Abstract
Objective
The aim was to compare the vitamin D levels in patients with Graves disease (GD) with the general population and to correlate the vitamin D levels with laboratory and clinical parameters in GD. Moreover, we examined the genetic variation in genes involved in the vitamin D metabolism and their association with GD.
Methods
The levels of vitamin D were compared in 292 patients with newly diagnosed GD and 2,305 controls. Single nucleotide polymorphisms (SNPs) in the vitamin D receptor (VDR), vitamin D binding protein (DBP), and 1-α-hydroxylase (CYP27B1) were examined for association with GD and/or Graves ophthalmopathy (GO) in 708 patients and 1,178 controls.
Results
Patients with GD had significantly lower vitamin D levels compared to controls (55.0 ± 23.2 vs. 87.2 ± 27.6 nmol/L, p < 0.001). In patients with GD (n = 219), there was no association between the levels of vitamin D at diagnosis and free thyroxine (fT4), free triiodothyronine (fT3), thyrotropin receptor antibodies (TRAb), GO at diagnosis, or relapse after terminating treatment with antithyroid drugs. Two SNPs in VDR were associated with GD: rs10735810 (OR = 1.36, 95% CI: 1.02–1.36, p = 0.02) and rs1544410 (OR = 1.47, 95% CI: 1.03–1.47, p = 0.02). There was no difference in the mean vitamin D level between genotypes in either rs10735810 or rs154410.
Conclusions
Patients with GD had lower vitamin D levels compared to the general population; however, the vitamin D levels did not affect the laboratory or clinical parameters of GD. SNPs in the VDR influenced the risk of GD through mechanisms other than reducing the vitamin D levels.
Keywords: Vitamin D, Graves disease, Graves ophthalmopathy, Vitamin D receptor
Introduction
In recent years, the extraskeletal effects of vitamin D have been extensively studied. Vitamin D deficiency is linked to a variety of autoimmune disorders, including autoimmune thyroid disease [1]. Genetic variation in genes involved in vitamin D metabolism has been associated with several autoimmune disorders including autoimmune thyroid disease [2, 3, 4, 5, 6, 7]. Several studies suggest that individuals with Graves disease (GD) have lower vitamin D levels than the general population [8, 9, 10, 11]; however, data on the relationship between the levels of vitamin D and clinical parameters in GD [12, 13] or therapeutic issues [14, 15, 16] are limited. The aim of the present study was to compare vitamin D levels in a large number of newly diagnosed patients with GD with those of the general population and to correlate the vitamin D levels at diagnosis with laboratory and clinical parameters in patients with GD. Moreover, we examined the genetic variation in genes involved in vitamin D metabolism and their association with GD. Single nucleotide polymorphisms (SNPs) in the vitamin D receptor (VDR), GC – vitamin D binding protein (DBP), and 1-α-hydroxylase (CYP27B1) were examined for association with GD and/or Graves ophthalmopathy (GO).
Materials and Methods
Comparison of Vitamin D Levels in Patients with GD and the General Population
In the epidemiological part of the study, the patients (n = 295) were recruited among subjects with newly diagnosed thyrotoxicosis referred to Skåne University Hospital, Malmö, Sweden. The diagnosis of GD was made by an endocrinologist, as described earlier [17]. None of the included patients had been started on treatment for thyrotoxicosis at the time of blood sampling. Patients using calcium and/or vitamin D supplements (n = 3) were excluded, leaving 292 patients for analysis. The controls were recruited from the Malmö Diet and Cancer Study (MDCS) [18]. Data on vitamin D were available in 3,414 individuals [19, 20]. Individuals with thyroid disease and parathyroid disease, using thyroid medication, and using calcium and/or vitamin D supplements were excluded, leaving a total of 2,305 controls. Vitamin D was analyzed with high-performance liquid chromatography (CV = 7.1% at 90 nmol/L and 8.5% at 70 nmol/L) in controls and liquid chromatography-tandem mass spectrometry (CV = 6% at 40 nmol/L and 4% at 120 nmol/L) in cases at the Department of Clinical Chemistry, Skåne University Hospital, which is an accredited laboratory. According to a recent study, there is a high correlation (r = 0.96) between these 2 methods regarding vitamin D analysis [21].
Correlation of Vitamin D Levels with Laboratory and Clinical Parameters in GD Patients
In the clinical part of the study, in 219 patients with GD with available data, the D vitamin levels were correlated with laboratory and clinical parameters at diagnosis, including (i) the levels of free thyroxine (fT4), free triiodothyronine (fT3), thyroid peroxidase antibodies (TPOAb), and thyrotropin receptor antibodies (TRAb); (ii) the presence of GO; and (iii) relapse within 1 year after terminating treatment with antithyroid drugs. Regarding the analysis of relapse, 139 patients were treated with antithyroid drugs as first-line therapy. The patients included in this analysis were those that completed an 18-month (±1 month) course of antithyroid drugs and were available for follow-up 12 months later. Patients who were switched to treatment with radioiodine or surgery during treatment, who interrupted the treatment earlier than at 18 months, who disappeared from follow-up, or who required prolonged treatment with antithyroid drugs or switch to alternative treatment after 18 months because of persistent active disease were excluded. We even excluded patients who relapsed with GD postpartum (n = 2) or after being given iodine contrast (n = 1), leaving a total of 100 patients for the analysis.
