Abstract
Prion diseases are a group of fatal neurodegenerative diseases associated with a protein-based infectious agent, termed prion. Compelling evidence suggests that natural transmission of prion diseases is mediated by environmental contamination with infectious prions. We hypothesized that several natural and man-made materials, commonly found in the environments of wild and captive animals, can bind prions and may act as vectors for disease transmission. To test our hypothesis, we exposed surfaces composed of various common environmental materials (i.e. wood, rocks, plastic, glass, cement, stainless steel, aluminum, and brass) to hamster-adapted 263K scrapie prions and studied their attachment and retention of infectivity in vitro and in vivo. Our results indicated that these surfaces, with the sole exception of brass, efficiently bind, retain, and release prions. Prion replication was studied in vitro using the protein misfolding cyclic amplification technology, and infectivity of surface-bound prions was analyzed by intracerebrally challenging hamsters with contaminated implants. Our results revealed that virtually all prion-contaminated materials transmitted the disease at high rates. To investigate a more natural form of exposure to environmental contamination, we simply housed animals with large contaminated spheres made of the different materials under study. Strikingly, most of the hamsters developed classical clinical signs of prion disease and typical disease-associated brain changes. Our findings suggest that prion contamination of surfaces commonly present in the environment can be a source of disease transmission, thus expanding our understanding of the mechanisms for prion spreading in nature.
Keywords: neurodegeneration, neurodegenerative disease, prion, prion disease, protein misfolding, Creutzfeldt-Jakob disease, chronic wasting disease, scrapie
Introduction
Transmissible spongiform encephalopathies, also known as prion diseases, are infectious and invariably fatal neurological disorders affecting various species of mammals, including sheep, deer, cattle, and humans (1, 2). The hallmarks of transmissible spongiform encephalopathies include spongiform degeneration of the brain and the accumulation of an infectious agent termed prion, which is composed of a misfolded version (PrPSc)2 of the physiological prion protein (PrPC) (3). Among the atypical features of PrPSc as an infectious agent (for reviews, see Refs. 2–4), it is possible to highlight its ability to bind to a wide variety of surfaces and to be highly resistant to conventional decontamination procedures. In humans, these properties have been linked to the iatrogenic transmission of Creutzfeldt-Jakob disease (CJD) through the use of contaminated electrodes or surgical materials (5, 6). Although exposure through the environment is believed to play an equivalent role in the transmission of animal diseases (7–11), the effect of prion interaction with specific surfaces on its infectivity remains largely undetermined.
The transmission of natural scrapie in sheep and goats and chronic wasting disease (CWD) in free-ranging deer has received considerable attention because of their extensive prevalence and elusive zoonotic potential (12, 13). Several lines of evidence have shown that environmental contamination may play an important role in prion transmission in these natural animal diseases. For example, farms containing scrapie-infected sheep have been recognized to pose a long-standing threat to naïve animals, even for as many as 16 years after removing the sick animals and careful decontamination (7–10). Also, prion disease can be transmitted by housing an asymptomatic prion-infected animal with naïve animals (14). Several studies have indicated that prions may enter the environment through different sources, including decaying carcasses, placenta, saliva, feces, and urine (for a review, see Ref. 15). Prions shed by sick and infected, but presymptomatic, animals may attach and accumulate on different surfaces that may subsequently transmit disease to naïve individuals. Various experimental (6, 8, 16–22), epidemiological, and anecdotal data (5, 10) suggest that prions attached to surfaces can persist for extended periods of time and remain infectious to appropriate hosts. Among the different environmental materials postulated to act as vectors for disease transmission, soil particles have been widely studied due to their high affinity to bind infectious prions (for reviews, see Refs. 23 and 24) and the significant quantities naturally ingested by ruminants. Importantly, prions bound to soil have been shown to remain infectious for prolonged periods of time (16, 19), and some reports even suggested that prion binding to soil increases infectivity (18). Besides soil, various other elements of the environment also have been proposed to act as vectors for disease transmission, including living organisms (such as plants (25), insects (26), and scavengers (27)) and inanimate objects (such as wood, plastic, and metals (22)).
Despite the reports showing prion interaction with various surfaces, prion binding and potential for disease transmission of different natural and man-made materials commonly present in the environment have not been comprehensively analyzed. The purpose of this study was to compare surfaces composed of different materials for their ability to bind, retain, and release PrPSc as well as to evaluate the infectious properties of prions bound to those materials. Our results show that diverse materials, including natural (rocks and wood) and man-made (stainless steel, plastic, glass, and aluminum) surfaces, efficiently bind and retain infectious prions. Strikingly, surfaces exposed to prions transmitted disease by simply placing them in the animal's cage, indicating that casual exposure to a prion-contaminated surface may result in infection. In contrast, brass surfaces showed a consistently poorer interaction with prions under several experimental conditions. Collectively, our results increase the mechanistic understanding of prion transmission in nature and the putative role of the environment while providing information necessary for establishing future guidelines and regulations to help prevent the spread of prion disease in farming and clinical settings.
Results
Binding and release of prions from environmental surfaces
To begin exploring the interaction of prions to diverse surfaces, we analyzed PrPSc binding to materials relevant in farming and/or clinical settings, including stainless steel, aluminum, brass, polypropylene, glass, cement, wood, and rocks. To obtain quantitative binding data, we used highly purified PrPSc from hamster brains infected with 263K hamster-adapted scrapie prions. For these studies, we radiolabeled the protein with 125-Iodine, a method that we have extensively used in previous studies and shown not to alter the biological and biochemical properties of PrPSc (28–31). Small beads (0.32 cm in diameter) made of different materials showed significant differences in their ability to capture purified/radiolabeled proteinase K (PK)-resistant PrPSc (125I-PrPSc) on their surfaces (Table 1). Specifically, cement was the most efficient material with 15.6% of the total 125I-PrPSc remaining bound to the beads after overnight incubation. Other materials that efficiently bound 125I-PrPSc were wood and rock with 3.6 and 1.6% of the initial material remaining bound to the surface, respectively. Aluminum, polypropylene, stainless steel, and glass also bound 125I-PrPSc but with lower efficiencies ranging from ∼0.15 to 0.56% (Table 1). Interestingly, surfaces made of brass did not show any apparent 125I-PrPSc binding.
Table 1.
