Abstract
Objectives
Parathyroid hormone (PTH) is a risk marker for hypoparathyroidism (hypoPTH). This study aimed to determine the predictive values of early PTH assays carried out at the moment of skin closure (PTH SC), to establish a treatment algorithm, identifying two threshold values. We assessed the reproducibility of this approach with two different immunoassay kits (hypoPTH) after total thyroidectomy, but its practical application is not consensual.
Study Design
We conducted a prospective descriptive study, including all patients who underwent a total thyroidectomy between March 2012 and November 2013. Postoperative PTH SC levels, corrected calcium on postoperative days, and occurrence of hypoPTH symptoms were collected.
Results
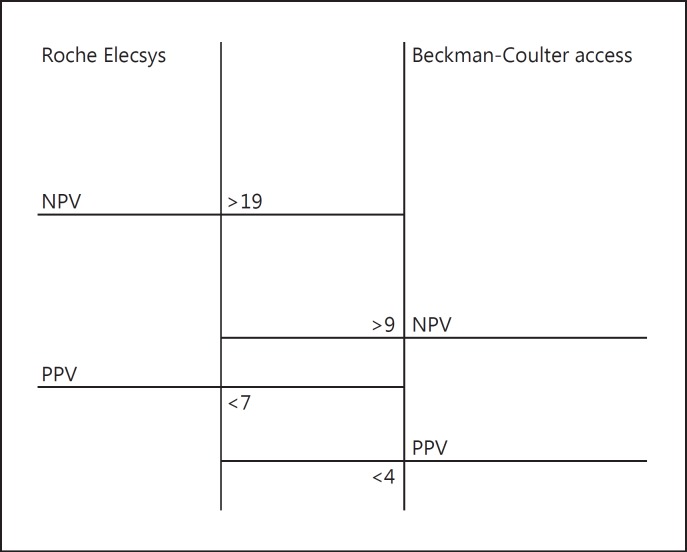
Of 257 patients, the rate of hypoPTH was 20%. Threshold values to obtain a 100% positive predictive value to identify patients for whom hypoPTH was absolutely certain were: PTH SC <7 ng/L for the Roche kit and PTH SC <4 ng/L for the Beckman-Coulter kit. Threshold values to obtain a 100% negative predictive value to identify patients for whom the absence of hypoPTH was absolutely certain were: PTH SC ≥19 ng/L for the Roche kit and PTH SC ≥9 ng/L the Beckman-Coulter kit.
Conclusions
A single serum PTH sampled at skin closure is a reliable test to predict hypoPTH after a total thyroidectomy. The use of a threshold based on a 100% negative predictive value enables patients with no risk of hypoPTH to be safely discharged within the first 24 h postoperatively without unnecessary calcium and vitamin treatment. This medication can be given promptly to patients at risk of hypoPTH to limit the occurrence of hypocalcaemia.
Keywords: Thyroidectomy, Hypoparathyroidism, Parathyroid hormone assay, Hypocalcaemia
Introduction
A total thyroidectomy is a common procedure, whose most frequent postoperative complication is hypoparathyroidism (hypoPTH). Medically, hypoPTH can cause disabling symptoms that may prove to be life-threatening [1]. Economically, it entails additional costs to the admission by: a prolonged hospital stay, implementation of treatment, investigations, and additional consultations. It is thus essential to promptly identify and treat patients developing hypoPTH, in order to avoid the adverse effects and limit the resulting additional costs.
The value of determining serum parathyroid hormone (PTH) levels as a biological risk marker for hypoPTH occurrence is largely recognized, and the realization of this early assay (at the point of skin closure) appears to give results comparable to those taken several hours following the procedure [2, 3]. However, the practical application of this assay is not consensual. For some authors, this test presents too many false positives and negatives, which make its clinical use questionable. Indeed, there is a limited but unavoidable grey area, where the PTH assay is not reliable to distinguish the hypoparathyroid patient from unaffected patients.
