Abstract
Independent vs interdependent self-construal is a concept that reflects how people perceive the relationship between self and other people, which has been extensively examined across disciplines. However, little evidence on the whole-brain functional connectivity (FC) pattern of independent vs interdependent self-construal has been reported. Here, in a sample of 51 healthy participants, we used resting-state functional magnetic resonance imaging and voxel-based FC analysis (i.e. FC strength and seed-based FC) by measuring the temporal correlation of blood oxygen level-dependent signals between spatially separate brain regions to investigate the neural mechanism of independent vs interdependent self-construal. First, we found that FC strength of bilateral posterior cingulate cortex and precuneus, and left inferior frontal gyrus were positively correlated with the independent vs interdependent score. Seed-based FC analysis with these three regions as seeds revealed that, FC within default mode network and executive control network was positively correlated with the independent vs interdependent score. Negative correlation with independent vs interdependent score was shown in the connections between default mode network and executive control regions. Taking together, our results provide a comprehensive FC architecture of the independent vs interdependent self-construal and advance the understanding of the interplay between culture, mind and brain.
Keywords: connectome, self-construal, functional connectivity, default mode network, executive control network
Introduction
Humans, as social animals, need to develop the concept for the relationship between self and other people. One of the most popular relevant concepts is independent vs interdependent self-construal, which has been extensively studied across many fields since it was introduced by Markus and Kitayama (1991). Individuals with an independent self-construal, which is more prevalent in Western cultures (Markus and Kitayama, 1991, 2010), perceive that the self is separate from other people and the embedded contexts, making them likely to seek for uniqueness and personal choice (Kim and Markus, 1999). In contrast, individuals with an interdependent self-construal, which is more prevalent in East Asian cultures (Markus and Kitayama, 1991, 2010), perceive that the self is connected with significant others in the given context, making them likely to maintain social harmony and fulfill social obligation (Miller et al., 1990). These fundamental differences due to the self-construal affect people’s behaviors not only in the social domains (e.g. prosocial behavior) but also in the non-social domains (e.g. self-regulation; for a review, see Cross et al., 2011; Ishii et al., 2014; Senzaki et al., 2014).
With the advances of non-invasive in vivo neuroimaging technology, researchers started to examine the brain mechanisms associated with the independent vs interdependent self-construal via different approaches (for a review, see Kitayama and Uskul, 2011). Task-based functional magnetic resonance imaging (fMRI) studies converged to support notable associations between the independent vs interdependent self-construal and the brain activities across multi-domains (for a review, see Kitayama and Uskul, 2011; Han, 2015). In the cognitive domain, previous studies found notable associations between the independent vs interdependent self-construal and medial prefrontal cortex (MPFC) activation for self-judgment tasks (Zhu et al., 2007; Chiao et al., 2009). Besides MPFC, which is a key region of default mode network (DMN), some regions in the executive control network (ECN) were found to be relevant to the self-related cognitive processing tasks (Uddin et al., 2005, for review, see Morin and Michaud, 2007), including the inferior parietal lobule and inferior frontal gyrus (IFG). Recently, a meta-analysis, including 35 task-based fMRI cultural neuroscience studies, revealed greater activation in the dorsal MPFC, lateral prefrontal cortex (LPFC), temporoparietal junction (TPJ) and dorsal LPFC among East Asians, who represent interdependent groups, but greater activation in the anterior cingulate cortex (ACC), ventral MPFC, bilateral insula and right temporal pole among Westerners, who represent independent groups, in the tasks that involve social cognitive/affective processes (Han and Ma, 2014).
Despite the observed notable associations between the independent vs interdependent self-construal and brain activities, most previous studies were task-based fMRI research. That means, these findings only demonstrate how the brain responses to a certain circumstance (i.e. demands of a certain task) differently among independent vs interdependent people. Contrasting to task-based fMRI, resting-state fMRI (R-fMRI), without relying on any specific task, can detect spontaneous or intrinsic brain activity (Biswal et al., 1995), which may reflect individuals’ chronic characteristics. Although previous studies have demonstrated some overlapping brain regions between the patterns during the self-related tasks and in the resting condition (Qin and Northoff, 2011; Murray et al., 2015; Davey et al., 2016; Huang et al., 2016; Northoff, 2016; Qin et al., 2016), significant dissociations between them have also been revealed (Whitfield-Gabrieli et al., 2011). It may suggest that intrinsic brain activity reflects a relatively different and more general pattern of chronic self-related concepts. A previous R-fMRI study has found that the interdependent self-construal score was positively correlated with the strength of functional connectivity (FC) between the ventral MPFC and the dorsal MPFC, while the independent self-construal score was positively correlated with the strength of FC between the ventral MPFC and the posterior cingulate cortex (PCC; Wang et al., 2013). These findings suggest that FC within the DMN (i.e. MPFC and PCC) may be associated with independent vs interdependent self-construal. However, it is still unclear whether the FC in other self-processing-related networks, such as the ECN, would also predict individuals’ independent tendency. A previous study using a self-recognition task showed that, self vs other condition induced activity in the inferior parietal loblue and IFG in the ECN, while other vs self condition induced activity in the MPFC and precuneus (PCu) in the DMN (Uddin et al., 2005). These results suggest that the DMN and the ECN showed different functions in self-related cognitive processing. However, how their relationship correlates with self-related cognitive processing remains unclear. Due to the lack of clear results regarding the relationship between the DMN and the ECN, we explored this question without any specific expectation in the present study.
Recently, combination of R-fMRI and graph theory (i.e. functional network or connectomices) allows revealing more comprehensive information of brain FC and topological organization (for review, see Bullmore and Sporns, 2009, 2012). Individual differences in the functional network have been connected to individual differences in intelligence (van den Heuvel et al., 2009) and personality traits (Adelstein et al., 2011; Kunisato et al., 2011; Dawes et al., 2012; Hahn et al., 2012, 2015).They act as a ′fingerprint′ for identifying individuals from a large group (Finn et al., 2015). However, whether and how the whole-brain functional network (especially the FC among the DMN and the ECN) is associated with the independent vs interdependent self-construal remains unclear.
To address these questions, we collected R-fMRI data and independent vs interdependent self-construal scores in 54 young healthy participants. A voxel-based graph theory analysis was used to explore the substantial contributions of the network nodal connectivity capacity to individual differences in independent vs interdependent self-construal.
Materials and methods
Participants
We recruited 54 participants from Sun Yat-sen University in China. Three participants were excluded (two were excluded for excessive motion in the MRI scan and one was excluded for missing behavioral data). Finally, 51 participants were included in the subsequent analyses. All participants were healthy adults (mean age = 22.22 ± 2.86 years old; 36 females). They had no history of neurological or psychiatric disorders, sensorimotor or cognitive impairment, or other anatomical injuries of brain. Before the experiment, all participants had given informed consent, and this study was approved by the Institutional Review Board in the Department of Psychology of Sun Yat-sen University.
MRI and behavioral data acquisition
All participants were scanned on a Siemens 3.0 Tesla MRI scanner (Siemens, Erlangen, Germany) at South China Normal University (Guangzhou, China).We used headphones and foam pads to avoid interference of scanner noise and reduce participants' head motion in the scan. Before scanning, participants were required to close their eyes, stay awake without thinking anything and keep their heads fixed during the data acquisition. Resting-state functional images were collected using echo-planar imaging sequence: repetition time (TR) =2000 ms, echo time (TE) = 30 ms, flip angle (FA) = 90°, field of view (FOV) = 224 × 224 mm2, slices = 32, matrix = 64 × 64, slice thickness = 3.5 mm and voxel size = 3.5 × 3.5 × 3.5 mm³. The total number of collected functional volumes was 240 for each participant. All participants confirmed that they had stayed awake during the scan. Structural images of T1-weighted images covering the entire brain were obtained in a sagittal orientation by employing magnetization prepared by rapid gradient echo sequence: TR = 2300 ms, TE = 3.24 ms, FA = 9°, FOV = 256 × 256 mm2, inversion time = 900 ms, matrix = 256 × 256, slices = 176, slice thickness = 1 mm and voxel size = 1 × 1 × 1 mm³.
