. doi: 10.1002/pon.648. [DOI] [PubMed] [Google Scholar]
- Yau E, Rohatiner A, Lister T, Hinds C. Long term prognosis and quality of life following intensive care for life-threatening complications of haematological malignancy. British Journal of Cancer. 1991;64:938–942. doi: 10.1038/bjc.1991.430. [DOI] [PMC free article] [PubMed] [Google Scholar]