Abstract
Goal-directed behavior and lifelong well-being often depend on the ability to control appetitive motivations, such as cravings. Cognitive reappraisal is an effective way to modulate emotional states, including cravings, but is often studied under explicit instruction to regulate. Despite the strong prediction from Self-Determination Theory that choice should enhance task engagement and regulation success, little is known empirically about whether and how regulation is different when participants choose (vs are told) to exert control. To investigate how choice affects neural activity and regulation success, participants reappraised their responses to images of personally-craved foods while undergoing functional neuroimaging. Participants were either instructed to view or reappraise (‘no-choice’) or chose freely to view or reappraise (‘yes-choice’). Choice increased activity in the frontoparietal control network. We expected this activity would be associated with increased task engagement, resulting in better regulation success. However, contrary to this prediction, choice slightly reduced regulation success. Follow-up multivariate functional neuroimaging analyses indicated that choice likely disrupted allocation of limited cognitive resources during reappraisal. While unexpected, these results highlight the importance of studying upstream processes such as regulation choice, as they may affect the ability to regulate cravings and other emotional states.
Keywords: craving regulation, cognitive reappraisal, choice, autonomy, self-determination
Introduction
The ability to control appetitive urges, such as cravings for food or drugs, or impulses to engage in risky sexual behavior, is an essential skill for health and well-being. Craving is an affective state characterized by strong appetitive motivation and can be regulated using various strategies (Giuliani and Berkman, 2015; Kober and Mell, 2015), including cognitive reappraisal or the reconstrual of a stimulus to change its affective meaning (Gross, 1998). Recent research has shown that cognitive reappraisal can be used to effectively reduce cravings for a variety of appetitive stimuli, including food (Siep et al., 2012; Giuliani et al., 2013; Yokum and Stice, 2013; Giuliani et al., 2014), drugs (Kober et al., 2010; Kober et al., 2010) and alcohol (Naqvi et al., 2015) and elicits activity in a network of regions, including dorsolateral (dlPFC), ventrolateral prefrontal cortex and dorsomedial prefrontal cortex (dmPFC) (for a meta-analysis, see Buhle et al., 2014). While the implementation of cognitive reappraisal has been studied extensively, much less is known about earlier stages in the emotion regulation process, including the decision to engage in regulation (Gross, 2015). As emotion regulation in the real-world typically begins with the decision to regulate, laboratory studies focusing exclusively on regulation implementation may actually misjudge individuals' emotion regulation abilities outside the lab where they might otherwise choose not to engage in emotion regulation in the first place, independent of ability. Indeed, previous research has indicated that regulation ability and frequency are only modestly related (McRae et al., 2012) if at all (Giuliani and Pfeifer, 2015).
Emotion regulation choice
Although this is a relatively new area, researchers have begun to investigate the process of choosing to engage in emotion regulation and factors affecting choice. Within the extended process model of emotion regulation (Gross, 2015), this antecedent stage is referred to as identification, and concerns the processes of forming an emotion regulation goal that ultimately leads to the decision to engage (or not engage) in regulation. Initial studies indicate that when given the choice whether to naturally view aversive images or engage in emotion regulation, individuals choose to regulate their emotions using cognitive reappraisal, though there are individual differences in frequency, and mean frequencies across individuals are lower than might be expected (Suri et al., 2015; Doré et al., 2017). For example, Suri et al. (2015) showed that when individuals were forced to make a choice between viewing aversive images and cognitively reappraising them, individuals chose to reappraise on approximately 40% of trials. However, when the forced choice was removed and the default option was to view (which may be more akin to the default in the real world), participants chose to reappraise relatively infrequently (approximately 10% of trials), demonstrating that although individuals do choose to use cognitive reappraisal to reduce negative affect, their choices are strongly influenced by the choice architecture.
However, whether and how choosing to regulate affects regulation implementation remains unknown. While the extended process model does not make explicit predictions regarding this relationship, it does posit that the strength of the emotion regulation goal formed during identification will affect the efficacy of implementation, with stronger regulation goals leading to more effective implementation. One factor that likely affects the strength of the regulation goal and the subsequent implementation process is the degree to which the decision to regulate is self-determined.
Choice supports autonomous self-regulation
Self-Determination Theory (Deci and Ryan, 2000) suggests that the degree to which a goal is autonomous will affect the level of intrinsic motivation to regulate. Indeed, environments and choice architectures that promote autonomy facilitate self-regulation and improve health and well-being (Deci and Ryan, 2000; Ng et al., 2012). Although it is difficult to manipulate autonomy in the laboratory, autonomy can be supported by providing individuals with choice. For example, one study showed that choice improved self-regulation on the Stroop task, by increasing intrinsic motivation and heightening attentional engagement (Legault and Inzlicht, 2013). In the context of emotion regulation, one functional neuroimaging (fMRI) study compared the neural and affective consequences of freely chosen reappraisal of aversive images (choice condition) and instructed reappraisal of aversive images (no choice condition; Kühn et al., 2014). In line with the findings from Legault and Inzlicht (2013), choice was associated with increased activity in regions associated with attention and control (e.g. dlPFC, dmPFC and posterior parietal cortex) and enhanced regulation success. However, it is unknown whether choice will similarly enhance emotion regulation in response to appetitive stimuli.
The present study
The present study integrates the extended process model of emotion regulation and Self-Determination Theory to investigate the relationship between regulation identification and implementation, and characterize whether choice enhances craving regulation at the behavioral and neural levels during reappraisal of appetitive stimuli. Participants were presented with images of personally craved foods and performed two actions: they either actively viewed the foods (‘look’) or reappraised their cravings for them (‘regulate’). Choice was manipulated by instructing participants on each trial whether to view or reappraise (‘no-choice’) or asking them to choose whether to view or reappraise (‘yes-choice’). We hypothesized that choice would increase intrinsic motivation to regulate, resulting in greater regulation success. As such, we expected an interaction between action (look vs regulate) and choice (yes vs no) on craving ratings, such that choice would increase regulation success. Neurally, we expected increased blood-oxygen-level-dependent (BOLD) signal in the frontoparietal control network (e.g. dlPFC, dmPFC and posterior parietal cortex) for the main effect of choice. Due to the lack of previously reported effects, we did not have strong hypotheses regarding regions involved in potential interactions between choice and action.
