Abstract
Rare bacteria can lead to infective endocarditis, which may lead to renal involvement as severe glomerulonephritis. We report our experience of a 12-year-old child who presented with infective endocarditis and blood culture-grown Gemella morbillorum - a rarely reported bacteria. The clinical picture was further complicated with severe glomerulonephritis. Renal biopsy was suggestive of crescentic glomerulonephritis. The child was managed with antibiotics, steroids, and plasmapheresis and responded well to the treatment. To our knowledge, this is the first report of G. morbillorum endocarditis with immune complex deposition and necrotizing glomerulonephritis in a child.
Keywords: Infective endocarditis, Crescentic glomerulonephritis, Gemella morbillorum, Plasmapheresis
Introduction
Renal complications of subacute bacterial endocarditis include glomerulonephritis, renal infarction, abscesses, and therapy-related or therapy-induced tubulointerstitial nephritis and acute tubular epithelial injury. Rare organisms may lead to infective endocarditis, which may further be complicated by severe renal involvement. We report the case of a previously healthy 12-year-old girl, who presented with the clinical finding of infective endocarditis and a positive blood culture growing Gemella morbillorum. The clinical picture was complicated with acute kidney injury requiring hemodialysis. Renal biopsy was suggestive of crescentic glomerulonephritis and responded to steroids, plasma exchange, and rituximab. There was residual valvular dysfunction which mandated valve replacement, but the child was discharged against medical advice after 35 days of hospitalization.
Case Report
A 12-year-old female child was admitted to our hospital emergency department because of generalized weakness. She had no medical history and was not on any chronic medications. Her initial manifestations were fever, chills, weight loss (unintentional), palpitations, and night sweats for the past 2 months. Three weeks prior to admission, she developed a nonproductive cough. There was no past history of recent travel or sick contacts, dental/gastrointestinal procedure, poor dental state, colon disease, congenital heart disease, or hematological malignancy.
On physical examination, the child was pale and averagely nourished. Her temperature was 38°C, heart rate was 142 beats/min, blood pressure was 117/57 mm Hg, respiratory rate was 21 breaths/min, and oxygen saturation was 99% while breathing ambient air. Cardiovascular examination revealed tachycardia with a high-pitched holosystolic murmur at the apex, radiating to the back. There was basal crepitation. The patient did not present with any vasculitic features such as purpura, and neurological examination was unremarkable.
Blood tests showed normal kidney function with serum creatinine of 48 µmol/L and urea of 2.8 mmol/L. Her hemoglobin was 5.2 g/dL. The cause of anemia was attributed to acute inflammation (based on iron indices/reticulocyte count/negative Coombs test). Urine examination showed microscopic hematuria and proteinuria. Complement C3 was low and C4 was normal. Renal ultrasound showed normal-sized echogenic kidneys.
Transthoracic echocardiography was performed on admission which showed severe mitral valve regurgitation due to the failure of coaptation with a significant gap between the anterior and posterior mitral valve leaflet with vegetation. The left atrium and left ventricle were dilated. Left ventricular ejection fraction was 72%, and there was no evidence of pericardial effusion.
The blood culture grew G. morbillorum which was sensitive to penicillin. A probable diagnosis of bacterial endocarditis was made and the patient was started on vancomycin at 10 mg/kg/dose every 6 h, gentamycin at 3 mg/kg/dose every 24 h, and penicillin at 20 million units/day every 6 h. Vancomycin and gentamycin were administered as per trough drug levels.
Her general condition continued to worsen in spite of antibiotics, and on the 3rd hospital day, she developed acute dyspnea with chest X-ray suggestive of pulmonary edema. Intra-alveolar hemorrhage was ruled out as the classical triad of hemoptysis, falling hematocrit, and diffuse infiltrates was absent. Diuretics were started. On the 7th hospital day, she developed acute kidney injury for which hemodialysis was required. The kidney ultrasound was unremarkable. The acute renal failure was initially ascribed to sepsis and possibly to acute tubulointerstitial nephritis related to antibiotics. These drugs, however, could not be stopped in view of the active ongoing infection.