The diagnosis of GO was made by an endocrinologist and/or ophthalmologist, as previously described [22]. Thyroid-stimulating hormone (TSH) (0.40–3.7 mIU/L), fT4 (12–22 pmol/L), fT3 (3.6–6.3 pmol/L), TPOAb (<34 kIU/L), and TRAb (<1.2 IU/L) were measured with electrochemiluminiscence immunoassay on Cobas (Roche) at the Department of Clinical Chemistry, Skåne University Hospital, Malmö.
Genetic Association between SNPs in Genes Involved in Vitamin D Metabolism and GD and/or GO
Genetic variation in genes involved in vitamin D metabolism was examined for association with GD and GO in 708 patients with (n = 245) or without (n = 459) ophthalmopathy and 1,178 sex-matched controls from Skåne University Hospital, Malmö. Controls without thyroid disease were recruited from the Malmö Preventive Project (MFM) [23] and MDCS [18] databases.
The following SNPs were chosen for analysis based on previous associations with autoimmune thyroid disease or other autoimmune disorders [6]: rs731236 (TaqI), rs7975232 (ApaI), rs10735810 (FokI), and rs1544410 (BsmI) in VDR; rs7041 and rs4588 in DBP; and rs10877012 and rs4646536 in CYP27B1. The minor allele frequency for all SNPs was >0.05. DNA was extracted from whole blood using the MaxiPrep Kit (QIAGEN, Sweden), and SNPs were genotyped using the Sequenom platform (MALDI-TOF). The genotyping success rate was >95%. In the GD patients with available data (n = 219), the genotype was correlated with the vitamin D levels at diagnosis of GD.
All statistical analyses were computed using IBM SPSS Statistics for Windows, version 22.0 (Armonk, NY, USA). All genetic analyses were performed using PLINK version 1.07 (http://pngu.mgh.harvard.edu/~purcell/plink/index.shtml) [24]. Logistic and linear regression with age, sex, smoking, and ethnicity as covariates was used for estimating SNP associations, and the data are presented as the odds ratios (OR) with 95% confidence intervals (CI). The p values are based on additive models for the genetic variants. Correction for multiple testing was performed using permutations.
All participants gave their informed consent and both MDCS (LU 51–90) and the current study (LU 328–01) were approved by the local ethics committee.
Results
Comparison of Vitamin D Levels in Patients with GD and the General Population
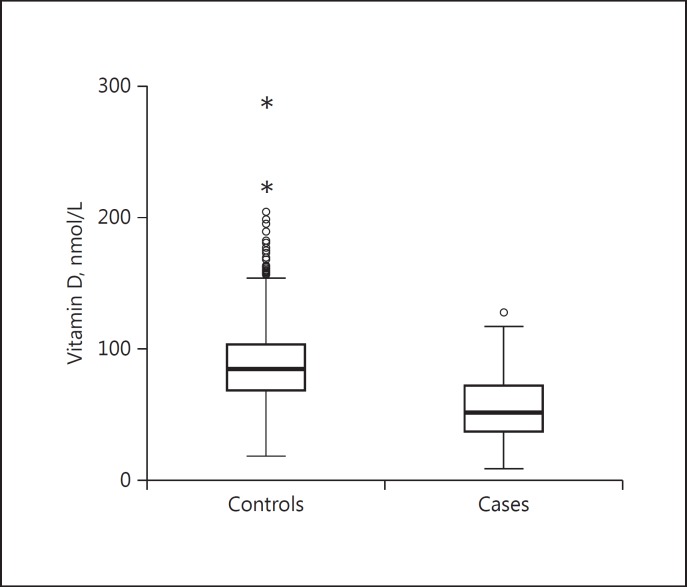
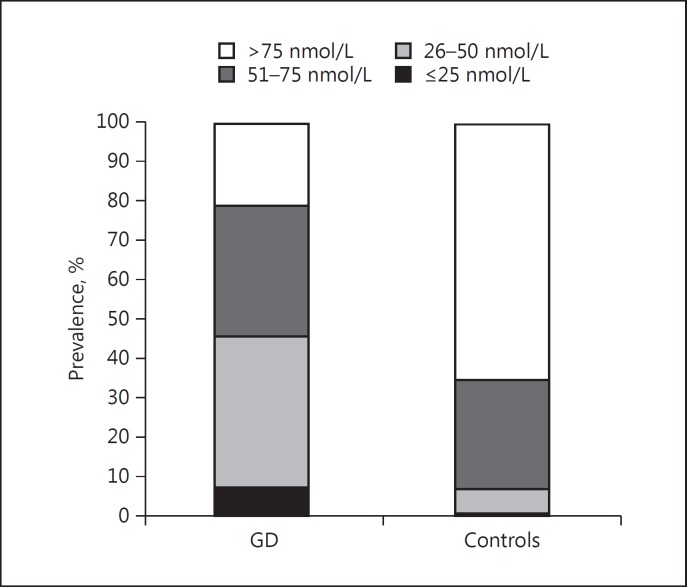
The characteristics of the cases and controls are presented in Table 1 (epidemiological part of the study) andTable 2 (genetic part of the study). Patients with GD had significantly lower vitamin D levels than controls (55.0 ± 23.2 vs. 87.2 ± 27.6 nmol/L, p < 0.0001) (Fig. 1). The prevalence of vitamin D deficiency (<25 nmol/L) and insufficiency (<50 nmol/L) was higher in GD patients compared to controls (Fig. 2). Due to the surprisingly high levels of vitamin D in the controls, we speculated that it might be caused by the fact that some subjects did not report the use of vitamin D supplements. Therefore, we performed analyses in which we only included subjects with vitamin D levels <150 nmol/L and <100 nmol/L, respectively, aiming