Materials and methods
Participants
Participants were 33 incoming college students (16 females, M = 18.12, s.d. = 0.34) recruited in the summer during freshman orientation at the University of Oregon, as part of a longitudinal study on health and well-being during the transition to college. Three participants were excluded from all analyses for failure to comply with instructions and one for indicating they disliked the food images. Two additional subjects were excluded from the univariate neural analyses because they exhibited excessive motion or did not complete the final run of the task. As follow-up multivariate analyses could still be performed on the participant missing the final task run, this participant was included in these analyses. This yielded a total of 29 participants for behavioral analyses, 27 for univariate neural analyses and 28 for multivariate neural analyses. This study was approved by the University of Oregon Institutional Review Board; all participants gave written informed consent and were compensated for their participation.
Procedure
Participants were presented with images of personally craved foods and completed a craving regulation task while in the MRI scanner. Prior to this, participants completed a structured training session to learn how to perform the craving regulation task and selected their top three ‘most craved’ foods from a list of 14 food categories (described below). Food craving was operationalized as having a strong desire to eat the food even when not hungry. To control for individual differences in hunger, participants reported their current hunger on a five-point scale (1 = not hungry at all, 5 = extremely hungry) and the time since their last meal. Body mass index (weight in kg/height in m2) was measured to control for individual differences in body mass. Participants were then situated in the MRI scanner and completed the craving regulation task (described below). To ensure task compliance, the experimenter interviewed participants after the first run of the task to help them improve their reappraisal strategy if they reported having difficulty and again after scanning to assess fidelity to the reappraisal instructions. Outside of the scanner, participants completed a short rating task in which they rated their craving for (i.e. the desire to eat) each of the food images they viewed while in the scanner. Participants also completed a number of survey measures as a part of the longitudinal study that will not be discussed further.
Stimuli
Stimuli were 84 appetizing images of food items based on participants’ food preferences. Participants chose their top three ‘most craved’ food categories from the following menu: barbeque, burgers, candy, cheese, chips, chocolate, cookies, doughnuts, French fries, fruit, fruit desserts, pasta, pizza and roasted vegetables. Each category contained 28 images independently rated for desirability (stimuli available via http://dsn.uoregon.edu/foodie).
Craving regulation task
Participants completed a craving regulation task (Giuliani et al., 2014; Giuliani and Pfeifer, 2015) that was modified to include an choice manipulation. Participants either actively viewed (‘look’ condition) or reappraised their craving for (‘regulate’ condition) the food images. On half of the trials, participants freely chose whether to look or regulate (‘yes-choice’ condition), and on the other half, participants were instructed whether to look or regulate (‘no-choice’ condition). Therefore, the task design was a 2 2 within-subjects repeated measures factorial with action (look, regulate) and choice (yes, no) as factors. To ensure a sufficient number of observations per condition, participants were instructed to choose to look approximately 50% of the time and to regulate the other 50%. They were reassured, however, that it was fine if their ratio was not exactly 50/50. They were also informed that their choices should be spontaneous (e.g. not alternating between the two actions). Descriptive analyses confirmed that participants were generally able to follow these instructions. The average percentage of regulation trials in the choice condition was 49.4% (s.d. = 5.4%; range = 38.1%–61.0%). More information regarding the relationship between percentage of regulation trials and outcome measures can be found in the Supplementary material.
On all look trials, participants were instructed to imagine that the food items were real and to consider how they would interact with them. On all regulate trials, participants were instructed to reappraise their craving for the foods by considering short- or long-term negative health consequences associated with consumption (e.g. stomach aches, weight gain, cavities), and participants were instructed to try to imagine how the health effects would feel physically. With the help of the experimenter, participants generated several negative health consequences so as to have multiple strategies to use while completing the task.
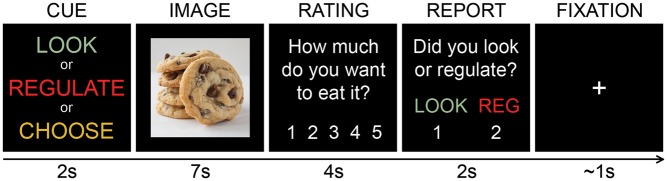
Each trial (see Figure 1) was 15 seconds long and consisted of the following events: cue (2 seconds), image presentation (7 seconds), craving rating (4 seconds) and action report (2 seconds). Inter-trial intervals were selected from a gamma distribution jitter (M = 1.01, s.d. = 0.26), and participants viewed a fixation cross during this period. On each trial, participants were cued about the instruction to look or regulate (no-choice condition) or to make a choice to look or regulate (yes-choice condition). The task consisted of three runs and each run consisted of 28 trials: 7 trials instructing participants to look, 7 trials instructing participants to regulate and 14 trials instructing participants to choose whether to look or regulate. To reduce potential image-related confounds (i.e. choosing to regulate on relatively less craved images and choosing to look on relatively more craved images) on choice trials, participants made their decision during the cue phase and were told that it was important to stick with their choice once made. After the cue, participants proceeded to look or regulate while viewing the food image, reported their craving for the food by rating how much they desired to eat the food item (1 = no desire, 5 = strong desire) and finally reported their instructed or chosen action. To minimize demand characteristics (e.g. reduced craving ratings on regulate trials), the experimenter stated that participants were not expected to be able to regulate well on every trial and stressed the importance of making honest craving ratings. Within each run, the trial order was optimized to maximize contrast estimation using a genetic algorithm (Wager and Nichols, 2003). Stimuli and trial order varied by subject, and run order was also counterbalanced across participants. Stimuli were presented using Psychtoolbox 3 (Brainard, 1997), and participants responded using a five-button box.
Fig. 1.
Task design. Each trial consisted of a 2 second cue period, followed by a 7 second image presentation during which participants looked or regulated while viewing the food image. Participants then had 4 seconds to rate their desire to eat the food and 2 seconds to report whether they looked or regulated on the trial. All trials ended with a jittered fixation cross for an average of 1 second.
Post-task craving ratings
Participants completed a rating task after the scan session to account for idiosyncratic reactions to stimuli (e.g. not liking some ice cream images due to the presence of a disliked topping). Participants were instructed to view the images afresh and rate their current craving, irrespective of their rating during the regulation task. Post-task ratings were centered within-subject to account for potential habituation effects.
Neuroimaging data acquisition
Data were acquired using a 3T Siemens Skyra scanner at the University of Oregon’s Lewis Center for Neuroimaging. High resolution anatomical volumes were acquired using a T1-weighted MP-RAGE pulse sequence and functional volumes were acquired using a T2*-weighted echo-planar sequence (voxel size = 2 mm3). Scan parameters are listed in Supplementary material.
