Abstract
Target of rapamycin (TOR) kinase controls cell growth and metabolism in response to nutrient availability. In the fission yeast Schizosaccharomyces pombe, TOR complex 1 (TORC1) promotes vegetative growth and inhibits sexual differentiation in the presence of ample nutrients. Here, we report the isolation and characterization of mutants with similar phenotypes as TORC1 mutants, in that they initiate sexual differentiation even in nutrient‐rich conditions. In most mutants identified, TORC1 activity is downregulated and the mutated genes are involved in tRNA expression or modification. Expression of tRNA precursors decreases when cells undergo sexual differentiation. Furthermore, overexpression of tRNA precursors prevents TORC1 downregulation upon nitrogen starvation and represses the initiation of sexual differentiation. Based on these observations, we propose that tRNA precursors operate in the S. pombe TORC1 pathway to switch growth mode from vegetative to reproductive.
Keywords: fission yeast, sexual differentiation, TORC1, tRNA precursor
Subject Categories: Metabolism, RNA Biology
Introduction
Target of rapamycin (TOR) is a widely conserved serine/threonine kinase in eukaryotes 1. TOR forms two distinct multi‐protein complexes, TORC1 (TOR Complex 1) and TORC2. It regulates a variety of cellular activities in response to environmental changes 2, 3, 4. TORC1 regulates cell growth by driving anabolic processes, such as protein synthesis and suppressing catabolic processes, such as autophagy. In mammals, growth factors and cellular energy stimulate TORC1 activity by Rheb GTPase through inhibition of the TSC1‐TSC2‐TBC1D7 complex. In response to amino acids, TORC1 is activated through Rag GTPases in a TSC‐independent pathway 5. Amino acids are recognized by CASTOR or Sestrin in the cytoplasm and by V‐ATPase or SLC38A9 in lysosomes, leading to activation of the Rag complex 6, 7, 8, 9, 10, 11, 12. Involvement of leucyl‐tRNA synthetase has also been reported in amino acid‐mediated activation of TORC1 13, 14, 15, 16.
The fission yeast Schizosaccharomyces pombe has two TOR homologs, Tor1 and Tor2 17, 18, 19. As in mammalian cells and budding yeast, S. pombe TOR forms two distinct complexes 20, 21, 22. TORC1, which contains Tor2 as a catalytic subunit, is essential for growth and represses sexual differentiation by sensing nitrogen supply. Schizosaccharomyces pombe cells proliferate by mitotic growth under nutrient‐rich conditions and enter sexual differentiation when starved of nutrients, especially nitrogen. Upon starvation, S. pombe cells arrest the mitotic cell cycle in the G1 phase and haploid cells conjugate with cells of the opposite mating type. Resulting diploid zygotes undergo meiosis and produce spores. Inactivation of TORC1 in tor2 mutants mimics nutrient starvation and results in the initiation of sexual differentiation, even in the presence of ample nutrients 20, 21, 22, 23, 24.
An increasing number of factors downstream of S. pombe TORC1 have been identified 25. We have shown that TORC1 phosphorylates Psk1, an S6 kinase homolog in S. pombe, which is a well‐known substrate of TORC1 in mammalian cells 26. Psk1 regulates the phosphorylation state of ribosomal protein S6 in response to nutrient availability 26, 27. In addition, we have demonstrated that TORC1 phosphorylates a key meiotic regulator, Mei2, which is also involved in the regulation of G1 arrest and conjugation. Phosphorylation of Mei2 by TORC1 induces its polyubiquitination and proteasomal degradation, eventually leading to suppression of sexual differentiation 28. However, Mei2 is not necessary for G1 arrest and conjugation, since cells lacking Mei2 are still able to conjugate, although with reduced efficiency compared to wild‐type cells. This indicates that Mei2 is not the sole target of TORC1 in the prevention of sexual differentiation in S. pombe. Meanwhile, it remains largely unknown how the extracellular nutrient‐level information is transmitted to TORC1. As in mammalian cells and budding yeast, Rag GTPases in S. pombe, namely Gtr1 and Gtr2, are known to contribute to TORC1 regulation in response to amino acids. However, unlike the key components of TORC1, such as Tor2 or Raptor Mip1, Gtr1 and Gtr2 are not essential for cell growth 29, suggesting that an additional regulatory pathway(s) may be involved in TORC1 signaling.
To gain insights into S. pombe TORC1 signaling pathways, we report the isolation of novel mutants that appear to phenocopy the TORC1 mutant, that is, mutants that initiate sexual differentiation ectopically under nutrient‐rich conditions. Intriguingly, most of the genes responsible for the mutant phenotype were involved in the expression or modification of tRNAs. We have characterized these factors and propose a novel mode of regulation of the TORC1 activity by tRNA precursors in response to nitrogen availability.
Results
Isolation of mutants that phenocopy TORC1 mutants
To identify novel factors involved in the TORC1 pathway, we screened for mutants that showed temperature sensitivity for growth and were derepressed for sexual differentiation at the restrictive temperature, similar to tor2‐ts mutants. We introduced mutations randomly in homothallic wild‐type cells and isolated mutants that could grow at 25°C, but not at 34°C. From these isolated mutants, we picked those that initiated sexual differentiation at 30°C under nutrient‐rich conditions. We obtained eight mutants and designated them hmt, standing for hypermating and temperature‐sensitive growth. We cloned the responsible genes by selecting plasmids from S. pombe genomic or cDNA libraries that could rescue their growth defect at the restrictive temperature (Fig 1A). Interestingly, five of the eight responsible genes (hmt1–hmt5) were annotated to encode tRNA‐related factors. The hmt1 (SPBC1773.10c/nrs1) and hmt2 (SPBC19C7.06/prs1) genes encode homologous proteins to aminoacyl‐tRNA synthetases for asparagine and proline, respectively. The hmt3 gene is identical to tad3, which encodes tRNA adenosine‐34 deaminase 30. The hmt4 gene is identical to rpc34, which encodes a subunit of RNA polymerase III 31. The hmt5 gene is identical to sfc4, which encodes a subunit of the RNA polymerase III‐specific general transcription factor IIIC 32. Meanwhile, hmt6 is identical to cts1, which encodes CTP synthetase (SPAC10F6.03c), and hmt7 is identical to pcm1, which encodes mRNA‐capping methyltransferase 33. The hmt8 gene is identical to pat1, which encodes a serine/threonine kinase that blocks the onset of meiosis 34, 35, 36. Partial inactivation of Pat1 has been shown to induce ectopic conjugation, although the molecular mechanism underlying this mistimed conjugation remains elusive 37.
Figure 1. Isolation of hypermating and temperature‐sensitive growth mutants.
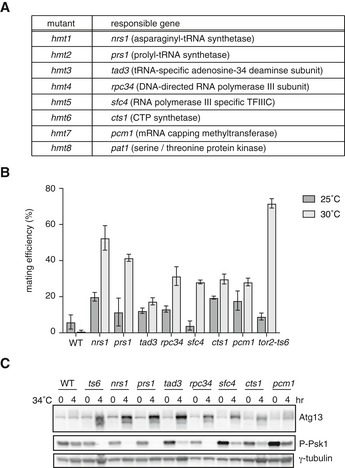
- The affected genes of hmt mutants.
- Mating efficiency of hmt mutants on nutrient‐rich medium. Cells of wild‐type (WT, JY450), nrs1/hmt1 (JS159), prs1/hmt2 (JS160), tad3/hmt3 (JS161), rpc34/hmt4 (JS162), sfc4/hmt5 (JS163), cts1/hmt6 (JS164), pcm1/hmt7 (JS165), and tor2‐ts6 (JV303) strains were grown on YE medium at 25 or 30°C for 5 days, and mating frequency was measured. Mean ± SD values of three independent measurements are shown (total n > 300).
- TORC1 activity in hmt mutants. Cells of wild‐type, tor2‐ts6, nrs1/hmt1, prs1/hmt2, tad3/hmt3, rpc34/hmt4, sfc4/hmt5, cts1/hmt6, and pcm1/hmt7 strains were grown in liquid YE medium at 25°C and then shifted to 34°C for 4 h. Cell extracts were subjected to Western blot analysis using anti‐Atg13 antibody and anti‐phospho‐S6 kinase antibody. γ‐tubulin is shown as a loading control.
We examined the phenotypes of these novel hypermating mutants in more detail. All hmt mutants initiated sexual differentiation including conjugation, meiosis, and sporulation under nutrient‐rich conditions at 30°C, in a similar fashion to the temperature‐sensitive tor2 mutant (tor2‐ts6, Figs 1B and EV1A). Several mutants (tad3, cts1, and pcm1) underwent sexual differentiation at both 25 and 30°C with similar efficiency, thereby suggesting that each mutant protein lost its ability to repress sexual differentiation even at the lower temperature, but maintained the essential functions for cell growth.
Figure EV1. Derepression of sexual differentiation in hmt mutants.