Behavioral data were collected before scanning. Participants completed a well-validated 24-item measurement of Independence/Interdependence scale (Singelis, 1994), with a 7-point Likert-scale ranging from 1 (strongly disagree) to 7 (strongly agree). The sample item for independence is, ‘I enjoy being unique and different from others in many respects’, and the sample item for interdependence is, ‘It is important for me to maintain harmony within my group’. The reliability for the scale was satisfactory (interdependence: α = 0.71; independence: α = 0.44; correlation between interdependence and independence: r = 0.557, P< 0.001). An average score of all items for independence and interdependence was calculated separately. Finally, following the method of previous studies (Chiao et al., 2010; Ma et al., 2014; Luo et al., 2015), we computed a score of independent (vs interdependent) self-construal, which was calculated by subtracting the interdependence score from the independence one. A higher score indicates that participants have a stronger tendency in independence than interdependence.
Image pre-processing
The Statistical Parametric Mapping (SPM8, http://www.fil.ion.ucl.ac.uk/spm) and Data Processing Assistant for Resting-State fMRI (Yan and Zang, 2010) were the main toolboxes used in preprocessing of the functional imaging data. We removed the first 10 volumes of functional images for stabilizing the signal that may be influenced by the factor of scanning machine or participants' adaption in the beginning. Then, we performed slice timing on the remaining volumes to correct the time delay during MRI scan. To correct for the head motion, we also realigned the volumes to the first volume and then checked the head motion of each participant. Two participants in the original set of 54 participants were excluded under the criterion of 2 mm and 2°. Then individual T1-weighted images were co-registered to the motion-corrected functional images using a linear transformation (Collignon et al., 1995). Next, we segmented these structural images into gray matter (GM), white matter (WM) and the cerebrospinal fluid (CSF) maps using a unified segmentation algorithm (Ashburner and Friston, 2005). The motion-corrected functional images were spatially normalized into the Montreal Neurological Institute (MNI) space using the normalization parameters estimated during unified segmentation and then re-sampled to 3-mm isotropic voxels. After the normalization, we smoothed the normalized functional images using Gaussian kernel (FHWM = 4 mm × 4 mm × 4 mm) and removed the linear trends. The signals whose frequency fell outside the range of 0.01∼0.08 Hz were filtered reserving the low-frequency information. At last, we regressed out the nuisance variables (Friston 24 head motion parameters, global signal, WM and CSF signals) from the original signal of each voxel. Due to the controversial role of global signal regression (Murphy et al., 2009; Saad et al., 2012; Yang et al., 2014), we further confirmed the results of subsequent analyses on the data without regressing out global signal and found consistent results (Supplementary Figure S1).
FC strength calculation
To find out the regions that are significantly correlated with the behavioral scores, we carried out nodal measurements that can directly and meaningfully reflect this correlation. Nodal FC strength (FCS) is a useful metric to show the FC property of a node (Zuo et al., 2012; Dai et al., 2015). We performed the FCS analysis in the following steps.
First of all, we calculated the Pearson’s correlations between the blood oxygenation level dependent (BOLD) signal series of each pair of voxels in the whole brain GM mask for each participant. GM mask was generated by thresholding (cutoff = 0.2) on the mean GM probability map of all participants and with non-zeros standard deviations of BOLD time series. After that, we performed the Fisher's Z-transformation on the Pearson’s correlation coefficients to improve the normality of the correlations. The detailed equation for computing the FCS of each voxel i in the GM-functional combined mask is shown below (Buckner et al., 2009; Zuo et al., 2012; Dai et al., 2015):
Where is the number of voxels in our defined cortical mask (here, = 63 309); is the Fisher's Z-transformed correlation coefficient between voxel i and j; is the Pearson’s correlation coefficient between voxel i and j, and is threshold of effective correlation coefficient (here, = 0.2) to rule out low spurious correlations. Moreover, in order to investigate both positive and negative FC patterns, the and in the equation were set as their absolute values. The absolute values here could retain the positive and negative information for the subsequent analyses. The FCS calculated here was the sum of the weighted absolute connectivity strength, so the higher FCS value of a voxel means the more and stronger connectivities it had.
In order to assess the relationship between FCS and independent (vs interdependent) self-construal scores and determine the region of interest (ROI) independent of the present data, we further defined ROIs based on a previous research of self-related process [MNI coordinates: MPFC: x/y/z = 2/60/−2; PCC: x/y/z = −6/−56/10 (Davey et al., 2016)] and a study of self-awareness [MNI coordinates: IFG: x/y/z = −54/30/9 (Binder et al., 1999)]. The ROIs were defined as spheres with radii of 10 mm centered at the peak voxel of significant clusters. Then, a partial correlation between FCS of these ROIs and independent (vs interdependent) self-construal scores was performed with age and gender as covariates.
To further examine the relationship between FCS metrics and independent (vs interdependent) self-construal scores in the whole brain, a voxel-wise partial correlation analysis between the participants' independent (vs interdependent) self-construal scores and FCS values was performed with age and gender as covariates. The statistical significance threshold was set at P< 0.01 and cluster size > 1863 mm3, corresponding to corrected P< 0.05. Multiple comparisons were corrected using Monte Carlo simulations using the AFNI AlphaSim program (http://afni.nimh.nih.gov/pub/dist/doc/manual/AlphaSim.pdf).
Seed-based FC analysis
In the FCS analysis we described earlier, we had determined three important regions (bilateral PCC/PCu and left IFG, see Results for more details) that were significantly correlated with participants’ independent (vs interdependent) self-construal scores. To further determine exactly which connections of these regions were correlated with the independent (vs interdependent) self-construal scores, it is necessary to conduct a whole-brain seed-based FC analysis on the three significant regions found in the FCS analysis.
At first, we generated ROI masks for the three significant regions. This process was performed by extracting the significant clusters of the resultant Pearson’s correlation map in the FCS analysis. Subsequently, we extracted the regional mean BOLD time series of ROI by averaging the BOLD time series of all the voxels within each ROI mask. Then we used the regional mean time series to calculate the Pearson’s correlation coefficients with the BOLD time series of all other voxels within the GM mask. The whole-brain GM mask we used was the same GM mask that was generated and used in the FCS analysis. Finally, in order to improve normality, we performed Fisher’s Z-transformation to convert the Pearson’s correlation coefficients into z-scores and obtained z-score maps indicating FC pattern of ROIs. All the seed-based FC computations were conducted in R-fMRI Data Analysis Toolkit (Song et al., 2011). So far, for each participant, we obtained three seed-based FC maps corresponding to the three seed regions.
For the purpose of investigating the relationship between the strength of FC and the independent (vs interdependent) self-construal scores, we performed a partial correlation analysis. To begin with, we hoped to retain the connectivities that were significantly not equal to zero for subsequent analysis. So we conducted a two-tailed one-sample t test on the original FC maps. Moreover, the significant FC maps contained both positive FC and negative FC. Hence, in order to examine the positive FC pattern and the negative FC pattern separately, we further divided the significant seed-based FC maps into positive FC maps and negative FC maps by creating positive and negative masks. Then we performed a Pearson’s correlation analysis between seed-based FC (positive or negative FC map) and the independent (vs interdependent) self-construal scores after controlling participants’ age and gender, which was performed within the positive and negative masks respectively. The statistical significance threshold was set at P< 0.01 and cluster size > 1404 mm3 for positive FC map and 1566 mm3 for negative FC map, corresponding to corrected P< 0.05. Multiple comparisons were corrected using Monte Carlo simulations. The same seed-based connectivity and correlation analyses were also performed for the three predefined ROIs based on previous studies as above (i.e. IFG, MPFC and PCC; Binder et al., 1999; Davey et al., 2016), which were independent with our data.
