Abstract
Purpose
We report 6 patients who received a hydroxyapatite (HA) orbital implant in the socket and developed chronic orbital inflammation unresponsive to conventional medical therapy.
Case Reports
We assisted 6 cases (4 males, 2 females) who received an HA orbital implant in the socket between 2015 and 2016 at King Khaled Eye Specialist Hospital, Riyadh, Saudi Arabia, and developed chronic orbital inflammation with chronic discharge, redness, and pain (onset from weeks to over 2 decades after surgery). Computed tomography evaluation indicated inflammation in the orbital tissues, and histological examination showed a foreign body granulomatous reaction mainly localized around and blanching the HA implant. The condition was unresponsive to usual medical treatment and was resolved immediately after implant removal.
Conclusions
Chronic inflammation can occur decades after placement of an HA implant in the orbit and can be successfully treated with implant removal.
Keywords: Hydroxyapatite, Integrated implant, Anophthalmic cavity, Chronic inflammatory reaction
Introduction
Hydroxyapatite (HA) implants were advocated in the 1980s to replace volume in anophthalmic sockets and to improve motility of the external prosthesis, based on the idea that these implants could be integrated to the host tissues and be coupled to the external prosthesis, transmitting the extraocular muscle movements to the prosthesis [1, 2, 3].
Twenty-six years after HA was introduced, multiple complications associated with the implants such as conjunctival thinning or dehiscence, implant exposure, infection, and chronic orbital pain were reported [1, 4, 5, 6, 7, 8], and enthusiasm with the HA implant has decreased.
A rarely reported complication of HA implants is chronic inflammatory reaction in the orbit [9, 10, 11]. In this case series, we are evaluating 6 anophthalmic socket carriers who received HA implants in the anophthalmic socket and developed chronic orbital inflammation unresponsive to conventional medical therapy.
Case Reports
We report 6 anophthalmic carriers (4 males, 2 females) who received HA orbital implants and developed unresponsive chronic orbital inflammation, treated between 2015 and 2016 at King Khaled Eye Specialist Hospital, Riyadh, Saudi Arabia.
Patient details are presented in Table 1. The patients had previously undergone enucleation (4 cases) or evisceration (2 cases). When patients underwent enucleation, the sphere was wrapped in donor sclera. All the HA implants were spherical, 18–20 mm in size, and none had a coupling peg. The main complaints at presentation to our hospital were conjunctival redness, copious thick discharge, tearing, and pain/discomfort. The onset of inflammation occurred between 3 weeks and 24 years after patients received the implants. Medical treatment using a topical antibiotic combined with steroids (Maxitrol®; Alcon Inc., Fort Worth, TX, USA) was partially effective in all cases. Patients developed conjunctival contraction with shortened fornix, symblepharon, and pain on palpation. Pyogenic granulomas and severe inflammation were observed in Case 2 and he was prescribed oral steroids. However, inflammation recurred when medication was discontinued. Two patients had implant exposure (Cases 3 and 5). In Case 3, implant exposure occurred 4 times and that was unsuccessfully repaired with tarsal flap, followed by scleral patch, amniotic membrane, and direct closure.
Table 1.
Demographics and ocular characteristics of patients with HA implants who developed chronic orbital inflammation unresponsive to conventional medical therapy
| Case | Gender | Age, years | Eye | Enucleation/evisceration | Cause of implantation | Implant size, mm | Symptoms | Elapse of time since implantation | Treatment | CT | Pathology |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 44 | RE | Evisceration | Trauma | 20 | Pain and chronic conjunctivitis | 6 months | HA removal and DMFG | HA implant with significant thickening along the preseptal area, eyelid edema, and no evidence of postseptal extension | |
| 2 | M | 36 | LE | Evisceration | Trauma | 20 | Persistent purulent conjunctivitis and severe discharge | 3 weeks | HA removal | Revealed HA implant surrounded by inflammation, involving also the retrobulbar fat and optic nerve sheath, with significant dirty fatty appearance as well as involvement of tendinous insertion of the extraocular muscles group | Multiple small “foreign bodies” surrounded by granulomatous reaction composed by giant cells and numerous eosinophils |
| 3 | M | 28 | RE | Enucleation and donor sclera | Trauma | 18 | Intermittent uncomfortable socket, with severe mucopurulent conjunctivitis and HA exposure 4 times | 24 months | HA removal | - | Multiple granulomas composed by foreign body reaction surrounding the exposed implant |
| 4 | M | 33 | RE | Enucleation and donor sclera | Trauma | 18 | Discharge and redness | 24 years | HA removal and DMFG | Preseptal soft tissue swelling affecting the right upper and lower eyelids including the medial canthal area and medial rectus muscle | Multiple granulomas with foreign body reaction and numerous eosinophils |
| 5 | M | 44 | LE | Enucleation and donor sclera | Trauma | 20 | Inflamed anophthalmic socket with a 6 mm exposed HA implant and contracted fornix | 10 years | HA removal | Inflamed socket mainly around the implant and affecting the anterior portion of the orbit | Intense fibrosis and granulomatous reaction |
| 6 | F | 9 | LE | Enucleation and donor sclera | Retinoblastoma | 20 | Continuous chronic discharge, redness and contracted fornix | 2 years | HA removal and DMFG | - | - |
RE, right eye; LE, left eye; DMFG, dermis fat graft; HA, hydroxyapatite; CT, computed tomography.