Autoimmune serological tests were done on the 8th hospital day. The results were obtained within 2 days and showed negative results for ANCA, ANA, anti-DsDNA, HIV, hepatitis panel, and rheumatoid factor.
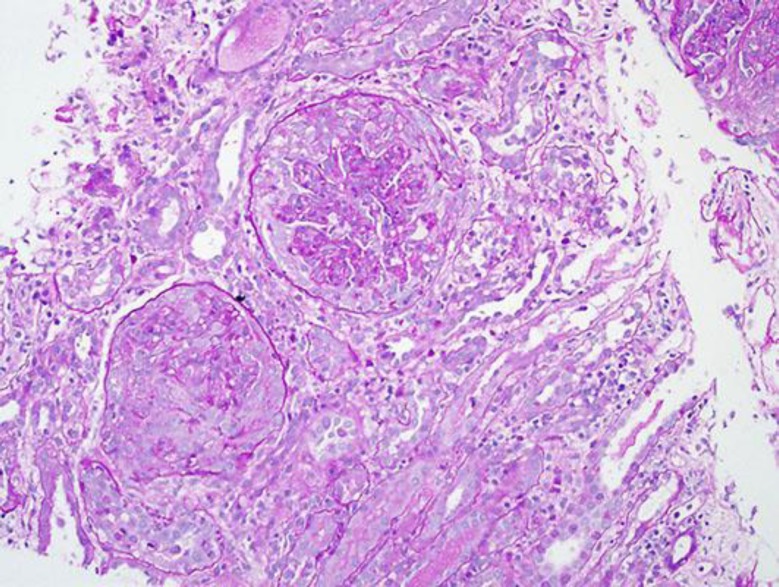
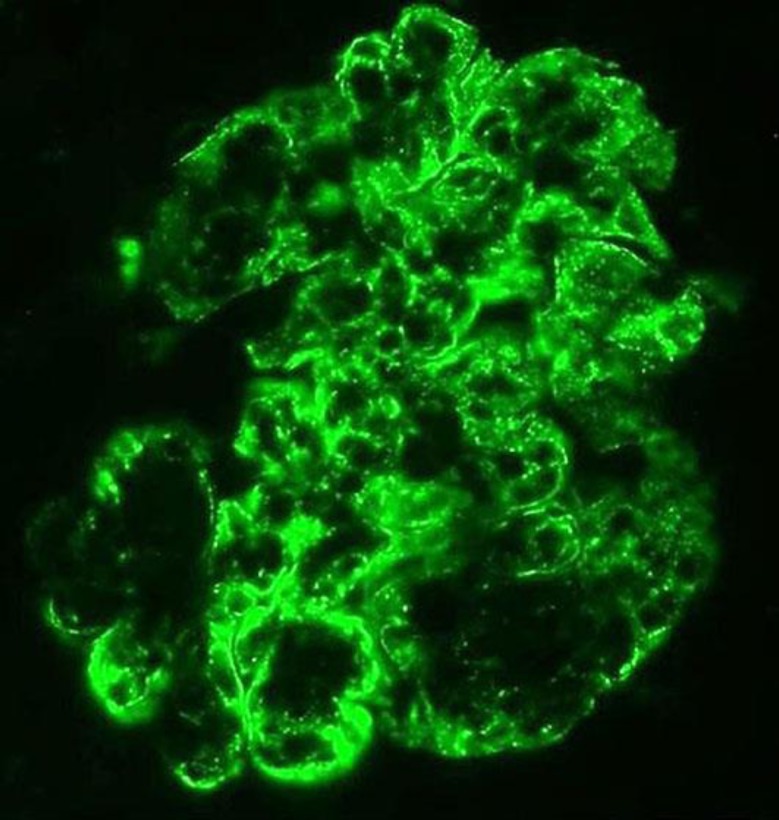
A renal biopsy was done on the 10th hospital day which showed 27 glomeruli of which 17 showed cellular crescents (Fig. 1). There was moderate acute tubular injury. The interstitium was unremarkable. Few arterioles were noted with no significant changes. Immunoflourescence showed mesangial and capillary wall staining for C3 (3+, diffuse and blotchy) (Fig. 2). There were no IgG, IgM, or IgA deposits. Electron microscopy showed slightly enlarged podocytes. A significant amount of subendothelial fine granular electron dense deposits consistent with immune complexes was noted in very patchy distribution. No significant intramembranous or subepithelial electron dense deposits were recognized. The mesangium was expanded by a significant amount of fine granular electron dense deposits consistent with immune complexes, also in patchy distribution.