Result
Functional connectivity strength
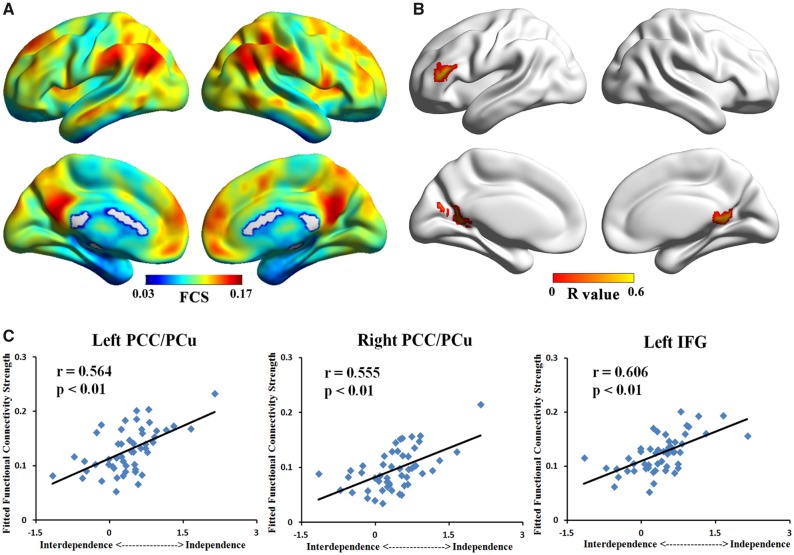
We first analyzed the whole-brain images based on the measure of FCS. We observed that the regions with high FCS were mainly distributed in PCC, PCu, lateral inferior parietal cortex and MPFC (Figure 1A). For visualization of FCS maps, we used the BrainNet Viewer (http://www.nitrc.org/projects/bnv/) (Xia et al., 2013). This pattern was consistent with previous studies of healthy adults (Buckner et al., 2009; Zuo et al., 2012; Dai et al., 2015).
Fig. 1.
Maps of whole-brain FCS analysis. (A) Mean FCS map across participants. (B) Correlation map showed that FCS of bilateral PCC/PCu and left IFG were positively correlated with independent (vs interdependent) self-construal score. (C) Scatter plot of peaks in three significant clusters (left PCC/PCu, right PCC/PCu and left IFG) showing positive correlation with independent (vs interdependent) self-construal score.
We then conducted whole-brain correlation analyses to test the association between FCS and independent (vs interdependent) self-construal scores across all participants. We found three clusters that were positively correlated with the independent (vs interdependent) self-construal scores (Figure 1B and Table 1). These significant regions were mainly distributed in the left PCC/PCu (peak r = 0.564, P< 0.05, corrected), right PCC/PCu (peak r = 0.555, P< 0.05, corrected) and left IFG (peak r = 0.606, P< 0.05, corrected). Correlations between peak voxels FCS and independent (vs interdependent) self-construal scores are presented in Figure 1C. Notably, the bilateral PCC/PCu in our results is the critical areas of the DMN and the IFG is the key region of the ECN. All significant regions were positively correlated with the independent (vs interdependent) self-construal scores, illustrating that high FCS was found in these regions among highly independent people.
Table 1.
Regions showing significant correlation with independent (vs interdependent) scores
| Brain regions | BA | Volume (mm3) | MNI coordinates (mm) |
R-score | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Left PCC/PCu | 30/17 | 2592 | −9 | −60 | 12 | 0.564 |
| Right PCC/PCu | 30/29 | 2241 | 27 | −48 | 6 | 0.555 |
| Left IFG | 45/46 | 2349 | −48 | 39 | 15 | 0.606 |
Notes: BA, Broadmann's area; x, y, z, coordinates of primary peak locations in the MNI space; R, the coefficient of Pearson’s correlation between region's FCS and independent (vs interdependent) self-construal scores; PCC, posterior cingulate cortex; PCu, precuneus; IFG, inferior frontal gyrus. P < 0.05, corrected for multiple comparisons.
Moreover, we calculated the association between FCS of IFG, MPFC and PCC, which defined independently based on the results of previous studies (Binder et al., 1999; Davey et al., 2016), and independent (vs interdependent) self-construal scores. The FCS values in these ROIs were extracted from each individual. Partial correlation analyses revealed that the correlations between independent (vs interdependent) self-construal scores and FCS were significant in PCC (r = 0.317, P = 0.008), and IFG (r = 0.291, P = 0.042), and marginally significant in MPFC (r = 0.271, P = 0.060).
Seed-based FC analysis
According to the whole-brain FCS results mentioned earlier, we found three regions (bilateral PCC/PCu and left IFG) that were positively correlated with the independent (vs interdependent) self-construal scores. In the next step, we defined these three clusters as seeds and calculated the voxel-wise seed-based FC, respectively. As mentioned before, we also divided the seed-based FC map into negative and positive connectivity map.
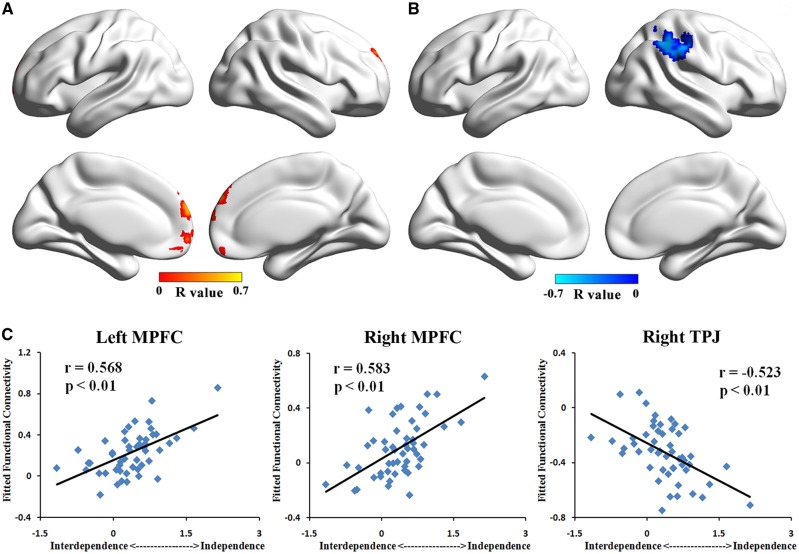
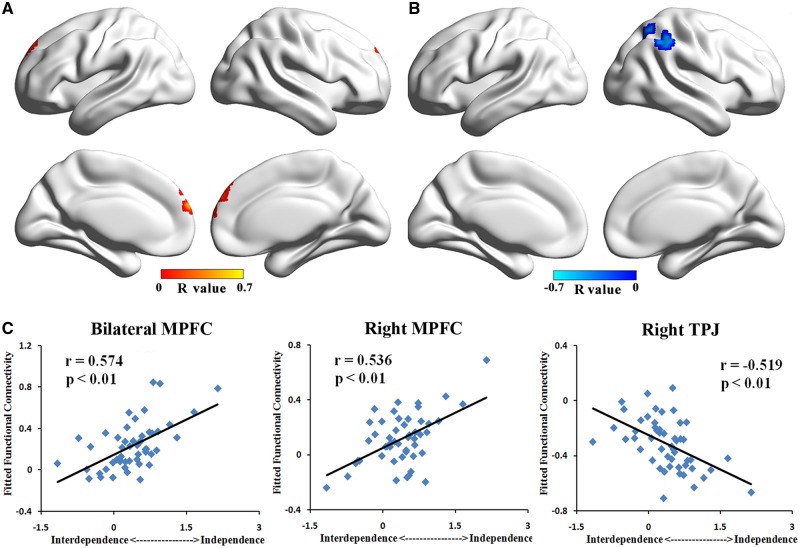
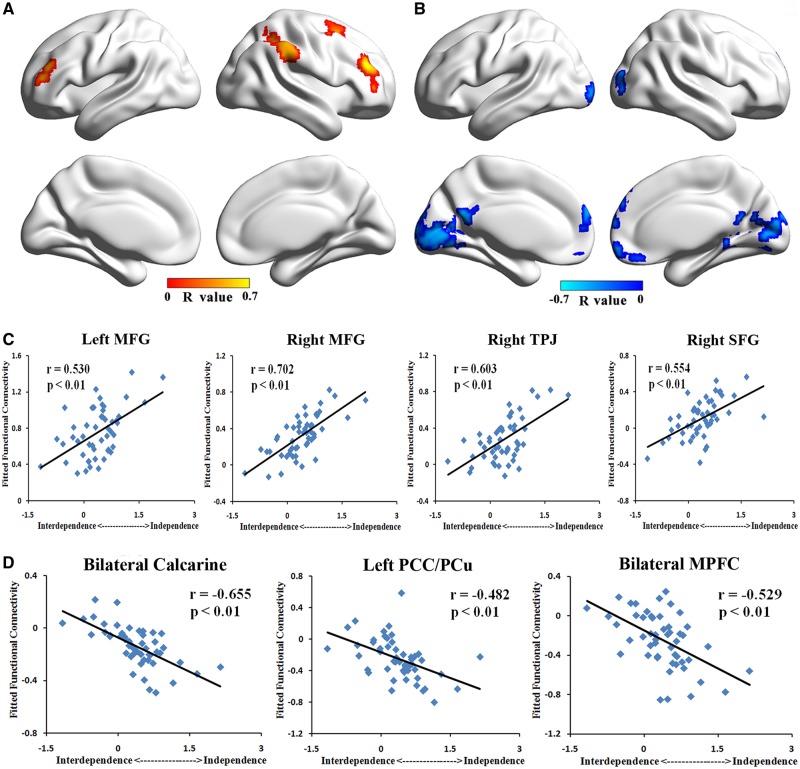
The correlation results of left PCC/PCu showed that the positive connectivity with bilateral MPFC was positively correlated with the independent (vs interdependent) self-construal scores (P< 0.05, corrected, Figure 2A and C and Table 2). In contrast, negative correlations with the independent (vs interdependent) self-construal scores were found in the negative connectivity from left PCC/PCu to right TPJ (P< 0.05, corrected, Figure 2B and C and Table 2). In the positive FC maps of right PCC/PCu, we found a positive correlations between connectivity to bilateral MPFC and the independent (vs interdependent) self-construal scores (P< 0.05, corrected, Figure 3A and C and Table 2). Besides, we found a negative correlation between connectivity to right TPJ and the independent (vs interdependent) self-construal scores in negative FC maps of right PCC/PCu (P< 0.05, corrected, Figure 3B and C and Table 2). As for FC maps of left IFG, positive correlations with the independent (vs interdependent) scores were observed