To avoid this pitfall and improve the clinical significance of this assay, we think that one should not define a single threshold value (based on a sensitivity and specificity compromise), but set two values which guarantee: first, a positive predictive value (PPV) of 100% and, second, a negative predictive value (NPV) of 100%.
To demonstrate the value of this approach, we conducted a study whose objectives were to:
Evaluate prospectively, on a large series of patients, the predictive values for hypoPTH occurrence, from early PTH assays after total thyroidectomy.
Assess the reproducibility of this approach using two second-generation immunoassay kits produced by the companies Roche and Beckman-Coulter.
Establish an original treatment algorithm for hypoPTH, based on the identification of a threshold value that guarantees a 100% NPV.
Materials and Methods
This study was conducted in a university hospital, whose ethics committee validated the study design and protocol (clinical trial: NCT02924532). This is a prospective descriptive study that included all consecutive patients who underwent a total thyroidectomy by extracapsular dissection with preservation of parathyroid glands between March 2012 and November 2013. In cases in whom an accidental parathyroidectomy occurred, the gland was immediately re-implanted between two infrahyoid muscular planes. In case of intraoperative thyroid cancer diagnosis, a unilateral or bilateral central neck dissection, possibly associated with a level 3 and 4 neck dissection, completed the procedure.
For each intervention, the following data were collected prospectively: age, sex, procedural indication, completion or not of a unilateral/bilateral central neck dissection, postoperative level of serum PTH at the moment of skin closure (PTH SC), and the day after the procedure (PTH D1), corrected calcium on day 1 postoperatively and the following days if the patient stayed in hospital, occurrence of perioral paraesthesia and/or cramps evaluated by a visual analogue scale (VAS), and implementation or not of hypocalcaemic treatment during hospitalization and upon discharge.
Exclusion criteria were: cases of partial to total thyroidectomy, patients whose postoperative data were not known, patients for whom the treatment protocol was not rigorously adhered to, and those presenting active pathologies or treatments that could modify calcium and phosphate metabolism.
HypoPTH was defined by the presence of clinical signs of hypocalcaemia (VAS >3 on the scale of paraesthesia and/or VAS >3 on the cramp scale), and/or serum corrected calcium less than 1.90 mmol/L the next day or 2 after the surgery. The patients that developed a hypoPTH were treated with calcium triphosphate (1.2 g, three times a day) and alfacalcidol (0.25 µg, three times a day) as soon as the diagnosis was made. Intravenous administration of calcium gluconate was given to very symptomatic patients, or in cases of severe hypocalcaemia of less than 1.80 mmol/L. Treatment of hypocalcaemia with vitamin and calcium supplementation was done blindly in all cases; thus, serum PTH levels did not bias this.
Two intact serum PTH immunoassay kits were used:
– Access PTH Intact Beckman-Coulter (Brea, CA, USA), whose intra-assay and inter-assay coefficient of variation, reference range, and functional sensitivity were respectively <2.6%, <5.8% (12–88 ng/L), and <4 ng/L.
– Elecsys 2010 Roche (Mannheim, Germany), whose intra-assay and inter-assay coefficient of variation, reference range, and functional sensitivity were respectively <2%, <3.4% (15–65 ng/L), and <6 ng/L (manufacturer data).
With each of these assays, PTH was measured according to the recommendations of the respective manufacturers using the Cobas 8000 modular analyser series (Roche, Mannheim, Germany). All measurements were done at the Biochemistry laboratory of our institution.
A descriptive analysis detailing the patient characteristics was performed. The variables were expressed by number, median, and percentage. A receiver operating characteristic (ROC) curve was established to determine the diagnostic threshold values of the PTH SC. The Student comparative test and χ2 analysis were used. The significance threshold level was set at 5%. The statistical analysis was performed with SAS version 9.3 (SAS Institute, Cary, NC, USA).