Fig. 1.
Two Glomeruli with cellular crescents. PAS, ×200.
Fig. 2.
Immunoflourescence showing C3 stain.
On the 12th hospital day, the patient received 3 days of pulse IV methylprednisolone 10 mg/kg/day, in combination with plasmapheresis, daily during the first 3 days and 3 times/week during the following 2 weeks. Methylprednisolone per os was subsequently given (1 mg/kg) with progressive tapering. Serum creatinine peaked at 438 µmol/L on the 9th hospital day. She required hemodialysis for 1 week. The creatinine improved by the 22nd hospital day to 54 µmol/L. Rituximab at 375 mg/m2 was given on the 25th hospital day as the family refused cyclophosphamide due to concerns of fertility and hair loss. Urine analysis showed proteinuria of 0.1 g/L and hematuria of 10 red blood cells/μL.
A repeat echocardiography 3 weeks after admission showed severe mitral valve insufficiency with lack of coaptation between the anterior leaflet and posteromedial leaflets of the mitral valve with accompanying rupture of a few chordae. The parents were offered valve replacement but this was refused by the family and the patient was discharged against medical advice on day 35 of hospitalization. The trend of C3 could not be documented as the patient took early discharge.
Discussion
The natural history of endocarditis-associated glomerulonephritis has changed significantly in parallel with the changing epidemiology of infective endocarditis. Similarly, pathological presentations are also different. To our knowledge, this is the first case of G. morbillorum endocarditis with immune complex deposition and necrotizing glomerulonephritis to be reported in the pediatric age group. G. morbillorum, previously known as Streptococcus morbillorum, is a facultative anaerobic, gram-positive coccus which belongs to the genus Gemella. This organism is found as part of the normal flora of the human oropharynx, genitourinary system, and gastrointestinal system and does not normally cause disease in humans [1]. Rare cases are reported in the literature of Gemella causing endocarditis, and it infects native valve endocarditis more than prosthetic valves.
Apart from endocarditis, Gemella has been reported to cause several other infections as well, such as peritonitis in a patient on continuous ambulatory peritoneal dialysis [2]. Vasishtha et al. (1996) reported 2 children who presented with septic shock due to Gemella, which proved fatal in one of them [3]. Omran et al. (1993) reported a case of endovascular infection and presumptive septic arthritis caused by G. morbillorum [4]. Farmaki et al. (2000) reported endocarditis secondary to G. morbillorum in a 9-year-old female child with no previous cardiac disease but who had recently been treated for dental problems [5]. Our index child, however, had never had any dental issues in the past. Nagashima et al. (2001) reported a 17-year-old girl who had congenital hydrocephalus and presented with shunt nephritis with fever, proteinuria, pancytopenia, and hypocomplementemia. Her blood and cerebrospinal fluid grew Gemella, and anti-neutrophil cytoplasmic autoantibody specific for proteinase 3 (PR3-ANCA) was detected in her serum. She was treated with steroids with poor response, but shunt removal improved her symptoms and decreased PR3-ANCA in the serum to an undetectable level [6]. Gemella has been reported by Gimigliano (2005) to cause endocarditis in a 10-year-old female child with complex congenital heart disease [7].
