Abstract
The relationship between pain expectancy and motor system plays a crucial role in the human defensive system. Here, we took advantage of the inhibitory modulation of the motor pathway to the muscle of the hand receiving painful stimuli, by recording motor-evoked potentials (MEPs) to Transcranial Magnetic Stimulation (TMS). We employed a classical conditioning paradigm in which neutral (visual and auditory) stimuli were conditioned by pairing either painful or not-painful stimuli (electric shocks) in separated groups. Only the Pain Group showed clear motor responses: i.e. a significant decrease in MEPs amplitude, with respect to the neutral condition, not only in conditioning stimuli, when actual shocks were paired with neutral stimuli, but also in conditioned stimuli, when shocks were only expected. Significant differences between the two groups suggest that the MEPs decrease is specific for pain expectancy and does not pertain to anticipation in general. Furthermore, in the Pain Group, a significant negative correlation between physiological responses to conditioned stimuli and the participants’ anxiety traits was found: the lower the MEPs amplitude, the higher the participants’ anxiety scores. The present findings suggest that, in order for defensive motor responses to occur, actual pain is not necessary; rather, anxiety-dependent pain expectancy can be sufficient.
Keywords: classical conditioning, pain expectancy, corticospinal excitability, TMS, defensive behaviors, anxiety traits
Introduction
In non-human mammal species, different types of avoidance and defensive behaviors may be adopted, according to the features of both the eliciting threatening stimulus and the situation in which it is encountered (Blanchard, 1997, 2011; Misslin, 2003; Canteras et al., 2010). For instance, when a part of the body comes in contact with painful stimuli, the simplest motor response is to withdraw the affected body part from the source of pain (Sherrington, 1910; Clarke and Harris, 2004). The animal model has been extensively used for investigating the relationship between non-human mammals’ and humans’ defensive systems (both physiology and behavioral expression), focusing on normal and psychopathology pattern and providing evidence for a congruence between the two systems (Blanchard et al., 2001).
In human, a physiological counterpart of defensive motor responses has been described in several studies demonstrating that the actual pain induces a modulation pattern on the primary motor cortex (M1) excitability. It is known that nociceptive fingertip stimulation inhibits voluntary electromyographic (EMG) activity of contracting muscles, the so-called cutaneous silent period (Kofler et al., 1998). The inhibitory effect of actual pain on the corticospinal excitability has been demonstrated also at rest condition, by using brain stimulation to evoke motor-evoked potentials (MEPs) and different methods to induce pain. Valeriani and colleagues showed the effect of short painful CO2 laser stimulation in modulating MEPs amplitude, recorded from both hands (Valeriani et al., 1999) and arms (Valeriani et al., 2001) muscles. By comparing effects on MEPs induced either by TMS, acting on cortical pyramidal neurons, or by anodal electrical stimulation, acting on corticospinal axons, a cortical origin of the inhibitory response has been proposed and interpreted as a “partial motor decerebration” mechanism which might promote spinal protective reflexes (Valeriani et al., 1999, 2001). Other studies, employing noxious electrical fingertip stimulation, found different modulation patterns on the EMG activity of the upper limb, showing inhibition of the hand muscles activity and facilitation on the arm muscles activity (Kofler et al., 1998; Urban et al., 2004). This observed inhibition/facilitation pattern has been interpreted as corresponding to the protective withdrawal reflex (Floeter et al., 1998), characterized by dropping of a painful stimulus (distal inhibition) and withdrawal of the hand from the stimulus (proximal facilitation) (Kofler et al., 1998; Urban et al., 2004; Zhang et al., 2016). Other lines of research showed that the observation of painful stimuli induces a corticospinal inhibition (i.e. freezing-like effect) in the observer similar to those recorded during the actual pain (e.g. Avenanti et al., 2005). This suggests that the observation of other’s pain may reflect the anticipation of pain in oneself and has been interpreted as the physiological basis of empathy (Singer and Frith, 2005) or, more recently, as the physiological counterpart of an embodiment phenomenon related to the sense of body ownership (Bucchioni et al., 2016).
Within the human defensive system, the importance of expectancy has been described as an adaptive behavior to predict the likelihood of aversive events. Indeed, the expectancy for a threatening event, associated with fear and anxiety, plays an important role in threat perception and risk assessment (Ploghaus et al., 1999, 2003; Simmons et al., 2006, 2011; Blanchard et al., 2011; Eilam et al., 2011; Sharvit et al., 2015) and modulates defensive responses (Sambo and Iannetti, 2013; Fossataro et al., 2016a; Bisio et al., 2017). Thus, physiological motor responses to aversive stimuli may depend not only on the objective magnitude of the stimulus itself but also on the subjective expectation of the related risk. Nonetheless, the role of expectancy for threatening stimuli in modulating motor defensive responses, as well as its relationship with subjective anxiety traits, has not been fully understood.
In the present study, we investigated whether expectancy for threatening stimuli (i.e. electric shock) could modulate the motor cortex excitability as during the exposure to actual shock. To this aim, we took advantage of the Pavlovian classical conditioning (LeDoux, 2014), known as a learning process in which innate responses to threatening stimuli come to be elicited in response to previously neutral stimuli; this is achieved by repeated pairings of the neutral stimulus with the threatening stimulus. It has been demonstrated that the classical conditioning evokes defensive behavior and autonomic responses (such as the Skin Conductance Response, SCR), automatically elicited in threatening situations (LeDoux, 1998, 2000, 2012, 2014; Büchel, 2000; Phelps and LeDoux, 2005; Zhang et al., 2016). Importantly, the conditioning paradigm has been shown to modulate motor components of threat-related responses (Aymard, 2000; Fendt and Koch, 2013; Löw et al., 2015; Wendt et al., 2017), such as the startle reflex (Skljarevski and Ramadan, 2002) and the nociceptive flexion reflex (Fendt and Koch, 2013).
By combining classical conditioning paradigm with MEPs recording to single-pulse TMS protocol (see Figure 1), we asked whether pain expectancy is able to induce changes in corticospinal excitability. Given the general agreement on the inhibitory modulation of the distal muscles during pain perception (irrespective of different interpretations, see above), we focused on the distal (inhibitory) component of the defensive motor responses. We predicted that a significant decrease of the MEPs amplitude recorded from distal muscles, with respect to the neutral condition, should be present not only during conditioning trials, when neutral stimuli were actually paired with electric shocks, but also during conditioned trials when electric shocks were only expected. In order to investigate whether the corticospinal inhibition may be specific for pain expectancy or may pertain to anticipation in general, we compared the results of two groups of subjects taking part in the same conditioning paradigm with the only difference that in the Pain Group high intensity painful stimuli were used, while in the No-Pain Group low intensity no-painful stimuli were used. Since anticipation is an important component of anxiety (Simmons et al., 2011) and fear responses in conditioning paradigms are known to be modulated by subjective anxiety traits (Lissek et al., 2005; Soliman et al., 2010; Indovina et al., 2011; Duits et al., 2015), the existence of correlations between the recorded MEPs and the participants’ anxiety traits was investigated.