Results
Of the 331 patients who underwent total thyroidectomy during the study period, 257 were included. 60 patients did not have PTH SC measured within the allocated times and 14 patients received routine alfacalcidol postoperatively without specific indications. The Beckman-Coulter kit was used for the first 132 patients (PTH Beckman-Coulter group) and the Roche kit was used for the last 125 patients (PTH Roche group). The clinical data from the two cohorts are presented in detail in Table 1.
Table 1.
Patient characteristics
| Roche kit (n = 125) | Beckmann-Coulter kit (n = 132) | Total (n = 257) | |
|---|---|---|---|
| Age, n (%) | |||
| ≥60 years | 54 (43) | 44 (33) | 98 (38) |
| <60 years | 72 (58) | 88 (67) | 160 (62) |
| Sex, n (%) | |||
| Man | 20 (16) | 41 (31) | 62 (24) |
| Woman | 106 (84) | 91 (69) | 197 (76) |
| Initial disease, n (%) | |||
| Graves' disease/thyroiditis | 34 (27) | 40 (30) | 74 (28) |
| Multinodular goitre | 71 (56) | 80 (60) | 151 (59) |
| Cancer | 22 (17) | 12 (10) | 34 (13) |
| Central neck dissection, n (%) | |||
| Yes | 13 (10) | 13 (10) | 26 (10) |
| No | 112 (90) | 119 (90) | 232 (90) |
There were 60 men (23%) and 197 women (87%). The median patient age was 54 years (min 43, max 64). The surgical indications ranged from Graves' disease or toxic goitre in 75 patients (29%), to multi-heteronodular goitre in 146 patients (57%), and thyroid cancer in 36 patients (14%). A central neck dissection was performed in 26 out of the 36 cancer cases, unilateral in 5 patients and bilateral in 21 patients. For the clinical data as a whole, there was no statistically significant difference observed between the group of patients analysed with either the Roche or the Beckman-Coulter kit (p > 0.05, χ2 test).
Postoperative hypoPTH was found in 45 patients, with 24 patients (19%) in the PTH group analysed by the Roche kit and 21 patients (17%) in the PTH group analysed by the Beckman-Coulter kit.
The average corrected calcium at day 1 was 2.06 mmol/L for the PTH Roche group and 2.18 mmol/L for the PTH Beckman-Coulter group, with no statistically significant differences between the groups (p > 0.05, Student test).
Thirty-five (76%) out of the 45 hypoparathyroid patients presented with clinical symptoms of hypocalcaemia. This was expressed for 23 patients by an association of cramps and paraesthesia (49%), for 10 patients as isolated paraesthesia (22%), and for 2 patients as isolated cramps (4%). Ten patients were judged to be hypoparathyroid biologically by serum corrected calcium <1.90 mmol/L despite the absence of clinical signs (24%).
The PTH SC values ranged from <6 to 149 ng/L for the PTH Roche group and between <4 and 140 ng/L for the PTH Beckman-Coulter group.
For both the PTH Roche group and the PTH Beckman-Coulter group, the median PTH SC level was 26 ng/L. In the 2 groups, there was a strong correlation between the serum levels of PTH SC and the serum levels of PTH D1 (Pearson correlation test, p < 0.05). In order to eliminate the assay results that were falsely lowered through blood test degradation, or a pre-analytical error, we checked and concluded that no discordance existed between the PTH SC values and the PTH D1 values of all the patients tested.
Amongst the patients in hypoPTH, the median level of PTH SC was 9 ng/L in the PTH Roche group and 5 ng/L in the PTH Beckman-Coulter group. For the patients that did not develop hypoPTH, the median values were 31 ng/L in the two groups. There was a statistically significant difference between the PTH SC level in those with hypoPTH and in the healthy subjects in both groups (Student test, p = 0.012).
To evaluate the diagnostic significance of the PTH SC values, an ROC curve was made for the 2 groups. The area under the curve for the PTH Roche kit was 0.945, with a 95% confidence interval of 0.908–0.983. The area under the curve for the PTH Beckman-Coulter kit was 0.956, with a 95% confidence interval of 0.89–1. From these ROC curves, we identified for each assay kits two threshold values that we judge clinically relevant.
