Abstract
Background
Radical prostatectomy (RP) is most commonly performed laparoscopically with a robot (robotic-assisted laparoscopic radical prostatectomy, R/PROST). Hysterectomy, which may be open hysterectomy (O/HYST), or laparoscopic (L/HYST), has been increasingly frequently done via robot (R/HYST). Small case series suggest increased corneal abrasions (CA) with less invasive techniques.
Methods
We identified RP (166,942), O/HYST (583,298), or L/HYST (216,890) discharges with CA in the Nationwide Inpatient Sample (2000–2011). For 2009–11, we determined odds ratios (OR) and 95% confidence intervals (CI) for CA, in R/PROST, non-R/PROST, L/HYST, O/HYST, and R/HYST. Uni- and multivariate models studied CA risk depending upon surgical procedure, age, race, year, chronic illness, and malignancy.
Results
In 2000–11, 0.18% RP, 0.13% L/HYST, and 0.03% O/HYST sustained CA. Compared to 17,554 non-R/PROSTs (34 abrasions, 0.19%) in 2009–11, OR was not significantly higher in 28,521 R/PROSTs (99, 0.35%; OR 1.508, CI 0.987–2.302, P < 0.057). CA significantly increased in L/HYST (70/51,323; 0.136%) vs O/HYST (70/191,199; 0.037%, OR 3.821, CI 2.594–5.630, P < 0.0001), further increasing in R/HYST (63/21, 213; 0.297%, OR 6.505, CI 4.323–9.788, P < 0.0001). For hysterectomy, risk of CA increased with age (OR 1.020, CI 1.007–1.034, P < 0.003), and number of chronic conditions (OR 1.139, CI 1.065–1.219, P < 0.0001). CA risk was likewise elevated in R/HYST with number of chronic conditions. Being African-American significantly decreased CA risk in R/PROST and in R/ or L/HYST.
Conclusions
L/HYST increased CA nearly 4-fold, and R/HYST about 6.5-fold vs O/HYST. Identifiable preoperative factors are associated with either increased risk (age, chronic conditions) or decreased risk (race).
Introduction
Prostate cancer, the most common non-skin male cancer in the United States, is also the second most frequent cancer death. Radical prostatectomy (RP) is conventional for clinically-localized cancer. Robotic-assisted laparoscopic radical prostatectomy (R/PROST) has advantages of shortened hospital stay, and decreased blood loss, morbidity, and post-operative analgesia compared to open prostatectomy (OP).1
Hysterectomy has been performed for decades, for both malignant and benign disease, and in one in three women by age 60.2 With faster recovery, less pain, and fewer overall complications, robotically-assisted laparoscopic hysterectomy (R/HYST) has increased dramatically, although without clear benefit for benign disease.3 In malignancy, initial outcomes of robotic surgery are encouraging.4,5
Retro- or prospective studies of anesthetic complications are lacking in R/HYST, but reported challenges of R/PROST are altered respiratory mechanics, laryngeal edema, and brachial plexus injuries.1 In a small study of 1,500 R/PROST patients, corneal abrasion (CA) was the most reported anesthesia-related complication (3%).6 Since R/HYST and R/PROST share steep head down positioning, pneumo-peritoneum, and a surgical learning curve, among other similarities, we hypothesized a similar incidence of eye-related complications.7,8
We aimed to determine the incidence of CA following prostatectomies and hysterectomies, to test the hypothesis that laparoscopic or robot assistance increases risk, and to examine risk factors. Rapid introduction of the robot for RP rendered it difficult to study the influence of laparoscopy vs robotic assistance on CA. However, hysterectomy, a similar procedure, is easily identifiable as open, laparoscopic, or robotic assisted. We used the Nationwide Inpatient Sample (NIS), a large administrative discharge database that allows study of low frequency events.9 CA is painful and disturbing, and has been targeted for anesthesia performance improvement as potentially preventable.10 With robotically-assisted surgery increasing, it is important to understand the increased risks of these unexpected, and painful perioperative complications.
Materials and Methods
Data Sources
Data were extracted for 2000–2011 from the NIS, the largest United States all-payer hospital inpatient database. With over 1,000 randomly selected hospitals in 44 states, it is an approximately 20% stratified sample, including public hospitals and academic medical centers. As part of the Healthcare Cost and Utilization Project (HCUP), NIS is maintained by the Agency for Health Care Research and Quality. Hospitals and discharges are weighted based on the number of hospitals and discharges in the database.i
A typical hospital discharge includes patient demographics (age, sex, race), diagnoses (principal and ≤ 14 secondary), procedures (principal and ≤14 secondary), charges, length of stay, discharge status, outcomes, and number of chronic conditions.ii Diagnosis and procedure data are coded using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). Quality control and reliability of the NIS have been examined each year since 2000.iii National estimates of essential health care parameters in the NIS were precise and accurate compared with the American Hospital Association Annual Survey, National Hospital Discharge Survey, and Medicare Inpatient data.iv Our Institutional Review Board deemed the study “exempt.”