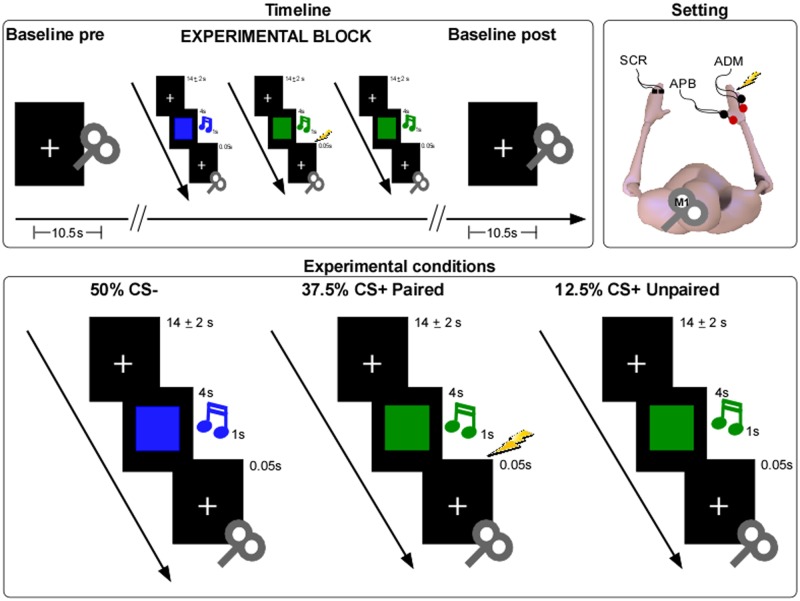
Fig. 1.
Schematic representation of the experimental protocol and design. Top right: a graphical representation of the experimental setting. Single-pulse TMS delivered over the participant’s left M1 and MEPs recorded from the APB and ADM muscles of the right hand; SCR responses recorded from the left hand in the Pain Group. Top left: a graphical representation of the experimental timeline. Before (Baseline Pre) and after (Baseline Post) each experimental block, five baseline conditions with a fixation cross was presented associated with TMS stimulation. In each block a total of 40 stimuli were presented in a pseudorandom order, 50% of them were neutral stimuli (CS) never followed by US (CS-), 37.5% were different CS paired with US (CS+ Paired; i.e. conditioning stimuli) and 12.5% were CS unpaired with US (CS+ Unpaired; i.e. conditioned stimuli). Bottom: a graphic representation of stimuli presented in each experimental condition. A blue square and a tone “A” associated with the TMS stimulation and never paired with electric shocks (CS- condition); green square and a tone “B” associated with TMS and electrical stimulation (CS+ Paired condition), which could be painful in the Pain Group or not painful in the No-Pain Group; green square and a tone “B” associated with TMS stimulation and not paired with electric shocks (CS+ Unpaired condition).
Materials and methods
Participants
Forty-two healthy volunteers participated in the study, half of the participants (19–29 years, mean ± SD 22.6 ± 2.43; 10 females) were assigned to the Pain Group (in which they receive painful electric shocks) and the other half (20–29 years, mean ± SD 23.66 ± 2.19; 15 females) to the No-Pain Group (in which no-painful electric shocks were delivered). All participants took part in the Main Experiment and only the Pain Group’s participants were involved in a Preliminary Experiment. All participants were right-handed, as assessed with the Edinburgh Handedness Inventory (Oldfield, 1971), naïve to the experimental procedure and before taking part in the study gave written informed consent. None of them had a history of neurological, major medical or psychiatric disorders and they were free from any contraindication to TMS (Rossi et al., 2009). The experimental procedure, according to the Declaration of Helsinki, was approved by local Ethics Committee of the University of Turin (3167, 01/02/2016).
Procedures
The experiment was programmed by using E-prime presentation software V2.0 (Psychology Software Tool Inc., USA) in order (a) to control sequence, timing and duration of the stimuli; (b) to trigger TMS pulses, EMG and SCR recording and electrical stimulation delivering. Participants were seated comfortable in front of a PC screen (17″ monitor; resolution 1280 × 720 pixels; refresh frequency 60 Hz) at a distance of ∼80 cm, with the head restrained by a comfortable pillow wrapping around the neck and supported by a fixed head rest; in order to avoid any muscles contractions, they were asked to keep resting their forearms on a pillow.
Preliminary Experiment
In order to verify the inhibitory effect of electric shocks on the corticospinal excitability, we compared two no-pain blocks (without electric shocks) to two pain blocks (with electric shocks delivering, see details in Stimulation and Recordings). In each block 6 MEPs were recorded for a total of 12 MEPs in each condition. Blocks were presented in counterbalanced order with half of the subjects starting with no-pain condition (ABBA) and the other half with pain condition (BAAB). In no-pain trials a fixation cross lasting 1050 ms was presented in the center of the screen and followed by TMS pulse over M1; in pain trials at 1000 ms from the fixation cross an electric shock was delivered and, according to a previous study (Urban et al., 2004), followed after 50 ms by a TMS pulse over M1.
Main Experiment
Experiment consisted of two separate blocks with a break of 20 min from each other in order to minimize habituation and to ensure that, after the first stimulation block, the corticospinal excitability came back to normal values. Visual and auditory neutral Conditioned Stimuli (CS) (i.e. coloured squares and sounds) may be paired or not with Unconditioned Stimuli (US) (i.e. electric shocks delivered to the right digit V, that according to the between-subjects conditions may be perceived as painful in the Pain Group and as not painful in the No-Pain Group). In each block a total of 40 stimuli were presented in a pseudorandom order, 50% of them were neutral stimuli (CS) never followed by US (CS-), 37.5% were different CS paired with US (CS+ Paired; i.e. conditioning stimuli) and 12.5% were CS unpaired with US (CS+ Unpaired; i.e. conditioned stimuli). A pseudorandom sequence was used to avoid that the CS+ Unpaired stimuli occurred in the first five trials and that more than two equal stimuli occurred in consecutive trials. In the CS- condition a visual stimulus (i.e. a blue square) accompanied by an auditory stimulus (i.e. a tone A, 10 097 Hz) were presented and never followed by US. In the CS+ Paired condition a visual stimulus (i.e. green square) accompanied by an auditory stimulus (i.e. a tone B, 9957 Hz) were always presented paired with an electrical stimulus (US) on the digit V of the right hand. Tones A and B were presented at the same intensity level (60 dB). According to a previous study (Urban et al., 2004), the US was delivered 50 ms before the TMS pulse in order to obtain the maximum inhibition over the target muscles. In the CS+ Unpaired condition a visual stimulus (i.e. green square) accompanied by the tone B were not paired with the US. All visual stimuli were presented for 4000 ms on a black background, accompanied by auditory stimuli of 1000 ms and spaced out by a fixation cross with a variable jittering (12 000–16 000 ms), chosen in order to have a variable time stimuli presentation. According to the experimental conditions, visual and auditory stimuli were followed (i) by a TMS pulse after 50 ms, in CS- and CS+ Unpaired trials and (ii) by an electric shock (lasting 200 μs) followed after 50 ms by a TMS pulse, in CS+ Paired trials (see Figure 1). In order to check for any corticospinal excitability change related to TMS per se or to the experimental block, five baselines with a fixation cross of 1050 ms in the center of the screen were presented, before (i.e. baseline pre) and after (i.e. baseline post) each block.
