Abstract
Affective science research on reward processing has primarily focused on monetary rewards. There has been a growing interest in evaluating the neural basis of social decision-making and reward processing. The present study employed a within-subject design and compared the reward positivity (RewP), an event-related potential component that is present following favorable feedback and absent or reduced following unfavorable feedback, during monetary and social reward tasks. Specifically, 114 participants (75 females) completed a monetary reward task and a novel social reward task that were matched on trial structure, timing, and feedback stimuli in a counterbalanced order. Results indicated that the monetary and social RewP were of similar magnitude, positively correlated and demonstrated comparable psychometric properties, including reliability and dependability. Across both the monetary and social tasks, women demonstrated a greater RewP compared with men. This study provides a novel methodological approach toward examining the electrocortical response to social reward that is comparable to monetary reward.
Keywords: event-related potential, monetary reward, reward positivity, social reward
Introduction
The evaluation of feedback is an important component of decision-making and reinforcement learning (Holroyd and Coles, 2002; Schultz, 2006). Event-related potentials (ERPs) are often used to examine this cognitive-affective process given their high temporal resolution. ERP studies have often utilized laboratory gambling or guessing tasks to measure the reward positivity (RewP) in response to feedback (Hajcak et al., 2006; Bernat et al., 2015; Novak and Foti, 2015). The RewP is a positive-going deflection in the ERP signal that peaks ∼250-350 ms after favorable feedback (e.g. winning money) and is absent or reduced following unfavorable feedback (e.g. losing money). The RewP is associated with behavioral and self-report measures of reward sensitivity (Bress and Hajcak, 2013) and the engagement of brain regions implicated in reward-processing, including the medial prefrontal cortex and striatum (Carlson et al., 2011; Foti et al., 2014).
Affective science research on reward processing has primarily focused on monetary outcomes; however, there has been growing interest in evaluating the neural basis of social decision-making and reward processing (e.g. Guyer et al., 2012; Bhanji and Delgado, 2014; Vrtička et al., 2014; Jarcho et al., 2015). Functional magnetic resonance imaging (fMRI) research suggests that there is a common neural system implicated in reward-learning for both non-social (e.g. monetary) and social rewards. For example, Izuma et al. (2008) found that both positive feedback regarding one’s reputation and receiving a monetary reward activated an overlapping aspect of the striatum. Indeed, the striatum is engaged during trial and error-based learning tasks (Daniel and Pollmann, 2014) and reward-based learning tasks (Lin et al., 2012) regardless of the type of reward received. Moreover, Hausler et al. (2015) found that the reward of scoring a goal in soccer vs winning money activated similar regions of prefrontal cortex and striatum. Some investigations have found dissociable neural networks for the processing of monetary and social reward (Rademacher et al., 2010; Chan and Cheung, 2016); however, many of these studies have employed affective images or smiling faces—stimuli which do not map well on to the actual experience of being rewarded. Together, these fMRI findings suggest that a common neural system is likely involved in reward processing for both nonsocial and social rewards.
ERP researchers have utilized many different tasks to examine the neural response to social feedback. For example, Kujawa et al. (2014) employed an ‘Island Getaway’ task, based on the television show ‘Survivor’, in which participants voted to remove players from an island, and received either acceptance or rejection feedback from peers. Results indicated that a larger RewP was elicited in response to acceptance relative to rejection feedback. Using a different social task, Sun and Yu (2014) also found a larger RewP in response to acceptance relative to rejection feedback. Finally, van der Veen et al. (2016) found that during a social task a larger RewP was elicited by acceptance feedback that was unexpected relative to expected. These data suggest that a RewP is elicited by both non-social (e.g. monetary) and social (e.g. acceptance) reward. The one study to compare the monetary and social RewP in the same participants found that monetary feedback elicited a larger RewP compared with social feedback (Flores et al., 2015). However, this investigation examined peak-to-peak amplitude, which is highly sensitive to noise (Clayson et al., 2013). Given this important limitation, it is still unclear whether the RewP elicited by monetary reward is comparable to that elicited by social reward.
This study employed a within-subject design and compared the electrocortical response to monetary and social reward. Specifically, 114 participants completed a monetary reward task (i.e. the doors task; Proudfit, 2015) and a novel social reward task in a counterbalanced order. One critical limitation to the extant literature comparing monetary and social reward processing has been the presence of several confounds between tasks (e.g. picture of money versus facial expression). To minimize potential confounds, this study employed monetary and social reward tasks that were matched on trial structure, timing and feedback stimuli. Given the existing research demonstrating that monetary and social reward engage similar neural substrates and electrocortical activation, we hypothesized that feedback indicating a favorable outcome (i.e. monetary gain, social acceptance) would elicit a larger RewP relative to feedback indicating an unfavorable outcome (i.e. monetary loss, social rejection). We also hypothesized that the magnitude of the RewP would not differ between the monetary and social reward tasks.
The RewP is a promising individual difference measure of reward sensitivity that has demonstrated good psychometric properties (Levinson et al., 2017; Luking et al., 2017), but supporting evidence has focused exclusively on the monetary RewP. To further elucidate the psychometric properties of the RewP, we examined the intra-individual correlation, reliability, and dependability of the monetary and social RewP. We hypothesized that the monetary and social RewP would be positively correlated with each other, and demonstrate comparable psychometric properties.
Research has indicated important sex differences in neural reactivity to emotional and motivational information (Stevens and Hamann, 2012). For example, one fMRI investigation examined whether men and women differed in neural activation in anticipation of two forms of reward: money and social approval (Spreckelmeyer et al., 2009). A wider network of brain areas were activated for monetary, compared with social, rewards in men. In contrast, anticipating both monetary and social rewards in women activated comparable brain regions. These results are consistent with previous evidence suggesting that emotional differences between men and women may vary depending on the type of stimulus or event (e.g. social or nonsocial) (Schirmer et al., 2013). However, the literature has been mixed regarding sex differences in the monetary RewP. Specifically, two investigations found a greater RewP in 9-year-old boys compared with girls (Kujawa et al., 2015) and adult men compared with women (Novak et al., 2016). However, another investigation reported a greater RewP in 16 to 17 year-old girls compared with boys (Santesso et al., 2011), while others have found no sex differences (Foti and Hajcak, 2009; Bress et al., 2012). The limited studies that have examined sex differences in the social RewP have also been mixed: one reported a greater social RewP in young adult women compared with men (van der Veen et al., 2016) and another reported no sex difference in the RewP in 10 to 15 year-old children (Kujawa et al., 2014). To further examine this issue, this study tested for sex differences in the monetary and social RewP. Given the mixed literature on sex differences in the RewP, we had no specific hypotheses for these analyses.