Binding and release of radiolabeled PrPSc from different materials
Values correspond to the average ± S.E. of two measurements and were normalized by acid precipitation for 125I-PrPSc to remove any radioactivity coming from free iodine-125. The values were estimated considering 100% the total amount of radioactive PrPSc added corrected for the experimentally estimated amount of free iodine bound to each of the beads. For initial binding (column 1), liquid was thoroughly removed (without washes), and material remaining bound to the beads was measured by radioactivity counting. The amount of radioactive remaining bound after five washes was experimentally measured and expressed as a percentage of the total amount of PrPSc loaded (column 2). The percentage of PrPSc released after washes (column 3) represents the amount of PrPSc released in all five washes expressed as a percentage of the amount of protein bound. The total amount released (column 4) corresponds to the percentage of PrPSc released from a surface after washing expressed as a percentage of the total amount of radioactive PrPSc added to the surface. This was estimated by mathematical calculation from the initial binding. ND, not detectable.
| Materials | Binding to surfaces |
Release from surfaces |
||
|---|---|---|---|---|
| Initial binding | Binding after washes | PrPSc released after washes | Total amount released | |
| % | % | |||
| Aluminum | 0.56 ± 0.05 | 0.42 ± 0.01 | 16.8 ± 3.4 | 0.094 |
| Brass | ND | ND | ND | ND |
| Cement | 15.6 ± 1.89 | 13.74 ± 1.77 | 8.6 ± 0.5 | 1.34 |
| Glass | 0.15 ± 0.04 | ND | 100.0 ± 14.3 | 0.15 |
| Polypropylene | 0.53 ± 0.03 | 0.38 ± 0.03 | 53.7 ± 0.8 | 0.28 |
| Rock | 1.6 ± 0.43 | 1.33 ± 0.08 | 29.6 ± 5.8 | 0.47 |
| Stainless steel | 0.22 ± 0 | ND | 100.0 ± 13.2 | 0.22 |
| Wood | 3.6 ± 0.76 | 3.57 ± 0.33 | 0.0 ± 15.1 | 0.0 |
The potential of a specific surface to act as a vector for prion transmission likely lies in its capacity to bind and subsequently release the infectious agent, which in turn depends on the nature of the material. To study the release of 125I-PrPSc from the surfaces, we performed five sequential washes with phosphate-buffered saline (PBS) of the contaminated surfaces (Table 1). Our analyses showed that stainless steel and glass released most of the bound 125I-PrPSc after serial washes with 85.3 and 67.8% of total material released after the first wash, respectively, and 100% released after the five washes. Polypropylene, rocks, and aluminum also released a high proportion of bound material (53.7, 29.6, and 16.8%, respectively) with most of it being released during the first washing. Cement and wood poorly released bound 125I-PrPSc with values of 8.6 and 0.0%, respectively (Table 1). As expected, no radioactivity was detected for washes involving 125I-PrPSc–treated brass. Taking into account PrPSc binding and release, we concluded that cement, wood, and rocks, perhaps the most environmentally relevant man-made and natural surfaces, were the materials that retained the largest amounts of PrPSc bound to them (Table 1).
Prions bound to a variety of inert surfaces are able to template PrPC to PrPSc conversion
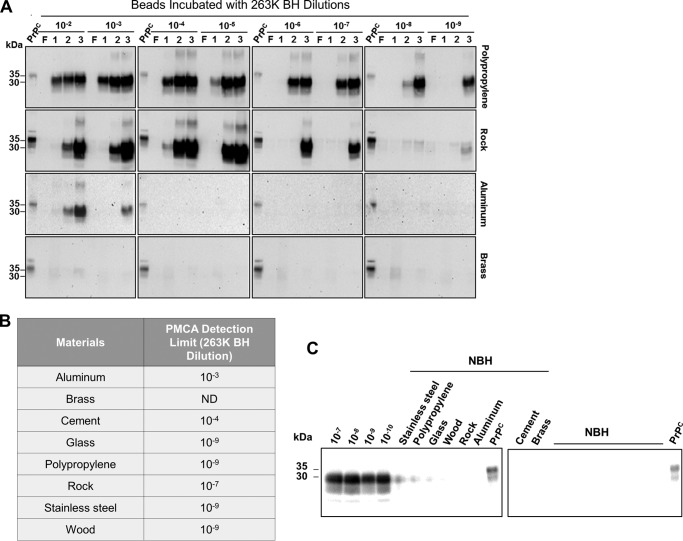
Protein misfolding cyclic amplification (PMCA) has a very high sensitivity to detect PrPSc and can be used in a broad variety of samples, including tissue homogenates and biological fluids as well as inert and biological surfaces (32, 33). To assess the ability of surface-bound prions to self-propagate, beads were incubated with serially diluted 263K prion–infected brain homogenate, extensively washed and air-dried, and then used to seed PMCA as described previously (32). Although the efficiency of prion replication was variable depending on the material tested, all surfaces, with the sole exception of brass, contained seeds competent for inducing PrPC misfolding (Fig. 1 and Fig. S1). Prion-contaminated polypropylene, glass, stainless steel, and wood beads showed the highest potential to retain seeds suitable for PMCA reactions because positive results were obtained with up to a 10−9 dilution of the 263K-affected brain extract (Fig. 1B). Contaminated rocks seeded PMCA reactions only when incubated with brain preparations diluted up to 10−7-fold. Cement and aluminum beads exhibited less potential as prion carriers because they were unable to sustain in vitro prion replication after being contaminated with brain dilutions higher than 10−4 and 10−3, respectively (Fig. 1B). Surprisingly, there was not a clear relationship between PrPSc binding and/or release from surfaces and their ability to retain prions competent for in vitro conversion of PrPC (compare Table 1 and Fig. 1B). Control experiments incubating each of the materials with normal hamster brain homogenate did not show any protease-resistant PrPSc signal, even after three rounds of PMCA (Fig. 1C).
Figure 1.
Ability of PrPSc attached to different surfaces to sustain in vitro prion replication. Serial dilutions of 263K brain homogenate (10−2–10−9) were incubated with either polypropylene, glass, stainless steel, wood, rock, cement, aluminum, or brass beads (0.32 cm diameter) for 16 h at room temperature. Water-washed and dried beads were mixed with PMCA substrate, and the presence of resulting PrPSc attached to each material was detected by PMCA. A, results of the polypropylene, rock, aluminum, and brass beads are shown as a representation of the different degrees of PMCA data obtained. Results with the rest of the materials are shown in Fig. S1. F, non-amplified control; 1, 2, and 3, PMCA rounds. B, summary of the last detectable dilution for each surface. ND, not detectable. C, as controls, 10% normal brain homogenate (NBH) was incubated with beads made of the different materials. After washing and drying, beads were added to PMCA tubes, and the reaction was carried out for three consecutive rounds of amplification. The blot displays representative results of the third PMCA round. As positive controls of PMCA amplification, various dilutions of 263K brain homogenate (10−7–10−10) are shown. In A and C, all samples, except for PrPC used as a migration control, were PK-treated. Numbers at the left of the blots indicate molecular mass markers. Data shown in this figure correspond to representative results obtained in two different experiments.
Previous data have shown that only 5 min of contact with prions is required for a stainless steel wire to become efficiently contaminated and able to transmit disease (21). Because exposure time of environmental materials to prions in nature conceivably fluctuates, we assessed the minimum time needed for surfaces to bind infectious prions suitable for PMCA amplification. Because of its high efficiency to bind seeding-competent PrPSc (Fig. 1), we chose to incubate polypropylene beads with a 10−7 dilution of 263K-infected brain homogenate for different time points ranging from 2 min to 16 h. Our results show that after only 2 min prions were bound to the polypropylene beads and were sufficient to plateau prion amplification by PMCA (Fig. S2).