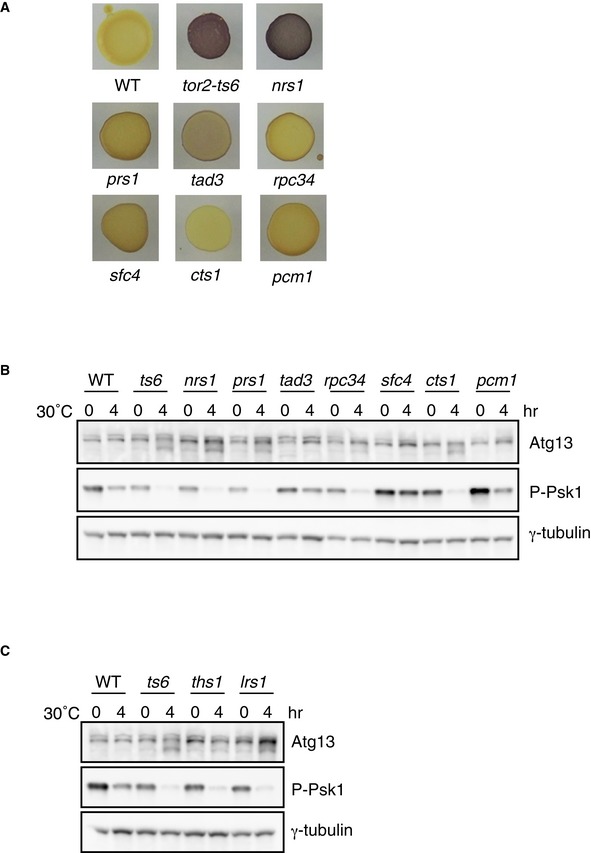
- Sporulation of hmt mutants. Cells of the wild‐type (WT, JY450), tor2‐ts6 (JV303), nrs1/hmt1 (JS159), prs1/hmt2 (JS160), tad3/hmt3 (JS161), rpc34/hmt4 (JS162), sfc4/hmt5 (JS163), cts1/hmt6 (JS164), and pcm1/hmt7 (JS165) strains were grown on nutrient‐rich YE medium at 30°C for 3 days and then exposed to iodine vapor, which stains sporulated cells dark brown.
- TORC1 activity in hmt mutants. Cells of wild‐type, tor2‐ts6, nrs1/hmt1, prs1/hmt2, tad3/hmt3, rpc34/hmt4, sfc4/hmt5, cts1/hmt6, and pcm1/hmt7 strains were grown in liquid YE medium at 25°C and subsequently shifted to 30°C for 4 h. Cell extracts were subjected to Western blot analysis using anti‐Atg13 antibody and anti‐phospho‐S6 kinase antibody. γ‐tubulin is shown as a loading control.
- TORC1 activity in ths1 (JS167) and lrs1 (JS168) mutants under the same conditions as in (B).
TORC1 activity is downregulated in most hmt mutants
Next, we examined TORC1 activity in hmt mutants. In S. pombe, the phosphorylation status of S6 kinase Psk1 or an autophagy regulator, Atg13, can be an indicator of TORC1 activity 25, 26. Accordingly, Atg13 migrated faster electrophoretically and phosphorylated Psk1 became undetectable in tor2‐ts6 cells shifted to the restrictive temperature of 34°C (Fig 1C). Except for pcm1/hmt7, decreased phosphorylation of Atg13 and Psk1 was evident in hmt mutant cells at the restrictive temperature, as seen in tor2‐ts6 cells (Fig 1C). Reduction in the phosphorylation of Atg13 and Psk1 was also observed at 30°C (Fig EV1B). These results indicate that TORC1 activity is downregulated in all novel hmt mutants except pcm1/hmt7 and that the products of these hmt genes are likely to function upstream of TORC1.
Inactivation of leucyl‐ or threonyl‐tRNA synthetase also induces ectopic sexual differentiation
Because hmt1 and hmt2 encode homologs of asparaginyl‐ and prolyl‐tRNA synthetase, respectively, we questioned whether mutations in other aminoacyl‐tRNA synthetase genes might induce sexual differentiation. Thus, we constructed temperature‐sensitive mutants of the genes encoding homologs of threonyl‐tRNA synthetase (ths1: SPBC25H2.02) and leucyl‐tRNA synthetase (lrs1: SPAC26F1.13c) and investigated their phenotypes (Fig 2A). Both ths1‐ts and lrs1‐ts cells initiated sexual differentiation under nutrition‐rich conditions at 30°C, as seen in the tor2‐ts6 and hmt cells; however, compared to the tor2‐ts6 cells, temperature sensitivity of the ectopic sexual differentiation phenotype was less prominent (Fig 2B and C). At restrictive temperatures, decreased phosphorylation of Atg13 and Psk1 was also observed in the ths1‐ts and lrs1‐ts cells, similar to that observed in the hmt1/nrs1 and hmt2/prs1 mutant cells (Figs 2D and EV1C). These results indicate that inactivation of leucyl‐ and threonyl‐tRNA synthetase can also mimic nitrogen‐starved conditions.
Figure 2. Loss of leucyl‐ and threonyl‐tRNA synthetase function leads to ectopic sexual development.
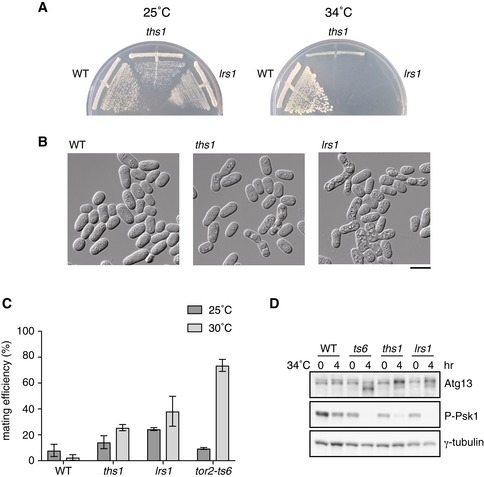
- Temperature‐sensitive growth of the ths1 and lrs1 mutant strains. Cells of wild‐type (JY450), ths1 (JS167), and lrs1 (JS168) strains were streaked on nutrient‐rich medium, YE, and incubated at either 25 or 34°C for 3 days.
- Ectopic induction of sexual differentiation in ths1 and lrs1 strains. Cells of the wild‐type, ths1, and lrs1 strains were examined microscopically after 3 days of incubation at 30°C on YE medium. Scale bar: 10 μm.
- Mating efficiency of ths1 and lrs1 strains on nutrient‐rich medium. Cells of the wild‐type, ths1, lrs1, and tor2‐ts6 (JV303) strains were grown on YE medium at 25 or 30°C for 5 days, and mating frequency was measured. Mean ± SD values of three independent measurements are shown (total n > 300).
- TORC1 activity in ths1 and lrs1 strains. Cells of wild‐type, tor2‐ts6, ths1, and lrs1 strains were grown in liquid YE medium at 25°C and subsequently transferred to 34°C for 4 h. Cell extracts were subjected to Western blot analysis using anti‐Atg13 antibody and anti‐phospho‐S6 kinase antibody. γ‐tubulin was used as a loading control.
hmt1/nrs1 and hmt2/prs1 mutants show reduced aminoacylation of corresponding tRNAs
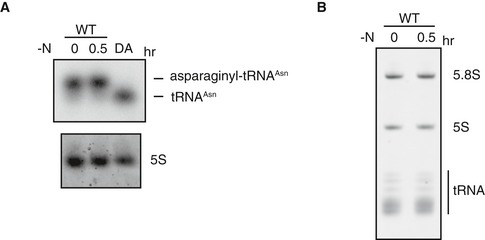
Because it was not previously demonstrated, we set out to confirm that hmt1/nrs1 and hmt2/prs1 indeed encode aminoacyl‐tRNA synthetases. In hmt1/nrs1 and hmt2/prs1 mutant cells, aminoacylation of corresponding tRNAs was impaired at the restrictive temperature (Fig 3A). By contrast, these tRNAs were properly aminoacylated in tor2‐ts6 cells.
Figure 3. Production and maturation of tRNA in hmt mutants.
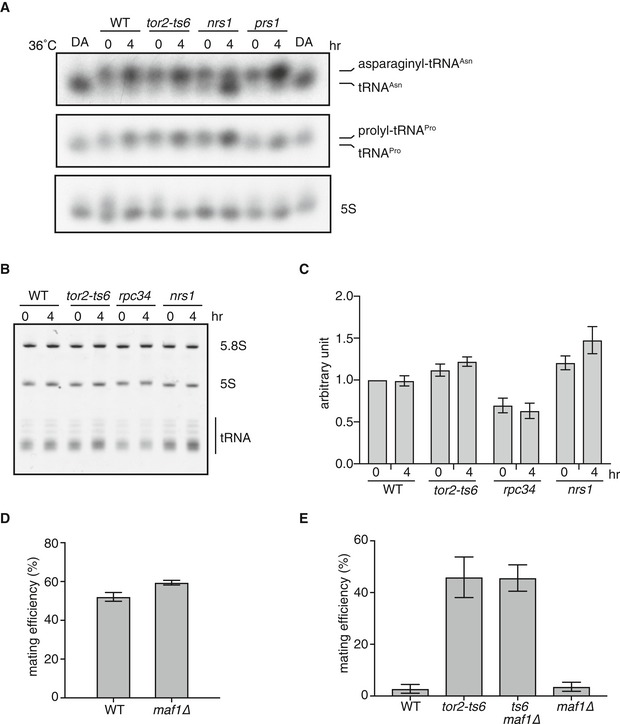
- Aminoacylation of tRNA in nrs1/hmt1 and prs1/hmt2 mutants. Cells of the wild‐type (JY450), tor2‐ts6 (JV303), nrs1/hmt1 (JS159), and prs1/hmt2 (JS160) strains were grown in liquid YE medium at 25°C and then shifted to 36°C for 4 h. RNA extracted in acidic conditions was analyzed by northern blot analysis with probes for tRNA‐Asn and tRNA‐Pro. The leftmost and rightmost lanes are deacylated wild‐type RNA (DA). 5S rRNA is shown as a loading control.