Note that participants assigned to the two groups (i.e. Pain Group and No-Pain Group) underwent the very same conditioning paradigm, with the only difference that in the No-Pain Group we recorded only MEPs (and not SCR) and the electrical stimulus was set at lower intensity compared to the one delivered in the Pain Group and deemed as not painful at all.
Stimulation and recordings
Magnetic stimulation
Both in Preliminary and Main Experiment, MEPs were elicited by a single pulse TMS (Magstim Rapid2; Magstim Co. Ltd, Whitland, UK) with a figure of eight-shaped coil positioned over the left M1. The intensity of magnetic pulses was set at 115% of the resting motor threshold (mean ± SD Pain Group: 63.9%±8.24%, range 54–78%; No-Pain Group: 62.02%±8.25%, range 49–75% of the maximum stimulator output), defined as the lower intensity of the stimulator output able to elicit 5 MEPs of 10 consecutive pulses with an amplitude of at least 50 μV (Brasil-Neto et al., 1992; Rossini et al., 1994).
Electrical stimulation
Transcutaneous electrical stimuli consisted in constant current square-wave pulses (DS7A, Digitimer) delivered to the right digit V, using a surface bipolar electrode attached to a Velcro strap. The stimulus duration was 200 μs and the delivering came ∼50 ms before the TMS pulse (accordingly to a previous study Urban et al., 2004). In the Pain Group’s participants, the means stimulation intensities were defined during the Preliminary Experiment (34.82 ± 10.63 mA, range 20–48 mA) and the same intensities were used in the Main Experiment. The stimulation intensity was initially set at 10-fold the perceptual threshold and then individually adjusted to elicit a painful sensation of 6.5/10 on a 0–10 Likert scale, where 0 is “not painful” and 10 is “the most painful imaginable.” Note that the electrical stimulus is subjectively perceived as painful. In the No-Pain Group’s participants, the stimulation intensity was adjusted and set at two-fold the individual perceptual threshold, estimated using the methods of limits (Gescheider, 1997), so that the elicited sensation was always classified as not painful (0/10 on the Likert scale). The mean stimulation intensity was 3.65 ± 1.09 mA, range 2.2–5 mA.
Electromyography recording
In both Preliminary and Main Experiment, EMG activity was simultaneously recorded (MP150, Biopac System, USA), from the right Abductor Digiti Minimi muscle (ADM) and the Abductor Pollicis Brevis muscle (APB), using two pairs of bipolar surface electrodes with the active electrode over the muscle belly and the reference electrode over the associated joint or tendon. Signals were amplified and digitalized with a sample rate of 10 kHz, filtered with a band-pass (10–500 Hz) and a notch (50 Hz) filter and stored for offline analysis.
Skin conductance response recording
In the Pain Group, SCR was recorded continuously during the Main Experiment, by using a Biopac system (MP150, USA). Two Ag-AgCl electrodes with constant voltage (0.5 V) where attached to the participant’s left hand on digit IV and III. Signal was amplified and digitalized with a sample rate of 1 kHz, band-stop filtered at 50 Hz and stored for offline analysis.
Self-report measure
After the TMS session of the Main Experiment, both Groups completed the trait scale of the State-Trait-Anxiety-Inventory (STAI) (Spielberger et al., 1970; Weiner and Craighead, 2010). The STAI-trait is a questionnaire of 20 items for assessing trait anxiety. Trait anxiety scale includes item related to the presence (e.g. “I worry too much over something that really doesn’t matter”) either to the absence of anxiety (e.g. “I am content; I am a steady person”). All items are rated on a four-point scale in terms of how often participants fell as described from 1 indicating “Almost Never” to 4 indicating “Almost Always” (items indicating absence of anxiety are reversed scored). Higher scores indicate greater anxiety.
Data analysis
MEPs analysis
EMG data of both Preliminary and Main Experiment were analyzed offline using AcqKnowlege software (Biopac Systems, Inc., Santa Barbara, CA) and SPSS statistical software v. 24 (IBM, Chicago, IL). Trials showing pre-activity (EMG signal greater than 50 µV) in the time window of 100 ms before the TMS pulse were excluded from the analysis. For both ADM and APB, peak-to-peak MEPs values were extracted and outlier values (±2 SD of the mean) were excluded from the analysis (no more than 2% of trials).
In the Preliminary Experiment, MEPs values of APB and ADM (raw data) were considered as dependent variables in a one-way multivariate analysis of variance (MANOVA) with “condition” (two levels: no-pain, pain) as within subjects factor.
In the Main Experiment, due to the not-normal distribution of the residuals, a natural Log(x + 1) transformation (Osborne, 2002) was applied to the raw data. First, we performed a baseline analysis in order to control for possible effects of TMS per se in modulating corticospinal excitability. To this aim, separately for each Experimental Group, baseline mean MEPs values of APB and ADM were considered as dependent variables in a 2 × 2 MANOVA with “block” (two levels: first, second) and “session” (two levels: pre, post) as within subjects factors. According to the negative results in the baseline analysis, the mean MEPs amplitude of the two blocks were used to normalize data.
In the principal analysis, a MEPs ratio (MEP ratio= MEPobtained/MEPbaseline) was calculated for APB and ADM. The obtained values were considered as dependent variables in a 3 × 2 MANOVA with “condition” (three levels: CS-, CS+ Paired and CS+ Unpaired) as within subjects factor and “group” (two levels: Pain and No-Pain Group) as between subjects factor. Post hoc comparisons were carried out by means of Bonferroni’s test. We checked for the equivalence of variance and the F-tests were not significant, suggesting that the equivalence of variance can be assumed and the two groups can be properly compared with the MANOVA model.