to exclude subjects using vitamin D supplements. Still, the patients had significantly lower vitamin D levels compared to controls: patients (n = 292): 55.0 ± 23.2 nmol/L vs. controls (n = 2248): 85.1 ± 24.2 nmol/L, p < 0.0001, in the <150 nmol/L group and patients (n = 279): 52.5 ± 20.4 nmol/L vs. controls (n = 1,646): 73.9 nmol/L, p < 0.0001 in the <100 nmol/L group. In subjects with vitamin D levels <150 nmol/L, the prevalence of vitamin D deficiency (≤25 nmol/L) was 7.5% in cases and 0.3% in controls, and that of insufficiency (26–50 nmol/L) was 38.4% in cases and 6.5% in controls. In subjects with vitamin D levels <100 nmol/L, the prevalence of vitamin D deficiency (≤25 nmol/L) was 7.5% in cases and 0.4% in controls, and that of insufficiency (26–50 nmol/L) was 38.4% in cases and 8.9% in controls. To overcome problems with differences between cases and controls, we performed subgroup analysis and logistic regression adjusting for confounders. The results were similar and the differences remained significant when we chose to analyze women (54.7± 22.8 vs. 89 ± 28.2 nmol/L, p < 0.0001) and men (56.4 ± 25.4 vs. 86 ± 27.1 nmol/L, p < 0.0001) separately and when we only analyzed individuals of Swedish origin, defined as born in Sweden (59.7 ± 22.3 vs. 88.7 ± 27.1 nmol/L, p < 0.0001). Logistic regression in all individuals, adjusted for sex, age, ethnicity, and smoking, also showed that vitamin D is negatively associated with GD (OR = 0.95, 95% CI: 0.95–0.96, p < 0.0001).
Table 1.
Characteristics of the subjects included in the epidemiological part of the study
| Cases | Controls | p | ||
|---|---|---|---|---|
| Patients | 292 | 2,305 | ||
| Sex | ||||
| Male | 46 (15.8) | 1,384 (60.0) | <0.001 | |
| Female | 246 (84.2) | 921 (40.0) | ||
| Age, years | 45.5±13.1 | 59.4±7.2 | <0.001 | |
| Smoking | ||||
| Yes | 121 (41.4) | 633 (27.5) | <0.001 | |
| No | 168 (57.5) | 1,671 (72.5) | ||
| Missing | 3 (1.1) | 1 (0.0) | ||
| Season for sampling | ||||
| Dec–Feb | 78 (26.7) | 554 (24.0) | 0.15 | |
| Mar–May | 73 (25.0) | 645 (28.0) | ||
| Jun–Aug | 58 (19.9) | 365 (15.9) | ||
| Sep–Nov | 83 (28.4) | 741 (32.1) | ||
| Ethnicity | ||||
| Swedish | 203 (69.5) | 2,067 (89.7) | <0.001 | |
| European | 58 (19.9) | 238 (10.3) | ||
| Other | 28 (9.6) | 0 (0.0) | ||
| Missing | 3 (1.0) | 0 (0.0) |
Values are n (%) or mean ± SD, as appropriate.
Table 2.
Characteristics of the subjects included in the genetic part of the study
| Cases, n | % | Controls, n | % | |
|---|---|---|---|---|
| Patients | 708 | 1,178 | ||
| Age at diagnosis/inclusion, years | 49+14 | 57+6 | ||
| Sex | ||||
| Male | 128 | 18.0 | 209 | 17.8 |
| Female | 580 | 82.0 | 967 | 82.1 |
| Missing | 2 | 0.1 | ||
| Ethnicity | ||||
| Swedish | 530 | 74.9 | 834 | 70.8 |
| European | 117 | 16.5 | 176 | 14.9 |
| Other | 58 | 8.2 | 83 | 7.1 |
| Missing | 3 | 0.4 | 85 | 7.2 |
| Smoking | ||||
| Yes | 277 | 39.1 | 343 | 29.1 |
| No | 402 | 56.8 | 799 | 67.8 |
| Missing | 29 | 4.1 | 36 | 3.1 |
| Ophthalmopathy | ||||
| Yes | 245 | 34.6 | 0 | 0 |
| No | 459 | 64.8 | 1,178 | 100.0 |
| Missing | 4 | 0.6 | 0 | 0 |
Fig. 1.
