Abstract
Irritative voiding symptoms (e.g. increased frequency and urgency) occur in many common pathologic conditions such as urinary tract infections and bladder outlet obstruction, and these conditions are well-established to have underlying inflammation that directly triggers these symptoms. However, it remains unclear as to how such diverse stimuli individually generate a common inflammatory process. Jürg Tschopp provided substantial insight into this conundrum when, working with extracts from THP-1 cells, he reported the existence of the inflammasome. He described it as a structure that senses multiple diverse signals from intracellular/extracellular sources and pathogens and triggers inflammation by the maturation and release of the pro-inflammatory cytokines interleukin-1β and interleukin-18. Recently, many of these sensors were found in the bladder and the nucleotide-binding domain, leucine-rich-containing family, pyrin domain-containing-3, has been shown to be a central mediator of inflammation in several urological diseases. In this review, we introduce the nucleotide-binding domain, leucine-rich-containing family, pyrin domaincontaining-3 inflammasome, highlight its emerging role in several common urologic conditions, and speculate on the potential involvement of other inflammasomes in bladder pathology.
Key Words: Inflammasomes, NLRP3, Bladder outlet obstruction, Urinary tract infection, Urothelial carcinoma, Bladder cancer
Introduction
The bladder lumen is a hostile environment. Under normal conditions chemicals or microbes in the urine are denied entry into the bladder wall by a glycosaminoglycan layer at the luminal surface of the urothelium. If this layer is compromised by invasive uropathogens, mechanical trauma (stones, catheters, etc.) or idiopathically in the case of interstitial cystitis, a host of potentially dangerous components gain access to the underlying tissue. Even with an intact barrier, the urothelium may be significantly altered in certain pathological conditions by physical changes such as elevated intravesical pressure, overstretch of tissues, hypoxia/reperfusion or products of metabolic dysregulation. The near ubiquitous response to this plethora of insults is the appearance of an inflammatory state. Despite the pervasive inflammation, there has been no clear understanding of the mechanism by which such diverse factors could trigger this common response. Inflammation in response to a wide range of agents is not unique to the bladder and in 2002 Jürg Tschopp began to shed light on this quandary in immune cells when his group discovered the existence of the inflammasome; a multimeric structure that senses diverse stress signals and triggers inflammation by the maturation and release of the pro-inflammatory cytokines interleukin-1β (IL-1β) and IL-18 [1]. Recently, the inflammasome has also emerged as a central mediator linking bladder insults to bladder inflammation and, ultimately, to bladder pathology [2,3].
Introduction to the Inflammasome
After the mechanical catharsis of micturition and the barrier provided by the glycosaminoglycan layer, the bladder's next line of defense is the innate immune system and the triggering of inflammation [4]. Cells that comprise the innate system rely on a set of receptors, collectively known as pattern recognition receptors, to detect relevant signals and initiate a response. These receptors are expressed by many different cell types including fibroblasts, myeloid immune cells, and epithelial cells [5]. The pattern recognition receptors are classified into 5 families: toll-like receptors (TLRs), C-type lectin receptors, retinoic acid-inducible gene-I (RIG-1)-like receptors, absent in melanoma 2 (AIM2)-like receptors, and nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs) [6].
NLRs are located intracellularly where they recognize 2 different types of signals, pathogen-associated molecular patterns (PAMPs) and danger (or damage) associated molecular patterns (DAMPs) [6]. PAMPs include common bacterial components and known virulence factors, such as lipopolysaccharide (LPS), flagellin, and hemolysins [5]. DAMPs are typically released by stressed, damaged or dying cells and include adenosine triphosphate (ATP), uric acid crystals, high-mobility group box 1, and heat-shock proteins [6]. A list of DAMPs and PAMPs particularly relevant to the urologic system is shown in table 1.
Table 1.
A list of the known DAMPs and PAMPs that trigger inflammasome activation in bladder pathology
| DAMPs | PAMPs |
|---|---|
| ATP | lipopolysaccharide |
| Uric acid (monosodium urate) | flagellin |
| High-mobility group box 1 | viral double-stranded RNA |
| Albumin | viral single-stranded RNA |
| Mitochondrial DNA | lipoteichoic acid |
| Cytosolic RNA | α-hemolysin |
| Heat-shock proteins | β-hemolysin |
| Mitochondrial cardiolipin | |
| Lysosomal cathepsin | |
| Acrolein | |
| Calcium oxalate |
The NLR protein family is large and diverse with over 20 known members in humans, many of which may contribute to the innate immune response in different ways. For example, some NLRs (NOD1, NOD2) stimulate transcription of NF-κB-regulated genes [7] whereas others assist the adaptive immune response by triggering transcription of major histocompatibility class II genes for antigen-presenting cells [8]. However, NLRs are best known for those that assemble into cytosolic multi-protein complexes called inflammasomes, which are typically named for the NLR involved. For example the inflammasome formed with nucleotide-binding domain, leucine-rich-containing family, pyrin domain-containing-3 (NLRP3) is known simply as the NLRP3 inflammasome. Formation of the inflammasome triggers the cleavage of pro-caspase-1 into active caspase-1. Caspase-1 cleaves pro-IL-1β and pro-IL-18 into their active forms (IL-1β and IL-18) along with gasdermin D, which triggers pyroptosis [9,10], a pro-inflammatory form of cell lysis. Pyroptosis in turn releases the mature IL-1β and IL-18 as well as intracellular DAMPs such as ATP, uric acid, and high-mobility group box 1 that further propagate inflammation [11,12]. Additionally, some other PPRs can form inflammasomes such as RIG-1 and AIM2 [13,14].
The NLRP3 Inflammasome
The best-studied member of the NLR family is NLRP3, which plays important roles in a wide variety of inflammatory pathologies. Unlike many other NLRs, NLRP3 is activated by both PAMPs and DAMPs [15,16,17] making it ideally suited to play a central role in the innate immune response. In an early tissue survey of NLRP3 expression, Kummer et al. [18] documented the presence of NLRP3 in the human bladder, and our lab has subsequently confirmed its expression in the rat and localized it to the urothelial layer [19].
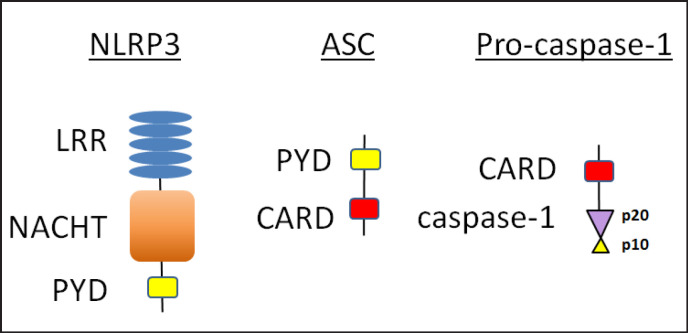
The domain structure of the components of the NLRP3 inflammasome is shown in figure 1 and is important to assembly. NLRP3 contains 3 separate domains; an N-terminal pyrin (PYD) domain that mediates homotypic binding, a nucleotide-binding and oligomerization (NACHT) domain that mediates ATP-dependent oligomerization, and a C-terminal leucine-rich repeat (LRR) domain that senses ligand (in the case of NLRP3 probably indirectly). While other NLRs also have caspase recruitment domains (CARDs) as part of their primary sequence, NLRP3 requires an adaptor protein to connect to pro-caspase-1. The adapter is known as apoptosis-associated speck-like protein containing a CARD (ASC) and consists of a PYD and a CARD. Pro-caspase-1 has a CARD and 2 subunits (p20 and p10) that become part of the final enzyme.
Fig. 1.
Domain structure of the components of the NLRP3 inflammasome. The NLRP3 inflammasome contains 3 components: 1) NLRP3 (the nod-like receptor), 2) ASC, and 3) procaspase-1. NLRP3 consists of 3 separate domains: a C-terminal LRR domain, a NACHT domain and an N-terminal PYD. ASC is composed of a PYD and a CARD, and is the adaptor protein that binds pro-caspase-1. Pro-caspase-1 has a CARD and 2 subunits (p20 and p10) which are cleaved to form active caspase-1 by the inflammasome.
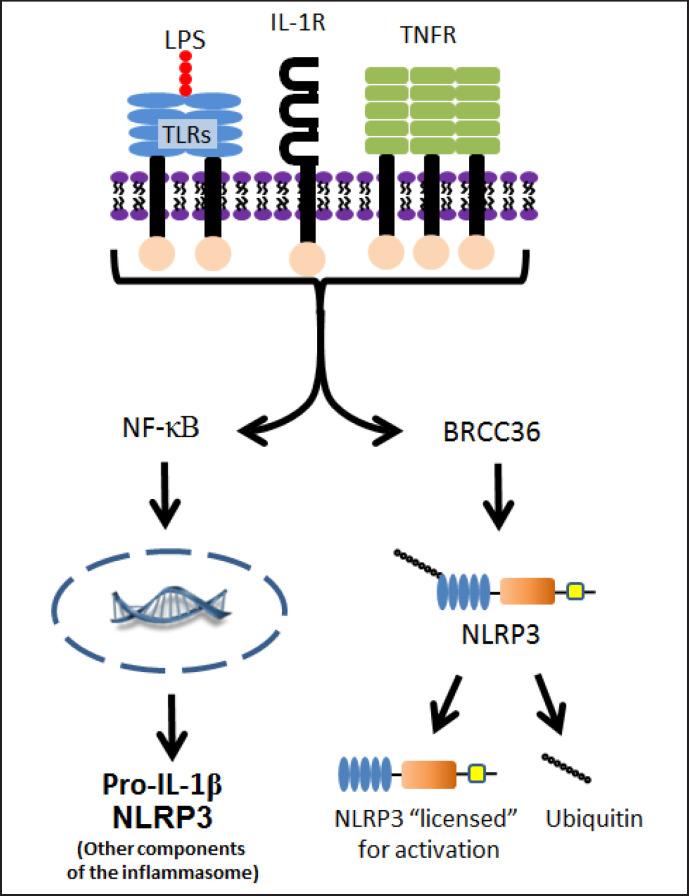
Activation of the NLRP3 inflammasome can occur by 3 different pathways termed the 1) canonical 2) non-canonical or 3) alternative pathways [11,20,21,22,23,24,25]. The canonical pathway requires 2 steps: priming and activation. Priming (fig. 2) begins when a DAMP or PAMP binds to a non-NLR receptor (typically a TLR) or there is signaling through endogenous cytokine receptors such as IL-1R and tumor necrosis factor receptor [26]. Signaling through NF-κB initiates production of de novo pro-IL-1β and NLRP3 [15,26,27] while signaling through BRCC36 deubiquitinates preexisting NLRP3 [28,29], licensing it to form an inflammasome.
Fig. 2.
Priming of the NLRP3 inflammasome in the canonical pathway. The canonical pathway of NLRP3 inflammasome activation requires a priming step. This occurs when a DAMP or PAMP (such as LPS) binds to a non-NLR receptor (such as TLR) or there is signaling though IL-1R or tumor necrosis factor receptor. The effect of this binding leads to further signaling through either NF-κB and/or BRCC36. Signaling through NF-κB leads to production of de novo pro-IL-1β and other components of the NLRP3 inflammasome while signaling through BRCC36 leads to deubiquination of existing NLRP3. Deubiquination then frees NLRP3 to be activated, i.e. “licenses” it for activation.