SCR analysis
In the Pain Group, SCR data were analyzed offline. For each participant of the Pain Group and each experimental condition the average peak-to-peak amplitude was extracted (as a difference between the minimum and the maximum value in a 10 s time window after the trigger coding for the stimulus delivering). Then, to obtain comparable measure among participants, the peak-to-peak responses were normalized within subjects and converted to Z-scores (Garbarini et al., 2014; Fossataro et al., 2016b). In order to test the effect of classical conditioning on the SCR, we performed a one-way repeated measure ANOVA with “condition” (three levels: CS-, CS+ Paired and CS+ Unpaired) as within participant factors. Post hoc comparisons were carried out by means of Bonferroni’s test.
Correlation analysis
In each experimental condition of the Main Experiment, Pearson’s r was used to investigate correlations (a) between the two acquired physiological measures (SCR and MEP ratio) in the Pain Group; and (b) between each physiological measure and the individual differences in anxiety traits, as measured by the STAI-trait. To account for multiple comparisons, the significance level (P value) was corrected using a false discovery rate (FDR) procedure (Benjamini and Hochberg, 1995). To test the possible presence of correlation effects across all conditions (that might not be significant when restricted to the subset of trials of one condition), we performed ANCOVA models, in which APB and ADM MEPs values were used as dependent variables (in separated analyses), with “conditions” (CS-, CS+ Paired and CS+ Unpaired) and “group” (Pain and No-Pain Group) as categorical predictors and the STAI-trait scores as covariate. Complementarily, to test for the specificity of a correlation effect for a specific condition, we compared correlations between conditions (i.e. co-correlation analyses) and we ran correlations with difference scores (i.e. calculating a delta between CS- and both CS+ Paired and CS+ Unpaired).
Results
MEPs results
Preliminary Experiment
In the Preliminary Experiment, the MANOVA model showed a significant overall effect of “condition” at multivariate tests (F(2, 19) = 18.322, P < 0.001). A significant effect of “condition” at the univariate tests was found in both muscles (APB: F(1, 20) = 38.363, P < 0.001; ADM: F(1, 20) = 8.55, P = 0.01). This means that the MEPs amplitude of pain condition was significantly reduced with respect to no-pain condition both in APB [mean (µV)±SD no-pain = 901.19 ± 587.06; pain = 287.22 ± 207.21] and ADM [mean (µV)±SD no-pain = 446.89 ± 366.59; pain = 321.05 ± 291.82]. See Figure 2. This suggests that our experimental paradigm is able to replicate the inhibitory effect of actual pain on the corticospinal excitability (Urban et al., 2004).
Fig. 2.
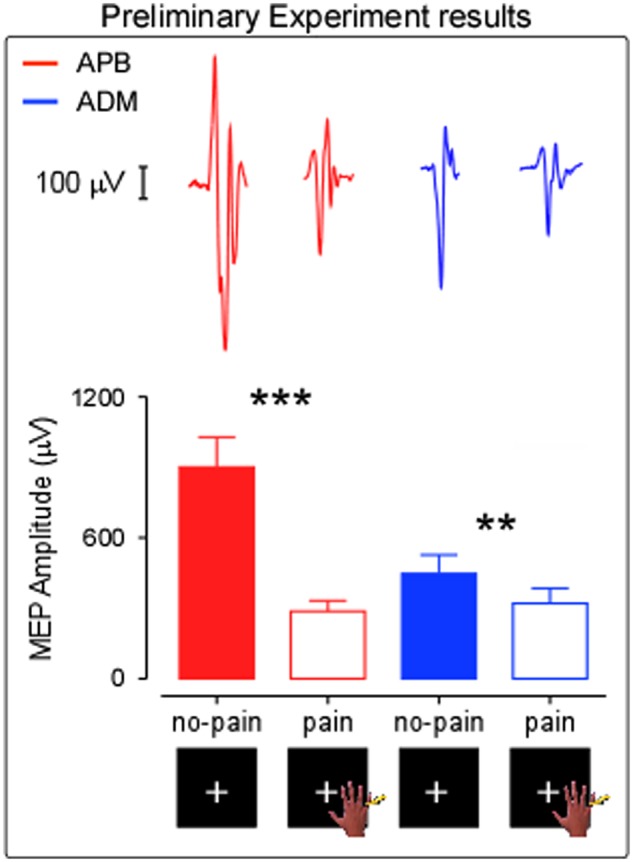
Preliminary Experiment results. Raw MEPs recorded from ADM and APB muscles in one representative subject are shown. Significant effect of condition in both muscles (APB; ADM). Error bars indicate sem. Asterisk indicates a significant comparison (**P < 0.005; ***P < 0.0005).
Main Experiment
In the baseline analysis, both in Pain and No-Pain Group, the MANOVA model did not show any significant results (see Tables 1 and 2). This suggests that TMS per se did not induce any change in corticospinal excitability and that the cortical excitability was unchanged in the second compared to the first experimental block.
Table 1.
Pain Group
| Block1 | Block2 | |
|---|---|---|
| Pre | APB: 2.7627±0.3942 | APB: 2.7537±0.4190 |
| ADM: 2.5046±0.3262 | ADM: 2.4741±0.3333 | |
| Post | APB: 2.6731±0.4383 | APB: 2.7032±0.3497 |
| ADM: 2.3445±0.4381 | ADM: 2.4251±0.4289 |
Table 2.
No-Pain Group
| Block1 | Block2 | |
|---|---|---|
| Pre | APB: 2.3431±0.414 | APB: 2.3865±0.312 |
| ADM: 2.2671±0.252 | ADM: 2.3572±0.232 | |
| Post | APB: 2.4233±0.396 | APB: 2.4212±0.522 |
| ADM: 2.3548±0.330 | ADM: 2.4349±0.410 |
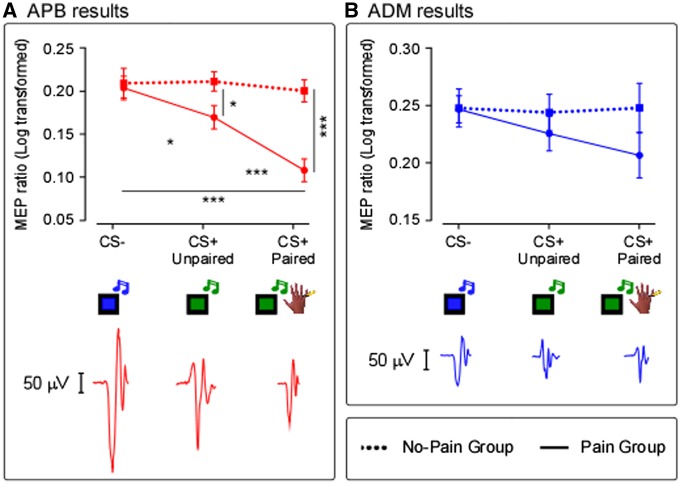
In the Main Experiment, the MANOVA revealed at multivariate tests a significant overall effect of “group” (F(2, 39) = 3.7, P = 0.034) and “condition” (F(4, 37) = 11.230, P < 0.001). Crucially, a significant interaction “group*condition” was found (F(4, 37) = 7.216, P < 0.001). Notably, this result is more driven by changes in MEPs amplitude recorded from APB than from ADM muscle. Indeed, at univariate tests a significant effect of “condition” (F(2, 80) = 20.702, P < 0.001) and a significant interaction “condition*group” (F(2, 80) = 13.718, P < 0.001) were found only in APB and not in ADM (always P > 0.1). See Figure 3A and B. In the Pain Group, post hoc comparisons showed that, in APB, the MEPs amplitude was significantly reduced in both CS+ Paired (P < 0.001) and CS+ Unpaired (P = 0.025) conditions with respect to CS- condition. Additionally, a significant difference between CS+ Paired and CS+ Unpaired was found (P < 0.001). Overall, in the Pain Group, the significant reduction of the MEPs amplitude in CS+ Unpaired condition compared to the CS- condition suggests that the mere expectancy of receiving painful stimuli can induce a significant modulation of the corticospinal excitability. In the No-Pain Group, post hoc comparisons did not show any significant differences between conditions (always P > 0.9), suggesting that no corticospinal modulation was found during unthreatening stimuli. Crucially, post hoc comparisons showed a significant difference between Pain and No-Pain Group in CS+ Paired (P < 0.001) and in CS+ Unpaired condition (P = 0.023) and not in CS- condition (P = 0.8). These results suggest that the inhibitory effect is specific for painful stimuli.