Computed tomography (CT) indicated inflammation localized around the HA implant, most significantly in the anterior aspect of the orbit and preseptal area, extending to the eyelids. Swab socket cultures were negative in all patients.
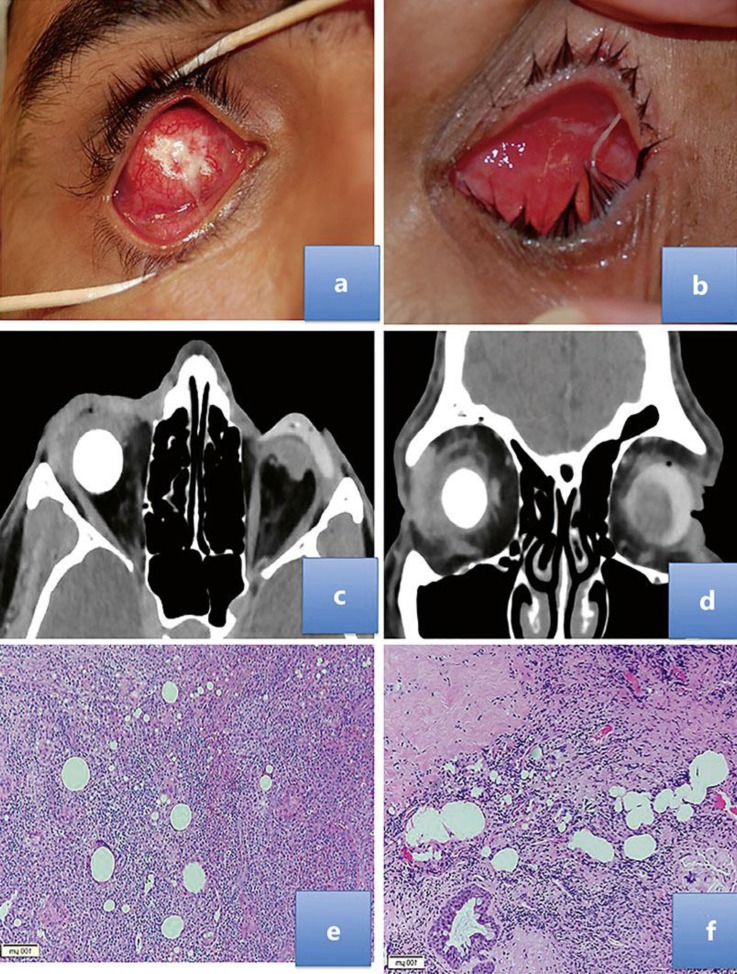
All patients underwent removal of the HA implant, and histological examination of the implant and surrounding tissue showed intense granulomatous reaction composed of giant cells, multiple small “foreign bodies” (likely small pieces of HA), and numerous eosinophils and fibrosis within the implant and surrounding the HA sphere. In the 2 cases of implant exposure (Cases 3 and 5), neutrophilic and lymphocytic reactions were observed in the anterior aspect of the socket (Fig. 1).