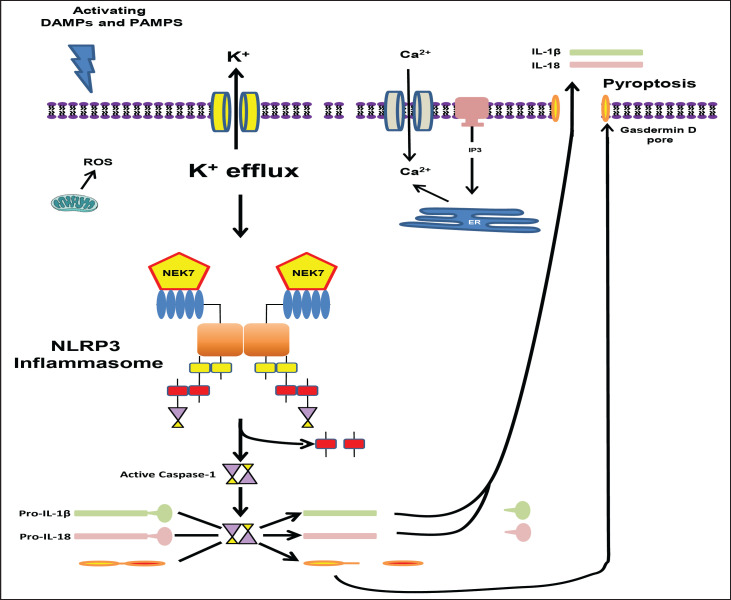
Canonical activation of NLRP3 (fig. 3) can be triggered in different models by different DAMPs and PAMPs. Given the large number of agents activating a single target, it seems unlikely to occur by direct ligand binding to NLRP3 and so there has been an intense study to find a common cellular event activating this inflammasome. Reactive oxygen species (ROS) were first proposed to be such an activator [30], but subsequent studies suggests they may dispensable [31]. Thus, the generation of ROS might best be thought of as a pathway to activation induced by certain DAMPs/PAMPs [32]. Likewise, Ca2+ signaling was suggested as a general requirement by several studies [33,34,35] but that too has been challenged [36]. Currently the prevailing theory is that K+ efflux represents a common denominator in the canonical pathway of NLRP3 activation [31,37,38]. However, how that may translate to NLRP3 assembly is unknown and K+ loss is not excusive to inflammasome activation but is a well-established event in apoptosis as well [39,40,41].
Fig. 3.
Activation of the NLRP3 inflammasome in the canonical pathway. The second step of the canonical pathway is activation. DAMPs and PAMPs trigger this step and lead to activation of the NLRP3 inflammasome. Proposed cellular events involved include ROS, Ca2+ signaling, and, most likely, K+ efflux. Regardless, NLRP3 inflammasome assembly is initiated and the catalytic domain of the never in mitosis gene a-related kinase 7 interacts with the LRR domain of NLRP3 causing oligomerization through binding of NACHT domains (the figure depicts only a dimer for simplicity). This leads to NLRP3 binding to ASC and ASC in turn binding to pro-caspase-1. Pro-caspase-1 molecules in close proximity lead autocatalytic cleavage of pro-caspase-1 to caspase-1 (induced proximity). Activated caspase-1 then cleaves pro-IL-1β and pro-IL-18 into their active forms (IL-1β and IL-18). Caspase-1 also cleaves gasdermin D whose N-terminus is responsible for forming a pore that results in pyroptosis and the release of IL-1β and IL-18.
Regardless of the trigger, once assembly is initiated, the catalytic domain of the never in mitosis gene A-related kinase 7 [42,43] interacts with the LRR domain of NLRP3 causing the NLRP3 to oligomerize through binding of NACHT domains. The NLRP3 oligomer then binds ASC, through homotypic binding of pyrin domains, which forms long filaments that bind multiple molecules of pro-caspase-1 (fig. 3 depicts only a dimer for simplicity). This large (up to 1 µm) structure is surprisingly stable and is often known as an ASC-speck. Typically only one of these form per cell and it incorporates all the ASC in that cell. Holding pro-caspase-1 molecules in close proximity in this speck results in an autocatalytic cleavage process known as induced proximity. This cleaves caspase-1 into its p20 and p10 subunits which associate with an additional set of p20 and p10 subunits to form an active tetramer. Both the active caspase-1 and the CARD domain dissociate from the speck and caspase-1 then cleaves pro-IL-1β and pro-IL-18 into their active forms (IL-1β and IL-18). Caspase-1 also cleaves gasdermin D [10] whose N-terminus is responsible for forming a pore in the cell membrane that results in pyroptosis [44,45,46].
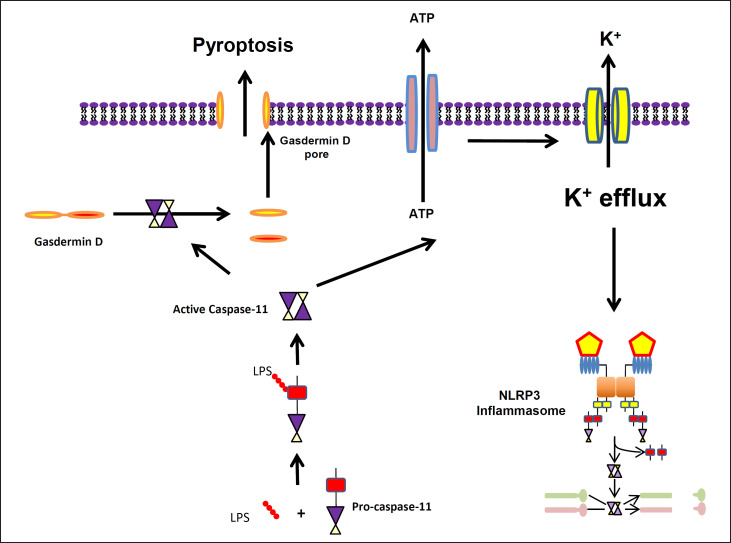
The NLRP3 inflammasome can also be activated by a non-canonical pathway (fig. 4) where LPS in the cytosol directly binds to the CARD domain of caspase-11 (in rodents; caspase-4 and caspase-5 in humans) [9,47,48]. This triggers the oligomerization and activation of caspase-11, which can directly cleave gasdermin D and cause pyroptosis, without promoting inflammasome formation or cytokine production. Caspase-11 can also lead to the release of ATP, which can stimulate K+ loss and the assembly of the NRP3 inflammasome, leading to the release of IL-1β and Il-18. Like the canonical pathway, K+ efflux is also characteristic of the noncanonical pathway. The importance and uniqueness of the non-canonical pathway have been highlighted by decreased mortality in caspase-11-deficient mice exposed to systemic administration of LPS [47,49].
Fig. 4.
Non-canonical pathway of NLRP3 inflammasome activation. NLRP3 activation in the noncanonical pathway requires activation of caspase-11 through direct binding to a cytosolic PAMP, like LPS, which then facilitates the oligomerization of the caspase. Caspase-11 can directly cleave gasdermin D and cause pyroptosis or can lead to the release of ATP that acts as a DAMP, stimulating K+ loss and the assembly of the NRP3 inflammasome with subsequent maturation of IL-1β and IL-18.
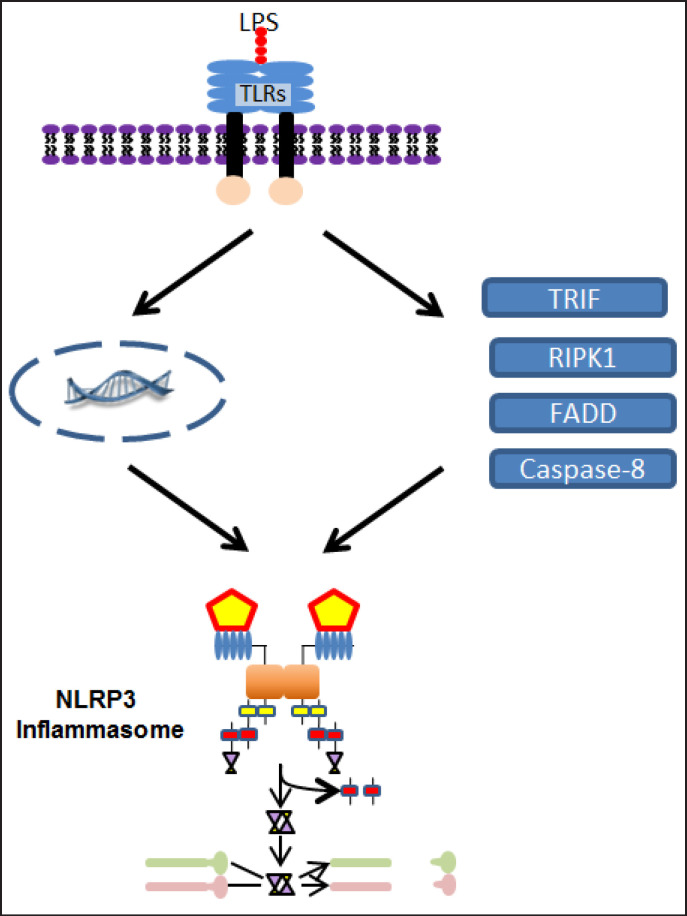
There is a third mechanism of NLRP3 activation (fig. 5), known currently as the alternative pathway that has been shown in human monocytes, although the extent of its deployment across cell types has not been thoroughly examined [23,24,25,50]. Similar to the canonical pathway, the alternative pathway relies on LPS binding to TLR4 to upregulate NLRP3 and pro-IL-1β production. However, unlike the canonical pathway, the efflux of K+ is not required [50]. Instead, TLR4 signaling leads to toll/ interleukin-1 receptor-domain-containing adapter-inducing interferon-β, receptor-interacting protein kinase 1, Fas-associated protein with death domain, and caspase-8 acting downstream to activate the NLRP3 inflammasome leading to cleavage of pro-IL-1β to IL-1β. Even though the inflammasome requires ASC and caspase-1 for this cleavage, there is no evidence for ASC speck formation or pyroptosis in this pathway [23,25].
Fig. 5.
Alternative pathway of NLRP3 inflammasome activation. The alternative pathway is the third mechanism of NLRP3 inflammasome activation. Similar to the canonical pathway's priming step, LPS binds to TLR4 that leads to upregulation of NLRP3 and pro-IL-1β through the NF-κB pathway. However, instead of the K+ efflux, NLRP3 inflammasome activation relies on toll/interleukin-1 receptor-domain-containing adapter-inducing interferon-β, receptor-interacting protein kinase 1, Fas-associated protein with death domain, and caspase-8 that are upregulated by the TLR4 signaling.
Urologic Pathology Dependent on Inflammasomes
The NLRP3 inflammasome was first discovered to mediate bladder inflammation in a cyclophosphamide-induced hemorrhagic cystitis model [51]. Since that time, inflammasomes have been implicated in both sterile and infectious cystitis, as well as malignancy. The following sections will discuss the role of inflammasomes in cyclophosphamide-induced hemorrhagic cystitis, bladder outlet obstruction (BOO), urinary tract infections (UTI), and bladder cancer.
Cyclophosphamide-Induced Hemorrhagic Cystitis
Cyclophosphamide is a widely used chemotherapeutic agent and acrolein, the metabolic byproduct of cyclophosphamide, is stored in the bladder prior to excretion where it damages the urothelium and can cause hemorrhagic cystitis. Fortunately, its occurrence in the patient population has been minimized primarily through the use of super hydration and 2-mercaptoethane sulfonate sodium, which chelates the acrolein in the urine and masks its toxic effects. However, it remains a useful experimental model to study the mechanisms of sterile bladder inflammation. Suggestive of a role for the inflammasome in this model, Smaldone et al. [52] reported an increase in urinary IL-1β and IL-18. Moreover, Ribeiro's group [53,54,55] used the IL-1 receptor antagonist anakinra in mice to directly demonstrate a causative role for IL-1β. Recently, our group inhibited NLRP3 in rats using glyburide, an FDA-approved diabetic medication and known inhibitor of the NLRP3 inflammasome [56]. Addition of this inhibitor diminished the changes associated with irritative voiding (increased frequency, decreased volume) along with reducing IL-1β and IL-18 levels and suppressing inflammation [51]. Haldar et al. [57] further refined the underlying mechanism by demonstrating that acrolein oxidizes mitochondrial DNA while epigenetically silencing DNA repair enzymes. The oxidized DNA then triggers NLRP3 inflammasome activation. Furthermore, IL-1β, acting downstream through insulin-like growth factor, induces bladder smooth muscle hyperplasia, a well-known change in this model that could be a contributor to the irritative voiding changes [57]. Taken together the findings of several investigators demonstrate that the NLRP3 inflammasome is critical in mediating biochemical, histological, and physiological inflammation during cyclophosphamide-induced hemorrhagic cystitis.