Fig. 3.
Main Experiment, MEPs results. The graph shows for both APB (A) and ADM (B) muscles. The mean MEPs amplitudes, expressed as percentage of the baseline and Log transformed, in the three experimental conditions (CS-; CS+ Unpaired; CS+ Paired) and in the two groups (Pain; No-Pain). Error bars indicate sem. Asterisk indicates a significant comparison (*P < 0.05; **P < 0.005; ***P < 0.0005). Raw MEPs recorded from APB and ADM muscles in one representative subject of the Pain Group are shown.
SCR results
A significant main effect of “condition” (F(2, 40)=32.34, P < 0.0001) was found. See Figure 4. Post hoc comparisons showed that the SCR amplitude was significantly greater in CS+ Paired condition compared to all other conditions, [mean (z-scores)±SD: CS+ Paired = 0.65 ± 0.27; CS+ Unpaired = −0.15 ± 0.34; CS- = −0.48 ± 0.17; P < 0.0001]. Crucially, the SCR amplitude in CS+ Unpaired condition was significantly enhanced compared to CS- condition [mean (z-scores)±SD: CS+ Unpaired = −0.15 ± 0.34; CS- = −0.48 ± 0.17; P < 0.002]. These data show that, when participants have learned the associations between CS and US, CS per se can elicit the physiological SCR typically triggered by US, suggesting that the pain expectancy may induce a significant SCR enhancement.
Fig. 4.
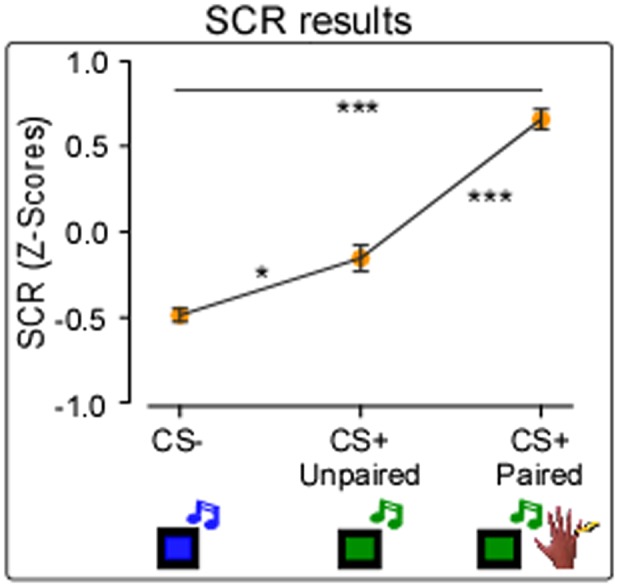
SCR results. The graph shows the mean SCR values, expressed in Z-scores, in Pain Group. Significant effect of condition (CS-; CS+ Unpaired; CS+ Paired). Error bars indicate sem. Asterisk indicates a significant comparison (*P < 0.05; **P < 0.005; ***P < 0.0005).
Correlation results
In the Main Experiment, no significant correlations between SCR and both MEPs ratio and STAI-trait scale were found.
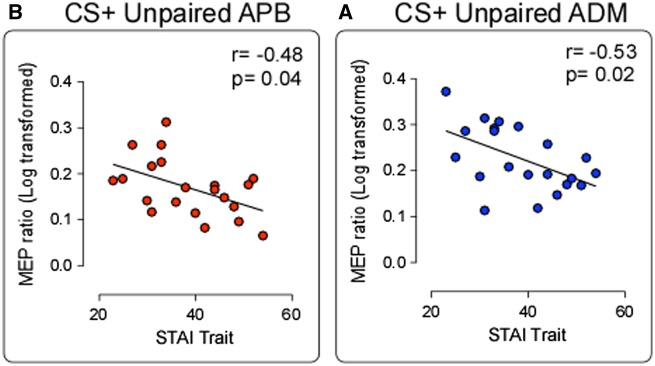
Crucially, only in the Pain Group, a significant negative correlation was found between STAI-trait scale and MEPs ratio in CS+ Unpaired condition in both muscles [ADM: r = −0.53; P = 0.007; P = 0.02 (after FDR correction), Figure 5A; APB: r = −0.48; P = 0.01; P = 0.04 (after FDR correction), Figure 5B]. These results suggest that the higher the level of anxiety trait, the lower the MEPs ratio in the CS+ Unpaired condition. No significant correlations were found between STAI-trait scale and MEPs ratio in CS- and CS+ Paired conditions. In the No-Pain Group, no significant correlations between STAI-trait scale and MEPs ratio were found.
Fig. 5.
Correlation results. Negative correlations between trait anxiety scores (STAI-Trait) and the corticospinal inhibition percentage for the CS+ Unpaired condition in ABP and ADM muscles.
In both APB and ADM ANCOVA models, no significant effects of the covariate STAI-trait in predicting the MEPs amplitude were found. These negative results rule out the possibility of correlation effects across all conditions. However, the lack of significant interaction between the covariate STAI-trait and the experimental conditions, as well as negative results in co-correlation analyses and in correlations with difference scores, make weaker the specificity of the correlation effect only found between STAI-trait and the MEPs amplitude in CS+ Unpaired condition.