in positive connectivity to bilateral middle frontal gyrus (MFG), right TPJ, and right superior frontal gyrus (SFG; P< 0.05, corrected, Figure 4A and C and Table 2). Moreover, in the negative FC maps of left IFG, negative correlations with the independent (vs interdependent) self-construal scores were found in the connectivity to bilateral MPFC, bilateral calcarine and left PCC/PCu (P< 0.05, corrected, Figure 4B and D and Table 2). Notably, the decrease in FC values of negative connectivity means the increase of their absolute value, which is the strength of the negative connectivity. Therefore, in the negative connectivity maps, a decrease in the strength of connectivity was associated with lower independent (vs interdependent) self-construal score. Combining with the positive connectivity results, we have obtained consistent results of all the seed-based FC maps, indicating that the enhancement of connectivity strength is significantly correlated with higher independent (vs interdependent) self-construal score. Consistent results were found in the analysis of three independently defined ROIs (i.e. IFG, MPFC and PCC).
Fig. 2.
Correlation result of left PCC/PCu’s FC pattern. (A) Correlation map of left PCC/PCu’s positive connectivity pattern showed that FCs to bilateral MPFC were positively correlated with independent (vs interdependent) self-construal score. (B) Correlation map of left PCC/PCu’s negative connectivity pattern showed that FC to right TPJ was negatively correlated with independent (vs interdependent) self-construal score. (C) Scatter plot of peaks in bilateral MPFC showing positive correlation with independent (vs interdependent) self-construal score and peak in right TPJ showing negative correlation with independent (vs interdependent) self-construal score.
Table 2.
Connectivities showing significant correlation with independent (vs interdependent) scores
| Brain regions | BA | Volume (mm3) | MNI coordinates (mm) |
R-score | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Seed: left PCC/PCu | ||||||
| Left MPFC | 10/9/11 | 4725 | −3 | 69 | 12 | 0.568 |
| Right MPFC | 9/10 | 2430 | 0 | 51 | 42 | 0.583 |
| Right TPJ/SMG | 40 | 7290 | 45 | −45 | 51 | −0.523 |
| Seed: right PCC/PCu | ||||||
| Bilateral MPFC | 10 | 1944 | 6 | 66 | 18 | 0.574 |
| Right MPFC | 9 | 2511 | 6 | 48 | 51 | 0.536 |
| Right TPJ | 40 | 4590 | 45 | −45 | 51 | −0.519 |
| Seed: left IFG | ||||||
| Right MFG | 45/46 | 5805 | 42 | 39 | 24 | 0.702 |
| Left MFG | 45/46 | 1512 | −42 | 45 | 12 | 0.530 |
| Right TPJ/SMG | 40 | 9288 | 48 | −51 | 57 | 0.603 |
| Right SFG/MFG | 8/6 | 2322 | 24 | 9 | 66 | 0.554 |
| Bilateral MPFC | 10 | 6615 | 9 | 69 | 18 | −0.529 |
| Bilateral Calcarine | 19/37 | 20,088 | 24 | −51 | 9 | −0.655 |
| Left PCC/PCu | 23/30 | 1890 | −6 | −57 | 21 | −0.482 |
Notes: BA, Broadmann’s area; x, y, z, coordinates of primary peak locations in the MNI space; R, the coefficient of Pearson’s correlation between regions’ FC and independent (vs interdependent) self-construal scores; PCC, posterior cingulate cortex; PCu, precuneus; IFG, inferior frontal gyrus; MPFC, medial prefrontal cortex; TPJ, temporoparietal junction; SMG, supramarginal gyrus; MFG, middle frontal gyrus; SFG, superior frontal gyrus. P < 0.05, corrected for multiple comparisons.
Fig. 3.
Correlation result of right PCC/PCu’s FC pattern. (A) Correlation map of right PCC/PCu’s positive connectivity pattern showed that FCs to bilateral MPFC were positively correlated with independent (vs interdependent) self-construal score. (B) Correlation map of right PCC/PCu’s negative connectivity pattern showed that FC to right TPJ was negatively correlated with independent (vs interdependent) self-construal score. (C) Scatter plot of peaks in bilateral MPFC showing positive correlation with independent (vs interdependent) self-construal score and peak in right TPJ showing negative correlation with independent (vs interdependent) self-construal score.
Fig. 4.
Correlation result of left IFG’s FC pattern. (A) Correlation map of left IFG’s positive connectivity pattern showed that FCs to bilateral MFG, right TPJ and right SFG were positively correlated with independent (vs interdependent) self-construal score. (B) Correlation map of left IFG’s negative connectivity pattern showed that FCs to bilateral calcarine, left PCC/PCu and bilateral MPFC were negatively correlated with independent (vs interdependent) self-construal score. (C) Scatter plot of peaks in bilateral MFG, right TPJ and right SFG showing positive correlation between their positive FCs and independent (vs interdependent) self-construal score. (D) Scatter plot of peaks in bilateral calcarine, left PCC/PCu and bilateral MPFC showing negative correlation between their negative FCs and independent (vs interdependent) self-construal score.
