Abstract
The molecular immunologist's dream is to elucidate a fundamental biochemical process that explains the basis of an affliction that affects millions of people, and that, precisely understood, might yield a rational approach to diagnosis, prevention, or therapy. In this issue of JBC, Ting et al. report proteomic, biochemical, and structural analyses that better explain how the antigen-presenting HLA-DR4 molecules bind citrullinated peptides to provoke rheumatoid arthritis (RA), a chronic autoimmune disease that affects 0.5–1% of the population.
Introduction
RA2 is characterized clinically by inflammation of the synovia, the joint capsule membranes, leading to bone destruction. Risk factors for RA, in addition to genetic markers described below, include sex (women > men), age (usually 40–60), and smoking. Although the molecular basis for the disease is poorly defined, diagnosis is often confirmed by blood tests indicative of inflammation (1). Historically, the most important diagnostic indicator was rheumatoid factor, a set of antibodies directed against self-immunoglobulin (2). However, this test has given way to detection of antibodies to citrullinated protein antigens (ACPAs) (3), which are observed in about 70% of RA patients, and may be identified even before other symptoms appear. But, what are citrullinated protein antigens, and where do ACPAs come from? Read on…
The most important genetic locus contributing to RA (of dozens that have been identified), is human leukocyte antigen–antigen D-related (HLA-DR) of the HLA-class II region, at chromosome 6p21.3. The locus encodes cell-surface glycoproteins expressed primarily on antigen-presenting cells (APC) that include dendritic cells, B cells, and macrophages. The heterodimers encoded by HLA-DRA and HLA-DRB consist of the essentially invariant α chain (∼33–35 kDa, two expressed alleles identified) paired with a highly polymorphic β chain (∼26–28 kDa, more than 1500 known expressed alleles). The function of HLA-DR, elucidated by immunological and structural studies over the past several decades, is to bind peptides generated from endocytosed proteins and to redirect them to the APC surface where they are detected by CD4+ T cells. HLA-DR binds peptides of variable length, with a core sequence of nine amino acids, anchored by side chains at positions 1, 4, 6, and 9 that interact with protein pockets correspondingly designated P1, P4, P6, and P9 (4). Polymorphic residues in the HLA-DR β chain contribute to the binding characteristics of particular HLA-DR molecules and thus determine the sequence motif of the peptides bound. Peptide side chains exposed to solvent contribute to a peptide/HLA-DR molecular surface that is recognized by T cells bearing clonally expressed receptors that are activated to produce cytokines and proliferate. T cells strongly reactive to self-peptide/HLA-DR complexes are negatively selected during T cell development in the thymus, a process designated “self-tolerance,” but peptide/HLA-DR complexes bearing peptides from foreign proteins derived from tumors, infectious agents, dysregulated expression, or post-translational modification (PTM) of self-proteins may be recognized by T cells leading to immune activation. The paper by Ting et al. (5) expands our understanding of the link between the antibodies found in the serum of RA patients (ACPAs) and specific HLA-DR-encoded proteins, i.e. HLA-DR4 heterodimers employing related HLA-DR β chains, HLA-DRB1*04:01/*04:04/*04:05, that possess the same “shared epitope,” due to the common protein sequence Q(K/R)RAA at residues 70–74. This paper examines HLA-DRB1*04:05 in new detail and extends our understanding of the relationship of peptide/HLA-DR4 to RA by reporting eight new X-ray structures.
Previous studies had shown that a common feature of HLA-DR4 molecules associated with RA was their ability to bind peptides in which a native arginine residue had been post-translationally modified by deimination to citrulline. Thus, early models, confirmed by HLA-DR4 peptide-binding studies and X-ray structures, were consistent with a view that HLA-DRB1*04:01 and *04:04 bind and present citrullinated peptides (6–8). The activation of T cells with specificity for citrullinated peptide/HLADR4 complexes provided “help” to B lymphocytes favoring their production of anti-citrulline antibodies. The pathogenesis proposed was that in the setting of HLA-DR4 expression, systemic inflammatory insults (such as smoking) result in the production of peptidyl-arginine deiminases (PADs) that then catalyze the conversion of arginine to citrulline in various tissue proteins.