To determine whether the nature of the different materials affected the PMCA assay, we prepared reactions containing distinct amounts of 263K prions that were supplemented with untreated beads of the different materials tested (Fig. 2). We observed that the presence of some materials such as aluminum, rock, cement, wood, and brass substantially affected PMCA efficiency when they were spiked with a 10−7 eq of 263K-containing brain homogenate. The interference was even more evident when PMCA reactions were seeded using a 10−9 dilution of the same infectious brain homogenate. In fact, the latter set of experiments showed that all materials partially affected PMCA amplification (as seen in the second PMCA round). The large negative influence of some surfaces (e.g. cement and aluminum) on the PMCA reaction may explain why these materials have a lower level of detection by PMCA (Fig. 1B), considering that these materials (especially cement) show high binding and release of PrPSc (Table 1).
Figure 2.
Interference of different materials on PMCA reactions. A, aliquots of PMCA substrate were spiked with different dilutions of 263K-infected brain homogenates (10−5–10−12) and submitted to incubation/sonication cycles. Additional aliquots of PMCA substrate were spiked with two high dilutions of 263K–infected brains (10−7 (B) and 10−9 (C)), mixed with untreated beads (0.32 cm in diameter) made of different materials, and immediately submitted to PMCA. Serial PMCA rounds were performed as described under “Experimental procedures.” All samples were PK-treated with the sole exception of PrPC used as a control for the electrophoretic mobility. Numbers at the left of each membrane depict molecular mass markers. Data shown in this figure correspond to representative results obtained in two different experiments.
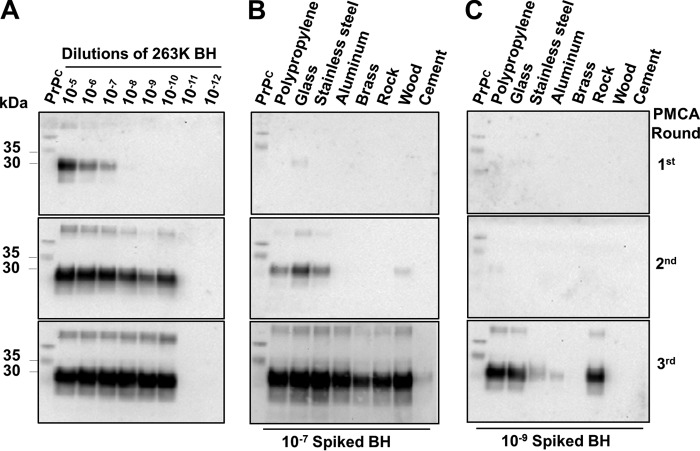
To investigate whether prions from other species are also able to bind to various materials and retain in vitro prion replication activity, we incubated the same surfaces with brain homogenate coming from RML prion–infected mice as well as natural prions of important biological and medical relevance (CWD from cervids and vCJD from humans). For these experiments, dilutions of brain homogenate from infected individuals (10−3, 10−5, and 10−7) were incubated for 16 h with beads composed of the distinct materials. After washing and drying, beads were used for PMCA in brain substrate from wildtype mice or transgenic mice expressing deer or human PrPC. As observed before with 263K, PrPSc derived from RML, CWD, and vCJD was able to attach to diverse materials and seed prion replication (Fig. 3). Although some common general trends were observed for the interaction of different prions with diverse materials, there were also some intriguing specific differences. The greatest interaction for all prions was seen with polypropylene, stainless steel, and wood, whereas the lowest was observed with brass (Figs. 1A and 3). Surprisingly, mouse RML prions did not appear to interact with materials made of cement, whereas vCJD prions showed a high interaction with these surfaces. Although vCJD and CWD prions attached to aluminum beads retained highly efficient prion replication, this was not the case for 263K hamster prions.
Figure 3.
Interaction of rodent, cervid, and human prions with different materials. To investigate whether prions from other species also interact with the materials under study, serial dilutions of RML, vCJD, and CWD brain homogenates (10−3, 10−5, and 10−7) were incubated either with polypropylene, glass, stainless steel, wood, rock, cement, aluminum, or brass beads (0.32 cm in diameter) for 16 h at room temperature. After thorough washing and drying, beads were mixed with respective PMCA substrate (wildtype mice or transgenic mice expressing human or deer PrPC) and subjected to three consecutive rounds of PMCA. Samples were analyzed by Western blotting using 6D11 or PRC1 anti-PrP antibody, and results of the third round of PMCA are shown. All samples were PK-treated with the sole exception of PrPC used as a control for the electrophoretic mobility. Numbers at the left of each membrane depict molecular mass markers. Numbers at the right indicate the dilution of brain homogenate used to incubate the beads.
Infectivity of prions bound to different surfaces
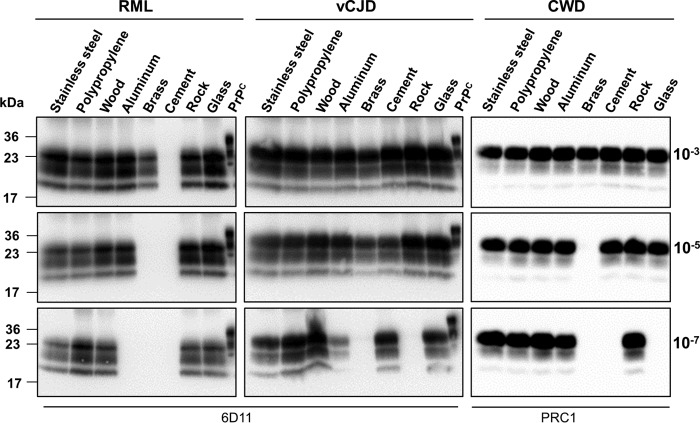
Because PrPSc binds surfaces and maintains its capacity to replicate in vitro, we evaluated the potential of the contaminated surfaces to cause prion disease. To explore this possibility, naïve Syrian hamsters were intracerebrally challenged with wire-shaped implants made of the same materials described in Fig. 1. It is important to note that, as in the in vitro experiments, surfaces were thoroughly washed and dried before being implanted into the animals. Hamsters inoculated with prions attached to polypropylene, aluminum, glass, stainless steel, and rocks manifested disease with a complete attack rate and at similar, relatively short, incubation periods (Fig. 4, A and B). A complete attack rate, albeit with a longer incubation period, was observed for hamsters treated with cement and wood implants. Surprisingly, considering the binding studies and the PMCA results, contaminated brass surfaces were also able to transmit disease but with an incomplete attack rate and a much longer incubation period (Fig. 4, A and B). All symptomatic animals showed classical clinical signs of 263K prions and harbored protease-resistant PrPSc in their brains (Fig. 4C), neither of which were observed in the control animals exposed to implants incubated with healthy brain homogenate or the single non-symptomatic animal from the brass contaminated group (sacrificed 500 days after treatment). The results obtained in the infectivity bioassay clearly show that surfaces made of different inert materials can act as reservoirs for infectious prions.
Figure 4.
In vivo assessment of prion transmission through contaminated surfaces implanted in the brain. The figure shows the survival curves (A), average incubation times and attack rates (B), and analysis of protease-resistant PrPSc (C) for hamsters intracerebrally (ic) challenged with implants exposed to 263K brain homogenates. Animals treated with implants incubated with normal brain homogenates (NBH) were also included as controls. All samples in C, except PrPC used as a migration control, were PK-treated. Numbers on the left of the blots represent molecular mass markers.