Regression analysis for the self-construal-related regions
We found that the self-construal related regions mainly located in the DMN (including PCC/PCu and MPFC) and the ECN (including IFG, TPJ, MFG and SFG). Further, we explored the contribution of the connectivity within the networks and the connectivity between the networks in explaining individuals’ independent (vs interdependent) self-construal scores. We averaged the value of the FC within the DMN (i.e. between PCC/PCu and MPFC), the ECN (i.e. between left IFG and bilateral MFG, right TPJ and SFG) and the FC between ECN and DMN (i.e. between right TPJ and bilateral PCC/PCu, and between left IFG and MPFC, calcarine, and PCC/PCu). We entered all these values into the regression as the predictors while entering participants’ age and gender as covariates. This FC pattern explained 71.8% of the variation in the independent (vs interdependent) self-construal scores (change in R2 = 0.718, F(3, 45) =39.57, P< 0.001). We also explored the unique contribution of each predictor. The unique variance explained by the averaged DMN value, the averaged ECN value, and the averaged connectivity value between DMN and ECN was 8.1% (R2 = 0.081, F(1, 45) = 13.39, P = 0.001), 8.8% (R2 = 0.088, F(1, 45) = 14.55, P< 0.001), and 0.1% (R2 = 0.001, F(1, 45) = 0.168, P = 0.684), respectively. The significance level of FC pattern remained consistent after repeating the regression analysis for the three independently defined ROIs (i.e. IFG, MPFC and PCC).
Discussion
In this study, we examined the neural mechanism of the independent vs interdependent self-construal and found that independent vs interdependent self-construal could be greatly decoded from the pattern of functional network connectivity recorded at the rest. Specifically, we found that the FCS in the bilateral PCC/PCu and left IFG was significantly positively correlated with individuals’ independent vs interdependent self-construal scores. Further using seed-based FC analysis, we found that the positive FC within DMN (i.e. between PCC/PCu and MPFC), and that within ECN (i.e. between left IFG and bilateral MFG, right TPJ and SFG) were both positively correlated with the individuals’ independent vs interdependent self-construal scores. The negative FC between DMN and ECN (i.e. between bilateral PCC/PCu and right TPJ and between left IFG and bilateral MPFC, calcarine and PCC/PCu) was negatively correlated with independent vs interdependent self-construal scores. Importantly, these related brain FC patterns jointly explained 71.8% of the variation in the independent vs interdependent self-construal scores.
Whole-brain FCS underlying individual differences in independent self-construal
In the current study, we found that the FCS in the bilateral PCC/PCu and left IFG was significantly positively correlated with individuals’ independent vs interdependent self-construal scores, which means the higher FCS values in these regions, the higher the independent vs interdependent self-construal score is. These findings were consistent with the previous work done by Wang et al. (2013).They found significantly stronger regional homogeneity, which reflects the degree of regional synchronization, in PCC/PCu among participants primed with an independent self than that among those primed with an interdependent self. Greater FCS in PCC, where is found to be important for self-reflection (Johnson et al., 2002) and episodic memory (Hassabis et al., 2007), may promote greater readiness state for high-level inference-based metalizing processes about self (Kelley et al., 2002; Northoff et al., 2006; Uddin et al., 2007; Zhu et al., 2007; Lombardo et al., 2010), which may eventually promote stronger independent self-construal that stresses on the self (Sui and Han, 2007; Ng et al., 2010; Wang et al., 2013).
In addition, we also found that the individuals with higher independent vs interdependent self-construal scores had greater FCS values in the left IFG. Prior work showed that the IFG played an important role in goal execution by controlling our selective attention (e.g. Chong et al., 2008; Paxton et al., 2008; Samanez-Larkin et al., 2009). The task-based fMRI studies found that the activities in the IFG were higher in high cognitive load conditions like introducing auditory or visual distracting cues (Stephan et al., 2003; Wais and Gazzaley, 2011). This implies that the IFG is responsible for attentional modulation that helps to filter task-irrelevant actions during ongoing tasks. The greater FCS values in the left IFG may indicate a greater readiness that allows individuals high in independent self-construal more able to focus on the task by distracting irrelevant cues. The notion was consistent with the findings in the behavioral tasks, in which Westerners from independent cultures were found to be more task-focused, focusing the task while ignoring the distracting irrelevant information, as compared with East Asians from interdependent cultures (e.g. Masuda and Nisbett, 2001; Doherty et al., 2008; Savani and Markus, 2012).
DMN connections underlying individual differences in independent self-construal
The findings showed that individuals with stronger independent vs interdependent self-construal had greater connectivities between PCC/PCu and MPFC, which are the key regions of DMN. Evidence in cultural neuroscience research converged to demonstrate the important role of MPFC in predicting individuals’ self-construal. For instance, Zhu et al. (2007) found that MPFC was important for self- vs other-judgment tasks. Specifically, Westerners from independent cultures had greater MPFC activation when they engaged in self-judgment tasks relative to mother-judgment tasks, which was also observed previously (Kelley et al., 2002; Lieberman et al., 2004; Heatherton et al., 2006); whereas this pattern was diminished among Chinese from interdependent cultures. In addition to the task-based fMRI evidence, similar findings showing that stronger FC in the DMN recorded at the rest were observed (Wang et al., 2013). Taking together, evidence converges to indicate that independent self-construal is highly associated with greater FC in the DMN, which is believed to play an important role in self-generated thoughts like mind-wandering and future thinking (Mason et al., 2007; Schacter et al., 2012) as well as creative idea production (Andrews-Hanna et al., 2014; Beaty et al., 2016).
ECN connections underlying individual differences in independent self-construal
In addition to the findings consistent with the previous work, the current research also discovered some new findings regarding the association between FC and individuals’ self-construal scores. Specifically, we found greater FC in the ECN (i.e. between left IFG and bilateral MFG, right TPJ and SFG) among individuals with stronger independent vs interdependent self-construal. Having similar functions with MFG, the TPJ is known for its involvement in self-agency and self-awareness tasks (Vogeley et al., 2001; Decety and Grezes, 2006), whereas the SFG is known for its involvement in facial self-awareness tasks (Sugiura et al., 2005; Uddin et al., 2005; Platek et al., 2006; Sui et al., 2012). These findings suggest that the ECN may play an important role in self-related processes, which may promote stronger independent vs interdependent self-construal. Supporting this notion, a study using a task related to theory of mind compared children (at the age of 8–11 years) from interdependent and independent cultures (Japanese and Americans) found that the right TPJ with stronger hemodynamic responses was observed in American children (Kobayashi et al., 2007).
The anti-correlation between DMN and ECN underlying individual differences in independent self-construal
We have also explored whether the relationship between DMN and ECN correlates with self-construal. Interestingly, we found that individuals with stronger independent vs interdependent self-construal had a stronger negative FC between DMN and ECN (i.e. between bilateral PCC/PCu and right TPJ and between left IFG and bilateral MPFC, Left MOG and PCC/PCu). Although previous research found the opposite activation patterns in inferior parietal lobule and IFG in the ECN, and MPFC and PCu in the DMN in self-recognition tasks (Uddin et al., 2005), there is still little work in the neuronal synchrony literature thoroughly discussing the role of anti-correlations between the large-scaled networks in the resting state. Some researchers proposed that positive correlations may indicate an integrative relationship, meaning that two networks work together to serve similar goals, whereas anti-correlations may indicate a differentiating relationship, meaning that two networks may serve competing goals (Fox et al., 2005; Buckner et al., 2013; Raichle, 2015). The DMN, involving regions that activate in the absence of external task demands (Greicius et al., 2003; Raichle, 2015), is known to be associated with internally-directed cognitive processes (Mason et al., 2007; Schacter et al., 2012) whereas the ECN is mainly engaged in the cognitive processes that involve externally-directed attention (Curtis and D'Esposito, 2003; Aron, 2007). The anti-correlations were also observed in the prior work (Fox et al., 2005) and the strength of the anti-correlations was found to correlate with other individual characteristics (e.g. cognitive performance; Ng et al., 2016). The weaker anti-correlation (or a rather positive correlation) between DMN and ECN among interdependent people may suggest that DMN and ECN network are more independent from each other among interdependent people. Although the role of anti-correlations between DMN and ECN in self-related processes was rarely studied, the current findings might suggest that interdependent people could more easily shift among different networks while independent people may be better at focusing on one specific task, which is required to be further examined for conclusive results.