Behavioral analysis
Multilevel modeling was used to test the effects of action and choice on self-reported craving ratings. Post-task craving ratings were included as a covariate to control for idiosyncratic reactions to stimuli. The model included the fixed effects of action, choice, action choice and post-task craving ratings, and the inclusion or exclusion of random effects was determined by sequentially removing effects that did not account for significant variance (see Supplementary material). Regulation success was defined as the mean difference in craving ratings between look and regulate conditions (look − regulate) and was calculated for each level of choice separately. Statistical analyses were performed in R 3.3.0 (R Core Team, 2016; https://www.r-project.org/) using the lme4 package (Bates et al., 2015). Behavioral data and related analysis scripts are available via the Open Science Framework (http://osf.io/e9cqv).
Univariate neural analysis
Images were preprocessed and analyzed using SPM12 (Wellcome Department of Cognitive Neurology; http://www.fil.ion.ucl.ac.uk/spm) with the following steps: realignment of functional images, coregistration of the anatomical image, manual reorientation of all images, and segmentation of the anatomical image. Segmented images for each subject were combined to form a group template using Dartel and flow fields were generated for each subject. Functional images were then spatially normalized to a Montreal Neurological Institute (MNI) standard using the Dartel template and individual flow fields, and smoothed using a 6 mm3 full-width at half maximum (FWHM) Gaussian smoothing kernel.
In first-level statistical analyses, event-related condition effects were estimated using a fixed-effects general linear model and convolving the canonical hemodynamic response function with stimulus events. Separate regressors were entered for conditions of interest (no-choice look, yes-choice look, no-choice regulate and yes-choice regulate) and modeled during the image presentation period. Additional regressors were added for the cue period, rating period and reporting period. Realignment parameters were transformed into five motion regressors, including absolute displacement from the origin in Euclidean distance and the displacement derivative for both translation and rotation, and a single trash regressor for images with >1 mm translation or rotation or visible motion artifacts (e.g. striping). These regressors were included as covariates of no interest. One participant was excluded from the group-level analysis for having >15% unusable volumes, which was more than 3 s.d. from the mean (M = 2.26%, s.d. = 4.08%). Additional regressors (covariates of no interest) were included as needed for trials in which participants failed to report whether they looked or regulated during yes-choice trials (N = 21, 0.87% of trials), or reported doing the opposite of the instruction during no-choice trials (N = 20, 0.83% of trials). All data were high-pass filtered at 128 seconds and modeled with a first-order autoregressive error structure. Linear contrasts for each condition of interest vs rest were estimated for each participant and used as inputs in second-level analyses.
A flexible factorial model was used to estimate second-level random effects. To determine the main effects of action, choice and their interaction, condition contrast images from each participant were used as inputs. This model was masked using a gray matter mask created by calculating the average of all subjects’ segmented grey matter maps, smoothing the average with a 6 mm3 FWHM Gaussian smoothing kernel and binarizing using the optimal thresholding protocol.
To correct for multiple comparisons, cluster-extent thresholding was implemented using AFNI version AFNI_16.1.06 (Cox, 1996). Smoothness was first estimated for each subject using AFNI’s 3dFWHMx tool with the spatial autocorrelation function and then averaged across subjects. To determine probability estimates of false-positive clusters given a random field of noise, Monte-Carlo simulations were conducted with AFNI’s 3dClustSim. To achieve a whole-brain familywise error rate of α = 0.05, a voxel-wise threshold of P < 0.001 and cluster extent of k > 108 was estimated (voxel dimensions = 2 mm3).
Multivariate neural analysis
To further explore differences in neural activity between yes-choice and no-choice trials, we conducted a follow-up analysis using multi-voxel pattern analysis (MVPA). For each participant, functional images were realigned, coregistered to the high-resolution anatomical image and smoothed using a 2mm3 FWHM Gaussian smoothing kernel in SPM12. The same first-level modeling procedure detailed above was followed, with the exception that models were run in native-space and each trial was entered in the model as a separate regressor (rather than grouped by condition). The resulting statistical maps for each trial were concatenated to create a beta-series (Rissman et al., 2004) and z-scored within run.
Classifier-based MVPA analyses were implemented in MATLAB 2014a (MathWorks; http://www.mathworks.com) using the Princeton MVPA Toolbox (Detre et al., 2006). To restrict the number of voxels, subject-specific masks were created using a standard parcellation atlas based on intrinsic connectivity from resting-state fMRI (Thomas Yeo et al., 2011). The frontoparietal network from this atlas was registered for each subject using FreeSurfer (Fischl, 2012; http://surfer.nmr.mgh.harvard.edu/) and binarized in SPM12. We then tested how well the trial-by-trial activation patterns in the frontoparietal network differentiated between look and regulate trials using a leave-one-out cross-validation procedure. During each cross-validation fold, a linear logistic regression classifier was trained to distinguish between look and regulate trials from two of three functional runs and then applied to the remaining run. This procedure was repeated so that each run served as a testing run, yielding three cross-validation accuracies for each subject. To test whether classification accuracy differed as a function of level of choice, this procedure was conducted separately for yes-choice and no-choice trials and accuracy was regressed on choice using multilevel modeling with subject intercepts as random effects.