Vitamin D levels in controls (n = 2,305, 87.2 ± 27.6 nmol/L) and patients with Graves disease (n = 292, 55.0 ± 23.2 nmol/L, p < 0.0001)
Fig. 2.
Prevalence of different vitamin D levels in patients with Graves disease (GD) and controls (<25 nmol/L: 7.5 vs. 0.3%, 26–50 nmol/L: 38.4 vs. 6.4%, 51–75 nmol/L: 33.2 vs. 27.9%, and >75 nmol/L: 20.9 vs. 65.5%, p < 0.0001).
Correlation of Vitamin D Levels with Laboratory and Clinical Parameters in GD Patients
We did not observe any correlation (Spearman) between the vitamin D levels and levels of fT4, fT3, TRAb, and TPOAb and relapse within 1 year after terminating treatment with antithyroid drugs. When comparing subjects with a vitamin D level <25 nmol/L to those with higher levels, we could not see any differences in the fT4, fT3, TRAb, and TPOAb levels or the frequency of GO. Regarding GO, GD patients without GO at diagnosis had the same vitamin D levels as those with GO (n = 254, 55.5 ± 22.9 nmol/L vs. n = 37, 52.1 ± 24.6 nmol/L, p = nonsignificant, ns). Logistic regression adjusting for sex, age, ethnicity, and smoking did not show any association between the vitamin D levels and GO at diagnosis of GD (OR = 0.99, 95% CI: 0.98–1.01, p = ns). We further analyzed the impact of vitamin D levels on the outcome of patients treated with antithyroid drugs (n = 100), defined as relapse of GD within 1 year of completing an 18-month course of antithyroid drugs. There was no difference in the vitamin D levels at baseline between individuals who achieved remission and those who relapsed (n = 78, 56.9 ± 21.8 nmol/L vs. n = 22, 63.1 ± 27 nmol/L, p = ns). Logistic regression, adjusting for sex, age, smoking, GO at diagnosis, and TRAb at diagnosis, did not show any association between the vitamin D levels at diagnosis and relapse after antithyroid drugs (OR = 1.02, 95% CI: 1.0–1.04, p = ns).
Genetic Association between SNPs in Genes Involved in Vitamin D Metabolism and GD and/or GO
The results are summarized in Table 3. Two SNPs in VDR were associated with GD: rs10735810 (p = 0.02, OR = 1.36, 95% CI: 1.02–1.36) and rs1544410 (p = 0.02, OR = 1.47, 95% CI: 1.03–1.47). There was no difference in the mean vitamin D levels between genotypes in either rs10735810 (AA 55.9 nmol/L, AG 56.1 nmol/L, GG 54.1 nmol/L, p = ns) or rs1544410 (AA 56.6 nmol/L, AG 57.5 nmol/L, GG 54.8 nmol/L, p = ns). Linear regression analysis in GD patients did not show any association with the vitamin D levels for rs10735810 and rs1544410, respectively. There was a borderline association between rs1544410 and fT4. Rs4588 was associated with the TRAb levels at diagnosis in the 150 patients with available data on TRAb. None of the SNPs was associated with the fT3 and TPOAb levels or GO at diagnosis.
Table 3.