Finally, aberrations in the brain’s reward system are central to several etiological models of depression (Russo and Nestler, 2013). Consistent with this perspective, a blunted RewP has been associated with more severe expression of depression symptoms and syndromes (Foti and Hajcak, 2009; Bress et al., 2012, 2015; Nelson et al., 2016). A growing number of studies have found that social rewards elicit depression-related differences in the brain’s reward system (Forbes, 2009; Silk et al., 2014; Olino et al., 2015). However, no study has examined, in the same sample of individuals, the association between depression symptoms and neural response to monetary and social rewards. Therefore, this study examined the association between the monetary and social RewP and individual differences in depression symptoms. We hypothesized that greater depression symptoms would be associated with a smaller RewP, but we had no specific hypothesizes regarding differences between monetary and social tasks.
Materials and methods
Participants
The sample included 114 undergraduate students who participated for course credit. The sample was college-aged (M = 20.47-years old; s.d. = 2.04), contained 75 (65.8%) females, and was racially/ethnically diverse (24.6% Asian, 6.1% Black, 47.4% Caucasian, 14.0% Latino and 7.9% ‘Other’). Informed consent was obtained prior to participation and all procedures were approved by the local Institutional Review Board.
Measures
Inventory of depression and anxiety symptoms. The Inventory of Depression and Anxiety Symptoms—Expanded Version; Watson et al., 2012) is a 99-item factor-analytically derived self-report inventory of empirically distinct dimensions of depression and anxiety symptoms. Each item assesses symptoms over the past two weeks on a five-point Likert scale ranging from 1 (‘not at all’) to 5 (‘extremely’). This study focused on the 10-item dysphoria scale (M = 19.05, s.d. = 6.45, Cronbach’s α = 0.84), which is a core symptom dimension of depression.
Stimuli
Social reward task stimuli consisted of 120 images of age-matched peers (60 females) compiled from multiple sources [National Institute of Mental Health’s Child Emotional Faces picture set (Egger et al., 2011), internet databases of non-copyrighted images, and photographs of college-aged individuals]. Variability in the appearance of the social stimuli was necessary in order to corroborate task deception, which suggested participants were being evaluated by actual peers. All images were cropped to a standardized size (3.5 in. width × 4.5 in. height), and occupied ∼8° of visual space horizontally and 10° vertically for participants seated ∼24 in. from the monitor. Each trial slide contained a pair of either male or female peers, pictured from their shoulders up, with a positive facial expression and a solid background.
Procedure
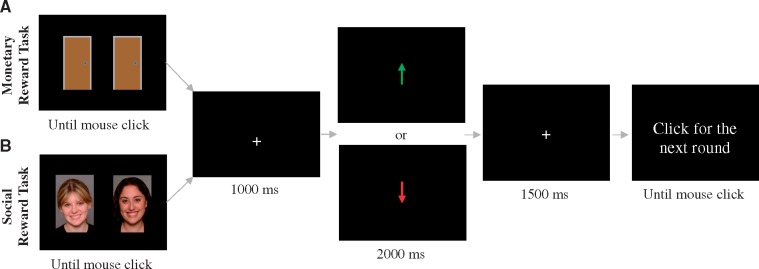
At the beginning of the experimental session, participants were told that they were completing a social evaluation study with peers at different universities across the USA. Participants were asked to provide a digital photo of them that was purportedly uploaded to a study database. Participants believed that once this photograph was uploaded, peers would receive a text message asking them to view the photo and indicate whether they thought they would ‘like’ or ‘dislike’ the participant. Participants were told that later in the lab session, after enough time had elapsed for the purported peers to have rated their photo, they would be asked to guess which peers ‘liked’ them. Participants were also told that they would be completing a monetary guessing task (see Figure 1 for task schematic). Next, participants completed a self-report questionnaire while an electroencephalography (EEG) cap was applied to their head. Finally, participants completed the monetary and social reward tasks in a counterbalanced order. At the end of the task participants were informally asked about their experience with the task, and nearly all participants reported task engagement and believing the veracity of the feedback.
Fig. 1.
In the monetary reward task, each trial began with the presentation of two identical doors. Participants were instructed to select the left or right door by clicking the left or right mouse button, respectively. Participants were told that they could either win $0.50 or lose $0.25 on each trial. These values were chosen in order to equalize the subjective value of the gains and losses (Tversky and Kahneman, 1981, 1992). The goal of the task was to guess which door contained the reward while attempting to earn as much money as possible. The image of the doors was presented until the participant made a selection. After stimulus offset, a fixation cross (+) was presented for 1000 ms, and then feedback was presented on the screen for 2000 ms. Correct selection of the rewarding door resulted in a monetary gain, indicated by a green arrow pointing upward (↑). Incorrect selection of the losing door resulted in a monetary loss, indicated by a red arrow pointing downward (↓). In actuality, feedback was pre-programmed to generate an equal number of gain and loss trials. The feedback stimulus was followed by a fixation cross presented for 1500 ms, immediately followed by the message ‘Click for next round’. This prompt remained on the screen until the participant responded with a button press to initiate the next trial. The social reward task was identical to the monetary reward task, except pictures of gender-matched peers (i.e. two male or two female faces) were presented instead of doors. Participants were instructed to select the individual they believed ‘liked’ them by clicking the left or right mouse button, respectively. Correct selection of the peer who purportedly provided ‘like’ feedback resulted in social acceptance, indicated by a green arrow pointing upward (↑). Incorrect selection of the peer who provided ‘dislike’ feedback resulted in social rejection, indicated by a red arrow pointing downward (↓). In actuality, feedback was pre-programmed to generate an equal number of acceptance and rejection trials.
Monetary reward task
The monetary reward task was administered using Presentation software (Neurobehavioral Systems, Inc., Albany, CA, USA) and was similar to the version used in previous studies (Proudfit, 2015). The task consisted of three blocks of 20 trials (60 total) (Figure 1A).
Social reward task
The social reward task was identical to the monetary reward task, except pictures of gender-matched peers (i.e. two male or two female faces) were presented instead of doors. There were an equal number of trials with male and female peers (30 each, 60 total) (Figure 1B).
EEG recording and processing
Continuous EEG was recorded using an elastic cap with 34 electrode sites placed according to the 10/20 system. Electrooculogram was recorded using four additional facial electrodes: two placed approximately 1 cm outside of the right and left eyes and two placed ∼1 cm above and below the right eye. All electrodes were sintered Ag/AgCl electrodes. Data were recorded using the ActiveTwo system (BioSemi, Amsterdam, The Netherlands). The EEG was digitized with a sampling rate of 1024 Hz using a low-pass fifth order sinc filter with a half-power cutoff of 204.8 Hz. A common mode sense active electrode producing a monopolar (non-differential) channel was used as recording reference.