Results
Behavioral results
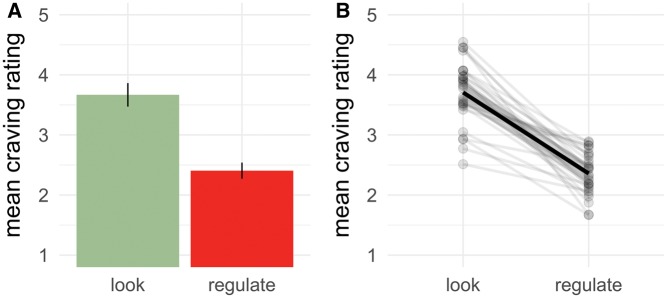
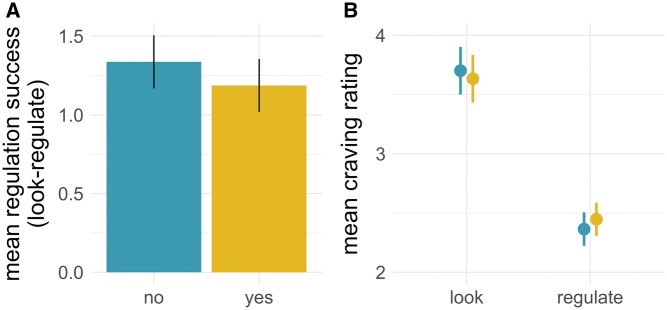
We used multilevel modeling to evaluate the effect of choice and action on self-reported craving ratings. All parameter estimates and relevant statistics can be found in Table 1. Consistent with previous findings, we found a significant main effect of action (see Figure 2), with lower ratings for food items on regulate trials (M = 2.36, s.d.= 0.98) than on look trials (M = 3.72, s.d. = 1.14). As expected, craving ratings on no-choice trials (M = 3.04, s.d. = 1.29) did not differ from yes-choice trials (M = 3.05, s.d. = 1.24) and the main effect of choice on craving ratings was not significant. The interaction between action choice was significant (Figure 3), but contrary to our predictions, the difference between look and regulate trials was lower for yes-choice trials (Mdiff = 1.29, s.d. = 0.59) than for no-choice trials (Mdiff = 1.42, s.d. = 0.60). Further, visual inspection revealed that choice affected both the look and regulate conditions, with cravings on yes-choice look trials rated lower than on no-choice look trials and higher on yes-choice regulate trials than on no-choice regulate trials (Figure 3B). Including hunger, last meal time, and body mass index did not improve model fit or change any of the results, χ2(3) = 1.79, P = 0.616.
Table 1.
Parameter estimates for fixed effects behavioral analysis
| Parameter | b | 95% CI | SE | df | t | P | |
|---|---|---|---|---|---|---|---|
| Intercept | 3.70 | 3.51 | 3.90 | 0.10 | 32.00 | 37.48 | <0.001 |
| Choice (yes) | −0.07 | −0.16 | 0.03 | 0.05 | 2261.57 | 1.37 | 0.171 |
| Action (regulate) | −1.34 | −1.57 | −1.10 | 0.12 | 33.75 | 11.43 | <0.001 |
| Average post-task rating | 0.39 | 0.33 | 0.46 | 0.03 | 28.34 | 11.83 | <0.001 |
| Choice action | 0.15 | 0.01 | 0.29 | 0.07 | 2256.06 | 2.14 | 0.032 |
Note. The reference group for choice is no and the reference group for action is look. Degrees of freedom (df) were calculated using the Satterthwaite approximation. CI, confidence interval.
Fig. 2.
(A) Parameter estimates for the fixed-effect of action from the multilevel model predicting self-reported craving ratings and (B) the raw subject means. Error bars and bands are 95% confidence intervals.
Fig. 3.
Parameter estimates from the multilevel model predicting self-reported craving ratings, plotted as (A) mean regulation success (look − regulate) for no- and yes-choice separately and (B) the interaction between action and choice (blue = no, yellow = yes). Error bars are 95% confidence intervals.
Univariate neural results
Main effect of choice
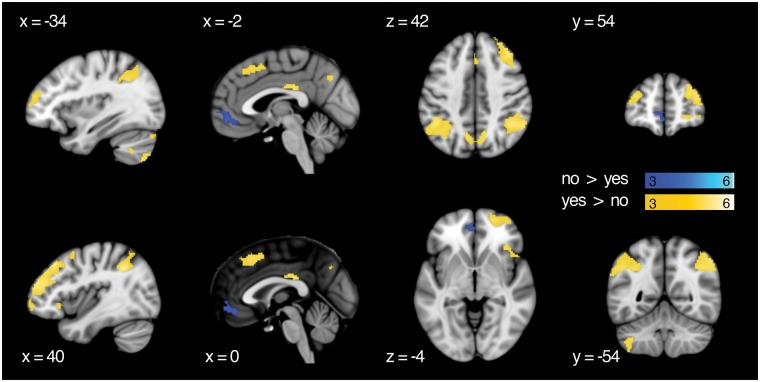
To investigate areas that showed relatively greater BOLD signal during implementation following choice, a contrast of yes > no was computed during the image presentation period (Figure 4). We observed increased BOLD signal in the frontoparietal control network, with significant clusters in bilateral posterior parietal cortex and lateral and medial prefrontal cortex. Additional clusters were found in left inferior temporal gyrus and left cerebellum. The reverse contrast, no > yes choice (Figure 4), revealed significant clusters of activation in bilateral ventromedial prefrontal cortex with a peak in left middle orbital gyrus. Table 2 shows the full results. Unthresholded statistical maps for this effect and all other effects reported in this article are available through NeuroVault (Gorgolewski et al., 2015; http://neurovault.org/collections/2427).
Fig. 4.
Univariate main effects for choice. Results are thresholded at P <0.001 and k = 108. Cluster extent (k) is measured in 2 mm3 voxels.
Table 2.
Regions, MNI coordinates, cluster extent, and peak t values for the main effects of yes > no choice and no > yes choice
| Contrast and region | MNI coordinates (x, y, z) | Extent (k) | Peak t | ||
|---|---|---|---|---|---|
| Yes > no | |||||
| R angular gyrus | 44 | −50 | 38 | 1037 | 6.03 |
| R inferior parietal lobule | 44 | −44 | 60 | 1037 | 4.02 |
| R middle frontal gyrus | 32 | 46 | 38 | 1952 | 5.88 |
| R middle frontal gyrus | 42 | 50 | 20 | 1952 | 5.41 |
| R middle orbital gyrus | 22 | 60 | −8 | 1952 | 4.58 |
| L inferior parietal lobule | −44 | −48 | 42 | 1013 | 5.23 |
| L superior parietal lobule | −32 | −64 | 50 | 1013 | 3.67 |
| L cerebellum (VII) | −40 | −72 | −52 | 771 | 5.06 |
| L cerebellum (VIII) | −40 | −48 | −46 | 771 | 4.59 |
| L cerebellum (Crus 1) | −42 | −80 | −26 | 771 | 4.07 |
| Bilateral PCC | 0 | −26 | 28 | 140 | 4.83 |
| R precuneus | 12 | −60 | 40 | 272 | 4.73 |
| L precuneus | −8 | −66 | 40 | 272 | 4.53 |
| L superior medial gyrus | 2 | 22 | 50 | 363 | 4.51 |
| L inferior temporal gyrus | −60 | −34 | −18 | 221 | 4.47 |
| L middle frontal gyrus | −32 | 54 | 18 | 265 | 4.41 |
| R IFG (p. orbitalis) | 36 | 26 | −6 | 142 | 4.39 |
| R superior frontal gyrus | 18 | 18 | 54 | 326 | 4.08 |
| R middle frontal gyrus | 38 | 10 | 52 | 326 | 3.88 |
| L inferior parietal lobule | −38 | −52 | 44 | 1013 | 3.37 |
| No > yes | |||||
| L mid orbital gyrus | −2 | 50 | −8 | 334 | 4.91 |
Note. Cluster family-wise error correction for α = 0.05 and P < 0.001 is k = 108. Cluster extent (k) is measured in 2 mm3 voxels.