Discussion
The present study investigates whether the threat-specific motor responses, occurring when participants receive painful stimuli, i.e. inhibitory modulation of the motor pathway to the muscle of the hand receiving pain, also pertain to pain expectancy. To this aim, we took advantage of classical conditioning procedure, in which visual and auditory stimuli were conditioned by pairing electrical stimuli, and we compared two groups of subjects receiving either painful or not painful electrical stimuli. According to our predictions, in the Pain Group, a significant decreased MEPs amplitude with respect to the neutral (CS-) condition was found both when participants received painful stimuli (CS+ Paired, conditioning stimuli) and when they were expecting to receive it (CS+ Unpaired, conditioned stimuli). The lack of effect in the No-Pain Group, as well as significant differences between the two groups, suggested that the MEPs decrease is specific for pain expectancy and do not pertain to anticipation in general. Furthermore, this inhibitory response during pain expectancy was inversely correlated with the participants’ anxiety traits: the lower the MEPs amplitude in CS+ Unpaired condition, the higher the participants’ anxiety scores.
Whit respect to the actual pain, the significant difference between the Pain Group and the No-Pain Group in CS+ Paired condition strongly support previous evidence on the M1 excitability modulation during pain perception (Farina et al., 2001; Le Pera et al., 2001; Farina et al., 2003; Urban et al., 2004). In particular, in keeping with previous study (Urban et al., 2004) and our preliminary experiment, only when the electrical stimulation was perceived as painful (i.e. Pain Group) both APB and ADM muscles, respectively showed 70% and 25% of MEPs amplitude decrease compared to the baseline.
What happens when actual pain was not present but only expected, as in CS+ Unpaired condition? Starting from a similar question, a previous study investigating the corticospinal excitability modulation by pain expectancy (Dubé and Mercier, 2011) found that corticospinal inhibition pertains only to actual pain and not to pain expectancy. On the contrary, in our Pain Group, a significantly decreased MEPs amplitude with respect to the CS- condition was found also during CS+ Unpaired condition, suggesting that corticospinal inhibition also pertains to pain expectancy. Several reasons can explain these different results. First, it can be matter of different painful stimulations, thermic vs electrical. Dubé and Mercier (2011) used thermic stimuli inducing “low-to-moderate short-lasting phasic pain” and, as reported by the authors, this intensity (2.8 at a 0–10 pain-rating scale) could be too weak to induce pain anticipation. On the contrary, in our experiment, the electrical stimuli were perceived as painful (>5 at a 0–10 pain-rating scale). Otherwise, it can be a matter of different experimental designs. In the study of Dubé and Mercier (2011), each block included a preliminary expectancy phase, followed by a painful thermal application phase, and the timing of the TMS pulse varied between these two phases. Conversely, the conditioning procedure employed here leads to an automatic association between neutral and threating stimuli, so that, as in the actual pain (CS+ Paired condition), in pain expectancy (CS+ Unpaired condition) defensive responses are automatically elicited and the timing of actual and expected pain is the same. Negative results of the No-Pain Group and significant difference between the two groups in CS+ Unpaired condition strongly supported the specificity of these motor responses for pain expectancy (i.e. they do not pertain to anticipation in general).
With respect to the difference between expected and actual pain, the present results showed, in the Pain Group, a similar trend in both recorded muscles (APB and ADM), with lower responses in CS+ Paired than in CS+ Unpaired conditions, although this difference reaches the significant level only in APB. Neuroimaging studies had found that a common network is activated at different thresholds during actual and expected pain; i.e. the activity observed during pain expectancy is about 30–40% of that observed during pain perception (Porro et al., 2003; Keltner et al., 2006; Atlas et al., 2010). This might explain the different intensity of the modulation exerted by pain-related areas on M1, greater for pain perception (CS+ Paired condition) and lower for pain expectancy (CS+ Unpaired condition). However, future studies are necessary to investigate the presence and the extant of functional connectivity with M1 during actual and expected pain, also exploring whether unexpected vs expected painful stimuli might have a different impact on corticospinal excitability.
Our conditioning procedure was able to modulate also the SCR, known to detect sympathetic responses to the expected noxious stimuli (Armel and Ramachandran, 2003; Guterstam et al., 2011; Garbarini et al., 2014; Romano et al., 2014; Zhang et al., 2016). In agreement with previous studies (Büchel, 2000; LeDoux, 1998, 2000, 2012, 2014; Phelps & LeDoux, 2005), higher SCR compared to the CS- condition was found not only when participants receive pain (CS+ Paired condition), but also when they expected to receive it (CS+ Unpaired condition). Although SCR and MEPs are similarly modulated by the experimental conditions, even if in the opposite direction, our data showed that these physiological parameters are not linearly correlated.
Importantly, in the Pain Group, MEPs amplitude in CS+ Unpaired condition was inversely correlated with the participants’ anxiety traits, as reported at the STAI-trait scale. This means that higher level of anxiety may significantly predict greater inhibitory motor responses to pain expectancy in CS+ Unpaired condition. Accordingly, in anxiety patients compared to controls, a robust increase of fear responses to conditioned safety cues has been demonstrated, suggesting an impaired ability to inhibit fear in the presence of safety cues and/or an increased tendency in anxiety disordered patients to generalize fear responses to safe stimuli resembling the conditioned danger cue (Lissek et al., 2005; Soliman et al., 2010;Indovina et al., 2011; Duits et al., 2015).
From an anatomical point of view, the amygdala-striatal system has been extensively described in the literature related to defensive mechanisms in both animal model and humans. According to consolidated evidence, this neural circuit involves transmission of threat input coming from both thalamus and somatosensory cortex to the amygdala and from there to the motor system that controls defensive behaviors (LeDoux, 1998, 2000, 2012, 2014; Phelps and LeDoux, 2005; Janak and Tye, 2015). However, a recent study (Zhang et al., 2016), investigating aversive learning process by combining physiological recordings and fMRI, proposed that the SCR and EMG effects, as those found in both CS+ Paired and CS+ Unpaired conditions of our experiment, might come from two distinct learning pathways: a “non-specific” amygdala-striatal system, which learns preparatory responses such as autonomic responses and facial expression; and a “specific” cerebellar system, which learns defensive motor responses of the body district receiving pain.
To conclude, the present findings suggest that, in order for the corticospinal excitability modulation to occur, actual pain is not necessary; rather, anxiety-dependent pain expectancy can be sufficient. This highlights the adaptive function of expectancy in motor system: anticipating the consequences of aversive stimuli, such as painful/threatening stimuli, allows the mobilization of the organism’s resources to prepare defensive or protective reactions.
Acknowledgements
The authors are grateful to all of the volunteers involved in the study. We are grateful to Nerina Martinelli and Tommaso Nessi for their contribution to data collection. This work has been funded by MIUR-SIR 2014 grant (RBSI146V1D), the San Paolo Foundation 2016 grant (CSTO165140) to F.G. and by the AXA Research Fund (AXA 12001) Ph.D. grant to G.B.
Conflict of interest. None declared.