EEG data were analyzed using BrainVision Analyzer (Brain Products, Gilching, Germany). Data were referenced offline to the average of left and right mastoids, band-pass filtered (0.1–30 Hz), and corrected for eye movement artifacts (Gratton et al., 1983). Feedback-locked epochs were extracted with a duration of 1000 ms, including a 200 ms pre-stimulus and 800 ms post-stimulus interval. The 200 ms pre-stimulus interval was used as the baseline. Epochs containing a voltage >50 μV between sample points, a voltage difference of 300 μV within a segment, or a maximum voltage difference of <0.50 μV within 100 ms intervals were automatically rejected. Additional artifacts were identified and removed based on visual inspection.
Feedback-locked ERPs were averaged separately for gain and loss feedback on the monetary task, and like and dislike feedback on the social feedback task. The ERP response to monetary and social feedback was scored as the mean amplitude from 250 to 350 ms following feedback at electrodes Fz, FCz and Cz, where the difference between favorable and unfavorable feedback was the greatest. The monetary and social RewP was quantified as the difference between gain and loss trials (i.e. gain–loss) and like and dislike trials (i.e. like–dislike), respectively.
Data analysis
Analyses were primarily conducted in IBM SPSS Statistics, Version 22.0 (Armonk, NY, USA). To compare the electrocortical response to monetary and social feedback, we conducted a Task (monetary vs. social) × Outcome [favorable (gain/like) vs unfavorable (loss/dislike)] × Location [Fz, FCz and Cz]) repeated measures analysis of variance (ANOVA). Greenhouse-Geisser epsilons (G-Gε) are reported for repeated measures analyses where assumptions of sphericity were violated.
Permutation tests were performed (Groppe et al., 2011) at each electrode to test the null hypothesis that the monetary and social RewP do not differ in spatial topography. Under the null hypothesis, the task labels for the difference scores should be interchangeable. Therefore, a null distribution of t scores was constructed by randomly shuffling monetary and social reward task labels, conducting paired t-tests for each electrode, and identifying the most extreme (positive or negative) t score. Ten thousand permutations were conducted, which resulted in a null distribution of t scores. Critical t scores (<−2.82 or >2.84) were then identified as values corresponding to the 2.5th and 97.5th percentiles. To test the difference at each electrode, t scores were calculated using the correct task labels and compared with the critical t scores.
The association between the ERP response to monetary and social feedback was examined using Pearson’s correlation coefficients and interclass correlation coefficients (ICCs; Shrout and Fleiss, 1979). The reliability of the ERP response to monetary and social feedback was examined using Generalizability (G) theory, split-half reliability and Cronbach’s α.
There were no sex differences in any demographic (Ps > 0.13), but more males (25 out of 39; 64.1%) completed the social task before the monetary task than females (29 out of 75; 38.7%), χ2 (1, n = 114) = 6.66, P = .010. Therefore, all analyses involving participant sex included task order (monetary first vs social first) as a dichotomous covariate. To test for the presence of sex differences, we conducted a Task (monetary vs social) × Outcome [favorable (gain/like) vs unfavorable (loss/dislike)] × Location (Fz, FCz and Cz) × Participant Sex (males vs females) mixed-measures ANOVA, with task, outcome and electrode as within-subject factors and sex as a between-subjects factor. Because the social reward task included an equal number of trials with feedback from male and female peers, we also examined whether the ERP response to social feedback differed as a function of peer and participant sex. To this end, we conducted an Outcome [favorable (like) vs unfavorable (dislike)] × Location (Fz, FCz and Cz) × Peer Sex (male vs female) × Participant Sex (male vs female) mixed-measures ANOVA, with outcome and peer sex as within-subject factors and participant sex as a between-subjects factor. Finally, to examine the association between individual differences in dysphoria symptoms and the RewP, we conducted two different analyses. For the monetary task, we conducted an Outcome [favorable (gain) vs unfavorable (loss)] × Location (Fz, FCz and Cz) × Dysphoria mixed-measures analysis of covariance (ANCOVA), with dysphoria symptoms included as the continuous covariate. For the social task, we conducted an Outcome [favorable (like) vs unfavorable (dislike)] × Location (Fz, FCz and Cz) × Peer Sex (male vs. female) × Dysphoria mixed-measures ANCOVA, with dysphoria symptoms included as the continuous covariate.
Results
Monetary and social reward
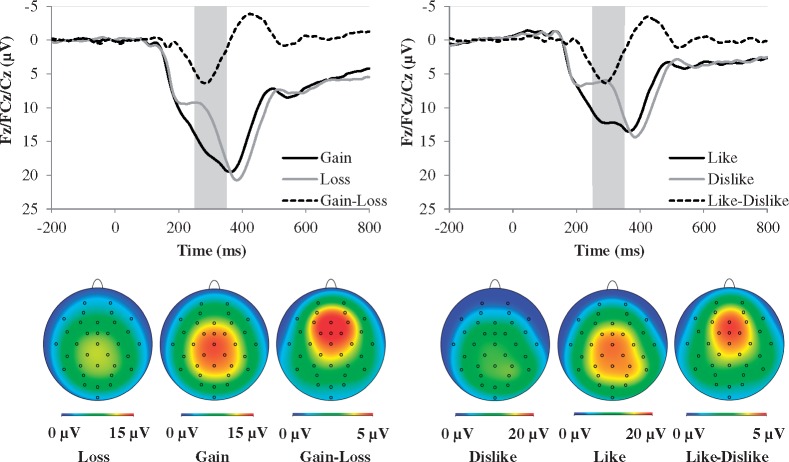
Figure 2 displays the grand average waveforms and scalp distributions for the ERP response to monetary and social feedback (see Table 1 for descriptive statistics). Results indicated main effects of task, F(1, 113) = 92.35, P < 0.001, ηp2 = 0.45, such that the electrocortical response during monetary trials was greater compared with social trials, outcome, F(1, 113) = 238.21, P < 0.001, ηp2 = 0.68, such that the electrocortical response to favorable feedback (i.e. monetary gain and social like) was greater compared with unfavorable feedback (i.e. monetary loss and social dislike), and location, F(1, 113) = 192.96, P < 0.001, ηp2 = 0.63, such that the electrocortical response at Cz was greater compared with FCz, F(1, 113) = 45.11, P < 0.001, ηp2 = 0.29 and Fz, F(1, 113) = 245.14, P < 0.001, ηp2 = 0.68, and was greater at FCz compared with Fz, F(1, 113) = 209.50, P < 0.001, ηp2 = 0.65.