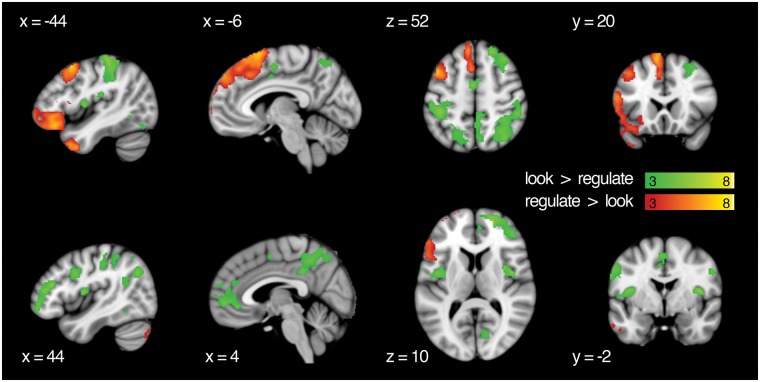
Main effect of action
To assess which areas of the brain had relatively stronger BOLD response when participants were reappraising their cravings and actively viewing food items, we computed contrasts for regulate > look and look > regulate. These results maps are visualized in Figure 5, and clusters that survived thresholding are reported in Table 3.
Fig. 5.
Univariate main effects for action. Results are thresholded at P <0.001 and k = 108. Cluster extent (k) is measured in 2 mm3 voxels.
Table 3.
Regions, MNI coordinates, cluster extent and peak t values for the main effects of regulate > look and look > regulate
| Contrast and region | MNI coordinates (x, y, z) | Extent (k) | Peak t | ||
|---|---|---|---|---|---|
| Regulate > look | |||||
| L post. med. frontal gyrus | −8 | 14 | 66 | 1796 | 7.49 |
| L superior frontal gyrus | −12 | 52 | 42 | 1796 | 6.63 |
| L superior medial gyrus | −8 | 34 | 52 | 1796 | 5.78 |
| L middle frontal gyrus | −44 | 10 | 54 | 782 | 6.88 |
| L IFG (p. orbitalis) | −48 | 34 | −12 | 2495 | 6.83 |
| L temporal pole | −40 | 14 | −40 | 2495 | 6.06 |
| L IFG (p. triangularis) | −54 | 18 | 16 | 2495 | 5.91 |
| R cerebellum (VII) | 32 | −76 | −44 | 1216 | 6.75 |
| R cerebellum (crus 2) | 10 | −82 | −26 | 1216 | 5.37 |
| L middle temporal gyrus | −66 | −36 | −2 | 167 | 4.38 |
| Look > regulate | |||||
| R IFG (p. triangularis) | 48 | 34 | 20 | 3284 | 6.02 |
| R middle orbital gyrus | 38 | 48 | −6 | 3284 | 5.87 |
| R superior frontal gyrus | 20 | 58 | 10 | 3284 | 5.22 |
| L postcentral gyrus | −50 | −22 | 22 | 3657 | 5.89 |
| L IFG (p. opercularis) | −60 | 6 | 32 | 3657 | 5.61 |
| L postcentral gyrus | −42 | −32 | 60 | 3657 | 5.48 |
| R intraparietal sulcus | 30 | −44 | 42 | 3125 | 5.53 |
| R rolandic operculum | 58 | −18 | 22 | 3125 | 5.37 |
| R angular gyrus | 34 | −66 | 52 | 3125 | 4.85 |
| R insula lobe | 42 | −2 | 12 | 181 | 5.42 |
| L insula lobe | −42 | −2 | 12 | 259 | 4.89 |
| L post. med. frontal gyrus | −2 | −4 | 54 | 189 | 4.88 |
| R MCC | 10 | −38 | 42 | 769 | 4.79 |
| R precuneus | 6 | −66 | 50 | 769 | 3.85 |
| L precuneus | −2 | −46 | 62 | 769 | 3.59 |
| R inferior temporal gyrus | 58 | −40 | −12 | 554 | 4.74 |
| R cerebellum (crus 1) | 48 | −56 | −24 | 554 | 3.72 |
| R inferior temporal gyrus | 60 | −20 | −22 | 554 | 3.54 |
| R IFG (p. opercularis) | 46 | 8 | 32 | 516 | 4.74 |
| R IFG (p. opercularis) | 56 | 8 | 14 | 516 | 3.94 |
| L cerebellum (VIII) | −28 | −70 | −52 | 298 | 4.69 |
| R cerebellum (VIII) | 22 | −56 | −52 | 260 | 4.29 |
| R calcarine gyrus | 10 | −68 | 22 | 285 | 4.19 |
| L inferior temporal gyrus | −52 | −58 | −8 | 163 | 3.89 |
Note. Cluster family-wise error correction for α = 0.05 and P < 0.001 is k = 108. Cluster extent (k) is measured in 2 mm3 voxels.
Interaction between action and choice
No significant clusters of activation for either the positive or negative effect of the interaction survived thresholding. However, to explore sub-threshold interactions, we parcellated the brain into 353 clusters (Craddock et al., 2012) and calculated the average effect size for the interaction within each parcel. This map, as well as similar maps for the simple effects, has been uploaded to the collection for this article on NeuroVault.
Post hoc multivariate neural results
We expected that choice would increase engagement with the task, resulting in increased activity in attention- and control-related regions and greater regulation success. Though we observed increased activity in the frontoparietal network following choice, behavioral results indicated reduced rather than enhanced regulation success. Although seemingly at odds, one hypothesis consistent with these findings is that choice may disrupt concurrent allocation of cognitive resources that are bandwidth limited (Vohs et al., 2008). We reasoned that if choice disrupted cognitive resource allocation during implementation, then neural representations for look and regulate would be less distinguishable in the yes-choice vs no-choice condition, mirroring the reduced self-reported regulation success in the choice condition. To test this hypothesis, we conducted post hoc analyses using MVPA. We measured classification accuracy of look vs regulate trials in the frontoparietal network and predicted lower classification accuracy on yes-choice relative to no-choice trials. Consistent with this prediction, we observed significantly lower classification accuracy for yes-choice (M = 0.65, s.d. = 0.16) than for no-choice (M = 0.70, SD = 0.17) trials, t(137.17) = 2.48, P = 0.014. Parameter estimates and statistics are in Table 4 and visualized in Figure 6.