- Amount of total tRNA in hmt mutants. Cells of the wild‐type (JY450), tor2‐ts6 (JV303), rpc34/hmt4 (JS162), and nrs1/hmt1 (JS159) strains were grown in liquid YE medium at 25°C and then shifted to 30°C for 4 h. Total RNA was separated, followed by ethidium bromide staining.
- Quantification of the amount of total tRNA in the wild‐type, tor2‐ts6, rpc34/hmt4, and nrs1/hmt1 cells. Data show the mean ± SD from three biological replicates, including the one in (B).
- Mating efficiency of the wild‐type (JY3) and maf1∆ (JS169) strains. Cells of each strain were incubated on SSA medium at 30°C for 3 days. Mean ± SD values of three independent measurements are shown (total n > 300).
- Mating efficiency of the wild‐type (JY3), tor2‐ts6 (JT360), tor2‐ts6 maf1∆ (JS170), and maf1∆ (JS169) strains on nutrient‐rich medium. Cells of each strain were incubated on YE medium at 30°C for 3 days. Mean ± SD values of three independent measurements are shown (total n > 300).
The hmt4/rpc34 gene was annotated to encode a subunit of RNA polymerase III, which is essential for tRNA transcription. In hmt4/rpc34 mutant cells, the total amount of tRNA was decreased (Fig 3B and C). This was not the case with tor2‐ts6 or hmt1/nrs1 cells.
Maf1 does not play a major role in regulation of sexual differentiation via the TORC1 pathway
We next investigated the possible involvement of Maf1, a repressor of RNA polymerase III, in the TORC1 nitrogen‐sensing pathway, because the hmt4/rpc34 and hmt5/sfc4 genes encode polymerase III‐related factors. Maf1 is known to be phosphorylated and inactivated by TORC1 in mammalian cells 38, 39, 40. It is also known that S. pombe Maf1 is phosphorylated in a TORC1‐dependent manner 41. We examined maf1‐deleted cells and found that they conjugated at the same frequency as wild‐type cells, suggesting that Maf1 does not significantly contribute to the onset of sexual differentiation (Fig 3D). Furthermore, deletion of maf1 had no impact on the ectopic depression of sexual differentiation in tor2‐ts6 cells (Fig 3E). These observations suggest that Maf1 is not a major regulator of the initiation of sexual differentiation; however, it is possible that Maf1 may play a role in other regulatory pathways present downstream of the TORC1 pathway.
The levels of precursor tRNAs are decreased upon nitrogen starvation
We examined whether quantity of tRNA changes in response to nitrogen starvation, which induces sexual differentiation. Consequently, we found that nitrogen starvation did not affect the aminoacylation level or the total amount of tRNA (Figs 4A, and EV2A and B). However, the level of tRNA precursor transcripts (pre‐tRNAs) for leucine was decreased upon nitrogen starvation (Fig 4A). tRNAs are transcribed as precursor molecules, which then undergo a series of post‐transcriptional processing steps to generate mature tRNAs 42. Because the amount of pre‐tRNA was much smaller than the amount of mature tRNA, it was difficult to detect pre‐tRNAs with probes for the coding regions of mature tRNAs. Hence, we used probes specifically designed to measure expression of the precursors of tRNA‐Leu‐CAA and tRNA‐Asn‐GTT (Fig 4B). We used a probe to detect the intron sequence of tRNA‐Leu‐CAA and a probe to detect the sequence encompassing the boundary of tRNA‐Met and tRNA‐Asn, which are transcribed as a single transcript. The levels of pre‐tRNA‐Leu‐CAA and pre‐tRNA‐Asn‐GTT were severely reduced under nitrogen starvation (Fig 4B). pre‐tRNA‐Leu‐CAA and pre‐tRNA‐Asn‐GTT expression started decreasing 15‐min post‐nitrogen deprivation and increased again after 4 h (Fig 4C). Expression levels of pre‐tRNAs recovered 15 min after nitrogen replenishment (Fig 4D). Reduction in these pre‐tRNAs upon nitrogen starvation was also confirmed by quantitative RT–PCR (Fig 4E and F).
Figure 4. Expression of pre‐tRNA is downregulated upon nitrogen starvation.
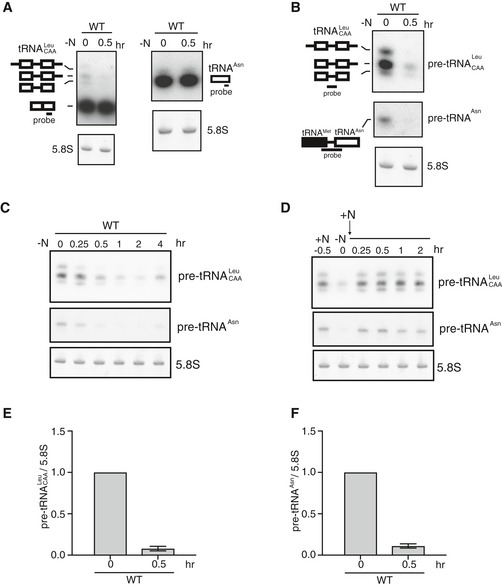
-
AExpression of tRNA‐Leu and tRNA‐Asn under nitrogen starvation in wild‐type cells (JY3). Total RNA was analyzed by northern blot analysis with probes for tRNA‐Leu and tRNA‐Asn, as indicated. The following graphic representations for tRNA species were used in (A) and (B): (dash/open square/dash/open square/dash (‐□‐□‐)) tRNA‐Leu precursor containing intron and unprocessed 5′ and 3′ ends; (open square/dash/open square/dash (□‐□‐)) tRNA‐Leu precursor with intron and unprocessed 3′ end; (open square/dash/open square (□‐□)) end‐matured intron containing pre‐tRNA‐Leu; (open square/open square (□□)) mature tRNA‐Leu; (closed square/dash/open square (■‐□)) tRNA‐Met and tRNA‐Asn; (open square (□)) mature tRNA‐Asn. 5.8S rRNA is shown as a loading control.
-
B, CExpression of pre‐tRNA‐Leu and pre‐tRNA‐Asn under nitrogen starvation. Total RNA was analyzed by northern blot analysis with probes for pre‐tRNA‐Leu and pre‐tRNA‐Asn, as indicated.
-
DExpression of pre‐tRNA‐Leu and pre‐tRNA‐Asn after replenishing nitrogen. After 30 min of nitrogen starvation (0 h), nitrogen was added, and total RNA was analyzed using northern blot analysis with probes for pre‐tRNA‐Leu and pre‐tRNA‐Asn.
-
E, FExpression of pre‐tRNA‐Leu and pre‐tRNA‐Asn analyzed using quantitative RT–PCR. Transcripts of pre‐tRNA‐Leu and pre‐tRNA‐Asn were quantified and normalized to 5.8S rRNA. Results represent the mean ± SD from three independent samples.
Figure EV2. The extent of aminoacylation and amount of total tRNA do not change under nitrogen starvation.
- Aminoacylation of tRNA‐Asn under nitrogen starvation. Wild‐type (JY3) cells were cultured in minimal medium, MM, and shifted to nitrogen‐deprived MM for 30 min. RNA extracted in acidic conditions was analyzed by northern blot analysis with probes for tRNA‐Asn. The rightmost lane is deacylated wild‐type RNA (DA). 5S rRNA is shown as a loading control.
- Amount of total tRNA under nitrogen starvation. Total RNA was separated, followed by ethidium bromide staining.
Precursor tRNAs are decreased in aminoacyl‐tRNA synthetase mutant cells
We then examined expression of pre‐tRNAs in hmt mutant cells shifted to the restrictive temperature under nutrient‐rich conditions. Expression of pre‐tRNAs for leucine and asparagine was rather constant after a temperature shift from 25 to 30°C in wild‐type cells (Fig 5A and B). By contrast, expression of pre‐tRNAs for leucine and asparagine was dramatically decreased when aminoacyl‐tRNA synthetase activity was compromised in lrs1 and nrs1 mutant cells, similar to wild‐type cells under nitrogen‐starved conditions. In tor2‐ts6 mutant cells, the expression of pre‐tRNA‐Leu decreased only marginally (Fig 5A and Table EV1). The expression of pre‐tRNA‐Asn decreased; however, this decrease was less severe than that observed in the lrs1 and nrs1 mutant cells (Fig 5B and Table EV1). These observations suggest that downregulation of pre‐tRNA expression precedes the initiation of sexual differentiation. They also suggest that expression of pre‐tRNAs is affected by the status of the subsequent aminoacylation step.