Population of interest and surgical procedure
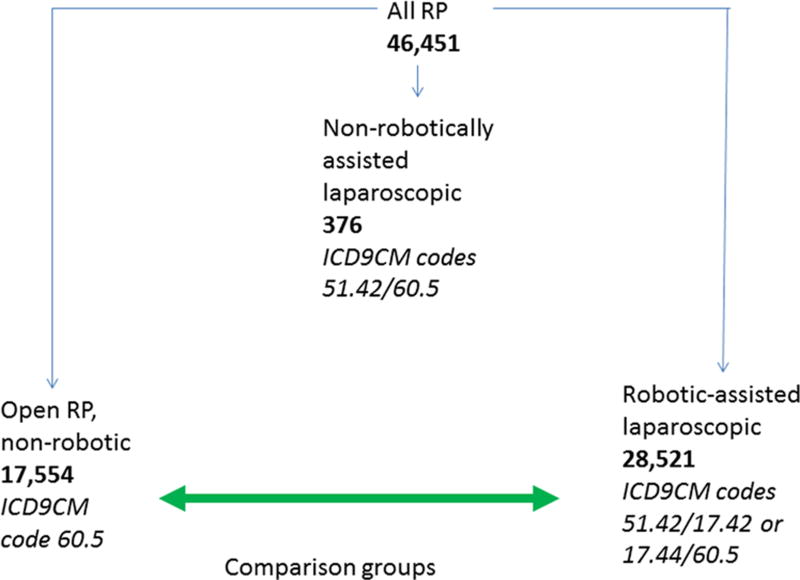
Discharges with RP, open hysterectomy (O/HYST), or laparoscopic hysterectomy (L/HYST) were identified using ICD-9-CM procedure codes (table 1), and confirmed with Encoder Pro.com (Optum, Salt Lake City, UT). While the L/HYST codes were specific, to identify laparoscopic RP required modifier code 51.42. However, most of the RPs in NIS from 2009–11 were either OP or robotic assisted, with few identifiable as laparoscopic, without robotic assistance. Because the number of laparoscopic, non-robotically assisted RPs in NIS was only 376, we eliminated these for insufficient sample size (fig. 1). As recognized by the National Center of Health Statistics and the Centers for Medicare and Medicaid Services, beginning October 1, 2008, the robot-assisted modifier codes (ICD-9-CM 17.42 and 17.44) were introduced to specifically identify R/PROST as well as R/HYST. Thus, discharges with procedure codes for both RP, and the robot-assisted modifiers were classified as R/PROST, while those that only had the procedure code for RP as non-R/PROST, i.e., OP (table 1 and fig. 1). L/HYST discharges that contained 17.42 or 17.44 codes were classified as R/HYST. Since the robotic modifier code was introduced in late 2008, we limited robotic modifier codes to 2009– 2011. At the time of our analysis, 2011 was the most current database. CA was identified as ICD-9-CM 371.20.
Table 1.
ICD-9-CM Procedure Codes for Prostatectomy, Laparoscopic, and Open Hysterectomy
| Procedure Code | Description |
|---|---|
| 60.5 | Radical prostatectomy |
| 68.31 | Laparoscopic supracervical hysterectomy |
| 68.41 | Laparoscopic total abdominal hysterectomy |
| 68.51 | Laparoscopic vaginal hysterectomy |
| 68.61 | Laparoscopic radical abdominal hysterectomy |
| 68.71 | Laparoscopic radical vaginal hysterectomy |
| 68.39* | Other and unspecified subtotal abdominal hysterectomy |
| 68.49* | Other and unspecified total abdominal hysterectomy |
| 68.59* | Other and unspecified vaginal hysterectomy |
| 68.69* | Other and unspecified radical abdominal hysterectomy |
| 68.79* | Other and unspecified radical vaginal hysterectomy |
The codes 68.39, 68.49, 68.59, 68.69, and 68.79 corresponded to open hysterectomy. Addition of the robotic modifier codes 17.42 and 17.44 allowed identification of discharges overlapping the 60.5 codes, and the laparoscopic hysterectomy codes, where robotic assistance was used. The code 51.42 was used for radical prostatectomy to identify laparoscopic assistance. See figure 1 for further explanation of the coding for prostatectomy.
ICD-9-CM = International Classification of Diseases, Ninth Revision, Clinical Modification
Figure 1.
