Abstract
Synchronous primary cancers involving the pancreas and kidney are extremely rare and poorly documented. We report the first case of this association treated with chemotherapy and tyrosine kinase inhibitor. A 70-year-old woman presented with a 2-month history of epigastric pain with weight loss of 12 kg. Two weeks previously, she had presented with jaundice and pelvic pain. A computed tomography (CT) scan of the body revealed the presence of an irregular mass in the body of the pancreas, encasing the celiac trunk, with dilatation of the biliary tract. CT also revealed a heterogeneously right renal mass with bone metastasis in the left acetabular cup and the left iliac wing. A biliary metallic prosthesis was performed with a pancreatic mass biopsy. Histology revealed a moderately differentiated pancreatic ductal adenocarcinoma. Another biopsy was performed in the right iliac wing. Pathological examination with immunohistochemistry confirmed the diagnosis of bone metastasis from clear cell renal cell carcinoma. The patient was treated with a combination of gemcitabine, sunitinib, and denosumab. She had a stabilization disease and a prolonged progression-free survival of 9 months. Side effects were manageable and included grade 2 fatigue and grade 2 hypertension. The patient died at 13 months from diagnosis after disease progression. This report suggests that the appropriate treatment for this association in metastatic or unresectable disease is chemotherapy for pancreatic cancer and tyrosine kinase inhibitor for kidney cancer. We also review the appropriate literature concerning that association.
Keywords: Synchronous primary cancers, Renal cell cancer, Pancreatic cancer, Gemcitabine, Sunitinib
Introduction
Multiple primary malignancies are uncommon (1–3% of all cancers), but synchronous multiple primary tumors are extremely rare, and synchronous primary tumors involving the kidney and pancreas are exceptional and poorly documented [1, 2]. Indeed, there are only a few reports of this situation in the English literature. These tumors occur essentially in the elderly, are typically smaller, and are more likely to be resectable [3]. We report an unusual case of a 70-year-old woman presenting synchronous renal cell cancer and pancreatic ductal adenocarcinoma, while also reviewing the literature data.
Case Presentation
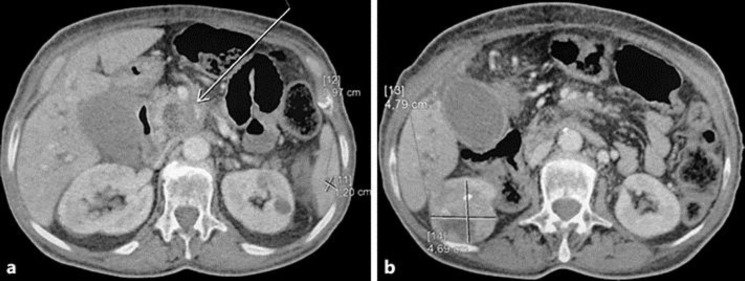
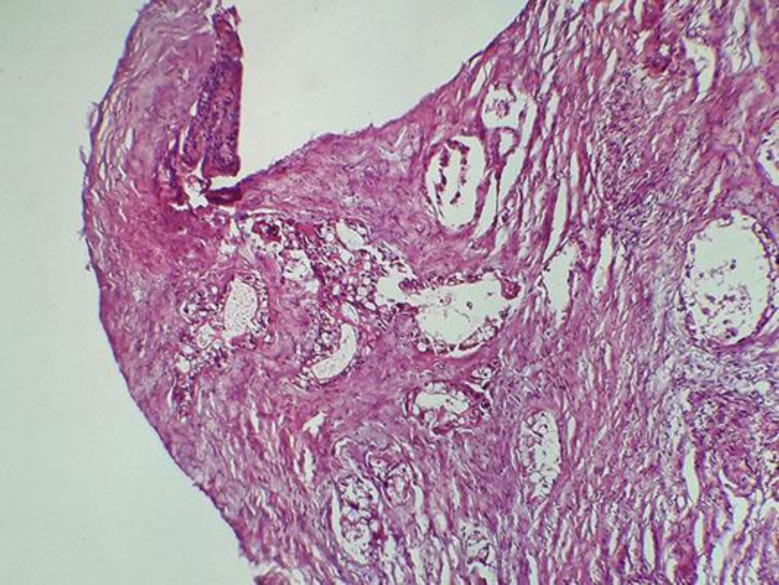
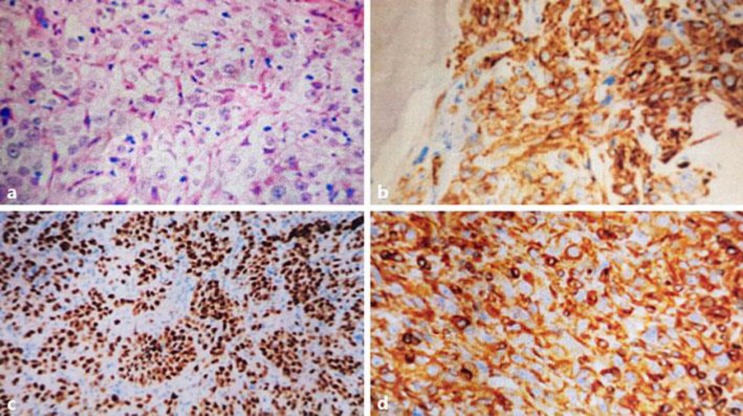