Fig. 1.
a Case 3 had multiple hydroxyapatite (HA) exposures showing dehiscence and exposure of the implant. b Case 5 with the HA implant and an extensive inflammatory reaction in the socket. c, d Case 1 had bilateral evisceration with the HA implant in the right socket and no implant in the left socket. The axial (c) and coronal (d) CT scans showed significant thickening along the right preseptal area with eyelid edema and mild bulkiness related to the right lacrimal gland. The implant is surrounded by slightly dirty fat and no evidence of localized collection. e Histopathology indicates fat necrosis, multiple “foreign bodies” surrounded by foreign body reaction, and numerous eosinophils. HE stain. ×100. f Intense fibrosis and extensive granuloma due to “foreign body.” HE stain. ×100.
After implant removal, all patients had a quiet painless socket with no redness and no discharge. At the same time of HA implant removal, three patients decided to undergo socket rehabilitation receiving a dermis fat graft, and no complications were detected in the postoperative period. The remaining patients refused to undergo any kind of anophthalmic socket reconstruction. At the last follow-up, all patients were doing fine and were able to hold external prosthesis.
Discussion
In this case series, we describe patients with HA implants who had chronic inflammation in the socket, noticed by chronic severe discharge, pain, and discomfort.
The chronic discharge in our cases was related to the chronic orbital inflammation but can be attributed to pyogenic granulomas, giant papillary conjunctivitis, or conjunctival cysts [12, 13]. Only Case 2 presented pyogenic granuloma without exposure of the implant. Cases 3 and 5 had exposure of the implant, which is likely the most frequent HA-associated complication [11], and in our cases exposure was not associated to pyogenic granuloma.
The dehiscence of the conjunctiva and exposure of the implant can provide a portal of entry for potential implant infection [14]. However, our exposure cases had negative implant culture swabs.
Treatment of exposure is difficult even with flaps or grafts. Persistent orbital discomfort, discharge, and the development of a pyogenic granuloma after HA implant should warn the ophthalmic surgeon of potential implant infection and can result in implant removal [1, 4, 5, 6, 7, 8] as occurred in our patients.
Numerous studies evaluated vascularization within HA implants [3, 10], but there are no reports that a CT scan can help to preclude the diagnosis of chronic peri-implant inflammation. The CT scan showed that the inflammatory reaction was mainly located around the implants. Further, histological evaluation of the explanted implants confirmed the image findings showing the inflammatory reaction in the implants and orbital tissues. The histological reaction was similar to that observed in experimental animal studies which received synthetic HA implants [9, 15], being composed mainly of chronic inflammatory cell infiltrate with predominant foreign body giant cell reaction inside and around the implant. This tissue response was also similar to that reported by Jordan et al. [11] who examined 15 explanted HA implants revealing a clinical pathological correlation without image confirmation, representing signs of acute or chronic inflammatory process, with or without necrosis and with or without identifiable microorganisms in the explanted HA implants.
Initially, our patients underwent unsuccessful clinical treatment to alleviate the pain, redness, and mucopurulent discharge, but remission did not occur because the inflammation was affecting the orbit. Case 2 improved with systemic steroids, confirming that the inflammation was in the orbital tissues.
Chronic inflammation resolved after implant removal in all the patients in this case series. This observation confirms that HA was causing the inflammation, and the definitive treatment for these cases is implant removal.
Chronic inflammation extended to the fornix, causing socket contraction with a shallow fornix and fibrosis. These signs prompted us to treat the patients after HA implant removal with a dermis fat graft, replacing the anterior surface and increasing the volume within the socket with the possibility of good outcomes.
In conclusion, chronic inflammation in the socket can be related to the presence of an HA implant. It can occur many years after HA implant placement and can successfully be treated with implant removal.
Statement of Ethics
This study adhered to the principles outlined in the Declaration of Helsinki. The institutional ethics committee board approved the study protocol, and informed consent was waived due to the retrospective nature of the study.
Disclosure Statement
The authors do not have a personal or family ownership or potential rights to more than 1% of the company or competing company or any interest in marketing any product, drug, instrument, or piece of equipment discussed in the manuscript. The authors state that they did not receive any funding to support this study.