References
- Armel K.C., Ramachandran V.S. (2003). Projecting sensations to external objects: evidence from skin conductance response. Proceedings. Biological Sciences/the Royal Society, 270(1523), 1499–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atlas L.Y., Bolger N., Lindquist M.A., Wager T.D. (2010). Brain mediators of predictive cue effects on perceived pain. Journal of Neuroscience, 30(39), 12964–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avenanti A., Bueti D., Galati G., Aglioti S.M. (2005). Transcranial magnetic stimulation highlights the sensorimotor side of empathy for pain. Nature Neuroscience, 8(7), 955–60. [DOI] [PubMed] [Google Scholar]
- Aymard C. (2000). Presynaptic inhibition and homosynaptic depression: A comparison between lower and upper limbs in normal human subjects and patients with hemiplegia. Brain, 123(8), 1688–702. [DOI] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing on JSTOR. Journal of the Royal Statistical Society, 57(1), 289–300. [Google Scholar]
- Bisio A., Garbarini F., Biggio M., Fossataro C., Ruggeri P., Bove M. (2017). Dynamic Shaping of the Defensive Peripersonal Space through Predictive Motor Mechanisms: When the “Near” Becomes “Far”. The Journal of Neuroscience, 37(9), 2415–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard D.C. (1997). Stimulus, environmental, and pharmacological control of defensive behaviors In M. E. Bouton & M. S. Fanselow (Eds.), Learning, motivation, and cognition: The functional behaviorism of Robert C. Bolles, (pp. 283–303). Washington: American Psychological Association. [Google Scholar]
- Blanchard D.C., Griebel G., Pobbe R., Blanchard R.J. (2011). Risk assessment as an evolved threat detection and analysis process. Neuroscience & Biobehavioral Reviews, 35(4), 991–8. [DOI] [PubMed] [Google Scholar]
- Blanchard D.C., Hynd A.L., Minke K.A., Minemoto T., Blanchard R.J. (2001). Human defensive behaviors to threat scenarios show parallels to fear- and anxiety-related defense patterns of non-human mammals. Neuroscience and Biobehavioral Reviews, 25(7–8), 761–70. [DOI] [PubMed] [Google Scholar]
- Brasil-Neto J.P., Pascual-Leone A., Valls-Sole J., Cohen L.G., Hallett M. (1992). Focal transcranial magnetic stimulation and response bias in a forced-choice task. Journal of Neurology, Neurosurgery & Psychiatry, 55(10), 964–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucchioni G., Fossataro C., Cavallo A., Mouras H., Neppi-Modona M., Garbarini F. (2016). Empathy or ownership? Evidence from corticospinal excitability modulation during pain observation. Journal of Cognitive Neuroscience, 28(11), 1760–71. [DOI] [PubMed] [Google Scholar]
- Büchel C. (2000). Classical fear conditioning in functional neuroimaging. Current Opinion in Neurobiology, 10(2), 219–23. [DOI] [PubMed] [Google Scholar]
- Canteras N.S., Resstel L.B., Bertoglio L.J., Carobrez A., de P., Guimarães F.S. (2010). Neuroanatomy of anxiety. Current Topics in Behavioral Neurosciences, 2, 77–96. [DOI] [PubMed] [Google Scholar]
- Clarke R.W., Harris J. (2004). The organization of motor responses to noxious stimuli. Brain Research. Brain Research Reviews, 46(2), 163–72. [DOI] [PubMed] [Google Scholar]
- Dubé J.A., Mercier C. (2011). Effect of pain and pain expectation on primary motor cortex excitability. Clinical Neurophysiology : Official Journal of the International Federation of Clinical Neurophysiology, 122(11), 2318–23. [DOI] [PubMed] [Google Scholar]
- Duits P., Cath D.C., Lissek S., et al. (2015). Updated meta-analysis of classical fear conditioning in the anxiety disorders. Depression and Anxiety, 32(4), 239–53. [DOI] [PubMed] [Google Scholar]
- Eilam D., Izhar R., Mort J. (2011). Threat detection: Behavioral practices in animals and humans. Neuroscience and Biobehavioral Reviews, 35(4), 999–1006. [DOI] [PubMed] [Google Scholar]
- Farina S., Tinazzi M., Le Pera D., Valeriani M. (2003). Pain-related modulation of the human motor cortex. Neurological Research, 25(2), 130–42. [DOI] [PubMed] [Google Scholar]
- Farina S., Valeriani M., Rosso T., et al. (2001). Transient inhibition of the human motor cortex by capsaicin-induced pain. A study with transcranial magnetic stimulation. Neuroscience Letters, 314(1–2), 97–101. [DOI] [PubMed] [Google Scholar]
- Fendt M., Koch M. (2013). Translational value of startle modulations. Cell and Tissue Research, 354(1), 287–95. [DOI] [PubMed] [Google Scholar]
- Floeter M.K., Gerloff C., Kouri J., Hallett M. (1998). Cutaneous withdrawal reflexes of the upper extremity. Muscle & Nerve, 21(5), 591–8. [DOI] [PubMed] [Google Scholar]
- Fossataro C., Gindri P., Mezzanato T., Pia L., Garbarini F. (2016a). Bodily ownership modulation in defensive responses: physiological evidence in brain-damaged patients with pathological embodiment of other’s body parts. Scientific Reports, 6(1), 27737.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossataro C., Sambo C.F., Garbarini F., Iannetti G.D. (2016b). Interpersonal interactions and empathy modulate perception of threat and defensive responses. Scientific Reports, 6(1), 19353.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbarini F., Fornia L., Fossataro C., Pia L., Gindri P., Berti A. (2014). Embodiment of others’ hands elicits arousal responses similar to one’s own hands. Current Biology, 24(16), R738–9. [DOI] [PubMed] [Google Scholar]
- Gescheider G.A. (1997). The classical psychofisical methods In: Psychophysics: The Fundamentals, 3rd edn, pp. 45–72. Mahwah: Lawrence Erlbaum Associates. [Google Scholar]
- Guterstam A., Petkova V.I., Ehrsson H.H. (2011). The illusion of owning a third arm. PLoS One, 6(2), e17208.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indovina I., Robbins T.W., Núñez-Elizalde A.O., Dunn B.D., Bishop S.J. (2011). Fear-conditioning mechanisms associated with trait vulnerability to anxiety in humans. Neuron, 69(3), 563–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janak P.H., Tye K.M. (2015). From circuits to behaviour in the amygdala. Nature, 517(7534), 284–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keltner J.R., Furst A., Fan C., Redfern R., Inglis B., Fields H.L. (2006). Isolating the modulatory effect of expectation on pain transmission: a functional magnetic resonance imaging study. Journal of Neuroscience, 26(16), 4437–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofler M., Glocker F.X., Leis A.A., et al. (1998). Modulation of upper extremity motoneurone excitability following noxious finger tip stimulation in man: a study with transcranial magnetic stimulation. Neuroscience Letters, 246(2), 97–100. [DOI] [PubMed] [Google Scholar]