Fig. 2.
ERP waveforms (top) and scalp distributions (bottom) for the monetary (left) and social (right) tasks. The shaded region of the waveforms shows the segment from 250 to 350 ms where the mean activity was scored at electrodes Fz, FCz and Cz. The monetary RewP is represented by the gain–loss difference, and the social RewP is represented by the like–dislike difference. ms, millisecond.
Table 1.
Descriptive and inferential statistics for the ERP response to monetary and social feedback
| Monetary reward task |
Social reward task |
Monetary vs social |
||||||
|---|---|---|---|---|---|---|---|---|
| Mean (s.d.) | 95% CI |
Mean (s.d.) | 95% CI |
Pearson’s r | ICC [95% CI] | |||
| Lower bound | Upper bound | Lower bound | Upper bound | |||||
| Gain/like feedback | 16.96 (7.52) | 15.56 | 18.35 | 12.03 (6.91) | 10.74 | 13.31 | 0.61** | 0.66 [0.23, 0.83] |
| Loss/dislike feedback | 12.36 (6.90) | 11.08 | 13.64 | 6.99 (6.87) | 5.71 | 8.27 | 0.55** | 0.60 [0.12, 0.79] |
| RewP | 4.59 (4.50) | 3.76 | 5.43 | 5.04 (4.89) | 4.13 | 5.95 | 0.26** | 0.41 [0.14, 0.59] |
Note. For ICCs, a two-way mixed-effects model (model 3 in Shrout and Fleiss, 1979) was conducted as a more conservative measure of absolute agreement. Qualitative cutoffs for ICCs are as follows: ICCs < 0.50: poor agreement, 0.50 < ICC < 0.75: moderate agreement, 0.75 < ICC < 0.90: good agreement, ICC > 0.90: excellent agreement; CI, confidence interval; ICC, intraclass correlation coefficient.
P < 0.01.
There were also Task x Location, F(2, 226) = 8.46, P = 0.001, G–Gε = 0.89, ηp2 = 0.07, and Outcome × Location interactions, F(2, 226) = 3.30, P = 0.04, ηp2 = 0.03. Follow-up analyses for the Task × Location interaction revealed that the electrocortical response during monetary trials was greater compared with social trials at electrodes Fz, F(1, 113) = 80.72, P < 0.001, ηp2 = 0.42, FCz, F(1, 113) = 85.13, P < 0.001, ηp2 = 0.43 and Cz, F(1, 113) = 100.49, P < 0.001, ηp2 = 0.47. To determine where these effects differed from each other, separate Task × Location ANOVAs were conducted for Fz vs FCz, Fz vs Cz and FCz vs Cz. These results revealed that the increased electrocortical response during monetary trials compared with social trials was greater at Cz compared with Fz, F(1, 113) = 13.02, P < 0.001, ηp2 = 0.10, and greater at FCz compared with Fz, F(1, 113) = 6.34, P = 0.013, ηp2 = 0.05, but it did not differ between Cz and FCz, F(1, 113) = 3.16, p = 0.08. Follow-up analyses for the Outcome × Location interaction revealed that the electrocortical response to favorable feedback was greater compared with unfavorable feedback at Cz, F(1, 113) = 73.77, P < 0.001, ηp2 = 0.40, FCz, F(1, 113) = 218.55, P < 0.001, ηp2 = 0.66 and Cz, F(1, 113) = 196.74, P < 0.001, ηp2 = 0.64. To determine where these effects differed from each other, separate Outcome X Location ANOVAs were conducted for Fz vs FCz, Fz vs Cz and FCz vs Cz. These results revealed that the increased electrocortical response to favorable feedback compared with unfavorable feedback was greater at Cz compared with FCz, F(1, 113) = 5.64, P < 0.019, ηp2 = 0.05, and Fz, F(1, 113) = 71.05, p < 0.001, ηp2 = 0.39, and greater at FCz compared with Fz, F(1, 113) = 116.51, P < 0.001, ηp2 = 0.51. There was no Task × Outcome, F(1, 113) = 0.01, P = 0.92, or Task × Outcome × Location interaction, F(1, 113) = 0.84, P = 0.42, suggesting no difference between the magnitude of the monetary RewP (i.e. gain–loss) and social RewP (i.e. like–dislike).
Permutation analyses indicated that no electrodes exceeded the critical t values, indicating that the monetary and social RewP did not differ anywhere on the scalp.
Psychometric properties
All psychometric properties were conducted on a pooling of electrodes Fz, FCz and Cz. Table 1 displays Pearson’s r and ICC values for the association between the ERP response to monetary and social gain feedback, monetary and social loss feedback, and their relative difference (i.e. the monetary and social RewP). Results indicated moderate to strong associations between the electrocortical response to monetary and social gain feedback, and the electrocortical response to monetary and social loss feedback. Results also indicated a statistically significant but weak association between the monetary and social RewP difference scores (i.e. gain–loss, like–dislike). The ICC analyses indicated moderate agreement between the electrocortical response to monetary and social gain feedback, and the electrocortical response to monetary and social loss feedback. However, results indicated poor agreement between the monetary and social RewP difference scores.
Table 2 displays split-half reliability, Cronbach’s α, and G theory dependability measures for the ERP response to monetary gain and loss and social like and dislike feedback. All three measures indicated strong reliability/dependability for the electrocortical response to monetary and social feedback. A Fischer r-to-z comparison of the split-half reliability for the monetary and social reward tasks indicated that the ERP response to monetary gain feedback was more reliable compared with the ERP response to social like feedback, z = 2.98, P = 0.001. However, the ERP response to monetary loss feedback did not differ from the ERP response to social dislike feedback, z = 1.50, P = 0.07. As shown in Figure 3, Cronbach’s α reached the acceptable range (> 0.70) earlier in the monetary compared with social tasks, but both reached an acceptable level by the 11th trial for all four types of feedback (i.e. monetary gain and loss, social like and dislike). Cronbach’s α was statistically greater during the monetary gain compared with social like feedback during trials 22–30 and was greater during the monetary loss compared with social dislike feedback during trials 25–27. Cronbach’s α of the monetary and social RewP difference scores was in the poor range and was greater during the monetary compared with social task for trials 6–14 and 22–27.
Table 2.