Animals can be infected by simple contact with prion-contaminated surfaces
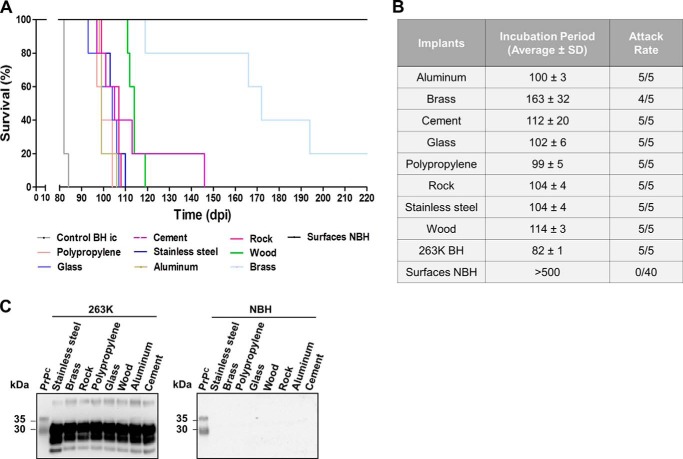
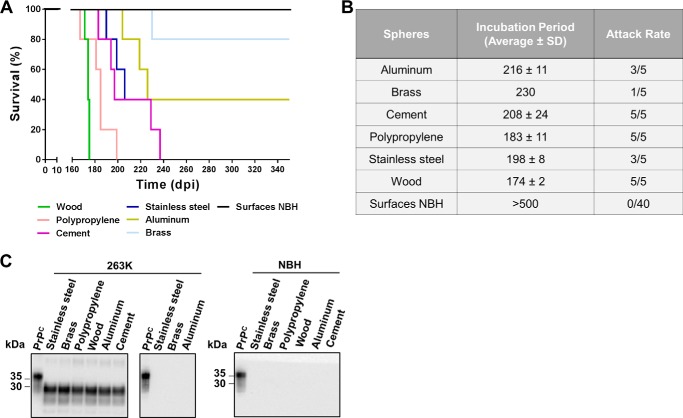
To mimic a more natural situation for disease transmission by environmental components, we contaminated large spheres (5.08 cm in diameter) with 263K prions and placed them in the cage of naïve experimental subjects for 4 weeks. Remarkably, many animals succumbed to prion disease when exposed to prions bound to various surfaces (Fig. 5, A and B). A complete attack rate was found for animals exposed to contaminated wood, polypropylene, and cement with average incubation periods ranging between 174 and 208 days. Stainless steel and aluminum contaminated spheres affected only 60% of the exposed animals with similar incubation periods as the aforementioned materials. In agreement with the previous bioassay, brass was the least efficient surface to transmit disease, affecting only one of five of the exposed animals. Unfortunately, we could not test materials made of rocks or glass because spheres of comparable size and dimensions were unavailable. As controls, spheres made by of same materials were exposed to brain extracts from healthy hamsters and placed in the cages of animals following the same schedule described for contaminated surfaces. As expected, protease-resistant PrPSc was not detected in any of the non-symptomatic subjects or the control animals similarly exposed to spheres treated with healthy brain homogenates (Fig. 5C). Altogether, these results indicate that animals can be effectively infected by simple exposure to prion-contaminated surfaces, but susceptibility did not correlate invariably with infectivity produced by intracerebral injection of the same contaminated material.
Figure 5.
Disease transmission by simple exposure to prion-contaminated surfaces. The figure shows the survival curves (A), average incubation times and attack rates (B), and biochemical assessments of protease-resistant PrPSc (C) for hamsters exposed to large spheres contaminated with 263K prions. Animals in contact with spheres exposed to normal brain homogenates (NBH) were also included as controls. The middle panel in C depicts brains from animals exposed to 263K-contaminated spheres but not developing clinical signs. All samples, except PrPC used as a migration control, were PK-treated. Numbers at the left of the blots in C represent molecular mass markers.
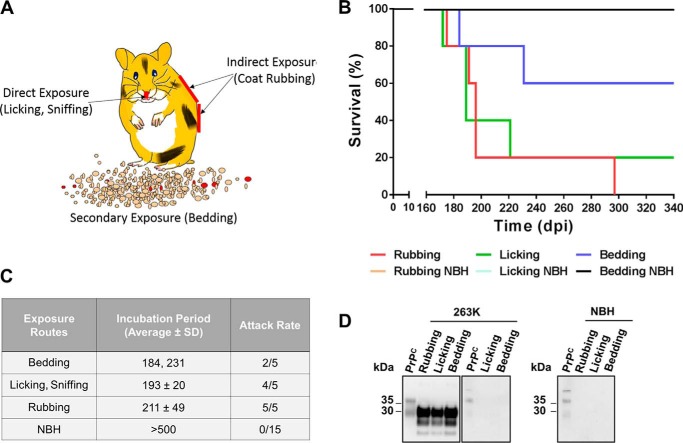
The route of infection for the prions bound to these large spheres is unknown, but we can envision at least three possibilities (Fig. 6A). (i) Prions may go directly from the surface to the animals and enter through the mucosal membranes of the nose and mouth when hamsters lick and smell the sphere. (ii) Prions may first move from the surface to the outside of the animals' body; for example, when hamsters rub their coats against the contaminated sphere. (iii) Prions may be transferred from the contaminated surface to other elements in the cage (e.g. bedding). To analyze the contribution of these different routes, we simulated animal rubbing and licking against contaminated surfaces as well as shedding of prions from contaminated materials to the bedding. For these experiments, we used polypropylene spheres because they are efficient disease vectors and chemically stable in aqueous environments. Animals rubbed against contaminated spheres showed the most efficient transmission of prion disease with an incubation period of 211 ± 49 days post-treatment and a complete attack rate (Fig. 6, B and C). Animals licking contaminated surfaces showed similar incubation periods (193 ± 20 days post-treatment) although with an incomplete attack rate (80%). Indirect contact with the prion-contaminated surface through bedding caused disease in two of five animals with incubation periods of 184 and 231 days post-treatment, suggesting that prions bound to one inert surface can actually spread to another. Non-symptomatic animals or control animals similarly exposed to spheres incubated with healthy brain homogenates did not show prion disease up to 500 days after treatment (Fig. 6C). Symptomatic animals showed the expected accumulation of protease-resistant PrPSc, whereas animals exposed to spheres incubated with normal brain homogenate did not show any PrPSc by Western blotting (Fig. 6D). Animals that were exposed to contaminated surfaces but did not develop clinical disease were negative for PrPSc by Western blotting (Fig. 6D, middle panel).
Figure 6.
Mimicking different modes of prion exposure to contaminated surfaces. A, schematic representation of three potential routes by which prions attached to large spheres may get access to the animals: (i) direct exposure through licking or sniffing the materials, (ii) indirect exposure through rubbing against the surfaces, and (iii) secondary exposure through transmission of prions from the surface to other components of the environment (e.g. bedding). Survival curves (B), average incubation periods and attack rates (C), and biochemical analysis (D) of protease-resistant PrPSc for hamsters exposed to 263K-contaminated polypropylene spheres mimicking rubbing, licking, and shedding of prions to bedding are shown. Data of animals challenged with spheres treated with normal brain extracts (NBH) are also included as controls. Analysis of 263K-treated asymptomatic animals is also shown (middle panel in D). All samples, except PrPC used as migration control, were PK-treated. Numbers at the left of blots in D represent molecular mass markers.