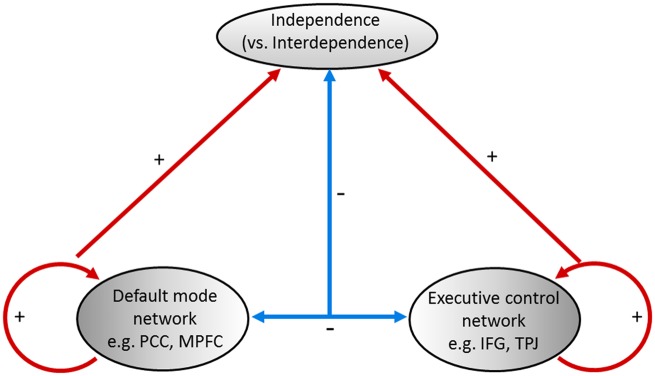
FC architecture underlying individual differences in independent self-construal
We found the evidence for the neural mechanism of the independent vs interdependent self-construal. As we can see from Figure 5, independent self-construal could be greatly predicted by the FC within (1) DMN, (2) ECN and (3) between DMN and ECN. These FC patterns explained 71.8% of the variation in the independent vs interdependent self-construal scores. Recently, cultural psychology research started to explore when people from independent vs interdependent cultures would acquire their cultural characteristics, i.e., when people would demonstrate their own culturally specific behaviors. The age emerging the cultural differences varied, ranging from 4-years-old to 11-years-old, across different behavioral tasks (e.g. Ji, 2008; Kuwabara and Smith, 2012; Ishii et al., 2014; Senzaki et al., 2014). Provided that the neural mechanism underpins the behavioral indicators (Misic and Sporns, 2016), and the high predictive power of neural mechanism suggested by the current study, using the neural indicators in the developmental cultural research may allow us to better detect when the sensitive period of acquiring cultural characteristics is. These findings would be important for understanding the interplay of culture, mind, and the brain (Kitayama and Uskul, 2011; Han, 2015). Moreover, the small unique variance explained by each FC predictor (i.e. DMN, ECN, and DMN and ECN) may imply that the interaction among multiple networks may matter more than any single network, which highlights the importance of investigating neural mechanism of multi-network interaction for understanding independent vs interdependent self-construal. Consistent to this argument, a meta-analysis study (Han and Ma, 2014) showed that cultural differences in social processes are indeed mediated by distinct brain networks. Thus, future research should pay attention to how multiple networks interact in addition to how each network solely works in order to depict the complexity of cultural mind.
Fig. 5.
Prediction model of the independent (vs interdependent) self-construal.
Further considerations
Some issues of the current study should be considered. First, participants of our study were mainly young adults (mean age: 22.22 ± 2.86 years old). As we know, aging could change the organization of human brain network (Damoiseaux et al., 2008; Ystad et al., 2011). Therefore, whether the patterns found in the current research could generalize to other age groups should be investigated in future studies. Second, to avoid the effects of head motion, we used regression method (Friston 24 head motion parameters) at the preprocessing step. Application of Friston-24 model could achieve the best reduction of motion effects among modeling-based approaches (Yan et al., 2013). However, effects of head motion might not be completely eliminated. Next, to improve prediction performance, models that combine data of R-fMRI and other neuroimaging techniques (e.g. structural MRI and diffusion tensor imaging) are encouraged in future research. Moreover, the Cronbach’s α of Independence/Interdependence scale (Singelis, 1994), a widely used scale for self-construal, was not highly satisfactory here, although a low value of Cronbach’s alpha was observed in many of past studies (e.g. Singelis et al., 1999; Ryder et al., 2000; Escalas and Bettman, 2005; Tsai et al., 2006; Kitayama et al., 2009). A more reliable measurement of independence vs interdependence may be needed in future studies to confirm the current findings. Finally, our analyses, suitable methods for investigating task-independent neural substrates, were correlational, which did not allow us to draw any causal conclusions. Further self-priming or transcranial magnetic stimulation studies may be needed for causal evidence.
Supplementary Material
Acknowledgement
This work was supported by the National Natural Science Foundation of China (NSFC) (No. 81601559, 31371129, 71701219, 61772569), Guangdong Provincial Natural Science Foundation of China (No. 2016A030310233), the Fundamental Research Funds for the Central Universities grant (Projects 16wkpy28 and 14wkpy71), Humanities and Social Sciences Foundation of the Ministry of Education of China (No. 16YJC190011).
Supplementary data
Supplementary data are available at SCAN online.
Conflict of interest. None declared.
References
- Adelstein J.S., Shehzad Z., Mennes M., et al. (2011). Personality is reflected in the brain's intrinsic functional architecture. PLoS One, 6(11), e27633.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna J.R., Smallwood J., Spreng R.N. (2014). The default network and self-generated thought: component processes, dynamic control, and clinical relevance. Annals of the New York Academy of Sciences, 1316(1), 29–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron A.R. (2007). The neural basis of inhibition in cognitive control. Neuroscientist, 13(3), 214–28. [DOI] [PubMed] [Google Scholar]
- Ashburner J., Friston K.J. (2005). Unified segmentation. Neuroimage, 26(3), 839–51. [DOI] [PubMed] [Google Scholar]
- Beaty R.E., Kaufman S.B., Benedek M., et al. (2016). Personality and complex brain networks: the role of openness to experience in default network efficiency. Human Brain Mapping, 37(2), 773–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder J.R., Frost J.A., Hammeke T.A., Bellgowan P.S., Rao S.M., Cox R.W. (1999). Conceptual processing during the conscious resting state. A functional MRI study. Journal of Cognitive Neuroscience, 11(1), 80–95. [DOI] [PubMed] [Google Scholar]
- Biswal B., Yetkin F.Z., Haughton V.M., Hyde J.S. (1995). Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magnetic Resonance in Medicine, 34(4), 537–41. [DOI] [PubMed] [Google Scholar]
- Buckner R.L., Krienen F.M., Yeo B.T. (2013). Opportunities and limitations of intrinsic functional connectivity MRI. Nature Neuroscience, 16(7), 832–7. [DOI] [PubMed] [Google Scholar]
- Buckner R.L., Sepulcre J., Talukdar T., et al. (2009). Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer's disease. The Journal of Neuroscience, 29(6), 1860–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore E., Sporns O. (2009). Complex brain networks: graph theoretical analysis of structural and functional systems. Nature Reviews Neuroscience, 10(3), 186–98. [DOI] [PubMed] [Google Scholar]
- Bullmore E., Sporns O. (2012). The economy of brain network organization. Nature Reviews Neuroscience, 13(5), 336–49. [DOI] [PubMed] [Google Scholar]
- Chiao J.Y., Harada T., Komeda H., et al. (2009). Neural basis of individualistic and collectivistic views of self'. Human Brain Mapping, 30(9), 2813–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiao J.Y., Harada T., Komeda H., et al. (2010). Dynamic cultural influences on neural representations of the self. Journal of Cognitive Neuroscience, 22(1), 1–11. [DOI] [PubMed] [Google Scholar]
- Chong T.T., Williams M.A., Cunnington R., Mattingley J.B. (2008). Selective attention modulates inferior frontal gyrus activity during action observation. Neuroimage, 40(1), 298–307. [DOI] [PubMed] [Google Scholar]
- Collignon A., Maes F., Delaere D., Vandermeulen D., Suetens P., Marchal G. (1995). Automated multi-modality image registration based on information theory. Information Processing in Medical Imaging, 3, 263–74. [Google Scholar]
- Cross S.E., Hardin E.E., Gercek-Swing B. (2011). The what, how, why, and where of self-construal. Personality and Social Psychology Review, 15(2), 142–79. [DOI] [PubMed] [Google Scholar]
- Curtis C.E., D'Esposito M. (2003). Persistent activity in the prefrontal cortex during working memory. Trends in Cognitive Sciences, 7(9), 415–23. [DOI] [PubMed] [Google Scholar]
- Dai Z., Yan C., Li K., et al. (2015). Identifying and mapping connectivity patterns of brain network hubs in Alzheimer's disease. Cerebral Cortex, 25(10), 3723–42. [DOI] [PubMed] [Google Scholar]