SNPs in genes involved in vitamin D metabolism and their association with Graves disease and vitamin D levels
| SNP | Allele | Allele frequency | OR (95% CI) | p | Difference in D Viamin per allele (95% CI) | p | Difference in fT4 per allele (95% CI) | p | Difference in TRAb per allele (95% CI) | p |
|---|---|---|---|---|---|---|---|---|---|---|
| rs731236 | G | 0.41 | 0.98 | 0.81 | −2.06 | 0.34 | 1.21 | 0.56 | −0.97 | 0.40 |
| (0.85 to 1.13) | (−6.28 to 2.15) | (−2.84 to 5.25) | (−3.22 to 1.27) | |||||||
| rs7975232 | C | 0.28 | 1.14 | 0.13 | −0.13 | 0.95 | 0.77 | 0.72 | 0.54 | 0.66 |
| (0.96 to 1.35) | (−4.56 to 4.28) | (−3.46 to 5.00) | (−1.84 to 2.92) | |||||||
| rs10735810 | A | 0.41 | 1.18 | 0.02 | 1.08 | 0.60 | 1.65 | 0.40 | 0.97 | 0.38 |
| (1.02 to 1.36) | (−2.94 to 5.10) | (−2.19 to 5.49) | (−1.17 to 3.12) | |||||||
| rs1544410 | A | 0.18 | 1.23 | 0.02 | 1.51 | 0.44 | 3.60 | 0.05 | −0.24 | 0.84 |
| (1.03 to 1.48) | (−2.33 to 5.35) | (−0.05 to 7.25) | (−2.44 to 1.97) | |||||||
| rs7041 | A | 0.42 | 0.96 | 0.62 | 1.92 | 0.18 | 2.61 | 0.15 | −1.28 | 0.22 |
| (0.83 to 1.12) | (−6.33 to 1.19) | (−0.99 to 6.21) | (−3.30 to 0.74) | |||||||
| rs4588 | T | 0.28 | 0.96 | 0.65 | −5.08 | 0.02 | −1.69 | 0.43 | −2.53 | 0.03 |
| (0.82 to 1.14) | (−9.41 to 0.75) | (−5.88 to 2.51) | (−4.85 to 0.21) | |||||||
| rs10877012 | T | 0.33 | 1.02 | 0.76 | 2.24 | 0.27 | −0.05 | 0.98 | −1.26 | 0.24 |
| (0.89 to 1.19) | (−1.71 to–6.20) | (−3.85 to 3.75) | (−3.34 to 0.83) | |||||||
| rs4646536 | G | 0.34 | 1.07 | 0.37 | −1.11 | 0.59 | −0.09 | 0.96 | −0.63 | 0.51 |
| (0.89 to 1.19) | (−5.06 to 2.86) | (−3.89 to 3.70) | (−2.77 to 1.51) |
Discussion
In this large study on vitamin D in GD, we found significantly lower levels of vitamin D in patients with GD at onset compared to controls. However, there was no correlation between the vitamin D levels and several laboratory and clinical parameters in GD. Two SNPs in VDR were associated with GD.
In the epidemiological part of the study, we found lower vitamin D levels in patients with GD compared to controls, a result that is supported by findings of other smaller studies [12, 13] and recent meta-analyses [10, 11]. The vitamin D levels were in the same range as previously reported in a Chinese study [12]. The strength of this part of the study is the large number of both cases and controls from one city, Malmö, in Southern Sweden. The drawback of this part of the study is that the cases and control samples were not collected at the same time and were not perfectly matched. However, there was no difference in the percentage of individuals recruited during different seasons between cases and controls. An advanced statistical analysis of the same material has previously shown the same results in matched cases and controls as in unmatched analysis controlled for confounders [19], as performed in this study. Analysis after removing individuals with very high vitamin D values confirmed significantly lower vitamin D levels in patients. Therefore, the cases and controls represent comparable populations.
There was no correlation between the vitamin D levels at diagnosis of GD with the levels of thyroid hormones, the levels of TRAb or TPOAb, or the presence of GO, suggesting that the level of vitamin D does not influence the disease severity at diagnosis. These results are in accordance with the observations of a Japanese study that found a significant association between serum vitamin D levels and thyroid volume, but not thyroid function or TRAb levels, in GD patients [13]. In a previous Chinese study of 35 patients with seropositive GD, 35 patients with seronegative GD, and 70 matched controls, the level of vitamin D was inversely correlated with the TRAb titer in TRAb-positive GD patients, but it was not correlated with the levels of TPOAb, thyroglobulin antibodies (TGAb), fT3, fT4, or TSH [12]. The discrepancy between the results regarding TRAb between ours and the Chinese study could be explained by the higher number of individuals in our study, ethnicity, 100% TRAb positivity in our patients, higher average TRAb titers in the Chinese study, and different method for analyzing TRAb. To the best of our knowledge, ours is the first study investigating the relationship between vitamin D levels and GO, showing no association between vitamin D levels with the presence of GO at the onset of GD.
Vitamin D levels at the diagnosis of GD were not correlated with relapse of GD after completing an 18-month course of antithyroid drugs. Unfortunately, we do not have data on the vitamin D levels at the time of termination of the antithyroid drugs or relapse. However, antithyroid drugs have not been shown to influence vitamin D levels in GD patients [13, 25], and a high correlation between individual levels of vitamin D measured on two separate occasions 3 years apart has previously been demonstrated [26]. Based on these results, the vitamin D levels at diagnosis of GD cannot be used for predicting relapse after termination of antithyroid drugs. In a Japanese study of 18 female patients in remission, 36 female patients without remission (discontinuing antithyroid drugs unattainable 4 years after therapy initiation and persistent TRAb positivity), and 49 healthy controls, the mean vitamin D levels were significantly lower in patients without remission than in those with remission [15]. However, the patients without remission were all TRAb positive, which per se is a strong prognostic factor of relapse, and the vitamin D levels do not add additional value in predicting relapse in this group.