Discussion
The mechanisms and routes responsible for the spread of natural prion diseases are unknown, but compelling evidence suggests that, at least in the case of animal diseases, environmental prion contamination plays an important role (7, 15, 24). Prion diseases have asymptomatic incubation periods lasting months, years, and even decades before clinical signs are observed. Therefore, infected animals are expected to release prions into the environment for extended periods of time where the infectious agent could accumulate because of its high resistance to inactivation and elimination (24). Progressive buildup of prions in the environment may result in exposure to increasingly larger quantities of infectious material, potentially leading to higher attack rates of exposed animals and perhaps also to greater chances for interspecies transmission. Because only limited data are currently available regarding how living and non-living objects could serve as vectors, an outstanding issue in the prion field is to elucidate which elements of the environment may be implicated in prion transmission. The animal diseases most likely to be influenced by environmental prion transmission are scrapie in sheep and CWD in cervids. Although some progress has been obtained in reducing traditional scrapie through selective breeding for resistant genotypes, the disease is still endemic in many countries (34). A more worrisome scenario is presented by CWD where the disease continues to spread geographically (35), currently affecting 24 states in the United States and two Canadian provinces and recently detected in a new cervid species in Norway (36).
Soil is perhaps the environmental element most studied for its interaction with prions (23, 24). Previous results showed that prions can bind various types of soil and remain infectious even after many years attached to soil (16–19). Given the potentially large role of environmental contamination in prion transmission, it is surprising that not many studies have been done to investigate the interaction of prions with other important elements of the environment. In this sense, Maddison et al. (7) reported that a scrapie-affected sheep farm had a widespread environmental contamination with prions, including seven of nine positive environmental swab samples taken from different surfaces (i.e. metal, plastic, and wood). The main goal of our study was to evaluate in a comprehensive manner the ability of prions to interact with various man-made and natural environmental materials. The studies were done using various methodologies to examine specifically the binding and release of PrPSc from diverse materials and the ability of prions bound to the surfaces to sustain the pathological conversion of PrPC in vitro and to transmit the disease in vivo. The in vivo infectivity experiments were done in a proof-of-concept paradigm by direct intracerebral implantation of contaminated materials as well as through a more relevant setting by simply exposing animals to prion-contaminated surfaces. The main conclusion of our study is that prions efficiently bind to surfaces composed of most of the materials, and PrPSc bound to them retains the ability to replicate in vitro and induce disease in vivo. Prion binding, retention, and release were confirmed for stainless steel, aluminum, polypropylene, glass, cement, wood, and rocks. The findings with wood and rocks are particularly worrisome because these materials are very abundant natural components in the woodlands and pastures of sheep and deer. The potential for a large contamination of rocks, wood, soil, and plants (23–25) suggests a major problem of environmental prion contamination in areas with a massive presence of prion-infected animals. Surprisingly, brass, a metal alloy composed of copper and zinc, showed poor efficiency to bind and retain infectious prions, thus representing a material that should be considered for surfaces known to be highly exposed to infectious prions. Nevertheless, even brass surfaces exposed to prions were able to transmit the disease experimentally, albeit with lower attack rates and longer incubation periods. Aside from brass, we were unable to rank the transmission potential of each material due to the variability between assays. Such variability can be attributed to different specific limitations of the experimental systems utilized. For example, the binding experiments using purified, radiolabeled PrPSc utilized a protein lacking the first 90 amino acids (a region that has been shown to play an important role in PrPC function and that can bind several ligands, including metal ions (37)). In contrast, the other procedures utilized the full-length protein. Besides, confounding degrees of interference for the materials on the efficiency of PMCA and potentially differing biological stabilities of the materials over time (especially when testing in vivo) may also introduce variability among distinct assays. The overall conclusion of our experiments is that distinct surfaces differ in their capacity to interact with infectious prions, which in turn may depend on the chemical properties of the material. Further studies need to be performed to understand how surface chemistry affects prion interaction. Knowledge gained from such studies might be useful to devise materials that may not attach to or retain infectious prions.
A particularly intriguing result coming from our study is that animals can be efficiently infected by simple exposure to prion-contaminated surfaces. This setting probably represents a situation closer to the environmentally mediated natural transmission of prions. Interestingly, our results indicate that infection rates and incubation times by this route are similar to our previous oral gavage bioassays (25). Although ingestion of surface-bound prions is the most likely route of environmental prion transmission, our data indicate that transmission can also occur by physical contact through rubbing or licking. Rubbing showed the greatest potential to infect, which may provide support for previous reports suggesting that scratching of sheep and goats on various surfaces in farms may be a primary route of transmission (8). Still the question remains of how prions move from the outside coat into the animal tissue. One plausible explanation is that prions shed from the contaminated surface to the coat of the animal will be ingested later while animals groom themselves or each other. In that sense, direct licking of the contaminated surface also caused disease, albeit with a lower attack rate. In addition, an interesting observation was that bedding in contact with prion-contaminated spheres was able to acquire the infectious agent and mediate disease transmission. This result, in addition to the rubbing simulation, indicate that infectious prions can relatively easily move from one surface to another, contributing to the spread of the infectious material in the environment. Moreover, we showed that prions can be released from the surfaces by washing; thus, a natural secondary method for the spread and transfer may be rain. This could rationally explain the PMCA detection of prions in the stream water samples from CWD endemic areas (38).
Overall, our results show that prions can bind to different surfaces and remain infectious. However, we do not know whether transmission requires the release of prions from the implants or whether initial conversion happens on the surface itself, in which case this would suggest that prion replication can occur in solid phase. Further testing of the properties of prions bound to surfaces is needed to better understand the role of these materials in natural prion transmission and to evaluate, for example, whether bound prions are more or less infectious than free prions. The information obtained in this study may provide important insight on the behavior and spread of prions in natural environments and help to set guidelines to prevent disease spread on farms and among free-ranging animals.
Experimental procedures
Preparation of biological samples
Syrian golden hamsters were experimentally infected by the intraperitoneal route with 263K prions. Brain tissue from clinically sick animals was collected. For control experiments, healthy Syrian golden hamsters were sacrificed, and the brain tissue was harvested. A 10% (w/v) brain homogenate (BH) was prepared in PBS plus Complete protease inhibitor mixture (Roche Applied Science) and centrifuged for 1 min at 800 × g. Samples were stored at −80 °C until use.