Figure 5. Expression of pre‐tRNA is downregulated in hmt mutants.
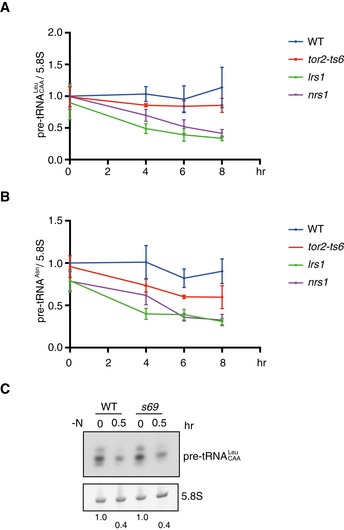
-
A, BExpression of pre‐tRNA‐Leu and pre‐tRNA‐Asn in hmt mutants. Cells of the wild‐type (JY450), tor2‐ts6 (JV303), nrs1/hmt1 (JS159), and lrs1 (JS168) strains were grown in liquid MM medium at 25°C and then shifted to 30°C after double dilution with MM. Total RNA for the indicated times was analyzed by quantitative RT–PCR. Transcripts of pre‐tRNA‐Leu (A) and pre‐tRNA‐Asn (B) were quantified and normalized to 5.8S rRNA. Results represent the mean ± SD from three independent samples.
-
CExpression of pre‐tRNA‐Leu under nitrogen starvation in the constitutively activated tor2 mutant cells. Cells of wild‐type (JS171) and tor2‐s69 (JS172) strains were cultured in minimal medium, MM, and shifted to nitrogen‐deprived MM for 30 min. Signal intensity of premature tRNA‐Leu, normalized to 5.8S rRNA, is shown at the bottom.
In activated tor2 mutant (tor2‐s69) cells, in which TORC1 activity was shown to be maintained upon nitrogen starvation 28, the amount of leucine pre‐tRNA was decreased under nitrogen starvation, as it was in wild‐type cells (Fig 5C). This observation suggests that the reduction in the amount of pre‐tRNA under nitrogen starvation is not mediated by downregulation of TORC1 activity.
Overexpression of precursor tRNA prevents sexual differentiation
The above results indicate that the amount of pre‐tRNA was decreased when sexual differentiation was induced either by nitrogen starvation in wild‐type cells or by inactivation of aminoacyl‐tRNA synthetase in hmt mutant cells. We then tested whether overexpression of pre‐tRNAs might repress sexual differentiation. For this purpose, we overexpressed Sla1 from a multicopy plasmid with a strong promoter. Sla1 is the S. pombe ortholog of the human La protein, which binds to the 3′ end of pre‐tRNA and facilitates its processing 43. It has been reported that overexpression of Sla1 increases the amount of pre‐tRNA 43. We also constructed a plasmid expressing pre‐tRNA‐Leu‐CAA from a promoter and a terminator for mRNA. We introduced a hammerhead ribozyme sequence immediately downstream of the pre‐tRNA‐Leu‐CAA sequence to remove the 3′ region, including the poly(A) tail. Hereafter, we call this construct pre‐tRNA‐Leu‐intr‐RZ (Fig 6A). We also constructed plasmids expressing pre‐tRNA‐Asn (tRNA‐Met‐tRNA‐Asn) and pre‐tRNA‐Pro‐CGG and labeled these constructs as pre‐tRNAMetAsn‐RZ and pre‐tRNA‐Pro‐intr‐RZ, respectively (Fig EV3A and D). Northern blot analysis revealed that the amount of pre‐tRNA‐Leu‐CAA increased with overexpression of Sla1, under both nutrient‐rich and nitrogen‐starved conditions (Fig 6B). Pre‐tRNA‐Leu‐intr‐RZ also increased the level of pre‐tRNA‐Leu‐CAA, but to a lesser extent than the overexpression of Sla1. Longer transcripts, which may have escaped trimming by ribozymes, were also detected in cells expressing pre‐tRNA‐Leu‐intr‐RZ. It remains unknown whether the longer transcripts are functional. Similarly, the level of pre‐tRNA‐Asn increased by overexpression of Sla1 (Fig EV3B). Pre‐tRNA‐MetAsn‐RZ increased the level of pre‐tRNA‐Asn about 1.2‐fold under nitrogen‐starved conditions. It is possible that both pre‐tRNA‐Asn and the longer transcripts encompassing pre‐tRNA‐Asn might function in the following observations.
Figure 6. Overexpression of pre‐tRNA prevents downregulation of TORC1 and inhibits sexual differentiation.
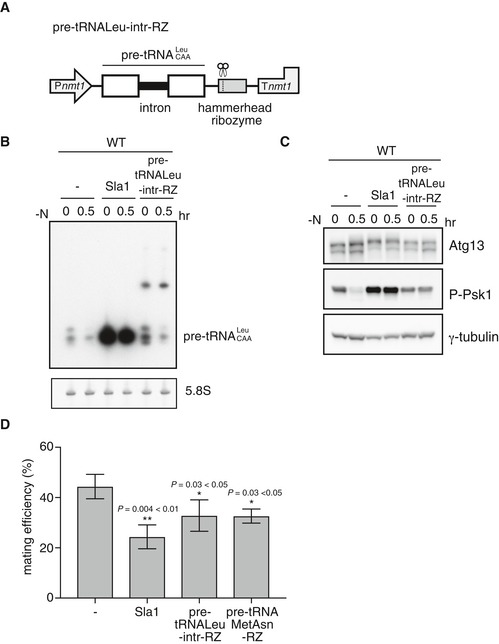
- Schematic diagram of pre‐tRNA‐Leu‐intr‐RZ. A thiamine‐repressible promoter and a terminator of the nmt1 gene are represented by Pnmt1 and Tnmt1, respectively.
- Overexpression of premature tRNA‐Leu. Wild‐type cells (JY450) carrying pREP1, pREP1‐sla1, or pREP1‐pre‐tRNA‐Leu‐intr‐RZ were grown in minimal medium, MM, and then shifted to nitrogen‐deprived MM for 30 min. Total RNA was analyzed by northern blot analysis with the probe for pre‐tRNA‐Leu. 5.8S rRNA is shown as a loading control.
- TORC1 activity in cells overexpressing pre‐tRNA‐Leu. TORC1 activity was analyzed by Western blot analysis with anti‐Atg13 antibody and anti‐phospho‐S6 kinase antibody under the same conditions as (B). γ‐tubulin is shown as a loading control.
- Mating efficiency of cells overexpressing pre‐tRNA. Wild‐type cells carrying pREP1, pREP1‐sla1, pREP1‐pre‐tRNA‐Leu‐intr‐RZ, or pREP1‐pre‐tRNAMetAsn‐RZ were incubated on SSA medium at 30°C for 1 day, and mating frequency was measured. Mean ± SD values of three independent measurements are shown (total n > 300). *P < 0.05; **P < 0.01 (Student's t‐test).
Figure EV3. Overexpression of pre‐tRNA‐Asn and pre‐tRNA‐Pro.
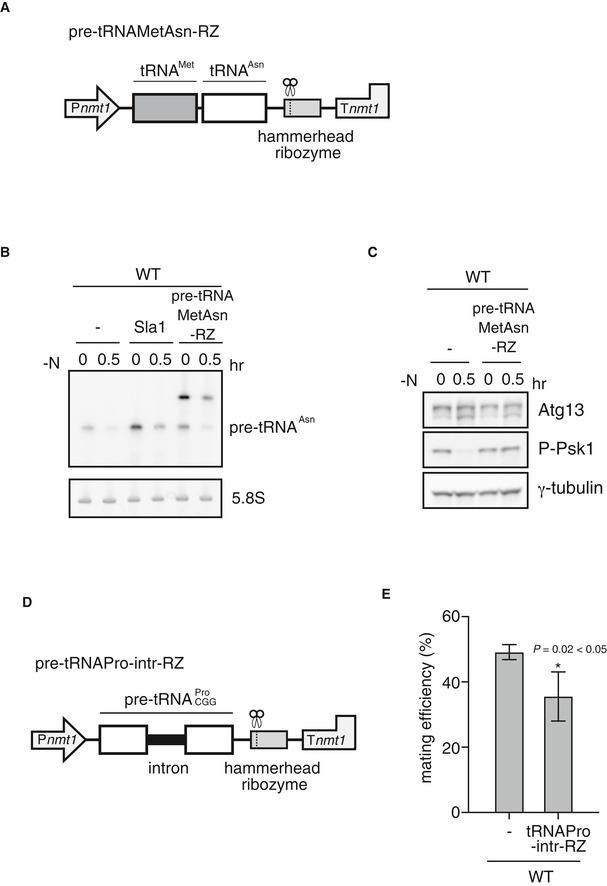
- Schematic diagram of pre‐tRNAMetAsn‐RZ. A thiamine‐repressible promoter and a terminator of the nmt1 gene are represented by Pnmt1 and Tnmt1, respectively.
- Overexpression of pre‐tRNA‐Asn. Wild‐type cells (JY450) carrying pREP1, pREP1‐sla1, or pREP1‐pre‐tRNAMetAsn‐RZ were grown in minimal medium (MM) and subsequently shifted to nitrogen‐deprived MM for 30 min. Total RNA was analyzed using northern blot analysis with a probe for pre‐tRNA‐Asn. 5.8S rRNA is shown as a loading control.