Table 4.
Parameter estimates for fixed effects of MVPA analysis
| Parameter | b | 95% CI | SE | df | t | P | |
|---|---|---|---|---|---|---|---|
| Intercept | 0.70 | 0.66 | 0.75 | 0.02 | 41.82 | 29.91 | <0.001 |
| Choice (yes) | −0.05 | −0.09 | −0.01 | 0.02 | 137.17 | 2.48 | 0.014 |
Note. The reference group for choice is no. Degrees of freedom (df) were calculated using the Satterthwaite approximation. CI, confidence interval.
Fig. 6.
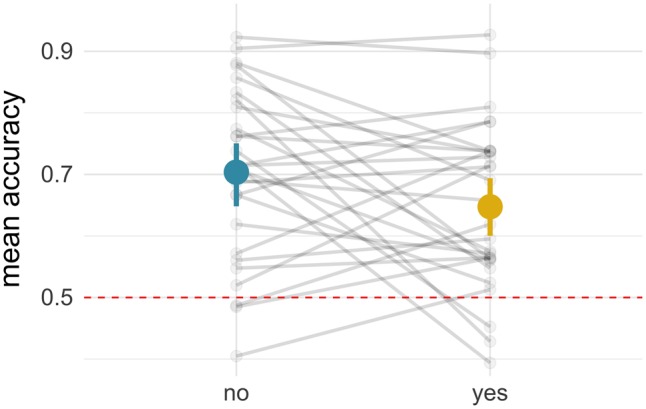
Mean group and subject classification accuracy from MVPA analyses classifying look and regulate trials, plotted separately for no- and yes-choice. Error bars are 95% confidence intervals and the dotted line at 50% represents chance accuracy.
Discussion
Our goal was to investigate whether and how choice affects appetitive regulation during a craving reappraisal task. As expected, reappraisal effectively reduced self-reported craving for personally craved foods. In line with previous studies, we also observed increased activity in regions associated with reappraisal (e.g. dlPFC, ventrolateral prefrontal cortex and dmPFC) and decreased activity in vmPFC, a region implicated in valuation and reward-processing (Hare et al., 2009; Kober et al., 2010; Giuliani et al., 2014). However, contrary to our prediction, choice slightly reduced rather than enhanced regulation success. This behavioral effect was not readily explainable by the univariate activation results. While choice was associated with relatively greater BOLD signal in the frontoparietal control network, there were no interactions at the whole-brain level that might explain the behavioral results. To reconcile the neural and behavioral findings, we hypothesized that choice may have disrupted allocation of cognitive resources during implementation. Consistent with this hypothesis, classifier-based MVPA demonstrated less differentiation between look and regulate trials in the yes-choice relative to no-choice condition.
Neural and behavioral effects of choice
Based on the theoretical premise that choice would enhance motivation for and engagement with the task, we expected to see increased BOLD signal in regions associated with attention and control following choice. In accordance, we replicated previous research showing increased activity in the frontoparietal control network during choice trials (Kühn et al., 2014). However, in contrast to Kühn et al. (2014), this activity was not accompanied by enhanced regulation success. Instead, choice slightly reduced regulation success on average.
One key difference between Kühn et al. (2014) and our study is that we used appetitive rather than aversive stimuli. Because appetitive stimuli like craved foods typically elicit approach tendencies rather than avoidance tendencies (Lang and Bradley, 2010), motivation to regulate affective responses likely differs between appetitive and aversive stimuli. This asymmetry may have made regulation more effortful in our study and could have undermined potential regulatory enhancement effects of choice. Although this has not been tested directly with emotional pictures, recent research has shown that affective context can modulate the effect of choice. For example, when both gains and losses are presented, individuals prefer choice in the gain, but not the loss condition (Leotti and Delgado, 2014).
Another potential explanation that reconciles these findings is that choice led to inefficient allocation of limited cognitive resources, such as attention and working memory. On choice trials, participants may have over-allocated attention to the decision during the choice phase (e.g. by tracking the number of times they chose to look and regulate) or equivocated about the decision during the implementation phase, resulting in the combination of increased activity in the frontoparietal network and reduced implementation efficacy. Consistent with this explanation, the follow-up MVPA analyses suggested that the neural representations for look and regulate trials were less differentiable. The pattern of the behavioral results also supports this conclusion, as choice reduced implementation efficacy for both look and regulate trials. That is, craving ratings were lower on yes-choice look trials than on no-choice look trials and higher on yes-choice regulate trials than on no-choice regulate trials. Together, these results support the hypothesis that, rather than enhancing task engagement and regulation success, in some contexts, choice may disrupt regulation. These findings are significant because the majority of research on cognitive reappraisal has focused narrowly on regulation per se without considering the effects of the antecedent choice to regulate, and therefore may misjudge cognitive regulation ability outside the lab when individuals must first choose to regulate their emotions.
Helpful and harmful effects of choice
Although the present manipulation of choice did not enhance regulation success, other laboratory studies have demonstrated positive effects of choice on self-regulation (Legault and Inzlicht, 2013; Kühn et al., 2014) and task performance (Murayama et al., 2015) and engagement (Leotti and Delgado, 2011; Legault and Inzlicht, 2013) more generally. Although several of these studies manipulated choice in a similar fashion, it is possible that choice in the context of this study may have felt burdensome rather than motivating (Schwartz, 2000; Vohs et al., 2008).
Indeed, although choice often promotes autonomy and intrinsic motivation, in certain contexts, choice can be detrimental. For example, individuals report decreased preference for choice in decisions involving unattractive or difficult options (Iyengar and Lepper, 2000; Botti and lyengar, 2004). For choice to enhance motivation, choices should feel volitional and self-determined (Reeve et al., 2003; Ryan and Deci, 2006). If individuals feel pressured or compelled to choose a particular option, or if the choice does not confer actual agency (i.e. the locus of perceived causality is external), the positive effects of choice can be undermined (Moller et al., 2006; Legault and Inzlicht, 2013; Sullivan-Toole et al., 2017). In our study, we asked participants to try to look and regulate approximately equally. While necessary to ensure there were sufficient trials per condition, this may have reduced participants experience of self-determination on choice trials. Further, because we sought to study the effect of choice on craving regulation in a normative sample and therefore did not explicitly recruit participants based on health- or diet-related goals, it is possible that choice in this context may not have been meaningful to all participants. Future research assessing the relationship between choice and craving regulation may benefit from a stronger choice manipulation to support autonomy, such as by providing more personally relevant choices or studying this relationship in individuals with explicit health or dietary concerns.