BOO
BOO is most often the result of benign prostatic hyperplasia (BPH) in older men, but may also occur after urethral stricture disease, pelvic organ prolapse, neurological trauma/disease, or in children with posterior urethral valves. BOO causes physical changes in the bladder, which leads to chronic inflammation [58,59,60,61,62,63] that evokes clinically bothersome, irritative voiding symptoms that are difficult to treat using available therapies. Our lab has explored the role of the NLRP3 inflammasome in a rat model of BOO in which the animals are treated with NLRP3 inhibitor glyburide before and during development of this disorder. We found that NLRP3 is activated in the acute period of BOO and is responsible for triggering the resulting inflammation and bladder hypertrophy [62]. Urodynamic studies of BOO rats demonstrated that glyburide prevented the increase in urinary frequency and decrease in void volume characteristic of irritative voiding symptoms seen during BOO in patients. Treatment with glyburide also led to a longer duration of voiding, leading us to conclude that blocking inflammation allowed the detrusor muscle to maintain a longer, stronger contraction, not surprising given that non-inflamed muscles can sustain a contraction longer then inflamed ones. Strong genetic evidence for the importance of the NLRP3/IL-1β pathway in BOO pathology was also provided by Kanno et al. [64] who found that inflammatory endpoints in obstructed mice were diminished in IL-1β−/- strains.
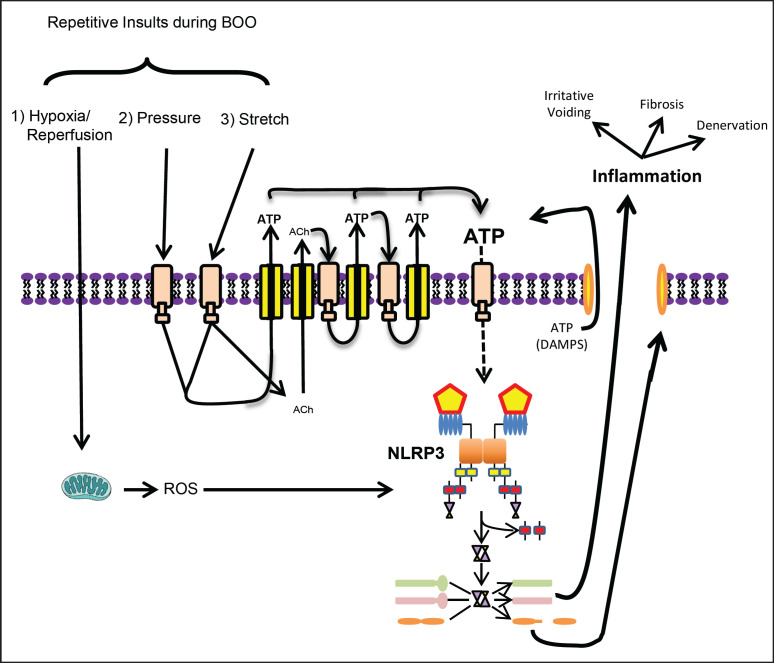
Inflammasomes, and NLRP3 in particular, can have very specific activating stimuli, although the exact mechanism of NLRP3 activation in response to BOO has not been defined. We propose that at least 3 potential inflammasome-activating insults occur repeatedly following obstruction. As shown in figure 6, those insults are hypoxia/reperfusion, increased pressure, and repetitive stretching. Both hypoxia and reperfusion are known to trigger the release of ROS and ROS is a well-known trigger for NLRP3 [11,21,65], if not a universal event. Due to incomplete emptying, inflammation, hypertrophy, and more, obstructed bladders are greatly enlarged compared to controls and undergo excessive distension during filling. This stretch reduces the diameter of blood vessels within the bladder wall, reducing blood flow and creating a mildly hypoxic environment [66]. Micturition relieves this partial occlusion resulting in reperfusion which brings a bolus of oxygen into the tissue that drives a burst of oxidative phosphorylation in the mitochondria and a surge of ROS, a well-known trigger for NLRP3 [11,21,65].
Fig. 6.
NLRP3 inflammasome activation in BOO. The NLRP3 inflammasomes is thought to be activated by at least 3 repetitive insults during BOO: hypoxia/reperfusion, high pressure and increased stretching. Hypoxia/reperfusion leads to production of ROS, a known trigger for the inflammasome. Increased pressure and repetitive stretch both release ATP, a known NLRP3 DAMP. Furthermore, bladder stretching releases acetylcholine that can act back on cholinergic receptors to trigger release of more ATP. The increase in extracellular ATP caused by pressure, stretch, and acetylcholine can drive yet another positive feedback loop known as ATP-dependent ATP release. The result is a tremendous amount of extracellular ATP, which then serves as a trigger for NLRP3 activation.
The potential also exists for NLRP3 to be activated by both increased pressure and stretch, which may exert their effects additively or synergistically through the release of ATP; a potent activator of NLRP3 [16,67]. Compared to a normal bladder, an obstructed bladder has a much higher pressure during micturition as the detrusor contracts with greater force to overcome the obstruction. Recently, Nagatomi's laboratory has used urothelial cells in culture to demonstrate that exposure to increased hydrostatic pressure directly stimulates AT P release [68] and NLRP3 activation [69]. The use of purinergic receptor antagonists demonstrated that the released ATP is directly responsible for triggering inflammasome activation (Jiro Nagatomi, personal communication, May 20, 2017) suggesting that pressure-induced release of ATP may play an important part in triggering NLRP3-mediated inflammation during BOO. Due to decreased bladder compliance secondary to BOO, the bladder also stretches much more during the storage phase, and increased stretch has been well-established in the literature to trigger the release of ATP [70,71,72,73,74]. Currently, no studies have investigated the ability of stretch to activate the NLRP3 inflammasome, although it is likely to be rich fodder for future studies. In addition to these 2 ATP-releasing mechanisms, the literature suggests the presence of an amplifying loop within the urothelia. Increased stretch stimulates the release of acetylcholine in addition to ATP [71,75,76,77]. While this acetylcholine may have effects on nearby nerves, it can also act on cholinergic receptors in the urothelia to trigger release of more ATP [78,79]. The increase in extracellular ATP caused by pressure, stretch, and acetylcholine can drive yet another positive feedback loop known as ATP-dependent ATP release [80]. The ultimate result is a substantial amount of ATP just outside the cell membrane. All of this ATP from the individual cell would be joined by ATP released from neighboring cells undergoing pyroptosis, magnifying this feed forward loop and propagating inflammation during BOO. While additional studies need to be performed to quantify the impact of such a feed forward loop, the literature clearly indicates that repetitive insults associated with BOO trigger an ongoing inflammatory process mediated by NLRP3 that evokes negative downstream events such as irritative voiding and fibrosis.
The harmful effects of an acute episode of BOO can typically be alleviated by simply removing the obstruction. However, in patients with chronic BOO (such as seen with BPH) de-obstruction is not always successful in restoring normal bladder function because the bladder has become decompensated and thus dysfunctional. The changes underlying decompensation are complex but certainly involve at least 2 major, potentially irreversible, changes to the bladder; fibrosis and denervation. Numerous studies in pulmonary, cardiac, and renal systems have demonstrated a link between the NLRP3 inflammasome and the induction of fibrosis [81,82,83,84] and we have recently shown a similar link in the bladder [61]. In those studies inhibition of NLRP3 with glyburide or IL-1β with a receptor antagonist (anakinra) blocked the appearance of fibrotic endpoints [85]. In vitro, urothelial cells exposed to IL-1β responded by producing collagens and cells harvested from BOO rats secreted more collagen than those taken from control animals. These findings suggest the existence of an IL-1β-mediated profibrogenic positive feedback loop within the urothelia that is active during BOO [61].
Loss of bladder nerve density during BOO has been found in many different species including man [86], pig [87], guinea pig [88], sheep [89], rabbit [90], and rat [91] and is speculated to be triggered by ROS [88,92], which we have noted is a common activator of NLRP3 [11,21,65]. In our rat model of BOO, inhibiting NLRP3 (glyburide) or blocking the IL-1 receptor (anakinra) attenuated the loss of neurons while in vitro experiments demonstrated a direct apoptotic effect of IL-1β on pelvic ganglionic neurons, suggesting 1 mechanism of BOO-induced denervation may be NLRP3/IL-1β triggered apoptosis. The combination of our studies on fibrosis and denervation suggest NLRP3 may be an important therapeutic target for preventing bladder deterioration and decompensation during the progression of BOO.
While the most common cause of BOO is BPH, it is interesting that BPH itself may be a result of inflammasome activation [93]. Clearly, infectious prostatitis has the potential to activate inflammasomes through PAMPs, but not all cases of prostatitis are infectious nor does infectious prostatitis necessarily cause BPH. Recently, Kashyap et al. [94] demonstrated that injection of formalin into the prostate upregulated NLRP1, caspase-1, and the cytokines IL-1β and IL-18. Thus, inflammasomes may mediate both infectious and sterile prostatitis, leading to BPH, BOO, and activation of NLRP3 within the bladder, eventually causing all of the deleterious results just discussed. Intriguingly, the main NLR involved in the prostate appears to be NLRP1, not NLRP3. NLRP1 has also been found in the bladder where its role remains unknown [95].
Uropathogenic E. coli UTIs
UTIs are the second most common forms of infection behind only the common cold and IL-1β is one of the first cytokines detected at the onset, suggesting an early role of inflammasomes in infectious cystitis [96]. While UTIs can be caused by various viral, mycobacterial, chlamydial, fungal, and schistosomal agents, by far the most common culprits are gram-negative, uropathogenic Escherichia coli (UPEC) [97]. UPEC produce LPS, flagellin, and α-hemolysin, which are well-known PAMPs that activate NLR inflammasomes in other tissues. Our lab has recently shown that both LPS and flagellin can trigger inflammasome activation when placed in the lumen of the bladder [51]. Moreover, injection of LPS into the bladder wall triggered irritative voiding reminiscent of UTIs and this pathological effect was blocked by glyburide [98], clearly showing that LPS activation of NLRP3 can evoke a cystitis analogous to what is seen during a UTI. Nagamatsu et al. [99] examined α-hemolysin expression in UPEC and found that α-hemolysin activated NLRP3 and triggered pyroptosis in human urothelia. This leads to exfoliation of urothelial cells, which is presumed to act as a host defense mechanism against UPEC expressing α-hemolysin. This approach is not without its downside however, as exposing the underlying transitional cells can allow the UPEC to penetrate further and form quiescent cellular reservoirs that can lead to chronic cystitis [99].
Perhaps unsurprisingly, given the nature of host-pathogen interactions, UPEC strains that avoid activation of NLRP3 show increased virulence or asymptomatic colonization [100]. For example, Waldhuber et al. [101] found that UPEC strain CFT073 avoids activation of the NLRP3 inflammasome by expressing Toll/IL-1 receptor-containing protein C (TcpC), a multifunctional protein that inhibits NLRP3 activation by binding both NLRP3 and pro-caspase-1, consequently suppressing the processing and activation of pro-caspase-1. TcpC also blocks signaling through the TLRs necessary for priming of NLRP3. Inhibition of TcpC expression resulted in greater activation of NLRP3 and elevated urinary IL-1β secretion compared to mice infected with wildtype UPEC strains [102]. Thus, NLRP3 may be a central player in the host-pathogen interaction during UPEC UTIs.