- TORC1 activity in cells overexpressing pre‐tRNA‐Asn. TORC1 activity was analyzed by Western blot analysis using the anti‐Atg13 antibody and anti‐phospho‐S6 kinase antibody under the same conditions as in (B). γ‐tubulin is shown as a loading control.
- Schematic diagram of pre‐tRNA‐Pro‐intr‐RZ.
- Mating efficiency of cells overexpressing pre‐tRNA‐Pro. Wild‐type cells carrying pREP1 or pREP1‐pre‐tRNA‐Pro‐intr‐RZ were incubated on SSA medium at 30°C for 2 days, and mating frequency was measured. Mean ± SD values of three independent measurements are shown (total n > 300). *P < 0.05 (Student's t‐test).
We measured TORC1 activity in pre‐tRNA‐overproducing cells by detecting the phosphorylation status of Atg13 and Psk1. Overproduction of pre‐tRNA prevented the decrease in TORC1 activity induced by nitrogen starvation (Figs 6C and EV3C). It should be noted that, in these experiments, cells were cultured in minimal medium to induce expression of Sla1 or pre‐tRNA‐RZ from plasmids, resulting in a slightly low TORC1 activity even before nitrogen depletion. Consistent with high TORC1 activity, pre‐tRNA overexpression repressed sexual differentiation (Figs 6D and EV3E). Thus, it appears that downregulation of pre‐tRNA expression is a prerequisite for the decrease in TORC1 activity in response to nitrogen starvation. Altogether, the above results suggest that a decrease in the amount of pre‐tRNA upon nitrogen starvation induces sexual differentiation by repressing TORC1 activity.
pre‐tRNA acts upstream of TORC1
We further examined the effect of pre‐tRNA overexpression to understand the relationship between pre‐tRNA and TORC1. Since Sla1 overexpression caused a growth defect in tor2‐ts6 cells (Fig EV4A), we used the pre‐tRNA‐Leu‐intr‐RZ plasmid for pre‐tRNA overexpression. TORC1 inactivation in tor2‐ts6 mutant cells abolished the effects of pre‐tRNA overexpression, which repressed sexual differentiation in wild‐type cells (Fig 7A). Conversely, TORC1 activity in the nrs1 and lrs1 mutant cells was restored by overexpression of the activated Tor2 mutant, namely Tor2‐s69 (Fig 7B). Further, derepression of sexual differentiation in hmt mutant cells was also suppressed by Tor2‐s69 overexpression (Fig EV4B). These observations at the genetic level support the hypothesis that changes in the level of pre‐tRNA affect TORC1 activity.
Figure EV4. Genetic analysis of overexpression of pre‐tRNA.
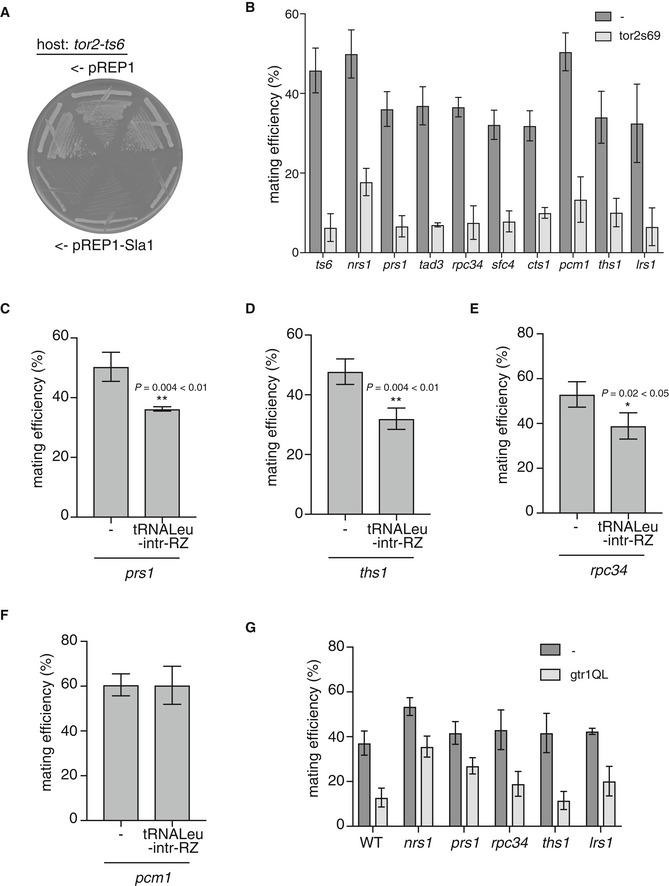
-
ASla1 overexpression in tor2‐ts6 mutant cells. tor2‐ts6 cells (JV303) carrying pREP1 or pREP1‐Sla1 were incubated on MM medium at 25°C for 4 days.
-
BMating efficiency of cells overexpressing constitutive active TORC1 mutant (Tor2‐s69). tor2‐ts6 (JV303), nrs1/hmt1 (JS159), prs1/hmt2 (JS160), tad3/hmt3 (JS161), rpc34/hmt4 (JS162), sfc4/hmt5 (JS163), cts1/hmt6 (JS164), pcm1/hmt7 (JS165), ths1 (JS167), and lrs1 (JS168) cells carrying pREP1 or pREP1‐tor2‐s69 were incubated on MM medium at 30°C for 2 days, and mating frequency was measured. Mean ± SD values of three independent measurements are shown (total n > 300).
-
C–FMating efficiency of cells overexpressing pre‐tRNA in prs1, ths1, rpc34, and pcm1 mutant cells. prs1, ths1, rpc34, and pcm1 cells carrying pREP1 or pREP1‐pre‐tRNA‐Leu‐intr‐RZ were incubated on MM medium at 30°C for 2 days (C and D) or 3 days (E and F), and mating frequency was measured. Mean ± SD values of three independent measurements are shown (total n > 300). *P < 0.05; **P < 0.01 (Student's t‐test).
-
GMating efficiency of cells overexpressing Gtr1QL in nrs1, prs1, rpc34, ths1, and lrs1 mutant cells. Wild‐type (WT), nrs1, prs1, rpc34, ths1, and lrs1 cells carrying pREP1 or pREP1‐Gtr1Q61L were incubated on MM medium at 30°C for 2 days, and mating frequency was measured. Mean ± SD values of three independent measurements are shown (total n > 300).
Figure 7. pre‐tRNA acts upstream of TORC1 to suppress sexual differentiation.
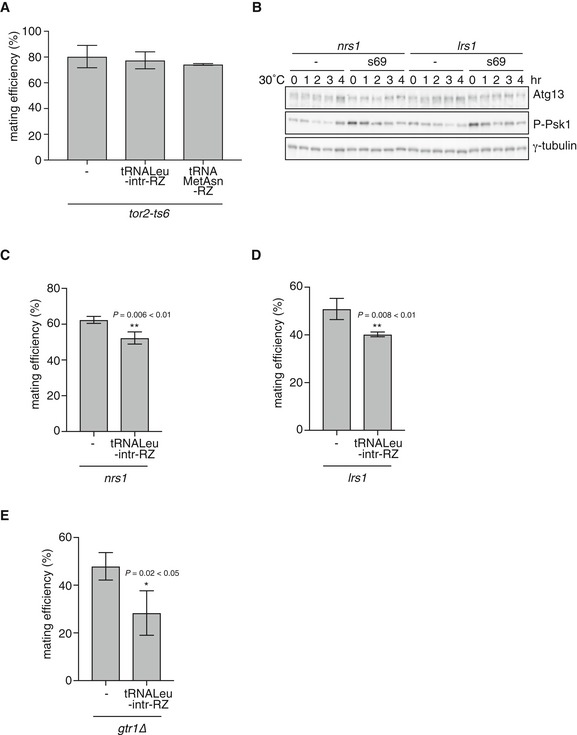
-
AMating efficiency of tor2‐ts6 cells overexpressing pre‐tRNA. tor2‐ts6 cells (JV303) carrying pREP1, pREP1‐pre‐tRNA‐Leu‐intr‐RZ, or pREP1‐pre‐tRNAMetAsn‐RZ were incubated on MM medium at 30°C for 2 days, and mating frequency was measured. Mean ± SD values of three independent measurements are shown (total n > 300).
-
BTORC1 activity in nrs1 and lrs1 mutants overexpressing constitutive active TORC1 mutant (Tor2‐s69). Cells of nrs1 (JS159) and lrs1 (JS168) strains carrying pREP1 or pREP1‐tor2‐s69 were grown in liquid MM medium at 25°C and subsequently shifted to 30°C. Cell extracts were subjected to Western blot analysis using anti‐Atg13 antibody and anti‐phospho‐S6 kinase antibody at the indicated time points. γ‐tubulin is shown as a loading control.