This study has several limitations. First, on choice trials, participants chose before viewing the food images. We did this to avoid confounding the decision to regulate with stimulus features (e.g. looking when food images were relatively more craved and regulating when food images were relatively less craved), even though it restricted ecological validity. Second, our task was not designed to assess how choice affected neural activity separately during the choice and implementation phases. Because regulation choices likely involve a host of cognitive processes, such as working memory to track previous decisions and effort calculations (Shenhav et al., 2013), we cannot rule out that these processes extended into the implementation phase. Indeed, this explanation would be consistent with the pattern of results indicating that choice disrupted implementation. Future studies may benefit from separating the choice and implementation phases to control for increases in cognitive load associated with choice (e.g. decision making and set shifting; Lo et al., 2012). It is possible that doing so would reduce the cognitive disruption and lead to enhanced regulatory success in the choice condition. However, it is important to note that implementation under the present conditions may more closely resemble the implementation process in the real-world. Third, to have sufficient trials per condition, participants were instructed to look and regulate approximately equally. This was necessary to ensure adequate power, but regulation frequency is likely an individual difference that should be investigated subsequently (see Supplementary material; McRae et al., 2012). Fourth, we did not measure affective experience, perceived effort or self-determination. Including these measures would help characterize the effects of choice on craving regulation. Fifth, we focused on cognitive reappraisal, but there are other effective regulatory strategies, such as mindfulness-based approaches, that require less effortful control (Westbrook et al., 2013; Kober and Mell, 2015). Because choice appears to have taxed limited cognitive resources, it may differentially affect such regulatory strategies and should be investigated in future studies. Finally, future studies should extend this work to include other outcomes measures, such as food choice (Hare et al., 2011; Hutcherson et al., 2012).
Conclusions
The present study is the first to investigate how choice affects appetitive regulation in the context of a craving reappraisal task. This study adds to the growing body of research on the cognitive regulation of appetitive motives, as well as emerging research on regulation choice. Contrary to the theoretical prediction that choice would increase task engagement and improve regulation, choice actually disrupted the implementation process, resulting in increased activity in the frontoparietal network and reduced regulation success. These unexpected results highlight the importance of considering upstream processes, such as regulation choice, when studying emotion regulation.
Supplementary Material
Acknowledgements
We would like to thank Norma Medina for her help with stimuli development and subject recruitment. Elliot Berkman is manager of Berkman Consultants, LLC.
Funding
This work was supported by the National Institutes of Health (DA035763 to J.H.P, and CA211224, CA17524, AG048840 to E.T.B.).
Supplementary data
Supplementary data are available at SCAN online.
Conflict of interest. None declared.
References
- Bates D., Mächler M., Bolker B., Walker S. (2015). Fitting linear mixed-effects models using lme4. Journal of Statistical Software, 67(1), 1–48. [Google Scholar]
- Botti S., lyengar S.S. (2004). The psychological pleasure and pain of choosing: when people prefer choosing at the cost of subsequent outcome satisfaction. Journal of Personality and Social Psychology, 87(3), 312–26. [DOI] [PubMed] [Google Scholar]
- Brainard D.H. (1997). The psychophysics toolbox. Spatial Vision, 10(4), 433–6. [PubMed] [Google Scholar]
- Buhle J.T., Silvers J.A., Wager T.D., et al. (2014). Cognitive reappraisal of emotion: a meta-analysis of human neuroimaging studies. Cerebral Cortex (New York, NY), 24(11), 2981–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R.W. (1996). AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research, 29(3), 162–73. [DOI] [PubMed] [Google Scholar]
- Craddock R.C., James G.A., Holtzheimer P.E., Hu X.P., Mayberg H.S. (2012). A whole brain fMRI atlas generated via spatially constrained spectral clustering. Human Brain Mapping, 33(8), 1914.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deci E.L., Ryan R.M. (2000). The “What” and “Why” of goal pursuits: human needs and the self-determination of behavior. Psychological Inquiry, 11(4), 227–68. [Google Scholar]
- Detre G., Polyn S.M., Moore C.D., et al. (2006). The Multi-Voxel Pattern Analysis (MVPA) toolbox. Presented at the Annual Meeting of the Organization for Human Brain Mapping, Florence, Italy.
- Doré B.P., Weber J., Ochsner K.N. (2017). Neural predictors of decisions to cognitively control emotion. The Journal of Neuroscience, 37(10), 2580–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B. (2012). FreeSurfer. NeuroImage, 62(2), 774–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliani N.R., Berkman E.T. (2015). Craving is an affective state and its regulation can be understood in terms of the extended process model of emotion regulation. Psychological Inquiry, 26(1), 48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliani N.R., Calcott R.D., Berkman E.T. (2013). Piece of cake. Cognitive reappraisal of food craving. Appetite, 64, 56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliani N.R., Mann T., Tomiyama A.J., Berkman E.T. (2014). Neural systems underlying the reappraisal of personally craved foods. Journal of Cognitive Neuroscience, 26(7), 1390–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliani N.R., Pfeifer J.H. (2015). Age-related changes in reappraisal of appetitive cravings during adolescence. NeuroImage, 108, 173–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorgolewski K.J., Varoquaux G., Rivera G., et al. (2015). NeuroVault.org: a web-based repository for collecting and sharing unthresholded statistical maps of the human brain. Frontiers in Neuroinformatics 9, https://doi.org/10.3389/fninf.2015.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J.J. (1998). Antecedent-and response-focused emotion regulation: divergent consequences for experience, expression, and physiology. Journal of Personality and Social Psychology, 74(1), 224.. [DOI] [PubMed] [Google Scholar]
- Gross J.J. (2015). Emotion regulation: current status and future prospects. Psychological Inquiry, 26(1), 1–26. [Google Scholar]
- Hare T.A., Camerer C.F., Rangel A. (2009). Self-control in decision-making involves modulation of the vmPFC valuation system. Science, 324(5927), 646–8. [DOI] [PubMed] [Google Scholar]
- Hare T.A., Malmaud J., Rangel A. (2011). Focusing attention on the health aspects of foods changes value signals in vmpfc and improves dietary choice. Journal of Neuroscience, 31(30), 11077–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutcherson C.A., Plassmann H., Gross J.J., Rangel A. (2012). Cognitive regulation during decision making shifts behavioral control between ventromedial and dorsolateral prefrontal value systems. The Journal of Neuroscience, 32(39), 13543–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyengar S.S., Lepper M.R. (2000). When choice is demotivating: can one desire too much of a good thing? Journal of Personality and Social Psychology, 79(6), 995–1006. [DOI] [PubMed] [Google Scholar]
- Kober H., Kross E.F., Mischel W., Hart C.L., Ochsner K.N. (2010). Regulation of craving by cognitive strategies in cigarette smokers. Drug and Alcohol Dependence, 106(1), 52–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kober, H. Mell, M.M. (2015) Neural Mechanisms Underlying Craving and the Regulation of Craving. In: Wilson, S. J., editor. The Wiley Handbook on the Cognitive Neuroscience of Addiction, Chichester, UK: John Wiley & Sons, Ltd.