A 70-year-old nonsmoking woman with no family history of pancreatic or renal diseases and without familial genetic syndromes presented with a 2-month history of epigastric pain and episodes of nausea/vomiting with weight loss of 12 kg. She had no digestive bleeding and no hematuria or other urinary symptoms. Two weeks previously, she had presented with yellowing of the eyes and skin with pelvic pain. Physical examination showed jaundice, epigastric tenderness, and pain of the left iliac wing. No palpable mass was revealed. Laboratory investigations showed an elevation of total bilirubin level (24 mg/dL) and direct bilirubin level (22 mg/dL), an increased serum carbohydrate antigen 19-9 level (560 IU/mL), and normal carcinoembryonic antigen level. Complete blood count and corrected calcium were normal. Computed tomography (CT) of the body revealed the presence of a 45 × 38 × 35 mm irregular and heterogeneous mass in the body of the pancreas, encasing the celiac trunk and the lower mesenteric vein (Fig. 1a). Celiac lymph nodes were present. CT also revealed the presence of a demarcated, heterogeneous, enhancing right renal mass 61 × 48 × 47 mm in size (Fig. 1b) with bone metastasis in the left acetabular cup and the left iliac wing. A biliary metallic prosthesis was performed with regression of jaundice. A CT-guided biopsy of the pancreas was performed; histology revealed a moderately differentiated pancreatic ductal adenocarcinoma (Fig. 2). Another biopsy was performed in the left iliac wing. Pathological examination showed tumor cells with abundant clear cytoplasm and irregular nuclei infiltrating the bone trabeculae. Results of immunohistochemical analysis confirmed the diagnosis of bone metastasis from clear cell renal cell carcinoma; the tumor cells were positive for cytokeratin 7 (CK7), vimentin, PAX8, and renal cell carcinoma antigen, but negative for CK20 (Fig. 3). In view of this association of a locally advanced pancreatic adenocarcinoma with metastatic renal cell cancer, the patient was treated with chemotherapy (gemcitabine 750 mg/m2 on days 1, 8, and 15; repeated every 4 weeks) in conjunction with tyrosine kinase inhibitor (sunitinib 37.5 mg/day; 4/6 weeks) and anti-RANK ligand (denosumab 120 mg every 4 weeks). The treatment was well tolerated with manageable side effects (grade 2 fatigue and grade 2 hypertension). The patient had a stabilization disease and a prolonged progression-free survival of 9 months. She died at 13 months from diagnosis after disease progression.
Fig. 1.
a Heterogeneous mass in the body of the pancreas. b Heterogeneous right renal mass.
Fig. 2.
Moderately differentiated pancreatic ductal adenocarcinoma. HE, ×20.
Fig. 3.
a Tumor cells with abundant clear cytoplasm and prominent nuclei infiltrating the bone trabeculae. HE, ×40. b–d Immunohistochemical analysis: positivity for renal cell carcinoma antigen (b), PAX8 (c), and vimentin (d).
Discussion
Multiple primary cancers (MPC) in the same patient are rare, but synchronous MPC are exceptional [1, 2]. Most synchronous tumors involve both the gastrointestinal and the genitourinary tract, followed by the breast and gastrointestinal tract or the breast and genitourinary tract [3].