Prion purification and radiolabeling
PrPSc was purified using detergent extraction and PK digestion as described previously (31). Briefly, 10% (w/v) brain homogenate was prepared in PBS supplemented with Complete proteinase inhibitor and 10% Sarkosyl (final concentration). After a 10-min incubation at room temperature, ⅓ volume of PBS and 0.1% Zwittergent 3-14 was added and centrifuged for 2 h at 200,000 × g at 20 °C. The pellet was resuspended in PBS, 10% NaCl, and 0.1% Zwittergent 3–14 using extensive sonication to obtain a homogeneous solution. The material was placed over a 20% sucrose cushion prepared in PBS, 10% NaCl, and 0.1% Zwittergent 3–14 and centrifuged for 3 h at 100,000 × g at 20 °C. The pellet was resuspended in PBS and 0.1% Zwittergent 3–14 and incubated with PK (100 μg/ml) for 2 h at 37 °C at 600 rpm. PK digestion was stopped with 10 mm Pefabloc SC. The final material was placed over a 20% sucrose cushion and centrifuged as explained above. The pellet was resuspended in water and centrifuged at 3000 × g for 5 min at room temperature. Supernatant was discarded, and the pellet was resuspended in sodium phosphate buffer (250 mm, pH 7.4) for radiolabeling. Only the protease-resistant core of PrPSc was found by silver staining of the final material, compared side by side with Western blotting. Resulting material was proven to amplify in PMCA conditions as well as remain infectious (31). Purified protease-resistant PrPSc was radiolabeled with iodine-125 (125I) using the Chloramine T method described elsewhere (30). Yield of radiolabeling was determined by the trichloroacetic acid (TCA) method. Radioactivity measurements were done in a COBRAII automatic γ counter (PerkinElmer Life Sciences).
Characteristics of the surfaces used for the studies
Surfaces utilized in these studies were of different materials and sizes depending on the specific experiments. The following materials were used: polypropylene (Small Parts, Logansport, IN), borosilicate glass (McMaster Carr, Elmhurst, IL), stainless steel (type 316; McMaster Carr), brass (type C260; Small Parts), aluminum (Alloy 2017; McMaster Carr), cement (Portland cement, Quikrete, Atlanta, GA), wood (birch; Woodworks Ltd., Haltom City, TX), and rocks (birdseye, KRC Rock, San Marcos, CA).
Assessment of binding and release of radiolabeled prions to different materials
Radiolabeled prions were spiked into 300 μl of 10% (w/v) healthy hamster BH and incubated with agitation (450 rpm) at room temperature for 14 h with beads (0.32 cm in diameter) made of stainless steel, brass, aluminum, glass, cement, wood, polypropylene, and rock. Beads were washed five times using PBS, and radioactivity was measured in the bead and the washing solution. Each wash was performed with 400 μl of PBS for 1 min at 500 rpm in a thermomixer. After each wash, beads and PBS were transferred to a new tube, and radioactivity was measured. Because the TCA value was 84%, we used free iodine (125I) as a negative control, which was spiked in a 10% (w/v) healthy BH and incubated with all the materials in the same way as described for radiolabeled prions.
Serial replication of prions in vitro by PMCA
PMCA substrate was made by homogenizing brains from healthy Syrian golden hamsters at a final concentration of 10% (w/v) as described previously (32). For the PMCA experiments with RML, CWD, and vCJD prions, we used wildtype mouse and transgenic mice expressing deer or human PrP, respectively. As positive controls for PMCA reactions, 10% (w/v) BH from prion-infected animals was serially diluted with PMCA substrate in 0.2-ml PCR tubes (USA Scientific, Ocala, FL). Tubes containing unseeded PMCA substrate were used as negative controls. Each 0.2-ml PMCA reaction tube contained a total volume of 120 μl and was supplemented with 3 Teflon beads (Hoover Precision Products, Cumming, GA). PMCA cycles were conducted in a microsonicator (Qsonica Model Q700, Newtown, CT) with incubations at 37 °C (29 min and 40 s) and a brief sonication (20 s at ∼260 watts). After 96 incubation/sonication cycles, 10 μl of each sample were transferred into tubes containing 90 μl of PMCA substrate. Subsequent PMCA rounds were performed until the detection limit was reached.
Exposure of surfaces to infectious prions for PMCA studies
For PMCA experiments, beads (0.32 cm in diameter) composed of polypropylene, glass, stainless steel, brass, aluminum, cement, and wood were utilized. Rock pieces of ∼0.4–0.5 cm in diameter were also included. Beads were washed with 2% Triton X-100, autoclaved, and incubated for 16 h at room temperature with 150 μl of serial dilutions (10−2–10−9 eq diluted in PBS) of BH from 263K-infected hamsters, RML-infected mouse, CWD-infected white-tailed deer, or vCJD-infected humans. Treatments with 10% healthy BH were used as controls. Nonspecifically bound particles were removed by three thorough washes with 1 ml of tap water, and beads were air-dried for 180 min. To amplify PrPSc bound to surfaces, each contaminated bead was placed in PMCA reaction tubes containing 120 μl of PMCA substrate and submitted to serial PMCA. Contaminated beads were only included in the first PMCA round.
To assess the attachment of prions to surfaces after different exposure times, polypropylene beads (0.32 cm in diameter) were incubated for 2 min, 10 min, 30 min, 60 min, 120 min, or 16 h in 10−7 diluted 263K BHs. After three thorough washes with tap water and air drying at room temperature, beads were analyzed by PMCA as described above.
Effect of surfaces on the PMCA assay
Beads made from different materials were incubated with 150 μl of 10% normal BH for 16 h at room temperature to block putative binding sites. Excess BH was removed by three washes with tap water. Dried beads were added to PMCA substrate mixed with 10−7–10−9 eq of 263K BH and analyzed by PMCA.
Proteinase K digestion and Western blotting
To detect protease-resistant PrPSc, PMCA samples and BH from treated animals were incubated in the presence of PK (50 μg/ml; Sigma-Aldrich) for 1 h at 37 °C with shaking (450 rpm) in an Eppendorf thermomixer (Eppendorf, Hamburg, Germany). PK digestion was stopped by adding SDS sample buffer (Invitrogen) and 33 mm dithiothreitol (DTT; Sigma-Aldrich) and boiling for 10 min. PK-resistant PrP fragments were fractionated by SDS-PAGE, electroblotted into Hybond-ECL nitrocellulose membranes (GE Healthcare), and probed with 6D11 (1:5,000; Covance, Princeton, NJ). For CWD prions, we used the PRC1 monoclonal antibody, kindly provided by Dr. Glenn Telling (Colorado State University). Immunoreactive bands were visualized by an enhanced chemiluminescence assay (ECL Prime, GE Healthcare) using an image analysis system (Bio-Rad).