The results of previous studies of genetic variation in VDR and association with GD are inconclusive. In a meta-analysis from 2008, ApaI, BsmI, and FokI were associated with susceptibility to GD in Asian populations, while ApaI, BsmI, TaqI, and FokI were not associated with GD in Caucasian populations [7]. Some studies found the association of BsmI and/or FokI with GD in Caucasians [27, 28], Japanese [29], and Chinese [30], while others did not [31]. As reviewed by Jolliffe et al. [6], BsmI and/or FokI were associated with other autoimmune disorders. In our study, the BsmI and FokI SNPs were associated with GD, but they did not affect the vitamin D status, which agrees with a recent genome-wide association study [32, 33]. The vitamin D status in these studies was influenced by genetic variation in the GC gene, and in our study we showed an association of rs4588 with the vitamin D levels. Rs4588 influences the binding affinity of DBP and serum and plasma vitamin D levels [34, 35]. Rs4588 SNP was also associated with TRAb levels in our study; however, due to the limited number of individuals with data on TRAb (n = 151), this result has to be interpreted with caution.
To understand the role of vitamin D in GD, it is important to determine whether lower vitamin D levels contribute to the development of GD. There are some animal data supporting this hypothesis. BALB/cJ mice given a vitamin D-deficient diet had lower preimmunization T4 levels and were more prone to developing persistent hyperthyroidism following immunization with the TSH receptor compared to mice fed regular chow. No differences in the TRAb levels were observed, suggesting that vitamin D directly modulated thyroid function in this animal model [36]. In our study, the lack of a significant association between vitamin D and TRAb would support the hypothesis that vitamin D deficiency might have a direct effect on the thyroid gland. However, as all clinical studies (including our own) that address this question have been cross-sectional in design, it is impossible to conclude whether the vitamin D status is directly involved in the pathogenesis or a consequence of the disease.
Further prospective studies designed to evaluate the role of vitamin D deficiency and the effects of its correction in patients with GD are needed.
Disclosure Statement
The authors declare that there is no conflict of interest regarding the publication of this article.
Acknowledgment
This work was supported by grants from Svenska Läkaresällskapet and ALF Region Skåne.
References
- 1.Kmiec P, Sworczak K. Vitamin D in thyroid disorders. Exp Clin Endocrinol Diabetes. 2015;123:386–393. doi: 10.1055/s-0035-1554714. [DOI] [PubMed] [Google Scholar]
- 2.Inoue N, Watanabe M, Ishido N, Katsumata Y, Kagawa T, Hidaka Y, Iwatani Y. The functional polymorphisms of VDR, GC and CYP2R1 are involved in the pathogenesis of autoimmune thyroid diseases. Clin Exp Immunol. 2014;178:262–269. doi: 10.1111/cei.12420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kurylowicz A, Badenhoop K. CYP27B1 gene polymorphism is associated with Graves’ disease in a Polish population study. Thyroid. 2005;15:1107–1108. doi: 10.1089/thy.2005.15.1107. [DOI] [PubMed] [Google Scholar]
- 4.Abd El Gawad SS, Abdul Samee ER, Metwali AA, Abd El Gawad MS. Vitamin D receptor gene polymorphism and its association with 1,25-dihydroxyvitamin D3 in patients with Graves disease in an Egyptian population: a pilot study. Endocr Pract. 2012;18:132–139. doi: 10.4158/EP11131.OR. [DOI] [PubMed] [Google Scholar]
- 5.Feng M, Li H, Chen SF, Li WF, Zhang FB. Polymorphisms in the vitamin D receptor gene and risk of autoimmune thyroid diseases: a meta-analysis. Endocrine. 2013;43:318–326. doi: 10.1007/s12020-012-9812-y. [DOI] [PubMed] [Google Scholar]
- 6.Jolliffe DA, Walton RT, Griffiths CJ, Martineau AR. Single nucleotide polymorphisms in the vitamin D pathway associating with circulating concentrations of vitamin D metabolites and non-skeletal health outcomes: review of genetic association studies. J Steroid Biochem Mol Biol. 2016;164:18–29. doi: 10.1016/j.jsbmb.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 7.Zhou H, Xu C, Gu M. Vitamin D receptor (VDR) gene polymorphisms and Graves’ disease: a meta-analysis. Clin Endocrinol. 2009;70:938–945. doi: 10.1111/j.1365-2265.2008.03413.x. [DOI] [PubMed] [Google Scholar]