Selection of discharges for radical prostatectomy
Radical prostatectomy (RP) discharges were segregated according to ICD-9-CM (International Classification of Diseases, Ninth Revision, Clinical Modification) codes. The ICD-9-CM codes and the number of discharges are shown. Because there were few cases of prostatectomy using laparoscopy alone, we narrowed our study to a comparison of open vs robotically-assisted laparoscopic prostatectomy
Comparisons of Laparoscopic, and Robotic Assisted Prostatectomies or Hysterectomies
We extracted all discharges containing R/PROST and non-R/PROST, R/HYST, L/HYST, and O/HYST in 2009–2011, and those that also had discharge codes corresponding to CA. We calculated CA incidence and the number needed to harm (NNH); also, we compared age, race, and number of chronic medical conditions between the groups, as we previously reported.9 Odds ratios (OR) were calculated by univariate and multivariable analysis. Moreover, within univariate and multivariable analysis, we assessed R/HYST and L/HYST within a categorical variable “Modified Hysterectomy” having two levels for each procedure variant. For hysterectomy, cases were segregated as malignant vs non-malignant using ICD-9-CM codes: 180 (carcinoma of the cervix), 182 (carcinoma of the uterus), and 183 (carcinoma of the fallopian tubes and broad ligament).
Statistical Analysis
Data were analyzed with STATA v13.0 (Stata Corporation, College Station, TX). All R/PROST and OP discharges, and separately, those discharges with diagnostic codes for CA were compared between groups for mean age and number of chronic conditions using an independent sample, 2-tailed t test, and proportions of distribution according to race (White, African American) using chi square, and P < 0.05 significant. For R/HYST, L/HYST, and O/HYST, 3-way comparisons used ANOVA with Bonferroni correction for three comparisons to compare mean age and mean number of chronic conditions; P < 0.05 was significant. Chi square assessed race distributions, and the presence of malignancy, and were corrected for multiple pairwise comparisons with P < 0.05/3 = 0.017.
To examine factors increasing CA risk in RP and hysterectomy, we first used univariate analysis. As prostatectomy and hysterectomy are distinct procedures on opposite genders, we maintained separate analyses. Age, number of chronic conditions, race, surgery year, malignancy diagnosis, and robotic or laparoscopic procedure were examined. For univariate analysis, P < 0.2 was the cutoff for entering parameters into the subsequent multiple regression analysis.
Collinearity tests were performed on remaining candidate parameters. Continuous, increasing variables including age and number of chronic conditions were compared through Pearson correlations, and discrete variables including malignant, robotic, laparoscopic, and race were compared using chi-square. Unpaired t-tests compared continuous and discrete variables. Correlations above 0.5 with P < 0.05 were deemed significant, as were P-values < 0.05 for unpaired t-tests and chi-2 tests.
Multiple logistic regression was performed using procedure modifiers, and the additional covariates age, number of chronic conditions, malignancy, race, and year in RP, and separately in hysterectomy. These models thus considered the impact of surgical technique on CA in the context of all the background covariates, effectively controlling for differences between group compositions. Forward and backward elimination yielded the same results. An area under the receiver operating characteristic (ROC) curve > 0.5 and Hosmer and Lemeshow P > 0.05 were the thresholds for confirming the goodness of fit, and validity of these models, respectively.
Results
The overall incidence of corneal abrasion in radical prostatectomy and hysterectomy
NIS contained 166,942 RP discharges from 2000–2011, with 295 CAs (0.18%, 1.8 per thousand, (table 2). The total number of CAs during this same time period increased; in 2000 there were < 10 discharges with CA (no more than 0.08%, 0.8 per thousand), while in 2011 there were 46 (0.28%, 2.8 per thousand), an increase of about 3-fold. (NIS does not permit reporting of research results with individual values < 10, hence, these are expressed as “<10”). We identified 216, 890 L/HYST discharges from 2000–2011, amongst them were 275 with CA, 0.13%, or 1.3 per thousand. There were less than 10 CAs in 2000 (≤ 0.09%, or 0.9 per thousand), and 55 (0.21%, 2.1 per thousand), an approximate 2-fold increase, in 2011 (table 2). The rate of CA in O/HYST remained stable during 2000–11, an overall rate of 0.03%, or 0.3 per thousand (table 2).
Table 2.