Reliability and dependability of ERP response to monetary and social feedback
| Measure | Gain/like feedback | Loss/dislike feedback | RewP | |
|---|---|---|---|---|
| Monetary Reward Task | Split–Half | 0.91 | 0.89 | |
| Cronbach’s α [95% CIs] | 0.91 [0.88, 0.93] | 0.88 [0.84, 0.90] | ||
| Adjusted α | 0.45 | |||
| Dependability [95% CIs] | 0.91 [0.88, 0.93] | 0.90 [0.87, 0.92] | ||
| Social Reward Task | Split–Half | 0.81 | 0.84 | |
| Cronbach’s α [95% CIs] | 0.84 [0.80, 0.88] | 0.84 [0.79, 0.88] | ||
| Adjusted α | 0.37 | |||
| Dependability [95% CIs] | 0.84 [0.80, 0.88] | 0.84 [0.80, 0.88] |
Notes. The RewP indicates the relative difference between the gain and like feedback and the loss and dislike feedback. Generalizability (G) theory measures of overall dependability were computed in MATLAB using the ERP Reliability Analysis Toolbox (Clayson and Miller, 2017). Internal consistency of the ERP response to monetary and social feedback was examined using two approaches derived from classical test theory. First, split-half reliability was examined by calculating the correlation between averages based on odd- and even-numbered trials, corrected using the Spearman-Brown prophecy formula (Nunnally et al., 1967). Second, Cronbach’s α, which is roughly equivalent to the mean of all possible split-half correlations, was examined for all trials. The overall internal reliability of the RewP difference score (i.e. gain–loss, like–dislike) was estimated using an adjusted α formula (Furr and Bacharach, 2013).
Fig. 3.
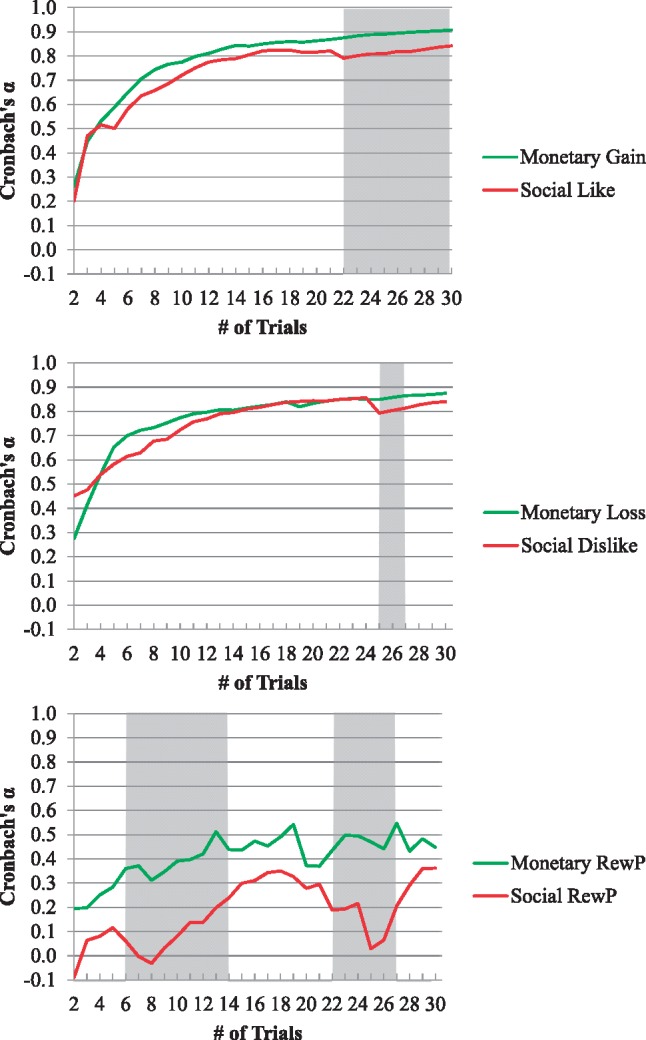
Cronbach’s α for the ERP response to monetary gain and social like feedback (top), monetary loss and social dislike feedback (middle), and the monetary RewP and social RewP (bottom) as a function of the number trials. The shaded regions show trials where Cronbach’s α significantly (P < 0.05) differed between the monetary and social RewP, which was determined using the cocron package in R (Diedenhofen and Musch, 2016).
Sex differences
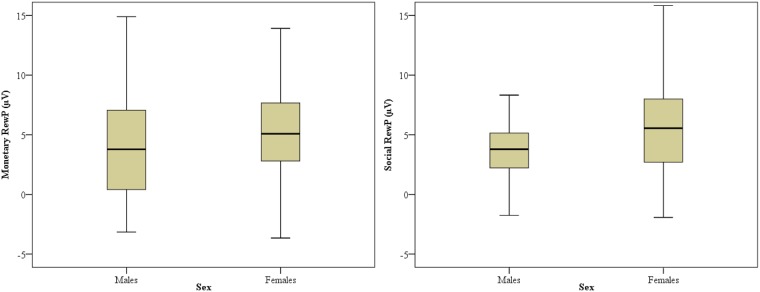
Figure 4 displays box and whisker plots of the monetary and social RewP in male and female participants. Results indicated an Outcome × Sex interaction F(1, 111) = 6.22, P = 0.014, ηp2 = 0.05, such that, across both monetary and social tasks, the RewP was greater in female participants relative to male participants. There was no Task × Outcome × Sex interaction, F(1, 112) = 0.54, P = 0.46, or any other main effects or interactions involving participant sex (Ps > 0.45).
Fig. 4.
Box and whisker plots for the monetary RewP (left) and social RewP (right) in male and female participants.
Dysphoria symptoms
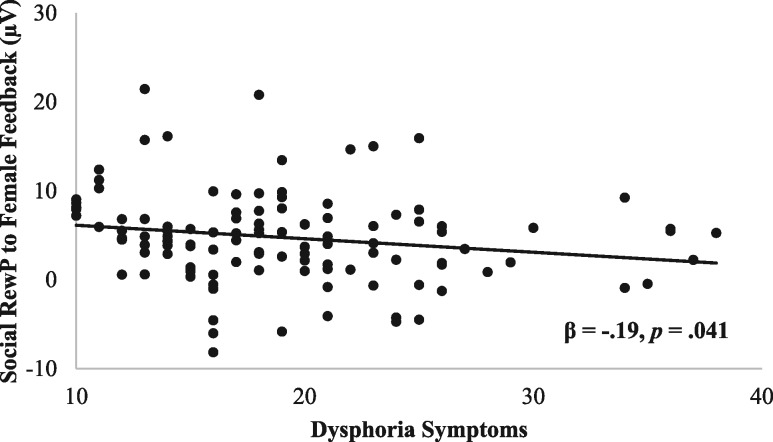
For the monetary task, there were no main effects or interactions involving dysphoria symptoms (Ps > 0.38). For the social task, results indicated a Peer Sex × Outcome × Dysphoria interaction, F(1, 112) = 4.01, P = 0.048, ηp2 = 0.034. Follow-up analyses were conducted by examining the association between dysphoria symptoms and the social RewP difference score (like–dislike) separately for male and female feedback. Results indicated that greater dysphoria symptoms were associated with a smaller social RewP to female feedback, β = −0.19, P = 0.041, but not male feedback, β = 0.04, P = 0.66 (Figure 5).