- Le Pera D., Graven-Nielsen T., Valeriani M., et al. (2001). Inhibition of motor system excitability at cortical and spinal level by tonic muscle pain. Clinical Neurophysiology, 112(9), 1633–41. [DOI] [PubMed] [Google Scholar]
- LeDoux J. (1998). Fear and the brain: where have we been, and where are we going? Biological Psychiatry, 44(12), 1229–38. [DOI] [PubMed] [Google Scholar]
- LeDoux J. (2000). Emotion circuits in the brain. Annual Review of Neuroscience, 23, 155–84. [DOI] [PubMed] [Google Scholar]
- LeDoux J. (2012). Rethinking the emotional brain. Neuron, 73(4), 653–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J. (2014). Coming to terms with fear. Proceedings of the National Academy of Sciences, 111(8), 2871–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S., Powers A.S., McClure E.B., et al. (2005). Classical fear conditioning in the anxiety disorders: a meta-analysis. Behaviour Research and Therapy, 43(11), 1391–424. [DOI] [PubMed] [Google Scholar]
- Löw A., Weymar M., Hamm A.O. (2015). When threat is near, get out of here. Psychological Science, 26(11), 1706–16. [DOI] [PubMed] [Google Scholar]
- Misslin R. (2003). The defense system of fear: behavior and neurocircuitry. Neurophysiologie Clinique/Clinical Neurophysiology, 33(2), 55–66. [DOI] [PubMed] [Google Scholar]
- Oldfield R.C. (1971). The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia, 9(1), 97–113. [DOI] [PubMed] [Google Scholar]
- Osborne J. (2002). Notes on the use of data transformations. Practical Assessment, Research & Evaluation, 8(6), 1–7. Retrieved from http://pareonline.net/getvn.asp?v=8&n=6. [Google Scholar]
- Phelps E.A., LeDoux J.E. (2005). Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron, 48(2), 175–87. [DOI] [PubMed] [Google Scholar]
- Ploghaus A., Becerra L., Borras C., Borsook D. (2003). Neural circuitry underlying pain modulation: expectation, hypnosis, placebo. Trends in Cognitive Sciences, 7(5), 197–200. [DOI] [PubMed] [Google Scholar]
- Ploghaus A., Tracey I., Gati S.J., Clare S., Menon R.S., Matthews P.M., Rawlins J.N.P. (1999). Dissociating Pain from Its Anticipation in the Human Brain. Science, 284(5422), 1979–81. [DOI] [PubMed] [Google Scholar]
- Porro C.A., Cettolo V., Francescato M.P., Baraldi P. (2003). Functional activity mapping of the mesial hemispheric wall during anticipation of pain. NeuroImage, 19(4), 1738–47. [DOI] [PubMed] [Google Scholar]
- Romano D., Gandola M., Bottini G., Maravita A. (2014). Arousal responses to noxious stimuli in somatoparaphrenia and anosognosia: clues to body awareness. Brain : A Journal of Neurology, 137(Pt 4), 1213–23. [DOI] [PubMed] [Google Scholar]
- Rossi S., Hallett M., Rossini P.M., Pascual-Leone A. (2009). Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clinical Neurophysiology : Official Journal of the International Federation of Clinical Neurophysiology, 120(12), 2008–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini P.M., Barker A.T., Berardelli A., et al. (1994). Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalography and Clinical Neurophysiology, 91(2), 79–92. [DOI] [PubMed] [Google Scholar]
- Sambo C.F., Iannetti G.D. (2013). Better safe than sorry? The safety margin surrounding the body is increased by anxiety. The Journal of Neuroscience, 33(35), 14225–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharvit G., Vuilleumier P., Delplanque S., Corradi-Dell’ Acqua C. (2015). Cross-modal and modality-specific expectancy effects between pain and disgust. Scientific Reports, 5(1), 17487.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrington C.S. (1910). Flexion-reflex of the limb, crossed extension-reflex, and reflex stepping and standing. The Journal of Physiology, 40(1–2), 28–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons A., Stein M.B., Strigo I.A., Arce E., Hitchcock C., Paulus M.P. (2011). Anxiety positive subjects show altered processing in the anterior insula during anticipation of negative stimuli. Human Brain Mapping, 32(11), 1836–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons A., Strigo I.A., Matthews S.C., Paulus M.P., Stein M.B. (2006). Anticipation of aversive visual stimuli is associated with increased insula activation in anxiety-prone subjects. Biological Psychiatry, 60(4), 402–9. [DOI] [PubMed] [Google Scholar]
- Singer T., Frith C. (2005). The painful side of empathy. Nature Neuroscience, 8(7), 845–6. [DOI] [PubMed] [Google Scholar]
- Skljarevski V., Ramadan N.M. (2002). The nociceptive flexion reflex in humans – Review article. Pain, 96, 3–8. [DOI] [PubMed] [Google Scholar]
- Soliman F., Glatt C.E., Bath K.G., et al. (2010). A genetic variant BDNF polymorphism alters extinction learning in both mouse and human. Science, 327(5967), 863–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger C.D., Gorsuch R.L., Lushene R.E. (1970). Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press. [Google Scholar]
- Urban P.P., Solinski M., Best C., Rolke R., Hopf H.C., Dieterich M. (2004). Different short-term modulation of cortical motor output to distal and proximal upper-limb muscles during painful sensory nerve stimulation. Muscle & Nerve, 29(5), 663–9. [DOI] [PubMed] [Google Scholar]
- Valeriani M., Restuccia D., Di Lazzaro V., et al. (1999). Inhibition of the human primary motor area by painful heat stimulation of the skin. Clinical Neurophysiology, 110(8), 1475–80. [DOI] [PubMed] [Google Scholar]
- Valeriani M., Restuccia D., Di Lazzaro V., et al. (2001). Inhibition of biceps brachii muscle motor area by painful heat stimulation of the skin. Experimental Brain Research, 139(2), 168–72. [DOI] [PubMed] [Google Scholar]
- Weiner I. B., Craighead W. E. (Eds.). (2010). The Corsini Encyclopedia of Psychology. Hoboken, NJ: John Wiley & Sons, Inc. [Google Scholar]
- Wendt J., Löw A., Weymar M., Lotze M., Hamm A.O. (2017). Active avoidance and attentive freezing in the face of approaching threat. NeuroImage, 158, 196–204. [DOI] [PubMed] [Google Scholar]
- Zhang S., Mano H., Ganesh G., Robbins T., Seymour B. (2016). Dissociable learning processes underlie human pain conditioning. Current Biology, 26(1), 52–8. [DOI] [PMC free article] [PubMed] [Google Scholar]