References
- 1.Chalasani R, Poole-Warren L, Conway RM, Ben-Nissan B. Porous orbital implants in enucleation: a systematic review. Surv Ophthalmol. 2007;52:145–155. doi: 10.1016/j.survophthal.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 2.Jordan DR, Brownstein S, Gilberg S, Coupal D, Kim S, Mawn L. Hydroxyapatite and calcium phosphate coatings on aluminium oxide orbital implants. Can J Ophthalmol. 2002;37:7–13. doi: 10.1016/s0008-4182(02)80092-8. [DOI] [PubMed] [Google Scholar]
- 3.Shields CL, Shields JA, Eagle RC, Jr, De Potter P. Histopathologic evidence of fibrovascular ingrowth four weeks after placement of the hydroxyapatite orbital implant. Am J Ophthalmol. 1991;111:363–366. doi: 10.1016/s0002-9394(14)72323-2. [DOI] [PubMed] [Google Scholar]
- 4.Ainbinder DJ, Haik BG, Tellado M. Hydroxyapatite orbital implant abscess: histopathologic correlation of an infected implant following evisceration. Ophthal Plast Reconstr Surg. 1994;10:267–270. [PubMed] [Google Scholar]
- 5.Badilla J, Dolman PJ. Methods of antibiotic instillation in porous orbital implants. Ophthal Plast Reconstr Surg. 2008;24:287–289. doi: 10.1097/IOP.0b013e318177ebc7. [DOI] [PubMed] [Google Scholar]
- 6.Bidar M, Hawes MJ, Dresner SC, Epstein G, Lucarelli MJ, Glover T, Fante RG, Migliori ME. Conjunctival melting syndrome associated with the use of bovine pericardium wrapping material. Ophthal Plast Reconstr Surg. 2003;19:257–261. doi: 10.1097/01.IOP.0000075794.80525.9D. [DOI] [PubMed] [Google Scholar]
- 7.Chee E, Kim YD, Woo KI, Lee JH, Kim JH, Suh YL. Inflammatory mass formation secondary to hydroxyapatite orbital implant leakage. Ophthal Plast Reconstr Surg. 2013;29:e40–e42. doi: 10.1097/IOP.0b013e3182696577. [DOI] [PubMed] [Google Scholar]
- 8.Schmitzer S, Simionescu C, Alexandrescu C, Burcea M. The anophthalmic socket - reconstruction options. J Med Life. 2014;7:23–29. [PMC free article] [PubMed] [Google Scholar]
- 9.Schellini SA, Marques ME, Padovani CR, Taga EM, Rossa R. Comparison of synthetic hydroxyapatite and porous polyethylene implants in eviscerated rabbit eyes. Ophthal Plast Reconstr Surg. 2003;19:136–139. doi: 10.1097/01.IOP.0000056028.98833.FF. [DOI] [PubMed] [Google Scholar]
- 10.Goldberg RA, Dresner SC, Braslow RA, Kossovsky N, Legmann A. Animal model of porous polyethylene orbital implants. Ophthal Plast Reconstr Surg. 1994;10:104–109. doi: 10.1097/00002341-199406000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Jordan DR, Brownstein S, Faraji H. Clinicopathologic analysis of 15 explanted hydroxyapatite implants. Ophthal Plast Reconstr Surg. 2004;20:285–290. doi: 10.1097/01.iop.0000131735.89093.22. [DOI] [PubMed] [Google Scholar]
- 12.Jones DF, Lyle CE, Fleming JC. Superior conjunctivoplasty-mullerectomy for correction of chronic discharge and concurrent ptosis in the anophthalmic socket with enlarged superior fornix. Ophthal Plast Reconstr Surg. 2010;26:172–175. doi: 10.1097/IOP.0b013e3181b8c49a. [DOI] [PubMed] [Google Scholar]
- 13.Jordan DR, Brownstein S, Jolly SS. Abscessed hydroxyapatite orbital implants. A report of two cases. Ophthalmology. 1996;103:1784–1787. doi: 10.1016/s0161-6420(96)30427-2. [DOI] [PubMed] [Google Scholar]
- 14.Kim JW, Kikkawa DO, Aboy A, Glasgow BJ. Chronic exposure of hydroxyapatite orbital implants: cilia implantation and epithelial downgrowth. Ophthal Plast Reconstr Surg. 2000;16:216–222. doi: 10.1097/00002341-200005000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Ranzani JJ, Rahal SC, Schellini SA, Marques ME, Taga EM. Repair of the anophthalmic cavity of rats with synthetic hydroxyapatite. Braz J Med Biol Res. 1997;30:1181–1186. doi: 10.1590/s0100-879x1997001000007. [DOI] [PubMed] [Google Scholar]