Group B Streptococcal UTIs
Inflammasomes have also been implicated in the host response to UTIs by gram-positive bacteria such as Streptococcus agalactiae, a Group B streptococcus (GBS) [103]. Their involvement was suspected when the initial stages of GBS UTIs were found to be characterized by secretion of IL-1β [104]. With uropathogenic GBS, β-hemolysin (a PAMP) activates the NLRP3 inflammasome by causing lysosomal leakage which permits the escape of GBS RNA (another, independent, PAMP) into the cytosol. Transfection of GBS RNA directly into the cytosol of GBS-infected cells increased IL-1β production while, conversely, introduction of RNase into this compartment decreased cytokine secretion [105]. Although bacterial RNA can clearly be important in inflammasome activation, it is not thought that NLRP3 is directly activated by the RNA. It has been hypothesized that the RNA could initiate the recruitment of mitochondrial antiviral signaling protein that mediates membrane damage through ROS production with subsequent release of intracellular K+ [106,107].
As might be expected, NLRP3-mediated inflammation in response to GBS is important for control of the infection. Costa et al. [103] infected mice with GBS and found that those lacking NLRP3 inflammasome components had increased mortality and an elevated GBS burden compared to controls. In another study, mice lacking IL-1 receptors were also found to be more susceptible to GBS infection compared to controls [108]. Finally, as was seen with UPEC [99], activation of NLRP3 results in urothelial exfoliation, exposing deeper layers and resulting in the formation of quiescent reservoirs leading to chronic UTIs. Thus, NLRP3 seems to be in a delicate balance where its activation is necessary for an adequate host response, but may also contribute to chronic colonization or infection. The therapeutic challenge, then, is to modulate the inflammatory response rather than eliminate it so that the advantageous mechanisms are preserved or enhanced while deleterious effects are minimized.
Bladder Cancer
While NLRP3 gene polymorphisms have been associated with melanoma, myeloma, and colorectal cancer [109,110,111], not until recently was there an investigation of the role of inflammasomes in bladder cancer [112]. Poli et al. [112] examined mRNA in urine from human subjects with and without bladder cancer for expression of inflammasome components and cytokeratin 20 mRNA (CK20), a marker of urothelial differentiation that is highly expressed in bladder cancer [113]. They found that compared to healthy controls, patients with bladder cancer on biopsy had an increased expression of NLRP3 and NLR family apoptosis inhibiting protein [NAIP; an important adaptor for the NLR containing a caspase recruitment domain 4 (NLRC4) inflammasome]. In addition, NLRP3 expression was increased in low-grade tumors, while NAIP expression was increased in high-grade tumors, although the significance of that is unknown. They also saw an increase in NLRP3 gene expression in patients with non-malignant inflammatory lesions on biopsy compared to healthy controls. The group concluded that concurrent analysis of NLRP3 or NAIP expression with CK20 enhanced the sensitivity and specificity of detection of bladder cancer. Therefore, while the mechanism of NLRP3 and NLRC4 inflammasomes in the genesis or proliferation of bladder cancer is unknown, their expression is present and may serve as a potential diagnostic biomarker.
Non-NLRP3 Inflammasomes in the Bladder and Their Potential Roles
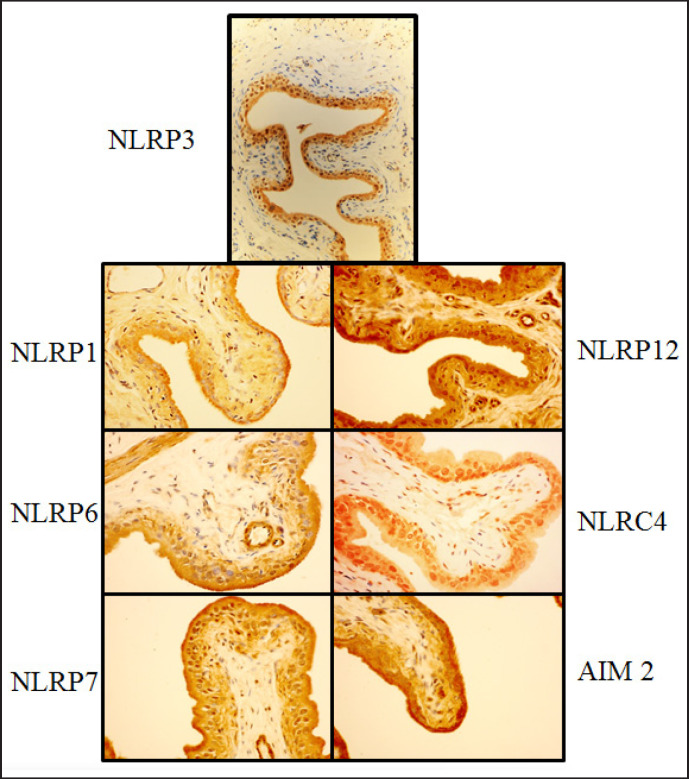
NLRP3 is the most studied inflammasome in the bladder and elsewhere. While most bladder pathology has been linked to this inflammasome, other inflammasomes may have potential roles as well. Indeed, many other NLRs that form inflammasomes, along with AIM2 which also forms an inflammasome, have been localized to the urothelia [95] (fig. 7) and thus may be important mediators in bladder inflammatory pathologies through unexplored mechanisms. As the RIG-I inflammasome has not been localized to the bladder urothelium, it was not included in the following section. Table 2 lists the inflammasome-forming PPRs that have been found in the bladder and their known and potential roles in the bladder, which will be discussed in more detail below.
Fig. 7.
Immunohistochemistry localizing inflammasomes to the urothelium. Antibody staining of rat bladders for NLRP1, NLRP3, NLRP6, NLRP7, NLRP12, NLRC4, and AIM2 and photographed at high magnification. Differential staining demonstrates that each inflammasome preferentially localizes to the urothelium. Reprinted with permission from [19].
Table 2.
Inflammasomes localized to the bladder urothelium and their potentially associated bladder pathologies
| Inflammasome | Bladder condition(s) associated with inflammasome | Potential bladder condition(s) associated with inflammasome |
|---|---|---|
| NLRP1 | UTI | |
| NLRP3 | cyclophosphamide-induced hemorrhagic cystitis BOO UTI bladder cancer |
|
| NLRP6 | UTI bladder stone formation radiation cystitis bladder cancer interstitial cystitis |
|
| NLRP7 | UTI | |
| NLRP12 | UTI interstitial cystitis |
|
| NLRC4 | bladder cancer | UTI |
| AIM2 | UTI |
Seven inflammasomes in the NLR and AIM receptor family have been localized to the bladder urothelium. This table lists the known and potentially associated bladder inflammation diseases mediated by the inflammasomes.
NLRP1 Inflammasome
NLRP1 was the first inflammasome to be described [1] and it plays a role in prostate inflammation [94]. It differs mechanistically from NLRP3 by undergoing an autolytic proteolysis necessary for activation [114]. It has been implicated in the inflammatory response to Bacillus anthracis[115], but, importantly, it has also been found to respond to muramyl dipeptide, which is a common peptidoglycan in gram positive and negative bacteria [116]. Therefore, the NLRP1 inflammasome may play a role in mediating UTI responses.
NLRP6 Inflammasome
The NLRP6 inflammasome differs from NLRP3 by not only activating proinflammatory cytokines, but by also having the potential to directly downregulate the NF-κβ and mitogen-activated protein kinase pathways [117,118]. NLRP6 is best known for playing two distinct roles in the gut: regulating intestinal microbiota and maintaining the protective mucus layer by facilitating autophagy in intestinal goblet cells [119]. While no PAMP has been found to specifically activate NLRP6 [95], its roles in the intestine may reflect its potential role in the bladder, which is known to have both asymptomatic biotic flora [120,121] and significant surface mucus [122]. Intriguingly, its potential role in regulating mucus may make it a useful therapeutic target since dysfunction of bladder surface mucins has been implicated in many bladder disorders including UTIs, calculus formation, radiation cystitis, carcinogenesis, and interstitial cystitis.
NLRP7 Inflammasome
Little is known about the mechanism of activation of NLRP7, but it has been shown to form an inflammasome in response to microbial acylated lipopeptides, common in bacteria such as pseudomonas, which is a prevalent UTI pathogen [123,124]. Furthermore, NLRP7-produced IL-1β and IL-18 prevent replication of bacteria inside human macrophages [125]. While macrophages are very different from urothelial cells, NLRP7 may play a role in controlling intracellular bacterial communities known to cause chronic cystitis.
NLRP12 Inflammasome
Another poorly understood NLR family member, NLRP12, seems to depend on a functional type III secretion (T3S) system for its activation [126]. As gram-negative bacteria, such as pseudomonas, are known to invade host cells using a T3S system [127], the NLRP12 inflammasome may play a role in gram-negative UTI. NLRP12 may also be involved in bladder disease associated with idiopathic sterile bladder inflammation, such as interstitial cystitis, for NLRP12 mutations have been associated with hereditary periodic fever syndromes, another unprovoked, self-limited inflammatory attack mediated by IL-1β [128,129].
NLRC4 Inflammasome
Even though it has been implicated as a biomarker in bladder cancer, the NLRC4 inflammasome is most commonly known to respond to macrophage invasion by gram-negative bacteria such as Salmonella typhimurium, Legionella pneumophila, and Pseudomonas aeruginosa[130,131,132]. Each of these bacteria send their virulence factors, such as flagellin and the inner-rod protein PrgJ, into the cell's cytoplasm through the bacterial T3S system [133]. These bacterial components are recognized in the cytosol by NAIPs, which then facilitate formation of the NLRC4 inflammasome. By drawing a comparison to its role in pulmonary pseudomonal infections [134], NLRC4 may be a central mediator in pseudomonal UTI. Furthermore, UPECs express aT3S system [135] and therefore may also interact with NLRC4. Finally, our lab has shown that placing flagellin, a well-established activator of NLRC4, in the lumen of the bladder activates caspase-1 activity [51] suggesting this system in the bladder is primed and ready to respond to invading bacteria.
AIM2 Inflammasome
Although AIM2 is not an NLR member, it does form an inflammasome and activate caspase-1. AIM2 is characterized by its Hin200 domains that bind to bacterial and viral double-stranded DNA to trigger inflammasome formation [14]. Therefore, the AIM2 inflammasome may respond to uropathogenic bacteria that release their DNA into the cytoplasm (such as through a T3S system or cytosolic bacteriolysis) or viruses that cause cystitis.
Additional Bladder Disorders
While inflammation is well understood to underlie bladder dysfunction in several common diseases, the role of the inflammasome as the central mediator has only recently been explored. In addition to the conditions just discussed, inflammasomes may be involved in other bladder pathologies like interstitial cystitis and diabetic bladder dysfunction, two common and debilitating diseases associated with bladder inflammation. There is also a potential role for inflammasomes in the inflammation caused by bladder stones. Indeed, monosodium urate is a well-known DAMP and a major component of bladder stones [136]. Our lab has shown that monosodium urate placed in the lumen of the bladder is sufficient to trigger inflammasome activation [51]. By understanding the role of each inflammasome in various pathological conditions, we may be able to target them with therapeutic agents to prevent these diseases and their symptoms.