- Kober H., Mende-Siedlecki P., Kross E.F., et al. (2010). Prefrontal–striatal pathway underlies cognitive regulation of craving. Proceedings of the National Academy of Sciences, 107(33), 14811–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn S., Haggard P., Brass M. (2014). Differences between endogenous and exogenous emotion inhibition in the human brain. Brain Structure and Function, 219(3), 1129–38. [DOI] [PubMed] [Google Scholar]
- Lang P.J., Bradley M.M. (2010). Emotion and the motivational brain. Biological Psychology, 84(3), 437–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legault L., Inzlicht M. (2013). Self-determination, self-regulation, and the brain: autonomy improves performance by enhancing neuroaffective responsiveness to self-regulation failure. Journal of Personality and Social Psychology, 105(1), 123–38. [DOI] [PubMed] [Google Scholar]
- Leotti L.A., Delgado M.R. (2011). The inherent reward of choice. Psychological Science, 22(10), 1310–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leotti, L.A., Delgado, M. R. (2014). The value of exercising control over monetary gains and losses. Psychological Science, 25(2), 596–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo B.C.Y., Lau S., Cheung S., Allen N.B. (2012). The impact of rumination on internal attention switching. Cognition and Emotion, 26(2), 209–23. [DOI] [PubMed] [Google Scholar]
- McRae K., Jacobs S.E., Ray R.D., John O.P., Gross J.J. (2012). Individual differences in reappraisal ability: links to reappraisal frequency, well-being, and cognitive control. Journal of Research in Personality, 46(1), 2–7. [Google Scholar]
- Moller A.C., Deci E.L., Ryan R.M. (2006). Choice and ego-depletion: the moderating role of autonomy. Personality and Social Psychology Bulletin, 32(8), 1024–36. [DOI] [PubMed] [Google Scholar]
- Murayama K., Matsumoto M., Izuma K., et al. (2015). How self-determined choice facilitates performance: a key role of the ventromedial prefrontal cortex. Cerebral Cortex, 25(5), 1241–51. [DOI] [PubMed] [Google Scholar]
- Naqvi N.H., Ochsner K.N., Kober H., et al. (2015). Cognitive regulation of craving in alcohol dependent and social drinkers. Alcoholism, Clinical and Experimental Research, 39(2), 343–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng J.Y.Y., Ntoumanis N., Thøgersen-Ntoumani C., et al. (2012). Self-determination theory applied to health contexts a meta-analysis. Perspectives on Psychological Science, 7(4), 325–40. [DOI] [PubMed] [Google Scholar]
- Reeve J., Nix G., Hamm D. (2003). Testing models of the experience of self-determination in intrinsic motivation and the conundrum of choice. Journal of Educational Psychology, 95(2), 375–92. [Google Scholar]
- Rissman J., Gazzaley A., D'Esposito M. (2004). Measuring functional connectivity during distinct stages of a cognitive task. NeuroImage, 23(2), 752–63. [DOI] [PubMed] [Google Scholar]
- Ryan R.M., Deci E.L. (2006). Self-regulation and the problem of human autonomy: does psychology need choice, self-determination, and will? Journal of Personality, 74(6), 1557–86. [DOI] [PubMed] [Google Scholar]
- Schwartz B. (2000). Self-determination: the tyranny of freedom. American Psychologist, 55(1), 79–88. [DOI] [PubMed] [Google Scholar]
- Shenhav A., Botvinick M.M., Cohen J.D. (2013). The expected value of control: an integrative theory of anterior cingulate cortex function. Neuron, 79(2), 217–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siep N., Roefs A., Roebroeck A., Havermans R., Bonte M., Jansen A. (2012). Fighting food temptations: the modulating effects of short-term cognitive reappraisal, suppression and up-regulation on mesocorticolimbic activity related to appetitive motivation. NeuroImage, 60(1), 213–20. [DOI] [PubMed] [Google Scholar]
- Sullivan-Toole, H., Richey, J.A., Tricomi, E. (2017). Control and Effort Costs Influence the Motivational Consequences of Choice. Frontiers in Psychology, 8, 675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suri G., Whittaker K., Gross J.J. (2015). Launching reappraisal: it’s less common than you might think. Emotion, 15(1), 73–7. [DOI] [PubMed] [Google Scholar]
- Vohs K.D., Baumeister R.F., Schmeichel B.J., Twenge J.M., Nelson N.M., Tice D.M. (2008). Making choices impairs subsequent self-control: a limited-resource account of decision making, self-regulation, and active initiative. Journal of Personality and Social Psychology, 94(5), 883–98. [DOI] [PubMed] [Google Scholar]
- Wager T.D., Nichols T.E. (2003). Optimization of experimental design in fMRI: a general framework using a genetic algorithm. NeuroImage, 18(2), 293–309. [DOI] [PubMed] [Google Scholar]
- Westbrook C., Creswell J.D., Tabibnia G., Julson E., Kober H., Tindle H.A. (2013). Mindful attention reduces neural and self-reported cue-induced craving in smokers. Social Cognitive and Affective Neuroscience, 8(1), 73–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas Yeo B.T., Krienen F.M., Sepulcre J., et al. (2011). The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of Neurophysiology, 106(3), 1125–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokum S., Stice E. (2013). Cognitive regulation of food craving: effects of three cognitive reappraisal strategies on neural response to palatable foods. International Journal of Obesity, 37(12), 1565–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.