- 8.Yamashita H, Noguchi S, Takatsu K, Koike E, Murakami T, Watanabe S, Uchino S, Yamashita H, Kawamoto H. High prevalence of vitamin D deficiency in Japanese female patients with Graves’ disease. Endocr J. 2001;48:63–69. doi: 10.1507/endocrj.48.63. [DOI] [PubMed] [Google Scholar]
- 9.Ma J, Wu D, Li C, Fan C, Chao N, Liu J, Li Y, Wang R, Miao W, Guan H, Shan Z, Teng W. Lower Serum 25-hydroxyvitamin D level is associated with 3 types of autoimmune thyroid diseases. Medicine. 2015;94:e1639. doi: 10.1097/MD.0000000000001639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang J, Lv S, Chen G, Gao C, He J, Zhong H, Xu Y. Meta-analysis of the association between vitamin D and autoimmune thyroid disease. Nutrients. 2015;7:2485–2498. doi: 10.3390/nu7042485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu MY, Cao B, Yin J, Wang DF, Chen KL, Lu QB. Vitamin D and Graves’ disease: a meta-analysis update. Nutrients. 2015;7:3813–3827. doi: 10.3390/nu7053813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang H, Liang L, Xie Z. Low vitamin D status is associated with increased thyrotropin-receptor antibody titer in Graves disease. Endocr Pract. 2015;21:258–263. doi: 10.4158/EP14191.OR. [DOI] [PubMed] [Google Scholar]
- 13.Yasuda T, Okamoto Y, Hamada N, Miyashita K, Takahara M, Sakamoto F, Miyatsuka T, Kitamura T, Katakami N, Kawamori D, Otsuki M, Matsuoka TA, Kaneto H, Shimomura I. Serum vitamin D levels are decreased and associated with thyroid volume in female patients with newly onset Graves’ disease. Endocrine. 2012;42:739–741. doi: 10.1007/s12020-012-9679-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li X, Wang G, Lu Z, Chen M, Tan J, Fang X. Serum 25-hydroxyvitamin D predict prognosis in radioiodine therapy of Graves’ disease. J Endocrinol Invest. 2015;38:753–759. doi: 10.1007/s40618-015-0252-4. [DOI] [PubMed] [Google Scholar]
- 15.Yasuda T, Okamoto Y, Hamada N, Miyashita K, Takahara M, Sakamoto F, Miyatsuka T, Kitamura T, Katakami N, Kawamori D, Otsuki M, Matsuoka TA, Kaneto H, Shimomura I. Serum vitamin D levels are decreased in patients without remission of Graves’ disease. Endocrine. 2013;43:230–232. doi: 10.1007/s12020-012-9789-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawakami-Tani T, Fukawa E, Tanaka H, Abe Y, Makino I. Effect of 1 α-hydroxyvitamin D3 on serum levels of thyroid hormones in hyperthyroid patients with untreated Graves’ disease. Metabolism. 1997;46:1184–1188. doi: 10.1016/s0026-0495(97)90214-6. [DOI] [PubMed] [Google Scholar]
- 17.Planck T, Shahida B, Sjogren M, Groop L, Hallengren B, Lantz M. Association of BTG2, CYR61, ZFP36, and SCD gene polymorphisms with Graves’ disease and ophthalmopathy. Thyroid. 2014;24:1156–1161. doi: 10.1089/thy.2013.0654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manjer J, Carlsson S, Elmstahl S, Gullberg B, Janzon L, Lindstrom M, Mattisson I, Berglund G. The Malmö Diet and Cancer Study: representativity, cancer incidence and mortality in participants and non-participants. Eur J Cancer Prev. 2001;10:489–499. doi: 10.1097/00008469-200112000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Almquist M, Bondeson AG, Bondeson L, Malm J, Manjer J. Serum levels of vitamin D, PTH and calcium and breast cancer risk-a prospective nested case-control study. Int J Cancer. 2010;127:2159–2168. doi: 10.1002/ijc.25215. [DOI] [PubMed] [Google Scholar]
- 20.Brandstedt J, Almquist M, Manjer J, Malm J. Vitamin D, PTH, and calcium and the risk of prostate cancer: a prospective nested case-control study. Cancer Causes Control. 2012;23:1377–1385. doi: 10.1007/s10552-012-9948-3. [DOI] [PubMed] [Google Scholar]
- 21.Enko D, Fridrich L, Rezanka E, Stolba R, Ernst J, Wendler I, Fabian D, Hauptlorenz S, Halwachs-Baumann G. 25-Hydroxy-vitamin D status: limitations in comparison and clinical interpretation of serum-levels across different assay methods. Clin Lab. 2014;60:1541–1550. doi: 10.7754/clin.lab.2014.131114. [DOI] [PubMed] [Google Scholar]
- 22.Traisk F, Tallstedt L, Abraham-Nordling M, Andersson T, Berg G, Calissendorff J, Hallengren B, Hedner P, Lantz M, Nystrom E, Ponjavic V, Taube A, Torring O, Wallin G, Asman P, Lundell G. Thyroid-associated ophthalmopathy after treatment for Graves’ hyperthyroidism with antithyroid drugs or iodine-131. J Clin Endocrinol Metab. 2009;94:3700–3707. doi: 10.1210/jc.2009-0747. [DOI] [PubMed] [Google Scholar]
- 23.Nilsson PM, Nilsson JA, Berglund G. Population-attributable risk of coronary heart disease risk factors during long-term follow-up: the Malmö Preventive Project. J Intern Med. 2006;260:134–141. doi: 10.1111/j.1365-2796.2006.01671.x. [DOI] [PubMed] [Google Scholar]