The work described by Ting et al. (5) advances our understanding of the contribution of citrullination to binding and presentation of peptides by extensive comparison of HLA-DRB1*04:01, *04:04, and *04:05. (These three differ from each other at only three residues—position 86 in P1, 71 in P4, and 57 in P9.) Earlier studies showed that the citrulline side-chain was accommodated by the basic P4 pocket of HLA-DRB1*04:01 and *04:04, but that HLA-DRB1*04:02 could also accommodate arginine at P4 (6). Here, these findings are extended in several ways. First, by mass spectrometry the authors identified some 2935 peptides that copurified with HLA-DRB1*04:05 and compared their nonamer core sequences with those previously described for HLA-DRB1*04:01 and *04:04. As expected, arginine is disfavored at peptide position 4. Strong preferences for peptide position 1 of tyrosine, phenylalanine, and isoleucine were noted; at position 6 of aspartic acid, asparagine, and threonine; and at position 9 of the acidic residues, aspartic acid and glutamic acid. Further comparisons with earlier work on HLA-DRB1*04:01 and *04:04 indicated that *04:05 was similar in the selection of peptides with position 1 to HLA-DRB1*04:01 corresponding to similarity of the P1 pocket. The selected peptides differed at position 9 consistent with P9 difference. When compared with DRB1*04:04, *04:05 selected peptides that differed at both 1 and 9.
An extensive series of quantitative binding experiments using some 34 native and citrullinated peptides to interrogate the affinity of the three allomorphs was then undertaken. Citrullinated peptides with the citrulline at position 4 consistently bound better than the homologous peptide with arginine at that position, and citrullination at other positions (2 and 3) had little effect. Differences in affinity of peptides for HLA-DRB1*04:01, *04:04, and *04:05 could be accounted for largely by differences in amino acid preferences of the different allomorphs for peptide positions 1 and 9, generally consistent with differences in the P1 and P9 pockets.
To clarify that the peptide binding preferences of the three DR4 allomorphs were due to differences in the specificities imposed by the pockets that interact with amino acids 1 and 9, eight new HLA-DRB1*04 structures, including five of DRB1*04:01 with different citrullinated peptides, two of DRB1*04:04 with two different citrullinated peptides, and one with DRB1*04:05 and a citrullinated peptide were determined crystallographically, revealing that position 4 citrulline is bound canonically by residues of the corresponding P4 pocket in each of the three allomorphs. The key role played by the P4 pocket of HLA-DRB1*04:05 in binding a citrullinated vimentin-derived peptide is illustrated in Fig. 1.
Figure 1.
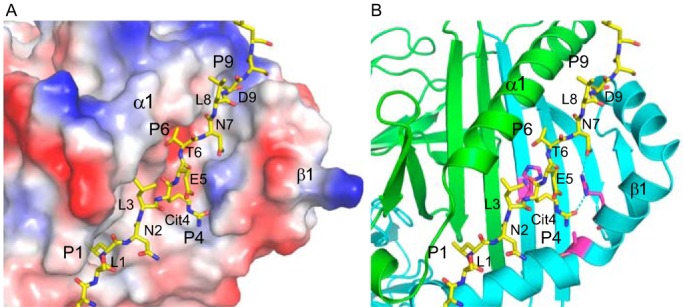
Coordination of citrulline at peptide position 4 with P4 pocket of HLA-DRB1*04:05. The X-ray structure (PDB entry: 6BIR) of the complex of HLA-DRB1*04:05 with the vimentin-421Cit(415–531) peptide (SSLNLXETNLDSL, in which X = citrulline, and the core nine-mer is underlined) is illustrated with the peptide in stick configuration and the HLA-DR in electrostatic surface representation (A) and showing HLA-DR α chain in green, HLA-DRB1*04:05 β chain in cyan, with key residues of the P4 pocket, His-13, Arg-71, and Ala-74 in magenta (B). HLA-DRA α1 domain and HLA-DRB1 β1 domain are indicated.
Although many more details of the effects of citrulline in non-anchor positions, or of particular amino acids throughout the peptide, may be gleaned from these extensive studies, the main points are clear—PTM by deimination of arginine, if this is in a position that can be accommodated in the P4 binding pocket (characterized by β71-lysine or -arginine and β13-histidine), will lead to binding by HLA-DRB1*04:01, *04:04, or *04:05. Differences in the affinity of binding and thus the specificity of the different allomorphs can be attributed to polymorphic residues at P1 and/or P9 pockets that exert differential specificity.