Biopsy findings in cases of infective endocarditis with glomerulonephritis have been reported to vary from focal, segmental, or diffuse proliferative glomerulonephritis to crescentic glomerulonephritis. Among these, crescentic glomerulonephritis has been found to be the most common finding. Tubulointerstitial disease usually parallels the degree of glomerular injury. C3 staining has been invariably seen in almost all cases, with a smaller percentage of samples showing IgG staining as well. As described in the literature, infection-related glomerulonephritis shows prominent C3 staining and detectable immune deposits by electron microscopy. C3 glomerulopathy could have been considered another differential diagnosis in view of proteinuria, hematuria, and low C3 levels, but the renal biopsy did not reveal a membranoproliferative glomerulonephritis pattern in our index case. Screening with C3NeF (C3 nephritic factor) and complement factor H was negative as well.
Treatment of glomerulonephritis associated with endocarditis is controversial as some authors advise the use of immunosuppressive therapy combined with antibiotics, while others suggest the use of antibiotics alone [8]. Susceptibility of the organism has been ascribed to various antibiotics including penicillin and ampicillin. Cases not responding to medical management may require surgical replacement of the valve.
In our patient, the isolated G. morbillorum was sensitive to penicillin, aminoglycosides, and macrolides. We used penicillin and gentamycin for the treatment of G. morbillorum endocarditis. The child improved clinically but had residual valvular dysfunction which necessitated valve replacement; however, this was refused by the family.
Conclusion
To our knowledge, this is the first case of rare bacteria such as G. morbillorum leading to infective endocarditis complicated with crescentic glomerulonephritis to be reported in an otherwise healthy child. Renal biopsy is indicated to guide the further management if the child develops features of acute nephritic syndrome.
Statement of Ethics
The published research complies with the guidelines for human studies and animal welfare regulations. All authors state that the subject gave informed consent and that the study protocol was approved by the institute's committee on human research.
Disclosure Statement
The authors have no conflicts of interest to declare. No funding was received for this study.
References
- 1.Ural S, Gul Yurtsever S, Ormen B, et al. Gemella morbillorum endocarditis. Case Rep Infect Dis. 2014;2014:456471. doi: 10.1155/2014/456471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guney I, Isik A, Altintepe L, Er C, Kurdoglu MG. Gemella morbillorum peritonitis in a CAPD patient. Perit Dial Int. 2009;29:674–675. [PubMed] [Google Scholar]
- 3.Vasishtha S, Isenberg HD, Sood SK. Gemella morbillorum as a cause of septic shock. Clin Infect Dis. 1996;22:1084–1086. doi: 10.1093/clinids/22.6.1084. [DOI] [PubMed] [Google Scholar]
- 4.Omran Y, Wood CA. Endovascular infection and septic arthritis caused by Gemella morbillorum. Diagn Microbiol Infect Dis. 1993;16(2):131–134. doi: 10.1016/0732-8893(93)90007-t. [DOI] [PubMed] [Google Scholar]
- 5.Farmaki E, et al. Gemella morbillorum endocarditis in a child. Pediatr Infect Dis J. 2000;19:751–753. doi: 10.1097/00006454-200008000-00015. [DOI] [PubMed] [Google Scholar]
- 6.Nagashima T, et al. Antineutrophil cytoplasmic autoantibody specific for proteinase 3 in a patient with shunt nephritis induced by Gemella morbillorum. Am J Kidney Dis. 2001;37(5):e38–e41. doi: 10.1016/s0272-6386(05)90002-4. [DOI] [PubMed] [Google Scholar]
- 7.Gimigliano F, et al. Gemella morbillorum endocarditis in a child. Pediatr Infect Dis J. 2005;24(2):190. doi: 10.1097/01.inf.0000153173.47612.ab. [DOI] [PubMed] [Google Scholar]
- 8.Boils CL, Nasr SH, Walker PD, Couser WG, Larsen CP. Update on endocarditis-associated glomerulonephritis. Kidney Int. 2015;87:1241–1249. doi: 10.1038/ki.2014.424. [DOI] [PMC free article] [PubMed] [Google Scholar]