In vivo evaluation of disease transmission with contaminated surfaces
Three approaches were followed: (i) Prion transmission of contaminated surfaces was first studied by intracerebral injection of 263K-contaminated implants into groups of five 4–6-week-old female Syrian golden hamsters (Harlan® Laboratories, Houston, TX). Implants of 0.5 mm in diameter and 5 mm long made of polypropylene (tips of gel loading tips with ends sealed; Fischer Scientific), borosilicate glass (glass fibers; Hampton Research, Aliso Viejo, CA), stainless steel (type 316), brass (type C260; McMaster Carr), aluminum (Alloy 1100; McMaster Carr), rock (birdseye), cement (Portland cement), and wood (birch toothpick, Diamond, Thermo Fisher Scientific, Waltham, MA) were washed in 2% Triton X-100 (Sigma-Aldrich), autoclaved, and incubated for 16 h at room temperature with 100 μl of 1% (w/v) 263K or healthy BH. Unbound material was removed by three thorough washes with 1 ml of tap water, and implants were air-dried. Treated implants were placed in the right hippocampus of anesthetized animals. (ii) To study the influence of simple external contact of animals with the contaminated surfaces for disease transmission, large spheres (5.08 cm in diameter) made of polypropylene (Balltec, Los Angeles, CA), stainless steel (type 316; CCR Products, West Hartford, CT), brass (type C260; CCR Products), aluminum (type 6061; United States Ball Corp., La Mirada, CA), cement (Portland cement), and wood (birch; McMaster Carr) were used. Rocks and glass materials were not used for this study due to unavailability in similar size and composition as the other materials. Spheres were washed with 2% Triton X-100, autoclaved, and incubated for 16 h at room temperature with 5 ml of 10% (w/v) 263K or healthy BH. Remnant BHs were saved and stored at −20 °C following incubations. Unbound material was removed by three thorough washes with 200 ml of tap water. Subsequently, groups of five 7–8-week-old female Syrian golden hamsters were exposed to the dried spheres that were treated as described. Following 1 week of contact, spheres were washed with tap water, incubated with the previously recovered BHs (as described above), and placed back in the cages. After a total exposure time of 4 weeks, spheres were removed from the cages. (iii) To determine the mechanism of transmission by contact with prion-contaminated surfaces, three different putative modes of exposure (rubbing, licking, or shedding of infectious particles onto bedding) were analyzed. For rubbing, polypropylene spheres (5.08 cm in diameter) exposed to 263K or healthy BH (as described above) were rubbed three times across the abdominal area, back, and legs of 7–8-week-old female Syrian golden hamsters. This procedure was repeated five times per week until completing 20 exposures. In a different experimental group, spheres treated with BH were exposed three times to the tongue and the nose of isoflurane-anesthetized animals. This procedure was repeated five times a week for 4 consecutive weeks. For secondary contamination through bedding, corn starch standard bedding (The Andersons Inc., Maumee, OH) was incubated for 1 week with polypropylene spheres treated with healthy or 263K BH. Balls were removed, and bedding was subsequently placed in the cage of the animals for an additional week. New sets of bedding were exposed to BH-treated spheres, and resulting bedding was used to replace the previous bedding. These cycles were repeated to complete an exposure time of 6 weeks. For all experiments listed in (iii), balls were exposed to 263K and healthy BH weekly. Treated hamsters were monitored at least twice a week, and the onset of clinical disease was measured as described (39). Disease was confirmed by biochemical analysis of brain samples as reported previously (39). Hamsters not showing clinical signs were sacrificed at 500 days post-treatment, and brains harvested for biochemical analysis of protease-resistant PrPSc.
Author contributions
S. P. designed the experiments, carried out the PMCA and in vivo studies, analyzed results, prepared the figures, and participated in writing the manuscript. R. M. helped with the experimental design and in vivo inoculation and participated in writing the manuscript. A. L. performed the PMCA experiments with RML, CWD, and vCJD. L. C.-M. modified the prion purification protocol, performed the binding experiments with radioactive material, and edited the manuscript. A. U. radiolabeled PrP and analyzed data from the radioactivity experiment. C. S. is the principal investigator and was responsible for coordinating research activity, data analysis, funding, and writing the final version of the manuscript.
Supplementary Material
Acknowledgments
We thank Andrea Flores-Ramirez for animal care and help with assessing prion-associated clinical signs and Dr. Charles Mays for critical reading of the manuscript. We also thank Dr. Glenn Telling (Colorado State University) for kindly providing transgenic mice expressing cervid or human PrPC and the PRC1 antibody.
This work was supported by National Institutes of Health Grants P01 AI07774 (to C. S.) and R01 AI132695 (to R. M.). Dr. Soto is inventor on several patents related to the PMCA technology and is currently founder, chief scientific officer, and vice president of Amprion Inc., a biotech company focusing on the commercial utilization of PMCA for diagnosis of various neurodegenerative diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This article contains Figs. S1 and S2.
- PrP
- prion protein
- CJD
- Creutzfeldt-Jakob disease
- CWD
- chronic wasting disease
- PK
- proteinase K
- PMCA
- protein misfolding cyclic amplification
- RML
- Rocky Mountain Laboratory
- vCJD
- variant CJD
- BH
- brain homogenate.
References
- 1. Collinge J. (2001) Prion diseases of humans and animals: their causes and molecular basis. Annu. Rev. Neurosci. 24, 519–550 10.1146/annurev.neuro.24.1.519 [DOI] [PubMed] [Google Scholar]
- 2. Aguzzi A., Sigurdson C., and Heikenwaelder M. (2008) Molecular mechanisms of prion pathogenesis. Annu. Rev. Pathol. 3, 11–40 10.1146/annurev.pathmechdis.3.121806.154326 [DOI] [PubMed] [Google Scholar]
- 3. Prusiner S. B. (1998) Prions. Proc. Natl. Acad. Sci. U.S.A. 95, 13363–13383 10.1073/pnas.95.23.13363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Soto C. (2012) Transmissible proteins: expanding the prion heresy. Cell 149, 968–977 10.1016/j.cell.2012.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bernoulli C., Siegfried J., Baumgartner G., Regli F., Rabinowicz T., Gajdusek D. C., and Gibbs C. J. Jr. (1977) Danger of accidental person-to-person transmission of Creutzfeldt-Jakob disease by surgery. Lancet 1, 478–479 [DOI] [PubMed] [Google Scholar]
- 6. Gibbs C. J. Jr., Asher D. M., Kobrine A., Amyx H. L., Sulima M. P., and Gajdusek D. C. (1994) Transmission of Creutzfeldt-Jakob disease to a chimpanzee by electrodes contaminated during neurosurgery. J. Neurol. Neurosurg. Psychiatry 57, 757–758 10.1136/jnnp.57.6.757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Maddison B. C., Baker C. A., Terry L. A., Bellworthy S. J., Thorne L., Rees H. C., and Gough K. C. (2010) Environmental sources of scrapie prions. J. Virol. 84, 11560–11562 10.1128/JVI.01133-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Konold T., Hawkins S. A., Thurston L. C., Maddison B. C., Gough K. C., Duarte A., and Simmons H. A. (2015) Objects in contact with classical scrapie sheep act as reservoir for scrapie transmission. Front. Vet. Sci. 2, 32 10.3389/fvets.2015.00032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hawkins S. A., Simmons H. A., Gough K. C., and Maddison B. C. (2015) Persistence of ovine scrapie infectivity in a farm environment following cleaning and decontamination. Vet. Rec. 176, 99 10.1136/vr.102743 [DOI] [PubMed] [Google Scholar]