Potential Treatments
As mentioned, our lab has used glyburide and anakinra, two FDA-approved medications, to inhibit the NLRP3 inflammasome and block the IL-1 receptor, respectively. Although glyburide is very useful experimentally, its hypoglycemic properties would probably limit widespread use in the general clinical population. Anakinra may be a more readily accepted anti-inflammasome medication, since it is already used for treating inflammatory diseases such as rheumatoid arthritis [137], but its current cost, side effect profile (including immunosuppression), and route of administration (subcutaneous) may limit its use as well. Besides these FDA-approved medications, the Chinese licorice Glycyrrhiza uralensis has been used for centuries as an anti-inflammatory treatment [138]. Recently, one of the active ingredients in this plant, isoliquirtigenin, has been found to be a naturally occurring NLRP3 inhibitor [139]. Not surprisingly, pharmaceutical companies are also developing a wide variety novel medications that target the NLRP3/ IL-1β pathway. These include small molecule inhibitors of NLRP3 such as AC-201 (TWi Biotechnology, Taiwan), MCC950 (Avistron, UK) and Parthenolide (Ashbury Biologicals, Canada) [140,141,142], caspase inhibitors such as Emricasan/IDN-6556 (Conatus Pharmaceuticals, USA) [143] and modified antibodies against IL-1β such as gevokizumab/XOMA 052 (Xoma Corporation, USA), HL 2351/GX P4, Handok, S. Korea) and Canakinumab/ Ilaris/ACZ885 (Novartis, Switzerland).
Conclusion
In both normal and pathological situations, the bladder is a hostile environment and source of many agents that activate inflammasomes to initiate inflammation. While the initial symptoms of inflammation that force a patient to seek a physician are typically irritative voiding patterns and feelings of urinary urgency, long-term or repetitive insults can lead to more dangerous and irreversible outcomes. Further explanation of the inflammasomes and their roles in urologic pathology will greatly enhance our understanding of urologic disease and provide possible therapeutic targets for intervention and management of the debilitating symptoms.
Abbreviations
AIM2 = absent in melanoma 2
ASC = apoptosis-associated speck-like protein containing a CARD
ATP = adenosine triphosphate
BOO = bladder outlet obstruction
BPH = benign prostatic hyperplasia
CARD = caspase recruitment domains
CK20 = cytokeratin 20
DAMP = danger (or damage) associated molecular pattern
FADD = Fas-associated protein with death domain
FDA = Food and Drug Administration
GBS = group B streptococcus
HMGB1= high-mobility group box 1
IGF = insulin-like growth factor
LPS = lipopolysaccharide
LRR = leucine-rich repeat
MAVS = mitochondrial antiviral signaling protein
MESNA = 2-mercaptoethane sulfonate sodium
MSU = monosodium urate
NACHT = nucleotide-binding and oligomerization
NAIP = NLR family apoptosis inhibiting protein
NEK = NIMA-related kinase
NIMA = never in mitosis gene a
NLR = NOD-like receptors
NLRP3 = NACHT, LRR and PYD domains-containing protein 3
NOD = nucleotide-binding and oligomerization domain
PAMP = pathogen-associated molecular pattern
PRR = pattern recognition receptor
PYD = pyrin domain
RIG-1 = retinoic acid-inducible gene-I
RIPK1 = receptor-interacting protein kinase 1
ROS = reactive oxygen species
TIR = toll/interleukin-1 receptor
TLR = toll-like receptor
TNFR = tumor necrosis factor receptor
TRIF = TIR-domain-containing adapter-inducing interferon-β toll/interleukin-1 receptor-containing adapter-inducing interferon-β
T3S = type III secretion
UPEC = uropathogenic Escherichia coli
UTI = urinary tract infection
References
- 1.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 2.Purves JT, Hughes FM., Jr Inflammasomes in the urinary tract: a disease-based review. Am J Physiol Renal Physiol. 2016;311:F653–F662. doi: 10.1152/ajprenal.00607.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hamilton C, Tan L, Miethke T, Anand PK. Immunity to uropathogens: the emerging roles of inflammasomes. Nat Rev Urol. 2017;14:284–295. doi: 10.1038/nrurol.2017.25. [DOI] [PubMed] [Google Scholar]
- 4.Mogensen TH. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin Microbiol Rev. 2009;22:240–273. doi: 10.1128/CMR.00046-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lamkanfi M, Dixit VM. Modulation of inflammasome pathways by bacterial and viral pathogens. J Immunol. 2011;187:597–602. doi: 10.4049/jimmunol.1100229. [DOI] [PubMed] [Google Scholar]
- 6.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 7.Lappas M. NOD1 and NOD2 regulate proinflammatory and prolabor mediators in human fetal membranes and myometrium via nuclear factor-kappa B. Biol Reproduct. 2013;89:14. doi: 10.1095/biolreprod.113.110056. [DOI] [PubMed] [Google Scholar]
- 8.Wilmanski JM, Petnicki-Ocwieja T, Kobayashi KS. NLR proteins: integral members of innate immunity and mediators of inflammatory diseases. J Leukoc Biol. 2008;83:13–30. doi: 10.1189/jlb.0607402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kayagaki N, Stowe IB, Lee BL, O'Rourke K, Anderson K, Warming S, Cuellar T, Haley B, Roose-Girma M, Phung QT, Liu PS, Lill JR, Li H, Wu J, Kummerfeld S, Zhang J, Lee WP, Snipas SJ, Salvesen GS, Morris LX, Fitzgerald L, Zhang Y, Bertram EM, Goodnow CC, Dixit VM. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature. 2015;526:666–671. doi: 10.1038/nature15541. [DOI] [PubMed] [Google Scholar]
- 10.Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, Zhuang Y, Cai T, Wang F, Shao F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660–665. doi: 10.1038/nature15514. [DOI] [PubMed] [Google Scholar]
- 11.Lamkanfi M, Dixit VM. Mechanisms and functions of inflammasomes. Cell. 2014;157:1013–1022. doi: 10.1016/j.cell.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 12.Fink SL, Cookson BT. Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cell Microbiol. 2006;8:1812–1825. doi: 10.1111/j.1462-5822.2006.00751.x. [DOI] [PubMed] [Google Scholar]
- 13.Poeck H, Bscheider M, Gross O, Finger K, Roth S, Rebsamen M, Hannesschläger N, Schlee M, Rothenfusser S, Barchet W, Kato H, Akira S, Inoue S, Endres S, Peschel C, Hartmann G, Hornung V, Ruland J. Recognition of RNA virus by RIG-I results in activation of CARD9 and inflammasome signaling for interleukin 1 beta production. Nat Immunol. 2010;11:63–69. doi: 10.1038/ni.1824. [DOI] [PubMed] [Google Scholar]
- 14.Hornung V, Ablasser A, Charrel-Dennis M, Bauernfeind F, Horvath G, Caffrey DR, Latz E, Fitzgerald KA. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009;458:514–518. doi: 10.1038/nature07725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, Fernandes-Alnemri T, Wu J, Monks BG, Fitzgerald KA, Hornung V, Latz E. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol. 2009;183:787–791. doi: 10.4049/jimmunol.0901363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mariathasan S, Weiss DS, Newton K, McBride J, O'Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM, Dixit VM. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 17.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 18.Kummer JA, Broekhuizen R, Everett H, Agostini L, Kuijk L, Martinon F, van Bruggen R, Tschopp J. Inflammasome components NALP 1 and 3 show distinct but separate expression profiles in human tissues suggesting a site-specific role in the inflammatory response. J Histochem Cytochem. 2007;55:443–452. doi: 10.1369/jhc.6A7101.2006. [DOI] [PubMed] [Google Scholar]
- 19.Hughes FM, Jr, Turner DP, Todd Purves J. The potential repertoire of the innate immune system in the bladder: expression of pattern recognition receptors in the rat bladder and a rat urothelial cell line (MYP3 cells) Int Urol Nephrol. 2015;47:1953–1964. doi: 10.1007/s11255-015-1126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo H, Callaway JB, Ting JP. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med. 2015;21:677–687. doi: 10.1038/nm.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sutterwala FS, Haasken S, Cassel SL. Mechanism of NLRP3 inflammasome activation. Ann N Y Acad Sci. 2014;1319:82–95. doi: 10.1111/nyas.12458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wen H, Miao EA, Ting JP. Mechanisms of NOD-like receptor-associated inflammasome activation. Immunity. 2013;39:432–441. doi: 10.1016/j.immuni.2013.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gaidt MM, Hornung V. Alternative inflammasome activation enables IL-1beta release from living cells. Curr Opin Immunol. 2016;44:7–13. doi: 10.1016/j.coi.2016.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He Y, Hara H, Nunez G. Mechanism and regulation of NLRP3 inflammasome activation. Trends Biochem Sci. 2016;41:1012–1021. doi: 10.1016/j.tibs.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gaidt MM, Ebert TS, Chauhan D, Schmidt T, Schmid-Burgk JL, Rapino F, Robertson AA, Cooper MA, Graf T, Hornung V. Human monocytes engage an alternative inflammasome pathway. Immunity. 2016;44:833–846. doi: 10.1016/j.immuni.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 26.Patel MN, Carroll RG, Galvan-Pena S, Mills EL, Olden R, Triantafilou M, Wolf AI, Bryant CE, Triantafilou K, Masters SL. Inflammasome priming in sterile inflammatory disease. Trends Mol Med. 2017;23:165–180. doi: 10.1016/j.molmed.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 27.Franchi L, Eigenbrod T, Nunez G. Cutting edge: TNF-alpha mediates sensitization to ATP and silica via the NLRP3 inflammasome in the absence of microbial stimulation. J Immunol. 2009;183:792–796. doi: 10.4049/jimmunol.0900173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Py BF, Kim MS, Vakifahmetoglu-Norberg H, Yuan J. Deubiquitination of NLRP3 by BRCC3 critically regulates inflammasome activity. Mol Cell. 2013;49:331–338. doi: 10.1016/j.molcel.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 29.Juliana C, Fernandes-Alnemri T, Kang S, Farias A, Qin F, Alnemri ES. Non-transcriptional priming and deubiquitination regulate NLRP3 inflammasome activation. J Biol Chem. 2012;287:36617–36622. doi: 10.1074/jbc.M112.407130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dostert C, Petrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320:674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Munoz-Planillo R, Kuffa P, Martinez-Colon G, Smith BL, Rajendiran TM, Nunez G. K+ efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity. 2013;38:1142–1153. doi: 10.1016/j.immuni.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harijith A, Ebenezer DL, Natarajan V. Reactive oxygen species at the crossroads of inflammasome and inflammation. Front Physiol. 