- Threshold value to obtain a 100% PPV. The clinical significance of this is to identify those patients for whom hypoPTH is absolutely certain.
- – For the PTH Roche kit, this threshold value was: PTH SC <7 ng/L. Retaining this threshold value, the test presented the following diagnostic characteristics: sensitivity = 25%, specificity = 100%, NPV = 85%, and PPV = 100%. In this group, we obtained these low values in 6 patients out of 125 (4.8%). Below this threshold, all the patients were hypoparathyroid postoperatively but a PTH SC above this threshold did not exclude a postoperative hypoPTH.
- – For the PTH Beckman-Coulter kit, this threshold value was: PTH SC <4 ng/L. For this threshold value, the test presented the following diagnostic characteristics: sensitivity = 29%, specificity = 100%, NPV = 88%, and PPV = 100%. In this group, we obtained these low values in 6 patients out of 132 (4.5%). Below this threshold, all the patients had postoperative hypoPTH but a PTH SC above this threshold did not exclude a postoperative hypoPTH.
- Threshold values to obtain a 100% NPV. The clinical significance of this value is to identify with certainty the patients that would not develop hypoPTH.
- – For the PTH Roche kit, this threshold value was: PTH SC ≥19 ng/L. Retaining this threshold value, the test provided the following diagnostic characteristics: sensitivity = 100%, specificity = 80.3%, NPV = 100%, and PPV = 54.5%. In this group, we obtained these high values in 81 patients out of 125 (65%). Of these, none developed hypoPTH but a PTH SC below this threshold did not confirm the occurrence of hypoPTH.
- – For the PTH Beckman-Coulter kit, this threshold value was: PTH SC ≥9 ng/L. Retaining this threshold value, the test provided the following diagnostic characteristics: sensitivity = 100%, specificity = 96%, NPV = 100%, and PPV = 80%. In this group, we obtained these high values in 107 patients out of 132 (81%). Of these, none developed hypoPTH but a PTH SC below this threshold did not confirm the occurrence of hypoPTH (Fig. 1).
Fig. 1.
Diagnostic accuracy study obtained for the Roche and Beckman-Coulter groups.
Between these two threshold values, there exists an area of diagnostic uncertainty. When the PTH SC values are found in this grey area, the test does not conclude with certainty the occurrence or absence of hypoPTH. In the PTH Roche group, 36 patients (28%) showed values in this grey zone between 7 and 19 ng/L. Among these, 17 showed symptoms of hypoPTH (47%) and 19 patients (53%) remained clinically and biologically asymptomatic. In the PTH Beckman-Coulter group, 19 patients (14%) showed values in this grey zone between 4 and 9 ng/L. Among them, 14 had symptoms of hypoPTH (74%) and 5 patients (26%) stayed clinically and biologically asymptomatic.
Discussion
Many studies have been conducted in order to define the clinical use of PTH assays for the early management of hypoPTH after total thyroidectomy. Our study shows results that are in line with literature data, regarding the rate of hypoPTH and the biochemical levels of calcium and postoperative PTH [4]. The value of this study is twofold. One, it confirms (in a large series of patients and using two different methods) the reliability of a PTH assay on skin closure (PTH SC) [5, 6, 7, 8]. Because of its pharmacokinetics, the PTH assay can be performed very promptly. The results are thus available a few hours after the surgery, which enables, whenever necessary, a prompt treatment of hypoPTH and brings an element of security in the case of ambulatory surgery [5, 6]. Two, for the first time in the literature, it enables to determine, not one threshold predictive value of the occurrence of hypoPTH, but two values that guarantee a 100% reliability of the clinical outcome, in order to adapt the therapeutic strategy.