Yearly rates of corneal abrasion in radical prostatectomy (RP), laparoscopic hysterectomy (L/Hyst), and open hysterectomy (O/Hyst) in the Nationwide Inpatient Sample (NIS) from 2000 to 2011
| 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | TOTAL | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RP | 12,045 | 12,487 | 13,833 | 12,290 | 11,482 | 11,185 | 13,512 | 16,042 | 17,991 | 15,614 | 13,770 | 16,691 | 166,942 |
| Corneal Abrasion | <10 | <10 | <10 | <10 | 12 | 17 | 30 | 40 | 39 | 47 | 40 | 46 | 295 |
| % | <0.08 | <0.08 | <0.07 | <0.08 | 0.10 | 0.15 | 0.22 | 0.25 | 0.22 | 0.30 | 0.29 | 0.28 | 0.18 |
| L/Hyst | 11,469 | 12,171 | 13,193 | 14,319 | 15,638 | 15,961 | 17,718 | 21,190 | 22,785 | 23,041 | 23,467 | 25,938 | 216,890 |
| Corneal Abrasion | <10 | 15 | <10 | <10 | <10 | 14 | 14 | 30 | 36 | 40 | 38 | 55 | 275 |
| % | <0.09 | 0.12 | <0.08 | <0.07 | <0.06 | 0.09 | 0.08 | 0.14 | 0.16 | 0.17 | 0.16 | 0.21 | 0.13 |
| O/Hyst | 28,493 | 26,733 | 28,698 | 27,401 | 32,420 | 31,847 | 44,981 | 89,109 | 82,417 | 73,168 | 63,073 | 54,958 | 583,298 |
| Corneal | <10 | <10 | <10 | <10 | <10 | <10 | 16 | 35 | 32 | 24 | 23 | 23 | 189 |
| % | 0.04 | 0.04 | 0.03 | 0.04 | 0.03 | 0.03 | 0.04 | 0.04 | 0.04 | 0.03 | 0.04 | 0.04 | 0.03 |
We used ICD-9-CM (International Classification of Diseases, Ninth Revision, Clinical Modification) codes to identify all discharges with RP, L/HYST, and O/HYST. Note that for results less than 10, NIS does not permit exact numerical reporting.
Patient characteristics and incidence of corneal abrasion in laparoscopy and/or robotic assistance vs open or non-robotically assisted surgery
In 2009–2011, of 28,521 discharges with R/PROST, there were 99 CAs (3.5 per thousand); whilst 17,554 discharges with OP had 34 CAs (1.9 per thousand, table 3). Among all discharges with R/PROST or OP, the latter had a greater mean number of chronic conditions (P < 0.0001 by unpaired t test), and a higher proportion of African-Americans (P < 0.0001 by chi square). Patients undergoing R/PROST and OP who developed CA were not significantly different in age, number of chronic conditions, or race, (table 3).
Table 3.
Characteristics, all Patients, and for those specifically with Corneal Abrasion in Prostatectomy or Hysterectomy, 2009–2011
| Open prostatectomy (OP) |
Robotic prostatectomy (R/Prost) |
P (OP vs R/Prost) |
Robotic Hysterectomy (R/Hyst) |
Laparoscopic Hysterectomy (L/Hyst) |
Open Hysterectomy (O/Hyst) ‡ |
P (3-way Hysterectomy comparisons, ANOVA) |
||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| All patients: Number of discharges | 17,554 | 28,521 | -- | 21,123 | 51,323 | 191,199 | -- | |||
|
| ||||||||||
| Mean age: all patients (95% CI) | 61.4 (61.3–61.5) | 61.5 (61.4–61.6) | 0.1165 | 50.7*† (50.6–50.9) | 46.0* (45.9–46.1) | 48.7 (48.6–48.7) | 0.0001 | |||
|
| ||||||||||
| Average # of chronic conditions: all patients (95% CI) | 3.4 (3.4–3.4) | 3.3 (3.2–3.3) | 0.0001 | 3.2*† (3.1–3.2) | 2.8* (2.8–2.9) | 3.0 (3.0–3.0) | 0.0001 | |||
|
| ||||||||||
| White (%): all | 11,772 (76.3) | 19,354 (77.3) | 0.0001 | 13,367 (72.7) | 32,282 (72.5) | 104,476 (62.7) | 0.0001 | |||
|
|
|
|
||||||||
| AA (%): all | 2,047 (13.3) | 2,772 (11.1) | 2,014 (11.0) | * | 5,036 (11.3) | * | 30,434 (18.3) | * | ||
|
| ||||||||||
| Benign (%):‡ all | 16,209 (76.7) | * | 48,332 (94.2) | 169,284 (88.5) | 0.0001 | |||||
|
|
|
|
||||||||
| Malignant (%): all | 4,914 (23.3) | † | 2,991 (5.8) | * | 21,915 (11.5) | |||||
|
| ||||||||||
| CA patients (per 1000) | 34 (1.9/1000) | 99 (3.5/1000) | 63 (3.0/1000) | 70 (1.4/1000) | 70 (0.4/1000) | |||||
|
| ||||||||||
| NNH for CA | 652 | 382 | 1002 | |||||||
|
| ||||||||||