2014;5:352. doi: 10.3389/fphys.2014.00352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee GS, Subramanian N, Kim AI, Aksentijevich I, Goldbach-Mansky R, Sacks DB, Germain RN, Kastner DL, Chae JJ. The calcium-sensing receptor regulates the NLRP3 inflammasome through Ca2+ and cAMP. Nature. 2012;492:123–127. doi: 10.1038/nature11588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murakami T, Ockinger J, Yu J, Byles V, McColl A, Hofer AM, Horng T. Critical role for calcium mobilization in activation of the NLRP3 inflammasome. Proc Natl Acad Sci USA. 2012;109:11282–11287. doi: 10.1073/pnas.1117765109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rossol M, Pierer M, Raulien N, Quandt D, Meusch U, Rothe K, Schubert K, Schöneberg T, Schaefer M, Krügel U, Smajilovic S, Bräuner-Osborne H, Baerwald C, Wagner U. Extracellular Ca2+ is a danger signal activating the NLRP3 inflammasome through G protein-coupled calcium sensing receptors. Nat Commun. 2012;3:1329. doi: 10.1038/ncomms2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Katsnelson MA, Rucker LG, Russo HM, Dubyak GR. K+ efflux agonists induce NLRP3 inflammasome activation independently of Ca2+ signaling. J Immunol. 2015;194:3937–3952. doi: 10.4049/jimmunol.1402658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Franchi L, Kanneganti TD, Dubyak GR, Nunez G. Differential requirement of P2X7 receptor and intracellular K+ for caspase-1 activation induced by intracellular and extracellular bacteria. J Biol Chem. 2007;282:18810–18818. doi: 10.1074/jbc.M610762200. [DOI] [PubMed] [Google Scholar]
- 38.Petrilli V, Papin S, Dostert C, Mayor A, Martinon F, Tschopp J. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ. 2007;14:1583–1589. doi: 10.1038/sj.cdd.4402195. [DOI] [PubMed] [Google Scholar]
- 39.Bortner CD, Hughes FM, Jr, Cidlowski JA. A primary role for K+ and Na+ efflux in the activation of apoptosis. J Biol Chem. 1997;272:32436–32442. doi: 10.1074/jbc.272.51.32436. [DOI] [PubMed] [Google Scholar]
- 40.Hughes FM, Jr, Bortner CD, Purdy GD, Cidlowski JA. Intracellular K+ suppresses the activation of apoptosis in lymphocytes. J Biol Chem. 1997;272:30567–30576. doi: 10.1074/jbc.272.48.30567. [DOI] [PubMed] [Google Scholar]
- 41.Bortner CD, Cidlowski JA. Cell shrinkage and monovalent cation fluxes: role in apoptosis. Arch Biochem Biophys. 2007;462:176–188. doi: 10.1016/j.abb.2007.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.He Y, Zeng MY, Yang D, Motro B, Nunez G. NEK7 is an essential mediator of NLRP3 activation downstream of potassium efflux. Nature. 2016;530:354–357. doi: 10.1038/nature16959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shi H, Wang Y, Li X, Zhan X, Tang M, Fina M, Su L, Pratt D, Bu CH, Hildebrand S, Lyon S, Scott L, Quan J, Sun Q, Russell J, Arnett S, Jurek P, Chen D, Kravchenko VV, Mathison JC, Moresco EM, Monson NL, Ulevitch RJ, Beutler B. NLRP3 activation and mitosis are mutually exclusive events coordinated by NEK7, a new inflammasome component. Nat Immunol. 2016;17:250–258. doi: 10.1038/ni.3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ding J, Wang K, Liu W, She Y, Sun Q, Shi J, Sun H, Wang DC, Shao F. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature. 2016;535:111–116. doi: 10.1038/nature18590. [DOI] [PubMed] [Google Scholar]
- 45.Sborgi L, Ruhl S, Mulvihill E, Pipercevic J, Heilig R, Stahlberg H, Farady CJ, Müller DJ, Broz P, Hiller S. GSDMD membrane pore formation constitutes the mechanism of pyroptotic cell death. EMBO J. 2016;35:1766–1778. doi: 10.15252/embj.201694696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aglietti RA, Estevez A, Gupta A, Ramirez MG, Liu PS, Kayagaki N, Ciferri C, Dixit VM, Dueber EC. GsdmD p30 elicited by caspase-11 during pyroptosis forms pores in membranes. Proc Natl Acad Sci USA. 2016;113:7858–7863. doi: 10.1073/pnas.1607769113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kayagaki N, Warming S, Lamkanfi M, Vande Walle L, Louie S, Dong J, Newton K, Qu Y, Liu J, Heldens S, Zhang J, Lee WP, Roose-Girma M, Dixit VM. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479:117–121. doi: 10.1038/nature10558. [DOI] [PubMed] [Google Scholar]
- 48.Shi J, Zhao Y, Wang Y, Gao W, Ding J, Li P, Hu L, Shao F. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature. 2014;514:187–192. doi: 10.1038/nature13683. [DOI] [PubMed] [Google Scholar]
- 49.Wang S, Miura M, Jung YK, Zhu H, Li E, Yuan J. Murine caspase-11, an ICE-interacting protease, is essential for the activation of ICE. Cell. 1998;92:501–509. doi: 10.1016/s0092-8674(00)80943-5. [DOI] [PubMed] [Google Scholar]
- 50.Gross CJ, Mishra R, Schneider KS, Médard G, Wettmarshausen J, Dittlein DC, Shi H, Gorka O, Koenig PA, Fromm S, Magnani G, Ćiković T, Hartjes L, Smollich J, Robertson AAB7, Cooper MA, Schmidt-Supprian M, Schuster M, Schroder K, Broz P, Traidl-Hoffmann C4, Beutler B, Kuster B, Ruland J, Schneider S, Perocchi F, Groß O. K+ efflux-independent NLRP3 inflammasome activation by small molecules targeting mitochondria. Immunity. 2016;45:761–773. doi: 10.1016/j.immuni.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 51.Hughes FM, Jr, Vivar NP, Kennis JG, Pratt-Thomas JD, Lowe DW, Shaner BE, Nietert PJ, Spruill LS, Purves JT. Inflammasomes are important mediators of cyclophosphamide-induced bladder inflammation. Am J Physiol Renal physiol. 2014;306:F299–308. doi: 10.1152/ajprenal.00297.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smaldone MC, Vodovotz Y, Tyagi V, Barclay D, Philips BJ, Yoshimura N, Chancellor MB, Tyagi P. Multiplex analysis of urinary cytokine levels in rat model of cyclophosphamide-induced cystitis. Urology. 2009;73:421–426. doi: 10.1016/j.urology.2008.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Leite CA, Alencar VT, Melo DL, Mota JM, Melo PH, Mourão LT, Wong DV, Magalhães PJ, Santos AA, Brito GA, Lima-Júnior RC, Cunha FQ, Ribeiro RA. Target inhibition of IL-1 receptor prevents Ifosfamide induced hemorrhagic cystitis in mice. J Urol. 2015;194:1777–1786. doi: 10.1016/j.juro.2015.07.088. [DOI] [PubMed] [Google Scholar]
- 54.Ribeiro RA, Freitas HC, Campos MC, Santos CC, Figueiredo FC, Brito GA, Cunha FQ. Tumor necrosis factor-alpha and interleukin-1beta mediate the production of nitric oxide involved in the pathogenesis of ifosfamide induced hemorrhagic cystitis in mice. J Urol. 2002;167:2229–2234. [PubMed] [Google Scholar]
- 55.Batista CK, Brito GA, Souza ML, Leitao BT, Cunha FQ, Ribeiro RA. A model of hemorrhagic cystitis induced with acrolein in mice. Braz J Med Biol Res. 2006;39:1475–1481. doi: 10.1590/s0100-879x2006001100011. [DOI] [PubMed] [Google Scholar]
- 56.Lamkanfi M, Mueller JL, Vitari AC, Misaghi S, Fedorova A, Deshayes K, Lee WP, Hoffman HM, Dixit VM. Glyburide inhibits the Cryopyrin/Nalp3 inflammasome. J Cell Biol. 2009;187:61–70. doi: 10.1083/jcb.200903124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Haldar S, Dru C, Choudhury D, Mishra R, Fernandez A, Biondi S, Liu Z, Shimada K, Arditi M, Bhowmick NA. Inflammation and pyroptosis mediate muscle expansion in an interleukin-1beta (IL-1beta)-dependent manner. J Biological Chemistry. 2015;290:6574–6583. doi: 10.1074/jbc.M114.617886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Metcalfe PD, Wang J, Jiao H, Huang Y, Hori K, Moore RB, Tredget EE. Bladder outlet obstruction: progression from inflammation to fibrosis. BJU Int. 2010;106:1686–1694. doi: 10.1111/j.1464-410X.2010.09445.x. [DOI] [PubMed] [Google Scholar]
- 59.Oka M, Fukui T, Ueda M, Tagaya M, Oyama T, Tanaka M. Suppression of bladder oxidative stress and inflammation by a phytotherapeutic agent in a rat model of partial bladder outlet obstruction. J Urol. 2009;182:382–390. doi: 10.1016/j.juro.2009.02.104. [DOI] [PubMed] [Google Scholar]
- 60.Deveaud CM, Macarak EJ, Kucich U, Ewalt DH, Abrams WR, Howard PS. Molecular analysis of collagens in bladder fibrosis. J Urol. 1998;160:1518–1527. [PubMed] [Google Scholar]
- 61.Hughes FM, Govada V, Sexton SJ, Purves J. Fibrosis in the bladder in response to outlet obstruction is triggered through the NLRP3 inflammasome and the production of IL-1β. Transl Androl Urol. 2016;5((Suppl 2)):AB279. doi: 10.1152/ajprenal.00128.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hughes FM, Jr, Hill HM, Wood CM, Edmondson AT, Dumas A, Foo WC, Oelsen JM, Rac G, Purves JT. The NLRP3 inflammasome mediates inflammation produced by bladder outlet obstruction. J Urol. 2016;195:1598–1605. doi: 10.1016/j.juro.2015.12.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lutolf R, Hughes FM, Purves J. NLRP3/ IL-1β mediates denervation during bladder outlet obstruction in rats. Transl Androl Urol. 2016;5((Suppl 2)):AB298. [Google Scholar]
- 64.Kanno Y, Mitsui T, Kitta T, Moriya K, Tsukiyama T, Hatakeyama S, Nonomura K. The inflammatory cytokine IL-1beta is involved in bladder remodeling after bladder outlet obstruction in mice. Neurourol Urodyn. 2016;35:3773–3778. doi: 10.1002/nau.22721. [DOI] [PubMed] [Google Scholar]
- 65.Abderrazak A, Syrovets T, Couchie D, El Hadri K, Friguet B, Simmet T, Rouis M. NLRP3 inflammasome: From a danger signal sensor to a regulatory node of oxidative stress and inflammatory diseases. Redox Biol. 2015;4:296–307. doi: 10.1016/j.redox.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yamaguchi O, Nomiya M, Andersson KE. Functional consequences of chronic bladder ischemia. Neurourol Urodyn. 2014;33:54–58. doi: 10.1002/nau.22517. [DOI] [PubMed] [Google Scholar]
- 67.Gombault A, Baron L, Couillin I. ATP release and purinergic signaling in NLRP3 inflammasome activation. Front Immunol. 2012;3:414. doi: 10.3389/fimmu.2012.00414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Olsen SM, Stover JD, Nagatomi J. Examining the role of mechanosensitive ion channels in pressure mechanotransduction in rat bladder urothelial cells. Ann Biomed Eng. 2011;39:688–697. doi: 10.1007/s10439-010-0203-3. [DOI] [PubMed] [Google Scholar]
- 69.Dunton CL, Hughes FM, Purves J, Nagatomi J. In vitro evaluation of hydrostatic pressure on ATP release and Caspase-1 activation in rat urothelial cells. Transl Androl Urol. 1916;5((Suppl 2)):AB276. [Google Scholar]
- 70.Chen S, Peng C, Wei X, Luo D, Lin Y, Yang T, Jin X, Gong L, Li H, Wang K. Simulated physiological stretch increases expression of extracellular matrix proteins in human bladder smooth muscle cells via integrin alpha4/alphav-FAK-ERK1/2 signaling pathway. World J Urology. 2017;35:1247–1254. doi: 10.1007/s00345-016-1993-1. [DOI] [PubMed] [Google Scholar]