- Damoiseaux J.S., Beckmann C.F., Arigita E.J., et al. (2008). Reduced resting-state brain activity in the “default network” in normal aging. Cerebral Cortex, 18(8), 1856–64. [DOI] [PubMed] [Google Scholar]
- Davey C.G., Pujol J., Harrison B.J. (2016). Mapping the self in the brain's default mode network. Neuroimage, 132, 390–7. [DOI] [PubMed] [Google Scholar]
- Dawes C.T., Loewen P.J., Schreiber D., et al. (2012). Neural basis of egalitarian behavior. Proceedings of the National Academy of Sciences of the United States of America, 109(17), 6479–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J., Grezes J. (2006). The power of simulation: imagining one's own and other's behavior. Brain Research, 1079(1), 4–14. [DOI] [PubMed] [Google Scholar]
- Doherty M.J., Tsuji H., Phillips W.A. (2008). The context sensitivity of visual size perception varies across cultures. Perception, 37(9), 1426–33. [DOI] [PubMed] [Google Scholar]
- Escalas J.E., Bettman J.R. (2005). Self-construal, reference groups, and brand meaning. Journal of Consumer Research, 32(3), 378–89. [Google Scholar]
- Finn E.S., Shen X., Scheinost D., et al. (2015). Functional connectome fingerprinting: identifying individuals using patterns of brain connectivity. Nature Neuroscience, 18(11), 1664–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M.D., Snyder A.Z., Vincent J.L., Corbetta M., Van Essen D.C., Raichle M.E. (2005). The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA, 102(27), 9673–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius M.D., Krasnow B., Reiss A.L., Menon V. (2003). Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences of the United States of America, 100(1), 253–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn T., Dresler T., Ehlis A.C., et al. (2012). Randomness of resting-state brain oscillations encodes Gray's personality trait. Neuroimage, 59(2), 1842–5. [DOI] [PubMed] [Google Scholar]
- Hahn T., Notebaert K., Anderl C., et al. (2015). Reliance on functional resting-state network for stable task control predicts behavioral tendency for cooperation. Neuroimage, 118, 231–6. [DOI] [PubMed] [Google Scholar]
- Han S. (2015). Understanding cultural differences in human behavior: a cultural neuroscience approach. Current Opinion in Behavioral Sciences, 3, 68–72. [Google Scholar]
- Han S., Ma Y. (2014). Cultural differences in human brain activity: a quantitative meta-analysis. Neuroimage, 99, 293–300. [DOI] [PubMed] [Google Scholar]
- Hassabis D., Kumaran D., Maguire E.A. (2007). Using imagination to understand the neural basis of episodic memory. The Journal of Neuroscience, 27(52), 14365–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton T.F., Wyland C.L., Macrae C.N., Demos K.E., Denny B.T., Kelley W.M. (2006). Medial prefrontal activity differentiates self from close others. Social Cognitive and Affective Neuroscience, 1(1), 18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z., Obara N., Davis H. H t., Pokorny J., Northoff G. (2016). The temporal structure of resting-state brain activity in the medial prefrontal cortex predicts self-consciousness. Neuropsychologia, 82, 161–70. [DOI] [PubMed] [Google Scholar]
- Ishii K., Miyamoto Y., Rule N.O., Toriyama R. (2014). Physical objects as vehicles of cultural transmission: maintaining harmony and uniqueness through colored geometric patterns. Personality and Social Psychology Bulletin, 40(2), 175–88. [DOI] [PubMed] [Google Scholar]
- Ji L.J. (2008). The leopard cannot change his spots, or can he? Culture and the development of lay theories of change. Personality and Social Psychology Bulletin, 34(5), 613–22. [DOI] [PubMed] [Google Scholar]
- Johnson S.C., Baxter L.C., Wilder L.S., Pipe J.G., Heiserman J.E., Prigatano G.P. (2002). Neural correlates of self-reflection. Brain 125(Pt 8), 1808–14. [DOI] [PubMed] [Google Scholar]
- Kelley W.M., Macrae C.N., Wyland C.L., Caglar S., Inati S., Heatherton T.F. (2002). Finding the self? An event-related fMRI study. Journal of Cognitive Neuroscience, 14(5), 785–94. [DOI] [PubMed] [Google Scholar]
- Kim H., Markus H.R. (1999). Deviance or uniqueness, harmony or conformity? A cultural analysis. Journal of Personality and Social Psychology, 77(4), 785. [Google Scholar]
- Kitayama S., Park H., Sevincer A.T., Karasawa M., Uskul A.K. (2009). A cultural task analysis of implicit independence: comparing North America, Western Europe, and East Asia. Journal of Personality and Social Psychology, 97(2), 236–55. [DOI] [PubMed] [Google Scholar]
- Kitayama S., Uskul A.K. (2011). Culture, mind, and the brain: current evidence and future directions. Annual Review of Psychology, 62, 419–49. [DOI] [PubMed] [Google Scholar]
- Kobayashi C., Glover G.H., Temple E. (2007). Cultural and linguistic effects on neural bases of ′Theory of Mind′ in American and Japanese children. Brain Research, 1164, 95–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunisato Y., Okamoto Y., Okada G., et al. (2011). Personality traits and the amplitude of spontaneous low-frequency oscillations during resting state. Neuroscience Letters, 492(2), 109–13. [DOI] [PubMed] [Google Scholar]
- Kuwabara M., Smith L.B. (2012). Cross-cultural differences in cognitive development: attention to relations and objects. Journal of Experimental Child Psychology, 113(1), 20–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman M.D., Jarcho J.M., Satpute A.B. (2004). Evidence-based and intuition-based self-knowledge: an FMRI study. Journal of Personality and Social Psychology, 87(4), 421–35. [DOI] [PubMed] [Google Scholar]
- Lombardo M.V., Chakrabarti B., Bullmore E.T., et al. (2010). Atypical neural self-representation in autism. Brain, 133(2), 611–24. [DOI] [PubMed] [Google Scholar]
- Luo S., Ma Y., Liu Y., et al. (2015). Interaction between oxytocin receptor polymorphism and interdependent culture values on human empathy. Social Cognitive and Affective Neuroscience, 10(9), 1273–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y., Bang D., Wang C., et al. (2014). Sociocultural patterning of neural activity during self-reflection. Cognitive and Affective Neuroscience, 9(1), 73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markus H.R., Kitayama S. (1991). Culture and the self: implications for cognition, emotion, and motivation. Psychological Review, 98(2), 224. [Google Scholar]
- Markus H.R., Kitayama S. (2010). Cultures and selves: a cycle of mutual constitution'. Perspectives on Psychological Science, 5(4), 420–30. [DOI] [PubMed] [Google Scholar]
- Mason M.F., Norton M.I., Van Horn J.D., Wegner D.M., Grafton S.T., Macrae C.N. (2007). Wandering minds: the default network and stimulus-independent thought. Science, 315(5810), 393–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda T., Nisbett R.E. (2001). Attending holistically versus analytically: comparing the context sensitivity of Japanese and Americans. Journal of Personality and Social Psychology, 81(5), 922–34. [DOI] [PubMed] [Google Scholar]
- Miller J.G., Bersoff D.M., Harwood R.L. (1990). Perceptions of social responsibilities in India and in the United States: moral imperatives or personal decisions? Journal of Personality and Social Psychology, 58(1), 33–47. [DOI] [PubMed] [Google Scholar]
- Misic B., Sporns O. (2016). From regions to connections and networks: new bridges between brain and behavior. Current Opinion in Neurobiology, 40, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin A., Michaud J. (2007). Self-awareness and the left inferior frontal gyrus: inner speech use during self-related processing. Brain Research Bulletin, 74(6), 387–96. [DOI] [PubMed] [Google Scholar]