| Mean age: CA (95% CI) | 62.4 (60.3–64.4) | 61.8 (60.5–63.2) | 0.6846 | 54.0 (50.8–57.2) | 52.0 (49.3–54.7) | 54.9 (52.0–57.7) | 0.354 | |||
|
| ||||||||||
| Average # of chronic conditions: CA (95% CI) | 3.3 (2.6–3.9) | 3.2 (2.8–3.7) | 0.9371 | 3.5 ** (3.0–4.0) | 3.5 £ (3.1–4.0) | 4.6 (4.0–5.2) | 0.0053 | |||
|
| ||||||||||
| White (%):‡ CA | 31 (96.9) | 75 (90.4) | 0.510 | 45 (84.9) | 49 (80.3) | 50 (86.2) | 0.635 | |||
|
|
||||||||||
| AA (%): CA | 0 (0.0) | 1 (1.2) | 1 (1.9) | 4 (6.6) | 2 (3.5) | |||||
|
| ||||||||||
| Benign (%):‡ CA | 40 (63.5) | 63 (90.0) | 56 (80.0) | 0.001 | ||||||
|
|
|
|||||||||
| Malignant (%): CA | 23 (36.5) | † | 7 (10.0) | 14 (20.0) | ||||||
p < 0.001 vs O/Hyst;
p < 0.001 vs L/Hyst;
P < 0.013 vs O/Hyst;
P < 0.018 vs O/Hyst:
reference group
Age and number of chronic conditions were compared between groups using unpaired t test or ANOVA with a Bonferroni correction for multiple comparisons. Race distribution was compared using Chi square. Pairwise comparisons between the groups used ANOVA with built-in Bonferroni and P < 0.05 significant; or chi square, corrected for multiple comparisons, with P < 0.017 significant between groups. The P values columns show statistical significance using unpaired t test, ANOVA, or chi square. Among all discharges with R/Prost or OP, OP had significantly greater mean number of chronic conditions and a higher proportion of African-Americans (AA). Among all Hyst, R/Hyst patients were older, had higher number of chronic conditions, a higher proportion of Whites, and of malignancy. Patients undergoing R/Prost and OP who developed CA were not significantly different in age, number of chronic conditions, or race. Patients undergoing R/Hyst and L/Hyst who developed CA had fewer number of chronic conditions vs O/Hyst; there was a significantly higher proportion of patients with malignancy in those undergoing R/Hyst vs L/Hyst.
ANOVA = analysis of variance; CA = corneal abrasion; CI = 95% confidence interval; L/Hyst = laparoscopic hysterectomy; NNH= number needed to harm; O/Hyst = open hysterectomy; OP = open prostatectomy; R/Hyst= robotic assisted laparoscopic hysterectomy; R/Prost = robotic assisted laparoscopic radical prostatectomy.
In hysterectomy, among 191,199 O/HYST, there were 70 CAs (0.4 per thousand) while in L/HYST, there were 70 in 51,323 (1.4 per thousand), and when robotic assistance (R/HYST) was used, CA was in 63 of 21,123 (3.0 per thousand, table 3). R/HYST patients were significantly older and had greater average number of chronic conditions vs O/HYST and L/HYST, and contained a lower proportion of African-Americans compared to O/HYST (table 3). Moreover, L/HYST discharges were significantly younger, had lower average number of chronic conditions, a lower proportion of African-Americans, and lower proportion of malignancy compared to O/HYST. R/HYST had significantly higher proportions of malignancy diagnoses compared to the other two groups. Among those discharges with CA, those who underwent R/HYST or L/HYST had fewer chronic conditions compared to O/HYST (P < 0.013 and P < 0.018, respectively); by repeated pairwise chi square testing, those with CA who underwent R/HYST consisted of a higher proportion of diagnoses of malignancy compared to L/HYST (table 3).
Univariate analysis to identify risk factors for corneal abrasion
Univariate analysis (table 4) identified factors increasing risk for inclusion in multivariable analysis; terms with P < 0.2 were considered significant for inclusion in the final models (table 5). For RP, being African American conferred lower risk (OR 0.06, 95% CI 0.01–0.44, P < 0.005) while robotic use increased risk (OR 1.55, 95% CI 1.01–2.36, P < 0.043).
Table 4.