- 71.Dunning-Davies BM, Fry CH, Mansour D, Ferguson DR. The regulation of ATP release from the urothelium by adenosine and transepithelial potential. BJU Int. 2013;111:505–513. doi: 10.1111/j.1464-410X.2012.11421.x. [DOI] [PubMed] [Google Scholar]
- 72.Mochizuki T, Sokabe T, Araki I, Fujishita K, Shibasaki K, Uchida K, Naruse K, Koizumi S, Takeda M, Tominaga M. The TRPV4 cation channel mediates stretchevoked Ca2+ influx and ATP release in primary urothelial cell cultures. J Biol Chem. 2009;284:21257–21264. doi: 10.1074/jbc.M109.020206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ochodnicky P, Michel MB, Butter JJ, Seth J, Panicker JN, Michel MC. Bradykinin modulates spontaneous nerve growth factor production and stretch-induced ATP release in human urothelium. Pharmacol Res. 2013;70:147–154. doi: 10.1016/j.phrs.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 74.Young JS, Matharu R, Carew MA, Fry CH. Inhibition of stretching-evoked ATP release from bladder mucosa by anticholinergic agents. BJU Int. 2012;110:E397–401. doi: 10.1111/j.1464-410X.2012.10966.x. [DOI] [PubMed] [Google Scholar]
- 75.Moro C, Uchiyama J, Chess-Williams R. Urothelial/lamina propria spontaneous activity and the role of M3 muscarinic receptors in mediating rate responses to stretch and carbachol. Urology. 2011;78:1442e9–15. doi: 10.1016/j.urology.2011.08.039. [DOI] [PubMed] [Google Scholar]
- 76.Cheng Y, Mansfield KJ, Sandow SL, Sadananda P, Burcher E, Moore KH. Porcine bladder urothelial, myofibroblast, and detrusor muscle cells: characterization and ATP release. Front Pharmacol. 2011;2:27. doi: 10.3389/fphar.2011.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ferguson DR, Kennedy I, Burton TJ. ATP is released from rabbit urinary bladder epithelial cells by hydrostatic pressure changes-a possible sensory mechanism? J Physiol. 1997;505((Pt 2)):503–511. doi: 10.1111/j.1469-7793.1997.503bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Beckel JM, Birder LA. Differential expression and function of nicotinic acetylcholine receptors in the urinary bladder epithelium of the rat. J Physiology. 2012;590:1465–1480. doi: 10.1113/jphysiol.2011.226860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McLatchie LM, Young JS, Fry CH. Regulation of ACh release from guinea pig bladder urothelial cells: potential role in bladder filling sensations. Br J Pharmacol. 2014;171:3394–3403. doi: 10.1111/bph.12682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sun Y, Chai TC. Augmented extracellular ATP signaling in bladder urothelial cells from patients with interstitial cystitis. Am J Physiol Cell physiol. 2006;290:C27–34. doi: 10.1152/ajpcell.00552.2004. [DOI] [PubMed] [Google Scholar]
- 81.Bugyei-Twum A, Abadeh A, Thai K, Zhang Y, Mitchell M, Kabir G, Connelly KA. Suppression of NLRP3 inflammasome activation ameliorates chronic kidney disease-induced cardiac fibrosis and diastolic dysfunction. Sci Rep. 2016;6:39551. doi: 10.1038/srep39551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sayan M, Mossman BT. The NLRP3 inflammasome in pathogenic particle and fibre-associated lung inflammation and diseases. Part Fibre Toxicol. 2016;13:51. doi: 10.1186/s12989-016-0162-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gong W, Mao S, Yu J, Song J, Jia Z, Huang S, Zhang A. NLRP3 deletion protects against renal fibrosis and attenuates mitochondrial abnormality in mouse with 5/6 nephrectomy. Am J Physiol Renal Physiol. 2016;310:F1081–1088. doi: 10.1152/ajprenal.00534.2015. [DOI] [PubMed] [Google Scholar]
- 84.Gasse P, Riteau N, Vacher R, Michel ML, Fautrel A, Di Padova F, Fick L, Charron S, Lagente V, Eberl G, Le Bert M, Quesniaux VF, Huaux F, Leite-de-Moraes M, Ryffel B, Couillin I. IL-1 and IL-23 mediate early IL-17A production in pulmonary inflammation leading to late fibrosis. PloS One. 2011;6:e23185. doi: 10.1371/journal.pone.0023185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sexton SJ, Hughes FM, Purves JT.Fibrosis in the bladder in response to outlet obstruction is triggered through the NLRP3 inflammasome and the production of IL-1β. North Carolina Urology Association, Durham, ND 2016.
- 86.Harrison SC, Hunnam GR, Farman P, Ferguson DR, Doyle PT. Bladder instability and denervation in patients with bladder outflow obstruction. Br J Urol. 1987;60:519–522. doi: 10.1111/j.1464-410x.1987.tb05033.x. [DOI] [PubMed] [Google Scholar]
- 87.Speakman MJ, Brading AF, Gilpin CJ, Dixon JS, Gilpin SA, Gosling JA. Bladder outflow obstruction-a cause of denervation supersensitivity. J Urol. 1987;138:1461–1466. doi: 10.1016/s0022-5347(17)43675-5. [DOI] [PubMed] [Google Scholar]
- 88.de Jongh R, Dambros M, Haenen GR, den Hartog GJ, Bast A, van Kerrebroeck PE, van Koeveringe GA. Partial bladder outlet obstruction reduces the tissue antioxidant capacity and muscle nerve density of the guinea pig bladder. Neurourol Urodyn. 2009;28:461–467. doi: 10.1002/nau.20677. [DOI] [PubMed] [Google Scholar]
- 89.Nyirady P, Thiruchelvam N, Fry CH, Godley ML, Winyard PJ, Peebles DM, Woolf AS, Cuckow PM. Effects of in utero bladder outflow obstruction on fetal sheep detrusor contractility, compliance and innervation. J Urol. 2002;168:1615–1620. doi: 10.1016/S0022-5347(05)64530-2. [DOI] [PubMed] [Google Scholar]
- 90.Levin RM, Levin SS, Zhao Y, Buttyan R. Cellular and molecular aspects of bladder hypertrophy. Eur Urol. 1997;32((Suppl 1)):15–21. [PubMed] [Google Scholar]
- 91.Barendrecht MM, Chichester P, Michel MC, Levin RM. Effect of short-term outlet obstruction on rat bladder nerve density and contractility. Auton Autacoid Pharmacol. 2007;27:47–53. doi: 10.1111/j.1474-8673.2006.00393.x. [DOI] [PubMed] [Google Scholar]
- 92.Nomiya M, Andersson KE, Yamaguchi O. Chronic bladder ischemia and oxidative stress: new pharmacotherapeutic targets for lower urinary tract symptoms. Int J Urol. 2015;22:40–46. doi: 10.1111/iju.12652. [DOI] [PubMed] [Google Scholar]
- 93.Chughtai B, Lee R, Te A, Kaplan S. Role of inflammation in benign prostatic hyperplasia. Rev Urol. 2011;13:147–150. [PMC free article] [PubMed] [Google Scholar]
- 94.Kashyap M, Pore S, Wang Z, Gingrich J, Yoshimura N, Tyagi P. Inflammasomes are important mediators of prostatic inflammation associated with BPH. J Inflamm (Lond) 2015;12:37. doi: 10.1186/s12950-015-0082-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hughes FM, Jr, Turner DP, Todd Purves J. The potential repertoire of the innate immune system in the bladder: expression of pattern recognition receptors in the rat bladder and a rat urothelial cell line (MYP3 cells) Int Urol Nephrol. 2015;47:1953–1964. doi: 10.1007/s11255-015-1126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Agace W, Hedges S, Andersson U, Andersson J, Ceska M, Svanborg C. Selective cytokine production by epithelial cells following exposure to Escherichia coli. Infect Immun. 1993;61:602–609. doi: 10.1128/iai.61.2.602-609.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Foxman B. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Am J Med. 2002;113((Suppl 1A)):5S–13S. doi: 10.1016/s0002-9343(02)01054-9. [DOI] [PubMed] [Google Scholar]
- 98.Hughes FM, Jr, Kennis JG, Youssef MN, Lowe DW, Shaner BE, Purves JT. The NACHT, LRR and PYD domains-containing protein 3 (NLRP3) inflammasome mediates inflammation and voiding dysfunction in a lipopolysaccharide-induced rat model of cystitis. J Clin Cell Immunol. 2016;7:396. doi: 10.4172/2155-9899.1000396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nagamatsu K, Hannan TJ, Guest RL, Kostakioti M, Hadjifrangiskou M, Binkley J, Dodson K, Raivio TL, Hultgren SJ. Dysregulation of Escherichia coli alpha-hemolysin expression alters the course of acute and persistent urinary tract infection. Proc Natl Acad Sci USA. 2015;112:E871–880. doi: 10.1073/pnas.1500374112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Schaale K, Peters KM, Murthy AM, Fritzsche AK, Phan MD, Totsika M, Robertson AA, Nichols KB, Cooper MA, Stacey KJ, Ulett GC, Schroder K, Schembri MA, Sweet MJ. Strain- and host species-specific inflammasome activation, IL-1beta release, and cell death in macrophages infected with uropathogenic Escherichia coli. Mucosal Immunol. 2016;9:124–136. doi: 10.1038/mi.2015.44. [DOI] [PubMed] [Google Scholar]
- 101.Waldhuber A, Puthia M, Wieser A, Cirl C, Dürr S, Neumann-Pfeifer S, Albrecht S, Römmler F, Müller T, Zheng Y, Schubert S, Groß O, Svanborg C, Miethke T. Uropathogenic Escherichia coli strain CFT073 disrupts NLRP3 inflammasome activation. J Clin Invest. 2016;126:2425–2436. doi: 10.1172/JCI81916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Schubert S, Norenberg D, Clermont O, Magistro G, Wieser A, Romann E, Hoffmann C, Weinert K, Denamur E. Prevalence and phylogenetic history of the TcpC virulence determinant in Escherichia coli. Int J Med Microbiol. 2010;300:429–434. doi: 10.1016/j.ijmm.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 103.Costa A, Gupta R, Signorino G, Malara A, Cardile F, Biondo C, Midiri A, Galbo R, Trieu-Cuot P, Papasergi S, Teti G, Henneke P, Mancuso G, Golenbock DT, Beninati C. Activation of the NLRP3 inflammasome by group B streptococci. J Immunol. 2012;188:1953–1960. doi: 10.4049/jimmunol.1102543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ulett GC, Webb RI, Ulett KB, Cui X, Benjamin WH, Crowley M, Schembri MA. Group B Streptococcus (GBS) urinary tract infection involves binding of GBS to bladder uroepithelium and potent but GBS-specific induction of interleukin 1alpha. J Infect Dis. 2010;201:866–870. doi: 10.1086/650696. [DOI] [PubMed] [Google Scholar]
- 105.Gupta R, Ghosh S, Monks B, DeOliveira RB, Tzeng TC, Kalantari P, Nandy A, Bhattacharjee B, Chan J, Ferreira F, Rathinam V, Sharma S, Lien E, Silverman N, Fitzgerald K, Firon A, Trieu-Cuot P, Henneke P, Golenbock DT. RNA and beta-hemolysin of group B Streptococcus induce interleukin-1beta (IL-1beta) by activating NLRP3 inflammasomes in mouse macrophages. J Biol Chem. 2014;289:13701–13705. doi: 10.1074/jbc.C114.548982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kanneganti TD, Ozoren N, Body-Malapel M, Amer A, Park JH, Franchi L, Whitfield J, Barchet W, Colonna M, Vandenabeele P, Bertin J, Coyle A, Grant EP, Akira S, Núñez G. Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature. 2006;440:233–236. doi: 10.1038/nature04517. [DOI] [PubMed] [Google Scholar]