Fig. 5.
Scatterplot displaying the association between dysphoria symptoms and the social RewP (i.e. like–dislike difference score) elicited by female feedback, averaged across electrodes Fz, FCz and Cz.
Exploratory analyses were also conducted to determine whether this association was significant separately for female and male participants. In female participants, results indicated a Peer Sex × Outcome × Dysphoria interaction, F(1, 73) = 5.52, P = 0.022, ηp2 = 0.07; follow-up analyses indicated that greater dysphoria symptoms were associated with a smaller social RewP to female feedback, β = −0.23, P = 0.045, but not male feedback, β = 0.08, P = 0.49. To determine whether the ERP response to social gain or loss feedback contributed to this association, a follow-up linear regression was conducted that included both ERP responses as simultaneous independent variables. Results indicated that dysphoria symptoms were associated with the ERP response to gain, β = −0.31, P = 0.046, but not loss feedback, β = 0.24, P = 0.12, from female peers. In male participants, there were no main effects or interactions involving dysphoria symptoms (Ps > 0.26).
Discussion
This study compared the electrocortical response to monetary and social reward. Results indicated that the monetary and social RewP were of similar magnitude, and these electrocortical responses were positively correlated with each other. Moreover, the electrocortical response to both monetary and social feedback demonstrated comparable psychometric properties, including reliability and dependability. Across both the monetary and social tasks, women demonstrated a greater RewP compared with men. Finally, greater depression symptoms were associated with a smaller social RewP to feedback from female peers. Overall, the present study provides a novel methodological approach toward examining electrocortical response to social reward that is comparable to monetary reward.
This study suggests that a common neural system is at least partially involved in the generation of electrocortical responses to monetary and social reward. The overall ERP signal was greater during the monetary compared with social task, potentially indicating greater motivational salience of the monetary feedback. However, the relative difference between the favorable outcomes (i.e. monetary gain vs loss, social like vs dislike) did not differ between tasks and were correlated within-subject. ERPs provide high temporal resolution at the expense of poor spatial resolution, and these results do not provide concrete evidence regarding the specific neural circuit that is common across both types of reward. Nonetheless, variation in monetary RewP magnitude has been associated with activation in the striatum (Carlson et al., 2011; Foti et al., 2011, 2014), and studies examining brain regions that are activated across both social and non-social reward tasks have similarly indicated the striatum (Izuma et al., 2008; Lin et al., 2012; Daniel and Pollmann, 2014; Hausler et al., 2015). It is plausible that the striatum is a key region involved in general reward processing and approach-oriented behaviors (Delgado, 2007), while other brain regions are involved in higher-order processing of stimulus meaning and value (e.g. prefrontal cortex; McClure et al., 2004).
This study contributes to a growing literature on the psychometric properties of the RewP to monetary reward (Levinson et al., 2017; Luking et al., 2017), and provides novel findings in response to social reward. The reliability and dependability for all four individual ERP responses (i.e. gain, loss, like and dislike feedback) was in the acceptable range and statistically better for monetary compared with social feedback for two indices (split-half reliability, Cronbach’s α for a small number of trials). The monetary and social RewP ‘difference scores’ demonstrated a statistically significant but weak positive correlation. However, consistent with previous findings (Bress et al., 2015; Levinson et al., 2017), the RewP difference scores had poorer psychometric properties. The reliability of a difference score is known to be adversely affected when the constitute measures are strongly interrcorrelated or have unequal variances (Furr and Bacharach, 2013). Reliability indexes the amount of variance in the measure due to the phenomenon of interest and not error variance, and accumulating research on the RewP difference score suggests it has a relatively lower cap on the true score variance. Nonetheless, previous research has demonstrated that both the ERP response to gains alone and the monetary RewP difference score are correlated with depression symptoms, and this correlation is often stronger for the RewP difference score (Bress et al., 2015; Foti and Hajcak, 2009). One potential explanation for this finding is that a larger portion of the RewP true score variance could be related to depression compared with the true score variance of just the ERP response to gains, which contains greater error variance and variance for overlapping ERP components (e.g. P200, P300). This phenomenon has been demonstrated with other ERP components (e.g. error-related negativity and anxiety; Hajcak et al., 2017), suggesting that measures with modest internal consistency and reliability might contain adequate criterion validity.
The monetary and social RewP demonstrated different relationships with depression symptoms. Specifically, greater depression symptoms were associated with a smaller social RewP to feedback from female (but not male) peers, and exploratory analyses suggested this association was only present in the female and not male participants. It is important to note that this result should be interpreted with caution. Indeed, there were no a priori hypotheses regarding the sex-specific relationship between depression symptoms and the social RewP. Although the relationship achieved statistical significance (P = 0.045), some researchers have suggested that the threshold for statistical significance of new discoveries should be greater (e.g. P < 0.005, Benjamin et al., 2017). In addition, the sample contained nearly twice as many female participants compared with male participants. Thus, we urge caution in interpreting this relatively novel result. This study did not find an association between depression symptoms and the monetary RewP. Previous studies on depression and the monetary RewP have primarily been conducted in clinical or community samples (Foti and Hajcak, 2009; Bress et al., 2013, 2015), and it is possible that the use of a college student sample may have impacted this relationship. Furthermore, the inclusion of the social task might have influenced the ERP response to the monetary feedback task and its relationship with depression.
The novel social task used in this study included several strengths and weaknesses. Unlike most studies that relate monetary and social reward, the social task had similar psychometric properties, the same trial structure and number of trials as the monetary reward task. However, some differences are worth noting. For example, monetary gain feedback ($0.50) was twice the value of loss feedback ($0.25); there was no comparable contrast for social feedback. Thus, it is unclear whether the social RewP would have been larger if the ‘like’ feedback was calibrated to be twice as valuable as the ‘dislike’ feedback. Varying the desirability of peers based on participant valuations may help address this issue. Additionally, we did not formally assess deception after the social task. Although it is not clear whether all participants believed the social feedback, even imagined social feedback elicits differences in brain response to peer-based acceptance and rejection (Hsu et al., 2013). Finally, in the social task participants knew that for each pair of peers one was going to provide acceptance and the other rejection feedback. In contrast, monetary loss was only relevant if it was the feedback presented. Future studies should consider addressing these task differences to better compare the monetary and social RewP.