Univariate Analysis of Risk Factors for Corneal Abrasion, 2009–2011
| Coding of the Variables (0 or 1, as labeled) |
Prostatectomy* | Hysterectomy‡ | |||||
|---|---|---|---|---|---|---|---|
| OR | CI | P | OR | CI | P | ||
| Age | Continuous, increasing | 1.01 | 0.99–1.04 | 0.362 | 1.03 | 1.02–1.04 | 0.0001 |
| # of Chronic Conditions | Continuous, increasing | 1.00 | 0.90–1.10 | 0.927 | 1.20 | 1.11–1.26 | 0.0001 |
| Race | 0 Caucasian; 1 African-American | 0.06 | 0.01–0.44 | 0.005 | 0.19 | 0.09–0.42 | 0.0001 |
| Year | Continuous, increasing | 0.94 | 0.75–1.18 | 0.600 | 1.27 | 1.05–1.55 | 0.015 |
| Malignancy | 0 Benign; 1 Malignant | -- | -- | -- | 2.09 | 1.41–3.08 | 0.001 |
| Robotic prostatectomy (R/Prost) | 0 Prostatectomy, open; 1 R/Prost | 1.55 | 1.01–2.36 | 0.043 | |||
| Modified hysterectomy | 0 O/L/Hyst; 1 R/Hyst; 2 L/Hyst | ||||||
| R/Hyst (1) vs O/Hyst (0) | 7.78 | 5.23–11.57 | 0.0001 | ||||
| L/Hyst (2) vs O/Hyst (0) | 3.69 | 2.52–5.41 | 0.0001 | ||||
Open prostatectomy is the reference group;
Open hysterectomy is the reference group
Univariate analysis examined the impact of robotic surgery, laparoscopic surgery, age, race, chronic conditions, and calendar year of surgery as risk factors for CA in radical prostatectomy. For O/Hyst, L/Hyst and R/Hyst, we also included the presence of malignant disease. Race was coded as White or African American, as these were predominant in NIS. Description and coding of the terms appears in the 2nd column from the left. The terms from the univariate analysis with P < 0.2 were entered into the full model for multivariable analysis.
CA = corneal abrasion; CI = 95% confidence interval; L/Hyst = laparoscopic hysterectomy; NIS = Nationwide Inpatient Sample; O/Hyst = open hysterectomy; O/L/Hyst = open or laparoscopic hysterectomy; R/Hyst= robotic assisted laparoscopic hysterectomy; R/L/Hyst = robotic assisted or laparoscopic hysterectomy; R/Prost = robotic assisted laparoscopic radical.
Table 5.
Multivariable Analysis of Risk Factors for Corneal Abrasion in 2009–2011
| Reference | Covariate | Odds Ratio |
95% confidence interval |
Logistic Regression P-value |
Hosmer and Lemeshow P-value |
Area under ROC curve |
|---|---|---|---|---|---|---|
| Prostatectomy* | Robotic prostatectomy | 1.508 | 0.987–2.302 | 0.057 | 0.9435 | 0.6003 |
| Race | 0.062 | 0.009–0.446 | 0.006 | |||
| Hysterectomy‡ | Modified hysterectomy | R/Hyst (1) vs O/Hyst (0) | 0.6558 | 0.7850 | ||
| 6.505 | 4.323–9.788 | 0.0001 | ||||
| L/Hyst (2) vs O/Hyst (0) | ||||||
| 3.821 | 2.594–5.630 | 0.0001 | ||||
| Age | 1.020 | 1.007–1.034 | 0.003 | |||
| Malignant | 1.031 | 0.657–1.618 | 0.895 | |||
| # of chronic conditions | 1.139 | 1.065–1.219 | 0.0001 | |||
| Race | 0.275 | 0.128–0.589 | 0.001 | |||
| Year | 1.119 | 0.916–1.368 | 0.271 | |||
Open prostatectomy is the reference group;
Open hysterectomy is the reference group
The multivariable model, which considers all of the covariates (see Materials and Methods, Statistical Analysis subsection, paragraph 4, lines 1–5), used terms from the univariate model table 4 with P < 0.2. Results using either forward or backward elimination were the same. Among patients with prostatectomies, the risk of corneal abrasion was significantly less in African Americans compared to Whites. Among hysterectomy patients, robotic or laparoscopic procedure along with increasing age and number of chronic conditions was associated with higher risk for corneal abrasion; additionally, corneal abrasion was significantly less likely in African Americans compared to Whites. The Hosmer- Lemeshow and the area under the ROC curve values suggested good fit of the model.
ROC = receiver operating characteristic.
Robotic usage increased CA risk in hysterectomy (OR 7.78, 95% CI 5.23–11.57, P < 0.0001), as did laparoscopy (OR 3.69, 95% CI 2.52–5.41, P < 0.0001). Increasing age, chronic conditions, calendar year of surgery, and a malignant diagnosis all increased CA risk, while being African-American significantly lowered the risk of CA (table 4).
Prostatectomy and Hysterectomy: Risk factors for corneal abrasion by multivariable regression analysis
By multiple regression analysis that considered for prostatectomy the background covariates surgical procedure, age, number of chronic conditions, age, race, and year of surgery, robotic assistance did not significantly increase the risk of CA; for robotic assistance, OR was 1.508 (95% CI 0.987–2.302, P < 0.057). However, being African-American was a significant negative risk factor (OR 0.062, 95% CI 0.009–0.446, P < 0.006, table 5). Hosmer and Lemeshow P was 0.9435 and area under the ROC curve 0.6003.