The frequency of pancreatic cancer in association with another cancer is estimated to be between 1 and 20%, with malignancies predominately of the stomach, colon, genitourinary tract, and thyroid [3, 4]. Second malignancies reported to be associated with renal cell carcinoma include non-Hodgkin lymphoma, chronic lymphatic leukemia, multiple myeloma, and melanoma and cancers of the bladder, breast, prostate, lung, and rectum, with an incidence that varies from 5 to 27% [3, 4].
However, there has been only infrequent reporting of synchronous or metachronous tumors of the kidney and the pancreas [1, 2, 3, 4]. In 1969, Sasaki et al. [5] reported the first case of an association between renal and pancreatic cancer. Since then, only a few case reports have been published, with the largest series including 16 patients [3]. An analysis of 4,176 patients with renal cell cancer in the Connecticut Tumor Registry for the period between 1935 and 1982 showed that 5% of them developed a second primary malignancy (relative risk = 1.2; p < 0.05). There were only 6 cases (0.14%) of pancreatic cancer but there was no significant association (relative risk = 1) [6]. In a series of 763 patients who underwent surgery for renal cancer in the Memorial Sloan Kettering Cancer Center, there was a significantly increased incidence of bladder, prostate, and colorectal cancers and non-Hodgkin lymphoma, but not pancreatic cancer [4].
The rest of the publications concern only a few case reports (Table 1). Alexakis et al. [1] reported a series of 373 patients with pancreatic cancer, with 1 case of synchronous association with renal cell carcinoma. In 2004, Olgyai et al. [7] reported a new case of synchronous primary renal clear cell carcinoma and pancreatic ductal adenocarcinoma. Another case was reported by Nobili et al. [8] in 2006; the patient had 2 histologically different pancreatic neoplasms (intraductal papillary mucinous tumor and intraepithelial tumor), 1 papillary carcinoma of the kidney and 1 embryogenic duodenal anomaly. Recently, Müller et al. [3] reported 16 cases of renal cancer and metachronous (n = 10) or synchronous (n = 6) primary pancreatic tumors. The median survival of all patients was 12.6 months (25.7 and 12.2 months, respectively, for patients with synchronous and metachronous cancers).
Table 1.
Reported cases of double kidney-pancreas cancers
| Authors | Year | Type of study | Cases, n | Timing |
|---|---|---|---|---|
| Sasaki et al. [5] | 1969 | Case report | 1 | Synchronous |
| Kantor et al. [6] | 1986 | 4,176 patients with renal cell carcinoma in the Connecticut Tumor Registry | 6 | Synchronous |
| Rabbani et al. [4] | 2000 | 373 patients with pancreatic cancer in a single unit | 3 | Synchronous |
| Ismail et al. [2] | 2010 | Case report | 1 | Synchronous |
| Alexakis et al. [1] | 2003 | 373 patients with pancreatic cancer in a single unit | 2 | 1 synchronous and 1 metachronous |
| Olgyai et al. [7] | 2004 | Case report | 1 | Synchronous |
| Nobili et al. [8] | 2006 | Case report | 1 | Synchronous |
| Müller et al. [3] | 2012 | Analysis of patient registries from university departments of urology and visceral surgery; 1,178 patients with pancreatic tumors and 518 patients with renal carcinoma | 16 | 6 synchronous and 10 metachronous |
| Present case | 2017 | Case report | 1 | Synchronous |
Most papers reporting an association between pancreatic and renal cancer were based on a small cohort of patients, and few of them provided clinical details [1, 3, 4, 5, 6]. The majority of patients were males over 65 years, and treatment consisted of a nephrectomy/partial nephrectomy for renal cancer combined with pancreaticoduodenectomy/left resection/double bypass for pancreatic cancer. Chemotherapy was given in only 2 cases [1, 8]. Our patient was a 70-year-old woman with a metastatic renal cell carcinoma and locally advanced pancreatic adenocarcinoma. She is the first case reported to be treated with a tyrosine kinase inhibitor (sunitinib) in conjunction with chemotherapy and denosumab. The rationale for this combination was to exploit the antiproliferative and antiangiogenic effect of sunitinib to control both the pancreatic and renal cell carcinoma. Gemcitabine was used as the standard of care for pancreatic cancers and denosumab represents the reference therapy in bone metastasis.