- 107.Franchi L, Eigenbrod T, Munoz-Planillo R, Muñoz-Planillo R, Ozkurede U, Kim YG, Arindam C, Gale M, Jr, Silverman RH, Colonna M, Akira S, Núñez G. Cytosolic double-stranded RNA activates the NLRP3 inflammasome via MAVS-induced membrane permeabilization and K+ efflux. J Immunol. 2014;193:4214–4222. doi: 10.4049/jimmunol.1400582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Biondo C, Mancuso G, Midiri A, Signorino G, Domina M, Lanza Cariccio V, Mohammadi N, Venza M, Venza I, Teti G, Beninati C. The interleukin-1beta/CXCL1/2/neutrophil axis mediates host protection against group B streptococcal infection. Infect Immunity. 2014;82:4508–4517. doi: 10.1128/IAI.02104-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Verma D, Bivik C, Farahani E, Synnerstad I, Fredrikson M, Enerbäck C, Rosdahl I, Sö-derkvist P. Inflammasome polymorphisms confer susceptibility to sporadic malignant melanoma. Pigment Cell Melanoma Res. 2012;25:506–513. doi: 10.1111/j.1755-148X.2012.01008.x. [DOI] [PubMed] [Google Scholar]
- 110.Voronov E, Dotan S, Krelin Y, Song X, Elkabets M, Carmi Y, Rider P, Idan Cohen, Romzova M, Kaplanov I, Apte RN. Unique versus redundant functions of IL-1alpha and IL-1beta in the tumor microenvironment. Front Immunol. 2013;4:177. doi: 10.3389/fimmu.2013.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ungerback J, Belenki D, Jawadul-Hassan A, Fredrikson M, Fransén K, Elander N, Verma D, Söderkvist P. Genetic variation and alterations of genes involved in NFkappaB/TNFAIP3- and NLRP3-inflammasome signaling affect susceptibility and outcome of colorectal cancer. Carcinogenesis. 2012;33:2126–2134. doi: 10.1093/carcin/bgs256. [DOI] [PubMed] [Google Scholar]
- 112.Poli G, Brancorsini S, Cochetti G, Barillaro F, Egidi MG, Mearini E. Expression of inflammasome-related genes in bladder cancer and their association with cytokeratin 20 messenger RNA. Urol Oncol. 2015;33:505e1–7. doi: 10.1016/j.urolonc.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 113.Miettinen M. Keratin 20: immunohistochemical marker for gastrointestinal, urothelial, and Merkel cell carcinomas. Mod Pathol. 1995;8:384–388. [PubMed] [Google Scholar]
- 114.Finger JN, Lich JD, Dare LC, Cook MN, Brown KK, Duraiswami C, Bertin J, Gough PJ. Autolytic proteolysis within the function to find domain (FIIND) is required for NLRP1 inflammasome activity. J Biol Chem. 2012;287:25030–25037. doi: 10.1074/jbc.M112.378323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Boyden ED, Dietrich WF. Nalp1b controls mouse macrophage susceptibility to anthrax lethal toxin. Nat Genet. 2006;38:240–244. doi: 10.1038/ng1724. [DOI] [PubMed] [Google Scholar]
- 116.Faustin B, Lartigue L, Bruey JM, Luciano F, Sergienko E, Bailly-Maitre B, Volkmann N, Hanein D, Rouiller I, Reed JC. Reconstituted NALP1 inflammasome reveals two-step mechanism of caspase-1 activation. Mol Cell. 2007;25:713–724. doi: 10.1016/j.molcel.2007.01.032. [DOI] [PubMed] [Google Scholar]
- 117.Grenier JM, Wang L, Manji GA, Huang WJ, Al-Garawi A, Kelly R, Carlson A, Merriam S, Lora JM, Briskin M, DiStefano PS, Bertin J. Functional screening of five PYPAF family members identifies PYPAF5 as a novel regulator of NF-kappaB and caspase-1. FEBS Lett. 2002;530:73–78. doi: 10.1016/s0014-5793(02)03416-6. [DOI] [PubMed] [Google Scholar]
- 118.Anand PK, Malireddi RK, Lukens JR, Vogel P, Bertin J, Lamkanfi M, Kanneganti TD. NLRP6 negatively regulates innate immunity and host defence against bacterial pathogens. Nature. 2012;488:389–393. doi: 10.1038/nature11250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wlodarska M, Thaiss CA, Nowarski R, Henao-Mejia J, Zhang JP, Brown EM, Frankel G, Levy M, Katz MN, Philbrick WM, Elinav E, Finlay BB, Flavell RA. NLRP6 inflammasome orchestrates the colonic host-microbial interface by regulating goblet cell mucus secretion. Cell. 2014;156:1045–1059. doi: 10.1016/j.cell.2014.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hilt EE, McKinley K, Pearce MM, Rosenfeld AB, Zilliox MJ, Mueller ER, Brubaker L, Gai X, Wolfe AJ, Schreckenberger PC. Urine is not sterile: use of enhanced urine culture techniques to detect resident bacterial flora in the adult female bladder. J Clin Microbiol. 2014;52:871–876. doi: 10.1128/JCM.02876-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Xu W, Yang L, Lee P, Huang WC, Nossa C, Ma Y, Deng FM, Zhou M, Melamed J, Pei Z. Mini-review: perspective of the microbiome in the pathogenesis of urothelial carcinoma. Am J Clin Exp Urol. 2014;2:57–61. [PMC free article] [PubMed] [Google Scholar]
- 122.Parsons CL. The role of the urinary epithelium in the pathogenesis of interstitial cystitis/prostatitis/urethritis. Urology. 2007;69:9–16. doi: 10.1016/j.urology.2006.03.084. [DOI] [PubMed] [Google Scholar]
- 123.Sanchez-Gomez S, Ferrer-Espada R, Stewart PS, Pitts B, Lohner K, Martinez de Tejada G. Antimicrobial activity of synthetic cationic peptides and lipopeptides derived from human lactoferricin against Pseudomonas aeruginosa planktonic cultures and biofilms. BMC Microbiol. 2015;15:137. doi: 10.1186/s12866-015-0473-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mittal R, Aggarwal S, Sharma S, Chhibber S, Harjai K. Urinary tract infections caused by Pseudomonas aeruginosa: a minireview. J Infect Public Health. 2009;2:101–111. doi: 10.1016/j.jiph.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 125.Khare S, Dorfleutner A, Bryan NB, Yun C, Radian AD, de Almeida L, Rojanasakul Y, Stehlik C. An NLRP7-containing inflammasome mediates recognition of microbial lipopeptides in human macrophages. Immunity. 2012;36:464–476. doi: 10.1016/j.immuni.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Vladimer GI, Weng D, Paquette SW, Vanaja SK, Rathinam VA, Aune MH, Conlon JE, Burbage JJ, Proulx MK, Liu Q, Reed G, Mecsas JC, Iwakura Y, Bertin J, Goguen JD, Fitzgerald KA, Lien E. The NLRP12 inflammasome recognizes Yersinia pestis. Immunity. 2012;37:96–107. doi: 10.1016/j.immuni.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sato H, Frank DW. Multi-functional characteristics of the Pseudomonas aeruginosa type III needle-tip protein, PcrV; comparison to orthologs in other Gram-negative bacteria. Front Microbiol. 2011;2:142. doi: 10.3389/fmicb.2011.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jeru I, Duquesnoy P, Fernandes-Alnemri T, Cochet E, Yu JW, Lackmy-Port-Lis M, Grimprel E, Landman-Parker J, Hentgen V, Marlin S, McElreavey K, Sarkisian T, Grateau G, Alnemri ES, Amselem S. Mutations in NALP12 cause hereditary periodic fever syndromes. Proc Natl Acad Sci U S A. 2008;105:1614–1619. doi: 10.1073/pnas.0708616105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rigante D, Frediani B, Cantarini L. A Comprehensive overview of the hereditary periodic fever syndromes. Clin Rev Allergy Immunol. 2016:1–8. doi: 10.1007/s12016-016-8537-8. [DOI] [PubMed] [Google Scholar]
- 130.Mariathasan S, Newton K, Monack DM, Vucic D, French DM, Lee WP, Roose-Girma M, Erickson S, Dixit VM. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature. 2004;430:213–218. doi: 10.1038/nature02664. [DOI] [PubMed] [Google Scholar]
- 131.Zamboni DS, Kobayashi KS, Kohlsdorf T, Ogura Y, Long EM, Vance RE, Kuida K, Mariathasan S, Dixit VM, Flavell RA, Dietrich WF, Roy CR. The Birc1e cytosolic pattern-recognition receptor contributes to the detection and control of Legionella pneumophila infection. Nat Immunol. 2006;7:318–325. doi: 10.1038/ni1305. [DOI] [PubMed] [Google Scholar]
- 132.Miao EA, Ernst RK, Dors M, Mao DP, Aderem A. Pseudomonas aeruginosa activates caspase 1 through Ipaf. Proc Natl Acad Sci USA. 2008;105:2562–2567. doi: 10.1073/pnas.0712183105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Miao EA, Mao DP, Yudkovsky N, Bonneau R, Lorang CG, Warren SE, Leaf IA, Aderem A. Innate immune detection of the type III secretion apparatus through the NLRC4 inflammasome. Proc Natl Acad Sci USA. 2010;107:3076–3080. doi: 10.1073/pnas.0913087107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sutterwala FS, Mijares LA, Li L, Ogura Y, Kazmierczak BI, Flavell RA. Immune recognition of Pseudomonas aeruginosa mediated by the IPAF/NLRC4 inflammasome. J Exp Med. 2007;204:3235–3245. doi: 10.1084/jem.20071239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Miyazaki J, Ba-Thein W, Kumao T, Akaza H, Hayashi H. Identification of a type III secretion system in uropathogenic Escherichia coli. FEMS Microbiol Lett. 2002;212:221–228. doi: 10.1111/j.1574-6968.2002.tb11270.x. [DOI] [PubMed] [Google Scholar]
- 136.Miano R, Germani S, Vespasiani G. Stones and urinary tract infections. Urol Int. 2007;79((Suppl 1)):32–36. doi: 10.1159/000104439. [DOI] [PubMed] [Google Scholar]
- 137.Lopalco G, Rigante D, Giannini M, Galeazzi M, Lapadula G, Iannone F, Cantarini L. Safety profile of anakinra in the management of rheumatologic, metabolic and autoinflammatory disorders. Clin Exp Rheumatol. 2016;34:531–538. [PubMed] [Google Scholar]
- 138.Shibata S. A drug over the millennia: pharmacognosy, chemistry, and pharmacology of licorice. Yakugaku Zasshi. 2000;120:849–862. doi: 10.1248/yakushi1947.120.10_849. [DOI] [PubMed] [Google Scholar]
- 139.Honda H, Nagai Y, Matsunaga T, Okamoto N, Watanabe Y, Tsuneyama K, Hayashi H, Fujii I, Ikutani M, Hirai Y, Muraguchi A, Takatsu K. Isoliquiritigenin is a potent inhibitor of NLRP3 inflammasome activation and diet-induced adipose tissue inflammation. J Leukoc Biol. 2014;91:967–976. doi: 10.1189/jlb.3A0114-005RR. [DOI] [PubMed] [Google Scholar]
- 140.Salla M, Butler MS, Pelingon R, Kaeslin G, Croker DE, Reid JC, Baek JM, Bernhardt PV, Gillam EM, Cooper MA, Robertson AA. Identification, synthesis, and biological evaluation of the major human metabolite of NLRP3 inflammasome inhibitor MCC950. ACS Med Chem Lett. 2016;7:1034–1038. doi: 10.1021/acsmedchemlett.6b00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Juliana C, Fernandes-Alnemri T, Wu J, Datta P, Solorzano L, Yu JW, Meng R, Quong AA, Latz E, Scott CP, Alnemri ES. Anti-inflammatory compounds parthenolide and Bay 11–7082 are direct inhibitors of the inflammasome. J Biol Chem. 2010;285:9792–9802. doi: 10.1074/jbc.M109.082305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Shahid H, Singh JA. Investigational drugs for hyperuricemia. Expert Opin Investig Drugs. 2015;24:1013–1030. doi: 10.1517/13543784.2015.1051617. [DOI] [PubMed] [Google Scholar]
- 143.Barreyro FJ, Holod S, Finocchietto PV, Camino AM, Aquino JB, Avagnina A, Carreras MC, Poderoso JJ, Gores GJ. The pan-caspase inhibitor Emricasan (IDN-6556) decreases liver injury and fibrosis in a murine model of non-alcoholic steatohepatitis. Liver Int. 2015;35:953–966. doi: 10.1111/liv.12570. [DOI] [PubMed] [Google Scholar]