The upper threshold limit enables the early assurance of hypoPTH absence. In our work, this involved more than 3 patients out of 4, with 65% for the PTH Roche group and 81% of patients for the Beckman-Coulter group. For all these patients, a PTH SC value above this threshold value allowed a prompt discharge at days 0 and 1 postoperatively, and avoidance of a unnecessary vitamin D and calcium supplementation. There is clinical benefit: no morbidity related to the calcium and vitamin treatment [9, 10], and improved patient quality of life. There is also economic benefit: no supplementary medication, reduced hospital stay, and absence of supplementary blood tests [11].
The second lower threshold value allows early confirmation of hypoPTH. In our work, this concerned few patients: 4.5% of the Roche PTH group and 4.8% of the Beckman-Coulter group. This value thus has much fewer economic benefits. However, it enables anticipation of the initiation of the vitamin and calcium supplementation, saving time with regard to increasing serum calcium levels, and informing the patients appropriately and clearly. Furthermore, we think these values can predict the occurrence of long-term hypoPTH because there are no cases, in the known and studied literature, of permanent late hypoPTH without early hypoPTH.
In our study, 14–28% of patients presented with values between the two threshold values, in the “grey area.” For these patients, the question remains as to which pathway to follow. In light of our results and data in the literature, two options can be discussed:
Determination of serum calcium and/or the PTH levels at day 1 to discriminate the patients who are truly at risk of hypocalcaemia [12, 13]. This stance avoids unnecessary treatment of patients that will ultimately not develop hypoPTH (24 patients [9%] in our study), but it exposes (at the onset of symptoms) the patients that will really develop hypoPTH (31 patients [12%] in our study).
Systematically treat all patients in the “grey area” to maximally limit symptom development and speed up discharge. This is our preferred stance, as the treatment is inexpensive and can prevent adverse effects, subject to normal renal function. In the prophylactically treated patients, a PTH assay can be done at the follow-up consultation 1–2 weeks postoperatively. Most of the time, it enables the discontinuation of the calcium-vitamin supplementation, in the presence of a normal PTH value [5].
This study shows a clear difference between the threshold values obtained between the two assay kits Roche and Beckman-Coulter. We worked with two immunoassay kits because of a change of the biochemistry laboratory; actually, the study could have been strengthened further through randomization, but it was not feasible because the two kits were not available at the same time. The variability between the different techniques is known, and our study confirms the necessity to assess the threshold values for each kit [14]. To that effect, an Australian meta-analysis suggested not to rely on the laboratory standard thresholds but on the values tested on populations [15]. It is interesting to note that, whilst we normalize our results by using the correlation line established by Ten et al. [16], we found very similar threshold values between the two assay techniques. These data tend to confirm the existence of two possible threshold values, but whose values can differ (for technical reasons) depending on the assay methods adopted.
Conclusion
The use of two threshold values improves the predictive power of the postoperative PTH levels. Based on our study, a single serum PTH measured at skin closure enable to identify promptly and with a 100% reliability patients with no risk of hypoPTH. This would enable patients to be discharged home safely within the first 24 h, improving patient comfort and the cost-effectiveness of the care provided.
Disclosure Statement
The authors have no conflict of interest to declare.