In hysterectomy, malignancy or benign diagnosis was included in the multiple regression analysis, in addition to the above, same covariates as for prostatectomy. Both laparoscopic and robotic usage (assessed as two levels in the factor variable “Modified Hysterectomy”) significantly increased CA; for L/HYST, OR was 3.821 (95% CI 2.594–5.630, P < 0.0001), and in R/HYST, OR was 6.505 (95% CI 4.323–9.788, P < 0.0001, table 5). Additional factors that increased risk in hysterectomy included advancing age (OR 1.020, 95% CI 1.007–1.034, P < 0.003), and increasing number of chronic conditions (OR 1.139, 95% CI 1.065–1.219, P < 0.0001, table 5). Hosmer and Lemeshow P was 0.6558 and area under the ROC curve 0.7850. As in prostatectomy, race was a significantly negative risk factor for CA (table 5).
Results of forward and backward elimination in the models were identical. To further test the model stringency, we tested collinearity and correlation to examine interactional effects within RP and hysterectomy. The only significant interactions were malignancy and age, and modified hysterectomy and number of chronic conditions, for hysterectomy (Supplemental Table 1).
Discussion
Elucidating the mechanisms of CA was beyond the scope of this study, however several proposed etiologies of perioperative CA include exposure, reduced corneal hydration, and chemical or direct mechanical trauma.11 Exposed corneas during general anesthesia had a significantly higher abrasion rate vs eyes that were covered, and a longer duration of operating time, i.e., greater corneal exposure, increased the rate, with the peak incidence occurring between 90 and 150 min.12 The latter study, however, was performed almost 40 years ago, when risks of CA were not as well recognized, and eye protection methods were not as universally practiced as they are today, which has resulted in significant reduction in incidence of these injuries.13
Another potential mechanism of injury in RP and hysterectomy is the increase in intraocular pressure (IOP) in steep Trendelenberg position during R/PROST,14 L/HYST, and R/HYST. Surgeons request such positioning for surgical exposure and optimal robotic arm positioning. But high head down angles (40–45°) may not actually be necessary.15 Corneal or conjunctival edema may also occur from increased central venous pressure16 and raised IOP, causing further stress to the eye via direct fluid pressure on the globe, or pressure causing the eyes to tend to remain open. Further support for these hypotheses is that IOP increased when patients were positioned prone for spine surgery, which has also been associated with a higher incidence of CAs.17
About 80% of RPs have robotic assistance.18 As a result of rapid introduction of the robot, there were few cases in NIS of laparoscopy alone (fig. 1). Hence, our comparison was exclusively of open prostatectomy to robotically-assisted laparoscopic surgery. Accordingly, the relative contributions in RP of laparoscopy and the robot to CA risk cannot be determined using this database. Surgery in the presence of malignancy would be expected to be more technically difficult, but in our study, there was no impact upon the risk of CA in patients undergoing R/HYST or L/HYST.
An unexpected finding was the apparent protective effect against CA, in African Americans in both RP and hysterectomy. There may be previously unrecognized differences in anatomy or eye structure that render African Americans less likely to sustain CAs. Similar findings have been suggested elsewhere.19 Xian et al, e.g., noted that African-Americans had lower mortality vs Whites.20 Also, African Americans > 65 years had lower adjusted 30-day mortality than Whites after congestive heart failure, acute myocardial infarction, hip fracture, and gastrointestinal bleeding, which suggests a systemic vs disease specific effect.21,22 Our finding may warrant further study using methodology not available in the NIS.
Multivariable analysis indicated a significantly greater risk of CA with advancing age in those undergoing modified hysterectomy. While this could be explainable as a decrease in tear production with aging, it is surprising that the findings were not present for R/PROST.
There are study limitations. NIS depends on the accuracy of diagnoses and procedure codes. But the coding for hysterectomy and prostatectomy is straightforward, as is the diagnosis code for CA, hence, errors in these codes are likely not a significant factor. NIS does not provide anesthetic techniques or length of surgery. With no surgeon identification, the effect of surgeon volume and/or the learning curve cannot be assessed. Verification of diagnosis in each case is also not possible. Although diagnostic codes on discharge records are taken to reflect those acquired during hospitalization, it is also possible that some of them are preexisting conditions, in which case, the rates might be overestimated.9 However, CA is typically an injury from which there usually is complete recovery, and it is not likely the diagnosis code would be previously present on a subsequent discharge record.
As NIS lacks longitudinal data beyond the hospital discharge, we cannot assess the recovery from CA, its long-term impact, or increased medical costs. It would have been interesting to assess the impact of obesity, however, in less than ¼ of the L/HYST and RP cases in 2009–11 did we find specific body mass index codes. An important limitation of NIS in cancer studies is the lack of adjustment for tumor stage, clinical features, and pathological findings.18 Therefore, the relationship between these factors and difficulty, length, and other operative features, and CA, cannot be evaluated.