In conclusion, this study provides a novel experimental framework for testing how the brain responds to social vs nonsocial reward. This study adds to growing evidence of a common neural system involved in reward processing and decision-making for both social and nonsocial rewards. These findings have important implications for the design of future experiments evaluating decision-making and reinforcement learning and the examination of neural correlates of clinical phenomenology.
Acknowledgments
Conflict of interest. None declared.
References
- Benjamin D.J., Berger J.O., Johannesson M., et al. (2018). Redefine statistical significance. Nature Human Behaviour, 2(1), 6. [DOI] [PubMed] [Google Scholar]
- Bernat E.M., Nelson L.D., Baskin-Sommers A.R. (2015). Time-frequency theta and delta measures index separable components of feedback processing in a gambling task. Psychophysiology, 52(5), 626–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhanji J.P., Delgado M.R. (2014). The social brain and reward: social information processing in the human striatum. Wiley Interdisciplinary Reviews: Cognitive Science, 5(1), 61–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bress J.N., Foti D., Kotov R., Klein D.N., Hajcak G. (2013). Blunted neural response to rewards prospectively predicts depression in adolescent girls. Psychophysiology, 50(1), 74–81. [DOI] [PubMed] [Google Scholar]
- Bress J.N., Hajcak G. (2013). Self-report and behavioral measures of reward sensitivity predict the feedback negativity. Psychophysiology, 50(7), 610–6. [DOI] [PubMed] [Google Scholar]
- Bress J.N., Meyer A., Proudfit G.H. (2015). The stability of the feedback negativity and its relationship with depression during childhood and adolescence. Development and Psychopathology, 27, 1285–94. [DOI] [PubMed] [Google Scholar]
- Bress J.N., Meyer A., Hajcak G. (2015). Differentiating anxiety and depression in children and adolescents: evidence from event-related brain potentials. Journal of Clinical Child & Adolescent Psychology, 44(2), 238–49. [DOI] [PubMed] [Google Scholar]
- Bress J.N., Smith E., Foti D., Klein D.N., Hajcak G. (2012). Neural response to reward and depressive symptoms in late childhood to early adolescence. Biological Psychology, 89(1), 156–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson J.M., Foti D., Mujica-Parodi L.R., Harmon-Jones E., Hajcak G. (2011). Ventral striatal and medial prefrontal BOLD activation is correlated with reward-related electrocortical activity: a combined ERP and fMRI study. Neuroimage, 57(4), 1608–16. [DOI] [PubMed] [Google Scholar]
- Chan R.C.K., Li Z., Li K., et al. (2016). Distinct processing of social and monetary rewards in late adolescents with trait anhedonia. Neuropsychology, 30(3), 274–80. [DOI] [PubMed] [Google Scholar]
- Clayson P.E., Baldwin S.A., Larson M.J. (2013). How does noise affect amplitude and latency measurement of event-related potentials (ERPs)? A methodological critique and simulation study. Psychophysiology, 50(2), 174–86. [DOI] [PubMed] [Google Scholar]
- Clayson P.E., Miller G.A. (2017). Psychometric considerations in the measurement of event-related brain potentials: guidelines for measurement and reporting. International Journal of Psychophysiology, 111, 57–67. [DOI] [PubMed] [Google Scholar]
- Daniel R., Pollmann S. (2014). A universal role of the ventral striatum in reward-based learning: evidence from human studies. Neurobiology of Learning Memory, 114, 90–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado M.R. (2007). Reward-related responses in the human striatum. Annals of the New York Academy of Sciences, 1104, 70–88. [DOI] [PubMed] [Google Scholar]
- Diedenhofen B., Musch J. (2016). cocron: a web interface and R package for the statistical comparison of Cronbach’s alpha coefficients. International Journal of Internet Science, 11, 51–60. [Google Scholar]
- Egger H.L., Pine D.S., Nelson E., et al. (2011). The NIMH Child Emotional Faces Picture Set (NIMH-ChEFS): a new set of children’s facial emotion stimuli. International Journal of Methods in Psychiatric Research, 20(3), 145–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores A., Münte T.F., Doñamayor N. (2015). Event-related EEG responses to anticipation and delivery of monetary and social reward. Biological Psychology, 109, 10–9. [DOI] [PubMed] [Google Scholar]
- Forbes E.E. (2009). Where’s the fun in that? Broadening the focus on reward function in depression. Biological Psychiatry, 66(3), 199–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foti D., Carlson J.M., Sauder C.L., Proudfit G.H. (2014). Reward dysfunction in major depression: multimodal neuroimaging evidence for refining the melancholic phenotype. Neuroimage, 101, 50–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foti D., Hajcak G. (2009). Depression and reduced sensitivity to non-rewards versus rewards: evidence from event-related potentials. Biological Psychology, 81(1), 1–8. [DOI] [PubMed] [Google Scholar]
- Foti D., Weinberg A., Dien J., Proudfit G.H. (2011). Event-related potential activity in the basal ganglia differentiates rewards from nonrewards: temporospatial principal components analysis and source localization of the feedback negativity. Human Brain Mapping, 32(12), 2267–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furr R.M., Bacharach V.R.. 2013. Psychmetrics: An Introduction, 2nd. edn Thousand Oaks, CA: Sage Publications. [Google Scholar]
- Gratton G., Coles M.G.H., Donchin E. (1983). A new method for off-line removal of ocular artifact. Electroencephalography Clinical Neurophysiology, 55(4), 468–84. [DOI] [PubMed] [Google Scholar]
- Groppe D.M., Urbach T.P., Kutas M. (2011). Mass univariate analysis of event-related brain potentials/fields I: a critical tutorial review. Psychophysiology, 48(12), 1711–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer A.E., Choate V.R., Pine D.S., Nelson E.E. (2012). Neural circuitry underlying affective response to peer feedback in adolescence. Social Cognitive and Affective Neuroscience, 7(1), 81–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajcak G., Meyer A., Kotov R. (2017). Psychometrics and the neuroscience of individual differences: internal consistency limits between-subjects effects. Journal of Abnormal Psychology, 126(6), 823–34. [DOI] [PubMed] [Google Scholar]
- Hajcak G., Moser J.S., Holroyd C.B., Simons R.F. (2006). The feedback-related negativity reflects the binary evaluation of good versus bad outcomes. Biological Psychology, 71(2), 148–54. [DOI] [PubMed] [Google Scholar]
- Häusler A.N., Becker B., Bartling M., Weber B., Pessiglione M. (2015). Goal or gold: overlapping reward processes in soccer players upon scoring and winning money. PLoS One, 10(4), e0122798.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holroyd C.B., Coles M.G.H. (2002). The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychological Review, 109(4), 679–709. [DOI] [PubMed] [Google Scholar]