- Murphy K., Birn R.M., Handwerker D.A., Jones T.B., Bandettini P.A. (2009). The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage, 44(3), 893–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray R.J., Debbane M., Fox P.T., Bzdok D., Eickhoff S.B. (2015). Functional connectivity mapping of regions associated with self- and other-processing. Human Brain Mapping, 36(4), 1304–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng S.H., Han S., Mao L., Lai J.C. (2010). Dynamic bicultural brains: fMRI study of their flexible neural representation of self and significant others in response to culture primes. Asian Journal of Social Psychology, 13(2), 83–91. [Google Scholar]
- Ng K.K., Lo J.C., Lim J.K.W., Chee M.W.L., Zhou J. (2016). Reduced functional segregation between the default mode network and the executive control network in healthy older adults: a longitudinal study. Neuroimage, 133, 321–30. [DOI] [PubMed] [Google Scholar]
- Northoff G. (2016) How does the ′rest-self overlap′ mediate the qualitative and automatic features of self-reference? Journal of Cognitive Neuroscience, 7(1-4), 18. [DOI] [PubMed] [Google Scholar]
- Northoff G., Heinzel A., de Greck M., Bermpohl F., Dobrowolny H., Panksepp J. (2006). Self-referential processing in our brain—a meta-analysis of imaging studies on the self. Neuroimage, 31(1), 440–57. [DOI] [PubMed] [Google Scholar]
- Paxton J.L., Barch D.M., Racine C.A., Braver T.S. (2008). Cognitive control, goal maintenance, and prefrontal function in healthy aging. Cerebral Cortex, 18(5), 1010–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platek S.M., Loughead J.W., Gur R.C., et al. (2006). Neural substrates for functionally discriminating self-face from personally familiar faces. Human Brain Mapping, 27(2), 91–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin P., Grimm S., Duncan N.W., et al. (2016). Spontaneous activity in default-mode network predicts ascription of self-relatedness to stimuli. Social Cognitive and Affective Neuroscience, 11(4), 693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin P., Northoff G. (2011). How is our self related to midline regions and the default-mode network? Neuroimage, 57(3), 1221–33. [DOI] [PubMed] [Google Scholar]
- Raichle M.E. (2015). The brain's default mode network. Annuual Review of Neuroscience, 38, 433–47. [DOI] [PubMed] [Google Scholar]
- Ryder A.G., Alden L.E., Paulhus D.L. (2000). Is acculturation unidimensional or bidimensional? A head-to-head comparison in the prediction of personality, self-identity, and adjustment. Journal of Personality and Social Psychology, 79(1), 49.. [DOI] [PubMed] [Google Scholar]
- Saad Z.S., Gotts S.J., Murphy K., et al. (2012). Trouble at rest: how correlation patterns and group differences become distorted after global signal regression. Brain Connect, 2(1), 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanez-Larkin G.R., Robertson E.R., Mikels J.A., Carstensen L.L., Gotlib I.H. (2009). Selective attention to emotion in the aging brain. Psychology and Aging, 24(3), 519–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savani K., Markus H.R. (2012). A processing advantage associated with analytic perceptual tendencies: european Americans outperform Asians on multiple object tracking. Journal of Experimental Social Psychology, 48(3), 766–9. [Google Scholar]
- Schacter D.L., Addis D.R., Hassabis D., Martin V.C., Spreng R.N., Szpunar K.K. (2012). The future of memory: remembering, imagining, and the brain. Neuron, 76(4), 677–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senzaki S., Masuda T., Ishii K. (2014). When is perception top-down and when is it not? culture, narrative, and attention. Cognitive Science, 38(7), 1493–506. [DOI] [PubMed] [Google Scholar]
- Singelis T.M. (1994). The measurement of independent and interdependent self-construals. Personality and Social Psychology Bulletin, 20(5), 580–91. [Google Scholar]
- Singelis T.M., Bond M.H., Sharkey W.F., Lai C.S.Y. (1999). Unpackaging culture's influence on self-esteem and embarrassability: the role of self-construals. Journal of Cross-Cultural Psychology, 30(3), 315–41. [Google Scholar]
- Song X.W., Dong Z.Y., Long X.Y., et al. (2011). REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PLoS One, 6(9), e25031.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan K.E., Marshall J.C., Friston K.J., et al. (2003). Lateralized cognitive processes and lateralized task control in the human brain. Science, 301(5631), 384–6. [DOI] [PubMed] [Google Scholar]
- Sugiura M., Watanabe J., Maeda Y., Matsue Y., Fukuda H., Kawashima R. (2005). Cortical mechanisms of visual self-recognition. Neuroimage, 24(1), 143–9. [DOI] [PubMed] [Google Scholar]
- Sui J., Chechlacz M., Humphreys G.W. (2012). Dividing the self: distinct neural substrates of task-based and automatic self-prioritization after brain damage. Cognition, 122(2), 150–62. [DOI] [PubMed] [Google Scholar]
- Sui J., Han S. (2007). Self-construal priming modulates neural substrates of self-awareness. Psychological Science, 18(10), 861–6. [DOI] [PubMed] [Google Scholar]
- Tsai J.L., Knutson B., Fung H.H. (2006). Cultural variation in affect valuation. Journal of Personality and Social Psychology, 90(2), 288–307. [DOI] [PubMed] [Google Scholar]
- Uddin L.Q., Iacoboni M., Lange C., Keenan J.P. (2007). The self and social cognition: the role of cortical midline structures and mirror neurons. Trends in Cognitive Sciences, 11(4), 153–7. [DOI] [PubMed] [Google Scholar]
- Uddin L.Q., Kaplan J.T., Molnar-Szakacs I., Zaidel E., Iacoboni M. (2005). 'elf-face recognition activates a frontoparietal “mirror” network in the right hemisphere: an event-related fMRI study. Neuroimage, 25(3), 926–35. [DOI] [PubMed] [Google Scholar]
- van den Heuvel M.P., Stam C.J., Kahn R.S., Hulshoff Pol H.E. (2009). Efficiency of functional brain networks and intellectual performance. The Journal of Neuroscience, 29(23), 7619–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogeley K., Bussfeld P., Newen A., et al. (2001). Mind reading: neural mechanisms of theory of mind and self-perspective. Neuroimage, 14(1 Pt 1), 170–81. [DOI] [PubMed] [Google Scholar]
- Wais P.E., Gazzaley A. (2011). The impact of auditory distraction on retrieval of visual memories. Psychonomic Bulletin and Review, 18(6), 1090–7. [DOI] [PubMed] [Google Scholar]
- Wang C., Oyserman D., Liu Q., Li H., Han S. (2013). Accessible cultural mind-set modulates default mode activity: evidence for the culturally situated brain. Society for Neuroscience, 8(3), 203–16. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S., Moran J.M., Nieto-Castañón A., Triantafyllou C., Saxe R., Gabrieli J.D.E. (2011). Associations and dissociations between default and self-reference networks in the human brain. Neuroimage, 55(1), 225–32. [DOI] [PubMed] [Google Scholar]
- Xia M., Wang J., He Y. (2013). BrainNet Viewer: a network visualization tool for human brain connectomics. PLoS One, 8(7), e68910.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C.G., Cheung B., Kelly C., et al. (2013). A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. Neuroimage, 76, 183–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C.G., Zang Y.F. (2010). DPARSF: a MATLAB Toolbox for “Pipeline” Data Analysis of Resting-State fMRI. Frontiers in Systems Neuroscience, 4, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G.J., Murray J.D., Repovs G., et al. (2014). Altered global brain signal in schizophrenia. Proceedings of the National Academy of Sciences of the United States of America, 111(20), 7438–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ystad M., Hodneland E., Adolfsdottir S., et al. (2011). Cortico-striatal connectivity and cognition in normal aging: a combined DTI and resting state fMRI study. Neuroimage, 55(1), 24–31. [DOI] [PubMed] [Google Scholar]
- Zhu Y., Zhang L., Fan J., Han S. (2007). Neural basis of cultural influence on self-representation. Neuroimage, 34(3), 1310–6. [DOI] [PubMed] [Google Scholar]
- Zuo X.N., Ehmke R., Mennes M., et al. (2012). Network centrality in the human functional connectome. Cerebral Cortex, 22(8), 1862–75. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.