Although possible causes for such an association of MPC are not fully understood, several factors have been suspected. Indeed, environmental risk factors (cigarette smoking, alcohol abuse, and pollution), previous medical treatment (radiotherapy and/or chemotherapy), genetic predisposition, and hormonal factors seem to be implicated [9]. Studies examining genetic factors found that microsatellite instability was more frequently observed in MPC than in sporadic cancer [3]. Another study reported a mutation in codon 12 of the K-ras gene in a patient with synchronous tumors of the kidney and pancreas [2]. In our case, molecular investigations were not considered because of the absence of known familial genetic syndromes, but genetic analysis of synchronous tumors in different organs may provide information about the etiology of genetic changes that occur during carcinogenesis [1, 2, 3, 9].
In conclusion, a case of synchronous primary renal cell carcinoma and pancreatic ductal adenocarcinoma is extremely rare. In most cases, tumors are typically smaller, and surgery is the appropriate treatment. In metastatic or unresectable disease, treatment can consist of a biliary derivation followed by chemotherapy for pancreatic cancer and tyrosine kinase inhibitor for renal cancer. This exceptional tumor association needs more epidemiological details and molecular investigation. Indeed, there may be a genetic predisposition for this situation.
Statement of Ethics
The Ethics Committee of Mohammed V University Hospital approved the study.
Written informed consent was obtained from the deceased's daughter for publication of this case report.
Disclosure Statement
All authors have no conflicts of interest to disclose.
References
- 1.Alexakis N, Bosonnet L, Connor S, Ellis I, Sutton R, Campbell F, Hughes M, Garvey C, Neoptolemos JP. Double resection for patients with pancreatic cancer and a second primary renal cell cancer. Dig Surg. 2003;20:428–432. doi: 10.1159/000072711. [DOI] [PubMed] [Google Scholar]
- 2.Ismail TO, Janane A, Hajji F, Dekkak Y, Bekkali S, Sossa J, Chafiki J, Eloundou J, Lahrech Y, Qamous O, Qarro A, Jira H, Ghadouane M, Ameur A, Albouzidi A, Abbar M. Synchronous primary tumors of the kidney and pancreas: case report. Afr J Urol. 2010;16:128–131. [Google Scholar]
- 3.Müller SA, Pahernik S, Hinz U, Martin DJ, Wente MN, Hackert T, Leowardi C, Haferkamp A, Büchler MW, Schmied BM. Renal tumors and second primary pancreatic tumors: a relationship with clinical impact? Patient Saf Surg. 2012;6:18. doi: 10.1186/1754-9493-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rabbani F, Reuter VE, Katz J, Russo P. Second primary malignancies associated with renal cell carcinoma: influence of histologic type. Urology. 2000;6:399–403. doi: 10.1016/s0090-4295(00)00682-8. [DOI] [PubMed] [Google Scholar]
- 5.Sasaki E, Kushida S, Okinaka T, Sasaki K, Abe S. Case report of double cancer of the pancreas and the kidney with polyposis of the large intestine (in Japanese) Gan No Rinsho. 1969;15:203–206. [PubMed] [Google Scholar]
- 6.Kantor AF, McLaughlin JK, Curtis RE, Flannery JT, Fraumeni JF., Jr Risk of second malignancy after cancers of the renal parenchyma, renal pelvis, and ureter. Cancer. 1986;58:1158–1161. doi: 10.1002/1097-0142(19860901)58:5<1158::aid-cncr2820580530>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 7.Olgyai G, Haulik L, Oláh A. Double resection for synchronous pancreatic and renal cell cancer-case report (in Hungarian) Magy Seb. 2004;57:287–289. [PubMed] [Google Scholar]
- 8.Nobili C, Franciosi C, Degrate L, Caprotti R, Romano F, Perego E, Trezzi R, Leone BE, Uggeri F. A case of pancreatic heterotopy of duodenal wall, intraductal papillary mucinous tumor and intraepithelial neoplasm of pancreas, papillary carcinoma of kidney in a single patient. Tumori. 2006;92:455–458. doi: 10.1177/030089160609200518. [DOI] [PubMed] [Google Scholar]
- 9.Beisland C, Talleraas O, Bakke A, Norstein J. Multiple primary malignancies in patients with renal cell carcinoma: a national population-based cohort study. BJU Int. 2006;97:698–702. doi: 10.1111/j.1464-410X.2006.06004.x. [DOI] [PubMed] [Google Scholar]