References
- 1.Bilezikian JP, Khan A, Potts JT, et al. Hypoparathyroidism in the adult: epidemiology, diagnosis, pathophysiology, target-organ involvement, treatment, and challenges for future research. J Bone Miner Res. 2011;26:2317–2337. doi: 10.1002/jbmr.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reddy AC, Chand G, Sabaretnam M, et al. Prospective evaluation of intra-operative quick parathyroid hormone assay as an early predictor of post thyroidectomy hypocalcaemia. Int J Surg. 2016;34:103–108. doi: 10.1016/j.ijsu.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 3.Grodski S, Serpell J. Evidence for the role of perioperative PTH measurement after total thyroidectomy as a predictor of hypocalcemia. World J Surg. 2008;32:1367–1373. doi: 10.1007/s00268-008-9545-5. [DOI] [PubMed] [Google Scholar]
- 4.Noordzij JP, Lee SL, Bernet VJ, et al. Early prediction of hypocalcemia after thyroidectomy using parathyroid hormone: an analysis of pooled individual patient data from nine observational studies. J Am Coll Surg. 2007;205:748–754. doi: 10.1016/j.jamcollsurg.2007.06.298. [DOI] [PubMed] [Google Scholar]
- 5.Houlton JJ, Pechter W, Steward DL. PACU PTH facilitates safe outpatient total thyroidectomy. Otolaryngol Head Neck Surg. 2011;144:43–47. doi: 10.1177/0194599810390453. [DOI] [PubMed] [Google Scholar]
- 6.Jumaily JS, Noordzij P, Dukas AG, et al. Prediction of hypocalcemia after using 1- to 6-h postoperative parathyroid hormone and calcium levels: an analysed of pooled individual patient data from 3 observational studies. Head Neck. 2010;32:427–434. doi: 10.1002/hed.21199. [DOI] [PubMed] [Google Scholar]
- 7.Landry CS, Grubbs EG, Hernandez M, et al. Predictable criteria for selective, rather than routine, calcium supplementation following thyroidectomy. Arch Surg. 2012;147:338–344. doi: 10.1001/archsurg.2011.1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lang BH-H, Yih PC-L, Ng KK. A prospective evaluation of quick intraoperative parathyroid hormone assay at the time of skin closure in predicting clinically relevant hypocalcemia after thyroidectomy. World J Surg. 2012;36:1300–1306. doi: 10.1007/s00268-012-1561-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kato Y, Sato K, Sata A, et al. Hypercalcemia induced by excessive intake of calcium supplement, presenting similar findings of primary hyperparathyroidism. Endocr J. 2004;51:557–562. doi: 10.1507/endocrj.51.557. [DOI] [PubMed] [Google Scholar]
- 10.Pfleiderer AG, Ahmad N, Draper MR, et al. The timing of calcium measurements in helping to predict temporary and permanent hypocalcaemia in patients having completion and total thyroidectomies. Ann R Coll Surg Engl. 2009;91:140–146. doi: 10.1308/003588409X359349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Page C, Strunski V. Parathyroid risk in total thyroidectomy for bilateral, benign, multinodular goitre: report of 351 surgical cases. J Laryngol Otol. 2007;121:237–241. doi: 10.1017/S0022215106003501. [DOI] [PubMed] [Google Scholar]
- 12.de Andrade Sousa A, Salles JMP, Soares JMA, et al. Course of ionized calcium after thyroidectomy. World J Surg. 2010;34:987–992. doi: 10.1007/s00268-010-0415-6. [DOI] [PubMed] [Google Scholar]
- 13.Raffaelli M, De Crea C, Carrozza C, et al. Combining early postoperative parathyroid hormone and serum calcium levels allows for an efficacious selective post-thyroidectomy supplementation treatment. World J Surg. 2012;36:1307–1313. doi: 10.1007/s00268-012-1556-6. [DOI] [PubMed] [Google Scholar]
- 14.Souberbielle J-C, Boutten A, Carlier M-C, et al. Inter-method variability in PTH measurement: implication for the care of CKD patients. Kidney Int. 2006;70:345–350. doi: 10.1038/sj.ki.5001606. [DOI] [PubMed] [Google Scholar]
- 15.AES Guidelines 06/01 Group Australian Endocrine Surgeons Guidelines AES06/01. Postoperative parathyroid hormone measurement and early discharge after total thyroidectomy: analysis of Australian data and management recommendations. ANZ J Surg. 2007;77:199–202. doi: 10.1111/j.1445-2197.2007.04018.x. [DOI] [PubMed] [Google Scholar]
- 16.Ten BE, Van Veen MC, Vervloet MG, et al. Influence of four different PTH methods on the classification of chronic kidney disease patients according to the new KDIGO guideline. Clin Lab. 2012;58:719–724. [PubMed] [Google Scholar]