-
C, DMating efficiency of nrs1 and lrs1 mutant cells overexpressing pre‐tRNA. nrs1 cells carrying pREP1 or pREP1‐pre‐tRNA‐Leu‐intr‐RZ were incubated on MM medium at 30°C for 2 days (C). lrs1 cells carrying pREP1 or pREP1‐pre‐tRNA‐Leu‐intr‐RZ were incubated for 3 days (D). Mean ± SD values of three independent measurements are shown (total n > 300). **P < 0.01 (Student's t‐test).
-
EMating efficiency of gtr1∆ cells overexpressing pre‐tRNA. gtr1∆ cells (JS173) carrying pREP1 or pREP1‐pre‐tRNA‐Leu‐intr‐RZ were incubated on SSA medium at 30°C for 2 days, and mating frequency was measured. Mean ± SD values of three independent measurements are shown (total n > 300). *P < 0.05 (Student's t‐test).
Overexpression of pre‐tRNA also suppressed ectopic initiation of sexual differentiation in the hmt mutant cells that displayed reduced TORC1 activity (nrs1, lrs1, prs1, ths1, and rpc34) (Figs 7C and D, and EV4C‐E). The modest effect of pre‐tRNA overexpression suggests that the expression level of pre‐tRNA‐Leu from pre‐tRNA‐Leu‐intr‐RZ might not be enough in the hmt mutants. We cannot also exclude the possibility of defects other than in the pre‐tRNA‐TORC1 pathway in those mutants. In the pcm1/hmt7 mutant cells, which maintained TORC1 activity (Figs 1C and EV1B), pre‐tRNA overexpression had no impact on derepression of sexual differentiation, while overexpression of activated Tor2 suppressed ectopic sexual differentiation (Fig EV4B and F).
Next, we investigated whether the Rag GTPase Gtr1 was involved in the regulation of TORC1 activity via pre‐tRNA. It has been reported that leucyl‐tRNA synthetase signals leucine sufficiency to TORC1 via the Rag GTPases in budding yeast and mammalian cells 13, 14. The gtr1 deletion mutant cells carrying either the pre‐tRNA‐Leu‐intr‐RZ plasmid or the control empty vector showed slow growth and low mating efficiency (about 10%) after 1‐day incubation on sporulation medium. This observation was consistent with a previous observation that Gtr1 was required for the downregulation of TORC1 activity after nitrogen starvation 44. After 2‐day incubation, the mating efficiency of gtr1∆ cells expressing pre‐tRNA‐Leu‐intr‐RZ was lower than the control cells (Fig 7E). This indicates that Gtr1 is not essential for the maintenance of TORC1 activity by pre‐tRNA overexpression upon nitrogen starvation.
Overexpression of a GTP‐locked Gtr1 mutant (Gtr1QL) repressed sexual differentiation in both the wild‐type and hmt mutant cells (Fig EV4G), thereby suggesting that the presence of excess GTP‐bound form of Gtr1 led to the prevention of TORC1 downregulation upon nitrogen starvation. It is possible that the GDP‐bound Gtr1 might play some role in TORC1 downregulation, although it remains to be proven how S. pombe Gtr1 works in the TORC1 pathway.
Discussion
In this study, we isolated and characterized novel S. pombe mutants designated as hmt, which show a similar phenotype to the temperature‐sensitive tor2 mutants, namely ectopic initiation of the sexual differentiation program at the restrictive temperature. The hmt8/pat1 gene encodes a well‐characterized Ser/Thr kinase that phosphorylates and inactivates its critical target, Mei2 34, 35, 36, 45, 46, which is also downregulated by TORC1 28. The hmt7/pcm1 gene encodes an mRNA‐capping methyltransferase. Among all hmt mutants except for hmt8/pat1, which was not tested, only hmt7/pcm1 cells maintained the TORC1 activity after shifting to the restrictive temperature. This suggests that Hmt7/Pcm1 may function either downstream of or in parallel to TORC1. Further studies are needed to elucidate the relationship between Hmt7/Pcm1 and the TORC1 signaling pathway.
Except for hmt8/pat1, hmt7/pcm1, and hmt6/cts1, the hmt genes encode proteins involved in the expression or modification of tRNAs. TORC1 activity was reduced by impairment of any one of hmt genes except for hmt7/pcm1 and hmt8/pat1, the latter of which was not examined, suggesting that their products may have a positive effect on TORC1, either directly or indirectly. Two of the hmt genes encode aminoacyl‐tRNA synthetases. Two other aminoacyl‐tRNA synthetase mutants we constructed also showed the hmt phenotype. It is intriguing that the reduction in activity of a single aminoacyl‐tRNA synthetase appears to be sufficient to induce initiation of sexual differentiation through downregulation of TORC1. Leucyl‐tRNA synthetase senses the abundance of leucine and promotes TORC1 activity in budding yeast and mammalian cells 13, 14, 15, 16. In S. pombe, aminoacyl‐tRNA synthetases may be involved in a signaling pathway linking nitrogen availability to TORC1 activity.
Another possibility, which does not exclude the above possibility, is that tRNA precursors are key signaling molecules that positively regulate TORC1 activity. The following four observations support this hypothesis: (1) pre‐tRNAs were significantly reduced upon nitrogen starvation; (2) impairment of aminoacyl‐tRNA synthetase caused a reduction in pre‐tRNA quantity; (3) nitrogen starvation leads to a decrease in pre‐tRNA quantity in the activated tor2 mutant, which retains TORC1 activity after nitrogen starvation; and (4) overexpression of pre‐tRNAs prevents the downregulation of TORC1 upon nitrogen starvation and is inhibitory to sexual differentiation. We demonstrated that the ectopic initiation of sexual differentiation in tor2 mutant cells was not suppressed by the overexpression of pre‐tRNAs. Forced activation of TORC1 via Tor2‐s69 could prevent sexual differentiation in hmt mutants. These results also support the hypothesis that pre‐tRNAs act upstream of TORC1. In addition, observation (2) suggests that feedback regulation may operate on pre‐tRNA expression, responding to the status of a later step(s) in tRNA dynamics, although the precise mechanism remains to be identified. Since pre‐tRNAs were slightly decreased in tor2‐ts6 mutant cells, expression of pre‐tRNAs may be feedback‐regulated through the TORC1 pathway, for example, through Maf1.
A challenging question is how pre‐tRNAs function in the TORC1 signaling pathway. Our observations indicate that the Rag GTPase Gtr1 is not essential for pre‐tRNA‐meditated regulation of TORC1. We cannot exclude the possibility that pre‐tRNA molecules act directly on the TORC1 pathway. However, there may also be a specific factor(s) that interacts with pre‐tRNAs, for instance a protein involved in tRNA processing, which is liberated from pre‐tRNAs upon nitrogen starvation and negatively affects the TORC1 pathway. In mammalian cells, a reduction in histidyl‐tRNA synthetase leads to the suppression of S6 kinase, which is positively regulated by TORC1 47. In budding yeast, tRNAs released from the protein synthesis machinery have been proposed to inhibit TORC1 activity 48. These regulatory models seem to differ from the model that we propose, in which tRNA precursors play a significant role in positively regulating TORC1. It will be interesting and important to clarify whether tRNA‐ or pre‐tRNA‐mediated regulation of the TORC1 pathway is adopted in other eukaryotes.
The S. pombe TORC1 pathway is unique in that it positively regulates vegetative growth and concurrently suppresses sexual differentiation by sensing nitrogen availability. Deciphering the mode of action of pre‐tRNAs and testing conservation of similar regulation mechanisms in other organisms is required to substantiate our pre‐tRNA‐mediated regulation model.
Materials and Methods
Fission yeast strains, media, plasmids, and genetic methods
The S. pombe strains used in this study are listed in Table EV2. Complete medium YE, minimal medium SD, minimal medium MM, and synthetic sporulation medium SSA, were used 49, 50. Vectors carrying the thiamine‐repressible nmt1 promoter or its derivatives have been described previously 51, 52. The plasmid overexpressing Sla1 was constructed by cloning the PCR‐amplified sla1 ORF into pREP1. The plasmid carrying pre‐tRNA‐Leu‐intr‐RZ and pre‐tRNAMetAsn‐RZ was constructed by cloning the genomic sequence of tRNA‐Leu‐CAA (SPBTRNALEU.06) and tRNA‐Met‐tRNA‐Asn (SPBTRNAMET.05 and SPBTRNAASN.01) PCR‐amplified with a primer set carrying the hammerhead ribozyme sequence (Table EV3) into pREP1. The plasmid carrying pre‐tRNA‐Pro‐intr‐RZ was constructed by inserting the DNA fragment containing tRNA‐Pro‐CGG (SPATRNAPRO.02) and the hammerhead ribozyme sequence synthesized by the artificial gene synthesis service (FASMAC) into pREP1.
General genetic procedures for S. pombe have been described previously 49. Mutagenesis of S. pombe cells with N‐methyl‐N′‐nitro‐N‐nitroso‐guanidine was performed as previously described 45. To generate lrs1 or ths1 mutants, we first constructed strains carrying three copies of the HA tag and a kanamycin cassette on the chromosome downstream of the respective gene. DNA fragments that encompass the respective ORF were then amplified by error‐prone PCR using genomic DNA of each strain as a template. Wild‐type cells were transformed with the resulting DNA fragments.