- Hsu D.T., Sanford B.J., Meyers K.K., et al. (2013). Response of the μ-opioid system to social rejection and acceptance. Molecular Psychiatry, 18(11), 1211–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izuma K., Saito D.N., Sadato N. (2008). Processing of social and monetary rewards in the human striatum. Neuron, 58(2), 284–94. [DOI] [PubMed] [Google Scholar]
- Jarcho J.M., Romer A.L., Shechner T., et al. (2015). Forgetting the best when predicting the worst: preliminary observations on neural circuit function in adolescent social anxiety. Developmental Cognitive Neuroscience, 13, 21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa A., Arfer K.B., Klein D.N., Proudfit G.H. (2014). Electrocortical reactivity to social feedback in youth: a pilot study of the Island Getaway task. Developmental Cognitive Neuroscience, 10, 140–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa A., Proudfit G.H., Kessel E.M., Dyson M., Olino T., Klein D.N. (2015). Neural reactivity to monetary rewards and losses in childhood: longitudinal and concurrent associations with observed and self-reported positive emotionality. Biological Psychology, 104, 41–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson A.R., Speed B.C., Infantolino Z.P., Hajcak G. (2017). Reliability of the electrocortical response to gains and losses in the Doors task. Psychophysiology, 54(4), 601–7. [DOI] [PubMed] [Google Scholar]
- Lin A., Adolphs R., Rangel A. (2012). Social and monetary reward learning engage overlapping neural substrates. Social Cognitive and Affective Neuroscience, 7(3), 274–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luking K.R., Nelson B.D., Infantolino Z.P., Sauder C.L., Hajcak G. (2017). Internal consistency of fMRI and EEG measures of reward in late childhood and early adolescence. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 2(3), 289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure S.M., York M.K., Montague P.R. (2004). The neural substrates of reward processing in humans: the modern role of fMRI. Neuroscience, 10(3), 260–8. [DOI] [PubMed] [Google Scholar]
- Nelson B.D., Perlman G., Klein D.N., Kotov R., Hajcak G. (2016). Blunted neural response to rewards as a prospective predictor of the development of depression in adolescent girls. American Journal of Psychiatry, 173(12), 1223–30. [DOI] [PubMed] [Google Scholar]
- Novak B.K., Novak K.D., Lynam D.R., Foti D. (2016). Individual differences in the time course of reward processing: stage-specific links with depression and impulsivity. Biological Psychology, 119, 79–90. [DOI] [PubMed] [Google Scholar]
- Novak K.D., Foti D. (2015). Teasing apart the anticipatory and consummatory processing of monetary incentives: an event-related potential study of reward dynamics. Psychophysiology, 52(11), 1470–82. [DOI] [PubMed] [Google Scholar]
- Nunnally J.C., Bernstein I.H., Berge J.M.T.. 1967. Psychometric Theory. New York, NY: McGraw-Hill. [Google Scholar]
- Olino T.M., Silk J.S., Osterritter C., Forbes E.E. (2015). Social reward in youth at risk for depression: a preliminary investigation of subjective and neural differences. Journal of Child and Adolescent Psychopharmacology, 25(9), 711–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfit G.H. (2015). The reward positivity: from basic research on reward to a biomarker for depression. Psychophysiology, 52(4), 449–59. [DOI] [PubMed] [Google Scholar]
- Rademacher L., Krach S., Kohls G., Irmak A., Gründer G., Spreckelmeyer K.N. (2010). NeuroImage Dissociation of neural networks for anticipation and consumption of monetary and social rewards. Neuroimage, 49(4), 3276–85. [DOI] [PubMed] [Google Scholar]
- Russo S.J., Nestler E.J. (2013). The brain reward circuitry in mood disorders. Nature Review of Neuroscience, 14(9), 609–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santesso D.L., Dzyundzyak A., Segalowitz S.J. (2011). Age, sex and individual differences in punishment sensitivity: factors influencing the feedback-related negativity. Psychophysiology, 48(11), 1481–9. [DOI] [PubMed] [Google Scholar]
- Schirmer A. (2013). Sex Differences in Emotion In: Armony J., Vuilleumier P. editors. The Cambridge Handbook of Human Affective Neuroscience, pp. 591–610. Cambridge: Cambridge University Press. [Google Scholar]
- Schultz W. (2006). Behavioral theories and the neurophysiology of reward. Annual Review of Psychology, 57, 87–115. [DOI] [PubMed] [Google Scholar]
- Shrout P.E., Fleiss J.L. (1979). Intraclass correlations: uses in assessing rater reliability. Psychological Bulletin, 86, 420–8. [DOI] [PubMed] [Google Scholar]
- Silk J.S., Siegle G.J., Lee K.H., Nelson E.E., Stroud L.R., Dahl R.E. (2014). Increased neural response to peer rejection associated with adolescent depression and pubertal development. Social Cognitive and Affective Neuroscience, 9(11), 1798–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreckelmeyer K.N., Krach S., Kohls G., et al. (2009). Anticipation of monetary and social reward differently activates mesolimbic brain structures in men and women. Social Cognitive and Affective Neuroscience, 4(2), 158–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens J.S., Hamann S. (2012). Sex differences in brain activation to emotional stimuli: a meta-analysis of neuroimaging studies. Neuropsychologia, 50(7), 1578–93. [DOI] [PubMed] [Google Scholar]
- Sun S., Yu R. (2014). The feedback related negativity encodes both social rejection and explicit social expectancy violation. Frontiers in Human Neuroscience, 8, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tversky A., Kahneman D. (1992). Advances in prospect theory: cumulative representation of uncertainty. Journal of. Risk and Uncertainty, 5(4), 297–323. [Google Scholar]
- Tversky A., Kahneman D. (1981). The framing of decisions and the psychology of choice. Science, 211(4481), 453–8. [DOI] [PubMed] [Google Scholar]
- van der Veen F.M., van der Molen M.W.J.W., van der Molen M.W.J.W., Franken I.H.A. (2016). Thumbs up or thumbs down? Effects of neuroticism and depressive symptoms on psychophysiological responses to social evaluation in healthy students. Cognitive, Affective & Behavioral Neuroscience, 16(5), 836–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrtička P., Sander D., Anderson B., Badoud D., Eliez S., Debbané M. (2014). Social feedback processing from early to late adolescence: influence of sex, age, and attachment style. Brain Behaviour, 4(5), 703–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D., O’Hara M.W., Naragon-Gainey K., et al. (2012). Development and validation of new anxiety and bipolar symptom scales for an expanded version of the IDAS (the IDAS-II). Assessment, 19(4), 399–420. [DOI] [PubMed] [Google Scholar]