Although HLA-DRB1*04:01, *04:04, or *04:05 clearly has a propensity to bind and present citrullinated peptides due to the charge and size of the P4 pocket, absolute prediction of the affinity of peptide binding and the frame of the peptide presentation remains an imprecise venture. Understanding the rank order of peptide binding to particular HLA-DRB1 allomorphs, and, thus, extracting rational and possibly antigen-specific approaches to RA therapy, demands further experimentation. Perhaps reagents that could selectively interact with P4 or modify its binding specificity would change the propensity for interaction with citrullinated peptides—thus shifting the inflammatory balance in RA.
Acknowledgments
I thank Drs. P. Cohen, K. Natarajan, M. Greene, and D. L. Vogel for their thoughts and discussion.
The author declares that he has no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- RA
- rheumatoid arthritis
- ACPA
- anti-citrullinated protein antibody
- APC
- antigen-presenting cells
- MHC
- major histocompatibility complex
- PAD
- peptidyl-arginine deiminase
- PTM
- post-translational modification.
References
- 1. Aletaha D., Neogi T., Silman A. J., Funovits J., Felson D. T., Bingham C. O. 3rd, Birnbaum N. S., Burmester G. R., Bykerk V. P., Cohen M. D., Combe B., Costenbader K. H., Dougados M., Emery P., Ferraccioli G., et al. (2010) 2010 Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 62, 2569–2581 10.1002/art.27584 [DOI] [PubMed] [Google Scholar]
- 2. Pike R. M., Sulkin S. E., and Coggeshall H. C. (1949) Concerning the nature of the factor in rheumatoid-arthritis serum responsible for increased agglutination of sensitized sheep erythrocytes. J. Immunol. 63, 447–463 [PubMed] [Google Scholar]
- 3. Schellekens G. A., de Jong B. A., van den Hoogen F. H., van de Putte L. B., and van Venrooij W. J. (1998) Citrulline is an essential constituent of antigenic determinants recognized by rheumatoid arthritis-specific autoantibodies. J. Clin. Invest. 101, 273–281 10.1172/JCI1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stern L. J., Brown J. H., Jardetzky T. S., Gorga J. C., Urban R. G., Strominger J. L., and Wiley D. C. (1994) Crystal structure of the human class II MHC protein HLA-DR1 complexed with an influenza virus peptide. Nature 368, 215–221 10.1038/368215a0 [DOI] [PubMed] [Google Scholar]
- 5. Ting Y. T., Petersen J., Ramarathinam S. H., Scally S. W., Loh K. L., Thomas R., Suri A., Baker D. G., Purcell A. W., Reid H. H., and Rossjohn J. (2018) The interplay between citrullination and HLA-DRB1 polymorphism in shaping peptide binding hierarchies in rheumatoid arthritis. J. Biol. Chem. 293, 3236–3251 10.1074/jbc.RA117.001013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Scally S. W., Law S. C., Ting Y. T., Heemst J. V., Sokolove J., Deutsch A. J., Bridie Clemens E., Moustakas A. K., Papadopoulos G. K., van der Woude D., Smolik I., Hitchon C. A., Robinson D. B., Ferucci E. D., Bernstein C. N., et al. (2017) Molecular basis for increased susceptibility of indigenous North Americans to seropositive rheumatoid arthritis. Ann. Rheum. Dis. 76, 1915–1923 10.1136/annrheumdis-2017-211300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Klareskog L., Stolt P., Lundberg K., Källberg H., Bengtsson C., Grunewald J., Rönnelid J., Harris H. E., Ulfgren A. K., Rantapää-Dahlqvist S., Eklund A., Padyukov L., and Alfredsson L. (2006) A new model for an etiology of rheumatoid arthritis: Smoking may trigger HLA-DR (shared epitope)-restricted immune reactions to autoantigens modified by citrullination. Arthritis Rheum. 54, 38–46 10.1002/art.21575 [DOI] [PubMed] [Google Scholar]
- 8. Malmström V., Catrina A. I., and Klareskog L. (2017) The immunopathogenesis of seropositive rheumatoid arthritis: From triggering to targeting. Nat. Rev. Immunol. 17, 60–75 10.1038/nri.2016.124 [DOI] [PubMed] [Google Scholar]