Microscopy
Schizosaccharomyces pombe cells were observed under a microscope (Axioplan 2, Carl Zeiss, Oberkochen, Germany). Images were captured using a chilled CCD camera (CoolSNAP HQ2, Photometrics, Tucson, AZ, USA) and MetaMorph software (Molecular Devices, Sunnyvale, CA, USA).
The mating efficiency was calculated as the following ratio: 2 × number of asci and zygotes / (number of unmated cells + 2 × number of asci and zygotes).
Western blot analysis
In Figs 1C, 2D, and EV1B and C, cells (4–6 × 106 cells/ml before dilution) were shifted to restrictive temperatures after dilution with the same amount of fresh YE. In other experiments, cells (4–6 × 106 cells/ml) were shifted to restrictive temperatures (Fig 7B) or nitrogen starvation conditions (Figs 6C and EV3C) without dilution. Harvested cells were disrupted with glass beads in 20% trichloroacetic acid 53. The samples were separated by 10% SDS–polyacrylamide gel electrophoresis. A rabbit polyclonal antibody against Atg13 54, mouse monoclonal antibodies against phospho‐p70 S6 kinase (Thr389; 1A5; Cell Signaling Technology, Danvers, MA, USA), and γ‐tubulin (GTU‐88, Sigma‐Aldrich, St. Louis, MO, USA) were used.
RNA preparation, northern blot analysis, and quantitative RT–PCR
Cells (4–6 × 106 cells/ml) were shifted to restrictive temperatures or nitrogen starvation conditions. In Fig 5A and B, cells (4–6 × 106 cells/ml before dilution) were shifted to restrictive temperatures after dilution with the same amount of fresh MM. For detection of aminoacyl‐tRNA, RNA was prepared by disrupting the cells with glass beads in extraction buffer (0.1 M NaOAc, pH 4.7, 0.5 M NaCl, 10 mM EDTA, 1% SDS). Proteins were removed using 5:1 phenol:chloroform, pH 4.3–4.7 (Sigma‐Aldrich), and then, RNA was precipitated with 100% ethanol. Total RNA (4 μg) was separated on a 6.5% polyacrylamide gel containing 8 M urea. RNA was blotted on GeneScreen Plus membrane (PerkinElmer, Waltham, MA, USA) in 1× TAE. Aminoacyl‐tRNA was deacylated by treatment with 0.25 M Tris–HCl, pH 8.0 at 75°C for 10 min.
For detection of total tRNA and pre‐tRNA, RNA was extracted with hot acidic phenol. Total RNA (4 μg) was separated on a 10% TBE–Urea gel (Invitrogen, Waltham, MA, USA). In Figs 3A, 4A and B, 5C, 6B, and EV2A, RNA was blotted on GeneScreen Plus membrane (PerkinElmer) in 0.5× TBE. The membrane was hybridized in a buffer (5× SSC, 50% formamide, 5× Denhardt's, 0.1% SDS) with heat‐denatured carrier DNA and oligonucleotide probes (Table EV3) that were 5′‐end labeled with [γ‐32P]‐ATP, using T4 polynucleotide kinase (Takara, Shiga, Japan). In Figs 4C and D, and EV3B, RNA was blotted onto a Hybond N+ membrane (GE Healthcare, Buckinghamshire, UK). A DIG‐labeled probe was synthesized using the DIG Oligonucleotide 3′‐End Labeling kit (2nd Generation; Roche, Basel, Switzerland). The blots were incubated with the DIG‐labeled probe overnight at 42°C, and the signals were detected according to the manufacturer's protocol.
For quantitative RT–PCR, cDNA was synthesized by ReverTra Ace qPCR RT Master Mix (TOYOBO, Osaka, Japan) using total RNA extracted with hot acidic phenol and treated with DNase I (Turbo DNA‐free kit, Thermo Fisher Scientific, Waltham, MA, USA). Quantitative PCR was performed using a LightCycler 96 instrument (Roche) and SYBR Premix Ex Taq II (Tli RNase H Plus, Takara). Primer sequences are listed in Table EV3.
Author contributions
YO, TM, MY, and AY conceived the concept of this work and designed the study. YO, TM, AN, and AY performed experiments and analyzed data. YO, MY, and AY interpreted the results and wrote the manuscript with the help of all other authors.
Conflict of interest
The authors declare that they have no conflict of interest.
Supporting information
Expanded View Figures PDF
Table EV1
Table EV2
Table EV3
Review Process File
Acknowledgements
We thank Drs. Y. Kamada, H. Tatebe, and K. Shiozaki for helpful discussion. We also thank Dr. A. Nakashima for generously providing the Gtr1QL plasmid and insightful suggestions, and A. Nakade and the Center for Radioisotope Facilities, Okazaki Research Facilities, NINS, for providing technical support. This work was supported by JSPS KAKENHI Grant Numbers 15J40037 (YO), 16K18542 (YO), and 15H04333 (AY) and by the joint research program of the Biosignal Research Center, Kobe University (Grant Number: 281009).
EMBO Reports (2018) 19: e44867
References
- 1. Wullschleger S, Loewith R, Hall MN (2006) TOR signaling in growth and metabolism. Cell 124: 471–484 [DOI] [PubMed] [Google Scholar]
- 2. Soulard A, Cohen A, Hall MN (2009) TOR signaling in invertebrates. Curr Opin Cell Biol 21: 825–836 [DOI] [PubMed] [Google Scholar]
- 3. Loewith R, Jacinto E, Wullschleger S, Lorberg A, Crespo JL, Bonenfant D, Oppliger W, Jenoe P, Hall MN (2002) Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Mol Cell 10: 457–468 [DOI] [PubMed] [Google Scholar]
- 4. Saxton RA, Sabatini DM (2017) mTOR signaling in growth, metabolism, and disease. Cell 168: 960–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shimobayashi M, Hall MN (2016) Multiple amino acid sensing inputs to mTORC1. Cell Res 26: 7–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wolfson RL, Chantranupong L, Saxton RA, Shen K, Scaria SM, Cantor JR, Sabatini DM (2016) Sestrin2 is a leucine sensor for the mTORC1 pathway. Science 351: 43–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Saxton RA, Knockenhauer KE, Wolfson RL, Chantranupong L, Pacold ME, Wang T, Schwartz TU, Sabatini DM (2016) Structural basis for leucine sensing by the Sestrin2‐mTORC1 pathway. Science 351: 53–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chantranupong L, Scaria SM, Saxton RA, Gygi MP, Shen K, Wyant GA, Wang T, Harper JW, Gygi SP, Sabatini DM (2016) The CASTOR proteins are arginine sensors for the mTORC1 pathway. Cell 165: 153–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bar‐Peled L, Schweitzer LD, Zoncu R, Sabatini DM (2012) Ragulator is a GEF for the rag GTPases that signal amino acid levels to mTORC1. Cell 150: 1196–1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jung J, Genau HM, Behrends C (2015) Amino acid‐dependent mTORC1 regulation by the lysosomal membrane protein SLC38A9. Mol Cell Biol 35: 2479–2494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rebsamen M, Pochini L, Stasyk T, de Araujo ME, Galluccio M, Kandasamy RK, Snijder B, Fauster A, Rudashevskaya EL, Bruckner M, et al (2015) SLC38A9 is a component of the lysosomal amino acid sensing machinery that controls mTORC1. Nature 519: 477–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang S, Tsun ZY, Wolfson RL, Shen K, Wyant GA, Plovanich ME, Yuan ED, Jones TD, Chantranupong L, Comb W et al (2015) Metabolism. Lysosomal amino acid transporter SLC38A9 signals arginine sufficiency to mTORC1. Science 347: 188–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Han JM, Jeong SJ, Park MC, Kim G, Kwon NH, Kim HK, Ha SH, Ryu SH, Kim S (2012) Leucyl‐tRNA synthetase is an intracellular leucine sensor for the mTORC1‐signaling pathway. Cell 149: 410–424 [DOI] [PubMed] [Google Scholar]
- 14. Bonfils G, Jaquenoud M, Bontron S, Ostrowicz C, Ungermann C, De Virgilio C (2012) Leucyl‐tRNA synthetase controls TORC1 via the EGO complex. Mol Cell 46: 105–110 [DOI] [PubMed] [Google Scholar]
- 15. Tsun ZY, Bar‐Peled L, Chantranupong L, Zoncu R, Wang T, Kim C, Spooner E, Sabatini DM (2013) The folliculin tumor suppressor is a GAP for the RagC/D GTPases that signal amino acid levels to mTORC1. Mol Cell 52: 495–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yoon MS, Son K, Arauz E, Han JM, Kim S, Chen J (2016) Leucyl‐tRNA synthetase activates Vps34 in amino acid‐sensing mTORC1 signaling. Cell Rep 16: 1510–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Otsubo Y, Yamamato M (2008) TOR signaling in fission yeast. Crit Rev Biochem Mol Biol 43: 277–283 [DOI] [PubMed] [Google Scholar]
- 18. Kawai M, Nakashima A, Ueno M, Ushimaru T, Aiba K, Doi H, Uritani M (2001) Fission yeast tor1 functions in response to various stresses including nitrogen starvation, high osmolarity, and high temperature. Curr Genet 39: 166–174 [DOI] [PubMed] [Google Scholar]