- 10. Georgsson G., Sigurdarson S., and Brown P. (2006) Infectious agent of sheep scrapie may persist in the environment for at least 16 years. J. Gen. Virol. 87, 3737–3740 10.1099/vir.0.82011-0 [DOI] [PubMed] [Google Scholar]
- 11. David W. W., Walsh D. P., Farnsworth M. L., Winkelman D. L., and Miller M. W. (2011) Soil clay content underlies prion infection odds. Nat. Commun. 2, 200 10.1038/ncomms1203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Barria M. A., Telling G. C., Gambetti P., Mastrianni J. A., and Soto C. (2011) Generation of a new form of human PrPSc in vitro by interspecies transmission from cervid prions. J. Biol. Chem. 286, 7490–7495 10.1074/jbc.M110.198465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cassard H., Torres J. M., Lacroux C., Douet J. Y., Benestad S. L., Lantier F., Lugan S., Lantier I., Costes P., Aron N., Reine F., Herzog L., Espinosa J. C., Beringue V., and Andréoletti O. (2014) Evidence for zoonotic potential of ovine scrapie prions. Nat. Commun. 5, 5821 10.1038/ncomms6821 [DOI] [PubMed] [Google Scholar]
- 14. Safar J. G., Lessard P., Tamgüney G., Freyman Y., Deering C., Letessier F., Dearmond S. J., and Prusiner S. B. (2008) Transmission and detection of prions in feces. J. Infect. Dis. 198, 81–89 10.1086/588193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gough K. C., and Maddison B. C. (2010) Prion transmission: prion excretion and occurrence in the environment. Prion. 4, 275–282 10.4161/pri.4.4.13678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Seidel B., Thomzig A., Buschmann A., Groschup M. H., Peters R., Beekes M., and Terytze K. (2007) Scrapie agent (strain 263K) can transmit disease via the oral route after persistence in soil over years. PLoS One 2, e435 10.1371/journal.pone.0000435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Johnson C. J., Phillips K. E., Schramm P. T., McKenzie D., Aiken J. M., and Pedersen J. A. (2006) Prions adhere to soil minerals and remain infectious. PLoS Pathog. 2, e32 10.1371/journal.ppat.0020032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Johnson C. J., Pedersen J. A., Chappell R. J., McKenzie D., and Aiken J. M. (2007) Oral transmissibility of prion disease is enhanced by binding to soil particles. PLoS Pathog. 3, e93 10.1371/journal.ppat.0030093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brown P., and Gajdusek D. C. (1991) Survival of scrapie virus after 3 years' interment. Lancet 337, 269–270 10.1016/0140-6736(91)90873-N [DOI] [PubMed] [Google Scholar]
- 20. Mathiason C. K., Hays S. A., Powers J., Hayes-Klug J., Langenberg J., Dahmes S. J., Osborn D. A., Miller K. V., Warren R. J., Mason G. L., and Hoover E. A. (2009) Infectious prions in pre-clinical deer and transmission of chronic wasting disease solely by environmental exposure. PLoS One 4, e5916 10.1371/journal.pone.0005916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Flechsig E., Hegyi I., Enari M., Schwarz P., Collinge J., and Weissmann C. (2001) Transmission of scrapie by steel-surface-bound prions. Mol. Med. 7, 679–684 [PMC free article] [PubMed] [Google Scholar]
- 22. Zobeley E., Flechsig E., Cozzio A., Enari M., and Weissmann C. (1999) Infectivity of scrapie prions bound to a stainless steel surface. Mol. Med. 5, 240–243 [PMC free article] [PubMed] [Google Scholar]
- 23. Smith C. B., Booth C. J., and Pedersen J. A. (2011) Fate of prions in soil: a review. J. Environ. Qual. 40, 449–461 10.2134/jeq2010.0412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bartelt-Hunt S. L., and Bartz J. C. (2013) Behavior of prions in the environment: implications for prion biology. PLoS Pathog. 9, e1003113 10.1371/journal.ppat.1003113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pritzkow S., Morales R., Moda F., Khan U., Telling G. C., Hoover E., and Soto C. (2015) Grass plants bind, retain, uptake, and transport infectious prions. Cell Rep. 11, 1168–1175 10.1016/j.celrep.2015.04.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wisniewski H. M., Sigurdarson S., Rubenstein R., Kascsak R. J., and Carp R. I. (1996) Mites as vectors for scrapie. Lancet 347, 1114 [DOI] [PubMed] [Google Scholar]
- 27. VerCauteren K. C., Pilon J. L., Nash P. B., Phillips G. E., and Fischer J. W. (2012) Prion remains infectious after passage through digestive system of American crows (Corvus brachyrhynchos). PLoS One 7, e45774 10.1371/journal.pone.0045774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Banks W. A., Niehoff M. L., Adessi C., and Soto C. (2004) Passage of murine scrapie prion protein across the mouse vascular blood-brain barrier. Biochem. Biophys. Res. Commun. 318, 125–130 10.1016/j.bbrc.2004.04.009 [DOI] [PubMed] [Google Scholar]
- 29. Banks W. A., Robinson S. M., Diaz-Espinoza R., Urayama A., and Soto C. (2009) Transport of prion protein across the blood-brain barrier. Exp. Neurol. 218, 162–167 10.1016/j.expneurol.2009.04.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Urayama A., Morales R., Niehoff M. L., Banks W. A., and Soto C. (2011) Initial fate of prions upon peripheral infection: half-life, distribution, clearance, and tissue uptake. FASEB J. 25, 2792–2803 10.1096/fj.11-180729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Urayama A., Concha-Marambio L., Khan U., Bravo-Alegria J., Kharat V., and Soto C. (2016) Prions efficiently cross the intestinal barrier after oral administration: study of the bioavailability, and cellular and tissue distribution in vivo. Sci. Rep. 6, 32338 10.1038/srep32338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Morales R., Duran-Aniotz C., Diaz-Espinoza R., Camacho M. V., and Soto C. (2012) Protein misfolding cyclic amplification of infectious prions. Nat. Protoc. 7, 1397–1409 10.1038/nprot.2012.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Saá P., and Cervenakova L. (2015) Protein misfolding cyclic amplification (PMCA): current status and future directions. Virus Res. 207, 47–61 10.1016/j.virusres.2014.11.007 [DOI] [PubMed] [Google Scholar]
- 34. Melchior M. B., Windig J. J., Hagenaars T. J., Bossers A., Davidse A., and van Zijderveld F. G. (2010) Eradication of scrapie with selective breeding: are we nearly there? BMC Vet. Res. 6, 24 10.1186/1746-6148-6-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Uehlinger F. D., Johnston A. C., Bollinger T. K., and Waldner C. L. (2016) Systematic review of management strategies to control chronic wasting disease in wild deer populations in North America. BMC Vet. Res. 12, 173 10.1186/s12917-016-0804-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Benestad S. L., Mitchell G., Simmons M., Ytrehus B., and Vikøren T. (2016) First case of chronic wasting disease in Europe in a Norwegian free-ranging reindeer. Vet. Res. 47, 88 10.1186/s13567-016-0375-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hetz C., Maundrell K., and Soto C. (2003) Is loss of function of the prion protein the cause of prion disorders? Trends Mol. Med. 9, 237–243 10.1016/S1471-4914(03)00069-8 [DOI] [PubMed] [Google Scholar]
- 38. Nichols T. A., Pulford B., Wyckoff A. C., Meyerett C., Michel B., Gertig K., Hoover E. A., Jewell J. E., Telling G. C., and Zabel M. D. (2009) Detection of protease-resistant cervid prion protein in water from a CWD-endemic area. Prion. 3, 171–183 10.4161/pri.3.3.9819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Castilla J., Gonzalez-Romero D., Saá P., Morales R., De Castro J., and Soto C. (2008) Crossing the species barrier by PrPSc replication in vitro generates unique infectious prions. Cell 134, 757–768 10.1016/j.cell.2008.07.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.