- 24.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, Sham PC. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pantazi H, Papapetrou PD. Changes in parameters of bone and mineral metabolism during therapy for hyperthyroidism. J Clin Endocrinol Metab. 2000;85:1099–1106. doi: 10.1210/jcem.85.3.6457. [DOI] [PubMed] [Google Scholar]
- 26.Platz EA, Leitzmann MF, Hollis BW, Willett WC, Giovannucci E. Plasma 1,25-dihydroxy- and 25-hydroxyvitamin D and subsequent risk of prostate cancer. Cancer Causes Control. 2004;15:255–265. doi: 10.1023/B:CACO.0000024245.24880.8a. [DOI] [PubMed] [Google Scholar]
- 27.Stefanic M, Karner I, Glavas-Obrovac L, Papic S, Vrdoljak D, Levak G, Krstonosic B. Association of vitamin D receptor gene polymorphism with susceptibility to Graves’ disease in Eastern Croatian population: case-control study. Croat Med J. 2005;46:639–646. [PubMed] [Google Scholar]
- 28.Ramos-Lopez E, Kurylowicz A, Bednarczuk T, Paunkovic J, Seidl C, Badenhoop K. Vitamin D receptor polymorphisms are associated with Graves’ disease in German and Polish but not in Serbian patients. Thyroid. 2005;15:1125–1130. doi: 10.1089/thy.2005.15.1125. [DOI] [PubMed] [Google Scholar]
- 29.Ban Y, Taniyama M, Ban Y. Vitamin D receptor gene polymorphism is associated with Graves’ disease in the Japanese population. J Clin Endocrinol Metab. 2000;85:4639–4643. doi: 10.1210/jcem.85.12.7038. [DOI] [PubMed] [Google Scholar]
- 30.Chen RH, Chang CT, Chen HY, Chen WC, Tsai CH, Tsai FJ. Association between vitamin-D receptor gene FokI polymorphism and Graves’ disease among Taiwanese Chinese. J Clin Lab Anal. 2007;21:173–177. doi: 10.1002/jcla.20163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Collins JE, Heward JM, Nithiyananthan R, Nejentsev S, Todd JA, Franklyn JA, Gough SC. Lack of association of the vitamin D receptor gene with Graves’ disease in UK Caucasians. Clin Endocrinol. 2004;60:618–624. doi: 10.1111/j.1365-2265.2004.02015.x. [DOI] [PubMed] [Google Scholar]
- 32.Ahn J, Yu K, Stolzenberg-Solomon R, Simon KC, McCullough ML, Gallicchio L, Jacobs EJ, Ascherio A, Helzlsouer K, Jacobs KB, Li Q, Weinstein SJ, Purdue M, Virtamo J, Horst R, Wheeler W, Chanock S, Hunter DJ, Hayes RB, Kraft P, Albanes D. Genome-wide association study of circulating vitamin D levels. Hum Mol Genet. 2010;19:2739–2745. doi: 10.1093/hmg/ddq155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang TJ, Zhang F, Richards JB, Kestenbaum B, van Meurs JB, Berry D, Kiel DP, Streeten EA, Ohlsson C, Koller DL, Peltonen L, Cooper JD, O'Reilly PF, Houston DK, Glazer NL, Vandenput L, Peacock M, Shi J, Rivadeneira F, McCarthy MI, Anneli P, de Boer IH, Mangino M, Kato B, Smyth DJ, Booth SL, Jacques PF, Burke GL, Goodarzi M, Cheung CL, Wolf M, Rice K, Goltzman D, Hidiroglou N, Ladouceur M, Wareham NJ, Hocking LJ, Hart D, Arden NK, Cooper C, Malik S, Fraser WD, Hartikainen AL, Zhai G, Macdonald HM, Forouhi NG, Loos RJ, Reid DM, Hakim A, Dennison E, Liu Y, Power C, Stevens HE, Jaana L, Vasan RS, Soranzo N, Bojunga J, Psaty BM, Lorentzon M, Foroud T, Harris TB, Hofman A, Jansson JO, Cauley JA, Uitterlinden AG, Gibson Q, Jarvelin MR, Karasik D, Siscovick DS, Econs MJ, Kritchevsky SB, Florez JC, Todd JA, Dupuis J, Hypponen E, Spector TD. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet. 2010;376:180–188. doi: 10.1016/S0140-6736(10)60588-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arnaud J, Constans J. Affinity differences for vitamin D metabolites associated with the genetic isoforms of the human serum carrier protein (DBP) Hum Genet. 1993;92:183–188. doi: 10.1007/BF00219689. [DOI] [PubMed] [Google Scholar]
- 35.Lauridsen AL, Vestergaard P, Hermann AP, Brot C, Heickendorff L, Mosekilde L, Nexo E. Plasma concentrations of 25-hydroxy-vitamin D and 1,25-dihydroxy-vitamin D are related to the phenotype of Gc (vitamin D-binding protein): a cross-sectional study on 595 early postmenopausal women. Calcif Tissue Int. 2005;77:15–22. doi: 10.1007/s00223-004-0227-5. [DOI] [PubMed] [Google Scholar]
- 36.Misharin A, Hewison M, Chen CR, Lagishetty V, Aliesky HA, Mizutori Y, Rapoport B, McLachlan SM. Vitamin D deficiency modulates Graves’ hyperthyroidism induced in BALB/c mice by thyrotropin receptor immunization. Endocrinology. 2009;150:1051–1060. doi: 10.1210/en.2008-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]