- 19. Weisman R, Choder M (2001) The fission yeast TOR homolog, tor1+, is required for the response to starvation and other stresses via a conserved serine. J Biol Chem 276: 7027–7032 [DOI] [PubMed] [Google Scholar]
- 20. Matsuo T, Otsubo Y, Urano J, Tamanoi F, Yamamoto M (2007) Loss of the TOR kinase Tor2 mimics nitrogen starvation and activates the sexual development pathway in fission yeast. Mol Cell Biol 27: 3154–3164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hayashi T, Hatanaka M, Nagao K, Nakaseko Y, Kanoh J, Kokubu A, Ebe M, Yanagida M (2007) Rapamycin sensitivity of the Schizosaccharomyces pombe tor2 mutant and organization of two highly phosphorylated TOR complexes by specific and common subunits. Genes Cells 12: 1357–1370 [DOI] [PubMed] [Google Scholar]
- 22. Alvarez B, Moreno S (2006) Fission yeast Tor2 promotes cell growth and represses cell differentiation. J Cell Sci 119: 4475–4485 [DOI] [PubMed] [Google Scholar]
- 23. Uritani M, Hidaka H, Hotta Y, Ueno M, Ushimaru T, Toda T (2006) Fission yeast Tor2 links nitrogen signals to cell proliferation and acts downstream of the Rheb GTPase. Genes Cells 11: 1367–1379 [DOI] [PubMed] [Google Scholar]
- 24. Weisman R, Roitburg I, Schonbrun M, Harari R, Kupiec M (2007) Opposite effects of tor1 and tor2 on nitrogen starvation responses in fission yeast. Genetics 175: 1153–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Otsubo Y, Nakashima A, Yamamoto M, Yamashita A (2017) TORC1‐dependent phosphorylation targets in fission yeast. Biomolecules 7: 50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nakashima A, Otsubo Y, Yamashita A, Sato T, Yamamoto M, Tamanoi F (2012) Psk1, an AGC kinase family member in fission yeast, is directly phosphorylated and controlled by TORC1 and functions as S6 kinase. J Cell Sci 125: 5840–5849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nakashima A, Sato T, Tamanoi F (2010) Fission yeast TORC1 regulates phosphorylation of ribosomal S6 proteins in response to nutrients and its activity is inhibited by rapamycin. J Cell Sci 123: 777–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Otsubo Y, Yamashita A, Ohno H, Yamamoto M (2014) S. pombe TORC1 activates the ubiquitin‐proteasomal degradation of the meiotic regulator Mei2 in cooperation with Pat1 kinase. J Cell Sci 127: 2639–2646 [DOI] [PubMed] [Google Scholar]
- 29. Valbuena N, Guan KL, Moreno S (2012) The Vam6 and Gtr1‐Gtr2 pathway activates TORC1 in response to amino acids in fission yeast. J Cell Sci 125: 1920–1928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tsutsumi S, Sugiura R, Ma Y, Tokuoka H, Ohta K, Ohte R, Noma A, Suzuki T, Kuno T (2007) Wobble inosine tRNA modification is essential to cell cycle progression in G(1)/S and G(2)/M transitions in fission yeast. J Biol Chem 282: 33459–33465 [DOI] [PubMed] [Google Scholar]
- 31. Huang Y, Maraia RJ (2001) Comparison of the RNA polymerase III transcription machinery in Schizosaccharomyces pombe, Saccharomyces cerevisiae and human. Nucleic Acids Res 29: 2675–2690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Huang Y, Hamada M, Maraia RJ (2000) Isolation and cloning of four subunits of a fission yeast TFIIIC complex that includes an ortholog of the human regulatory protein TFIIICbeta. J Biol Chem 275: 31480–31487 [DOI] [PubMed] [Google Scholar]
- 33. Saha N, Schwer B, Shuman S (1999) Characterization of human, Schizosaccharomyces pombe, and Candida albicans mRNA cap methyltransferases and complete replacement of the yeast capping apparatus by mammalian enzymes. J Biol Chem 274: 16553–16562 [DOI] [PubMed] [Google Scholar]
- 34. Nurse P (1985) Mutants of the fission yeast Schizosaccharomyces pombe which alter the shift between cell proliferation and sporulation. Mol Gen Genet 198: 497 [Google Scholar]
- 35. Iino Y, Yamamoto M (1985) Negative control for the initiation of meiosis in Schizosaccharomyces pombe . Proc Natl Acad Sci USA 82: 2447–2451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. McLeod M, Beach D (1986) Homology between the ran1+ gene of fission yeast and protein kinases. EMBO J 5: 3665–3671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Beach D, Rodgers L, Gould J (1985) ran1+ controls the transition from mitotic division to meiosis in fission yeast. Curr Genet 10: 297–311 [DOI] [PubMed] [Google Scholar]
- 38. Shor B, Wu J, Shakey Q, Toral‐Barza L, Shi C, Follettie M, Yu K (2010) Requirement of the mTOR kinase for the regulation of Maf1 phosphorylation and control of RNA polymerase III‐dependent transcription in cancer cells. J Biol Chem 285: 15380–15392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Michels AA, Robitaille AM, Buczynski‐Ruchonnet D, Hodroj W, Reina JH, Hall MN, Hernandez N (2010) mTORC1 directly phosphorylates and regulates human MAF1. Mol Cell Biol 30: 3749–3757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kantidakis T, Ramsbottom BA, Birch JL, Dowding SN, White RJ (2010) mTOR associates with TFIIIC, is found at tRNA and 5S rRNA genes, and targets their repressor Maf1. Proc Natl Acad Sci USA 107: 11823–11828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Du W, Halova L, Kirkham S, Atkin J, Petersen J (2012) TORC2 and the AGC kinase Gad8 regulate phosphorylation of the ribosomal protein S6 in fission yeast. Biol Open 1: 884–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hopper AK (2013) Transfer RNA post‐transcriptional processing, turnover, and subcellular dynamics in the yeast Saccharomyces cerevisiae . Genetics 194: 43–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Van Horn DJ, Yoo CJ, Xue D, Shi H, Wolin SL (1997) The La protein in Schizosaccharomyces pombe: a conserved yet dispensable phosphoprotein that functions in tRNA maturation. RNA 3: 1434–1443 [PMC free article] [PubMed] [Google Scholar]
- 44. Ma N, Ma Y, Nakashima A, Kikkawa U, Furuyashiki T (2016) The loss of Lam2 and Npr2‐Npr3 diminishes the vacuolar localization of Gtr1‐Gtr2 and disinhibits TORC1 activity in fission yeast. PLoS ONE 11: e0156239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Iino Y, Yamamoto M (1985) Mutants of Schizosaccharomyces pombe which sporulate in the haploid state. Mol Cell Biol 198: 416 [DOI] [PubMed] [Google Scholar]
- 46. Watanabe Y, Shinozaki‐Yabana S, Chikashige Y, Hiraoka Y, Yamamoto M (1997) Phosphorylation of RNA‐binding protein controls cell cycle switch from mitotic to meiotic in fission yeast. Nature 386: 187–190 [DOI] [PubMed] [Google Scholar]
- 47. Iiboshi Y, Papst PJ, Kawasome H, Hosoi H, Abraham RT, Houghton PJ, Terada N (1999) Amino acid‐dependent control of p70(s6k). Involvement of tRNA aminoacylation in the regulation. J Biol Chem 274: 1092–1099 [DOI] [PubMed] [Google Scholar]
- 48. Kamada Y (2017) Novel tRNA function in amino acid sensing of yeast Tor complex1. Genes Cells 22: 135–147 [DOI] [PubMed] [Google Scholar]
- 49. Gutz H, Heslot H, Leupold U, Loprieno N (1974) Schizosaccharomyces pombe In Handbook of genetics, King RD. (ed), Vol. 1, pp 395–446. New York, NY: Plenum Publishing Corporation; [Google Scholar]
- 50. Moreno S, Klar A, Nurse P (1991) Molecular genetic analysis of fission yeast Schizosaccharomyces pombe . Methods Enzymol 194: 795–823 [DOI] [PubMed] [Google Scholar]
- 51. Maundrell K (1990) nmt1 of fission yeast. A highly transcribed gene completely repressed by thiamine. J Biol Chem 265: 10857–10864 [PubMed] [Google Scholar]
- 52. Basi G, Schmid E, Maundrell K (1993) TATA box mutations in the Schizosaccharomyces pombe nmt1 promoter affect transcription efficiency but not the transcription start point or thiamine repressibility. Gene 123: 131–136 [DOI] [PubMed] [Google Scholar]
- 53. Caspari T, Dahlen M, Kanter‐Smoler G, Lindsay HD, Hofmann K, Papadimitriou K, Sunnerhagen P, Carr AM (2000) Characterization of Schizosaccharomyces pombe Hus1: a PCNA‐related protein that associates with Rad1 and Rad9. Mol Cell Biol 20: 1254–1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kohda TA, Tanaka K, Konomi M, Sato M, Osumi M, Yamamoto M (2007) Fission yeast autophagy induced by nitrogen starvation generates a nitrogen source that drives adaptation processes. Genes Cells 12: 155–170 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Expanded View Figures PDF
Table EV1
Table EV2
Table EV3
Review Process File