In conclusion, in a large United States nationwide sample, rates of CA have increased from 2000–2011 for RP and hysterectomy. In 2009 to 2011, there was an approximately 4-times higher risk when laparoscopy was used for hysterectomy, and a 7-times higher risk when laparoscopic hysterectomy was robotically-assisted, compared to an open procedure. Thus both laparoscopy and robotic assistance appear to contribute independently to increasing the risk of CA for hysterectomy. Age, the number of chronic conditions, and race were factors influencing the risk of CA. In light of the widening indications and use of robotic assistance,23 clinicians should be vigilant and methods should be developed to lower the incidence of these eye injuries in the perioperative setting, and efforts to identify causative factors should be undertaken.
Supplementary Material
Final Boxed Summary Statement.
What we already know about this topic
Laparoscopic and robotic assisted procedures typically take longer than open procedures and a small study suggested that they are associated with an increased risk of corneal abrasions
What this article tells us that is new
In a review of nearly 1 million prostatectomy and hysterectomy cases from the National Inpatient Sample, corneal abrasion was not increased with robotic assisted prostatectomy
Compared to open hysterectomy, risk of corneal abrasion was increased nearly 4-fold with the laparoscopic technique and nearly 6.5 fold with the robotic technique
Acknowledgments
At the time this study was conducted, Dr. Sampat was a medical student at the Pritzker School of Medicine, The University of Chicago. Dr. Sampat was the recipient of the Calvin Fentress Medical Student Research Scholarship (Chicago, Illinois), which provided partial funding for the study. Additional funding was provided by departmental sources and by National Institutes of Health Grant (Bethesda, Maryland) RO1 EY10343 to Dr. Roth, and by a grant from the National Institutes of Health to the University of Chicago Institute for Translational Medicine (UL1 RR024999).
Footnotes
HCUP Nationwide Inpatient Sample (NIS). Healthcare Cost and Utilization Project (HCUP), 2000–2009. Rockville, MD: Agency for Healthcare Research and Quality. Available at: http://www.hcup-us.ahrq.gov/nisoverview.jsp. Accessed April 10, 2012.
HCUP Chronic Condition Indicator. Healthcare Cost and Utilization Project (HCUP). November 2011. Agency for Healthcare Research and Quality, Rockville, MD. www.hcup-us.ahrq.gov/toolssoftware/chronic/chronic.jsp. Accessed December 18, 2012.
NIS Comparison Reports, 1999–2009. Rockville, MD: Agency for Healthcare Research and Quality. Available at: http://www.hcup-us.ahrq.gov/db/nation/nis/nisrelatedreports.jsp. Accessed April 10, 2012.
Whalen D, Houchens R, Elixhauser A. 2004 HCUP Nationwide Inpatient Sample (NIS) Comparison Report. HCUP Methods Series Report #2007-03. Rockville, MD: U.S. Agency for Healthcare Research and Quality, 2007. Available at: http://www.hcup-us.ahrq.gov/reports/methods.jsp. Accessed April 10, 2012.
This work has been presented in part at the American Society of Anesthesiologists Annual Meeting 2012 in Washington, DC on October 16, 2012, and was accompanied by a press release and brief description of the findings.
Conflict of Interest Statement: Drs. Sampat, Glick, Lee, Tenney, and Eggener, and Messrs. Kunnavakkam and Parakati report no conflict of interest. Dr. Roth has served as an expert witness in cases of perioperative eye injuries on behalf of patients, physicians, and hospitals.
Contributor Information
Ajay Sampat, Department of Neurology, Northwestern Memorial Hospital, Chicago, Illinois.
Isaac Parakati, Department of Anesthesia and Critical Care, the University of Chicago Medicine, Chicago, Illinois.
Rangesh Kunnavakkam, Department of Health Studies, the University of Chicago, Chicago, Illinois.
David B. Glick, Department of Anesthesia and Critical Care, the University of Chicago Medicine, Chicago, Illinois.
Nita K. Lee, Department of Obstetrics and Gynecology, the University of Chicago Medicine, Chicago, Illinois.
Meaghan Tenney, Department of Obstetrics and Gynecology, the University of Chicago Medicine, Chicago, Illinois.
Scott Eggener, Section of Urology, Department of Surgery, the University of Chicago Medicine, Chicago, Illinois.
Steven Roth, Department of Anesthesia and Critical Care, the University of Chicago Medicine, Chicago, Illinois; the Center for Health and Social Sciences, the University of Chicago, Chicago, Illinois.
References
- 1.Awad H, Walker CM, Shaikh M, Dimitrova GT, Abaza R, O'Hara J. Anesthetic considerations for robotic prostatectomy: A review of the literature. J Clin Anesth. 2012;24:494–504. doi: 10.1016/j.jclinane.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 2.Ayala-Yáñez R, Olaya-Guzmán EJ, Haghenbeck-Altamirano J. Robotics in gynecology: Why is this technology worth pursuing? Clin Med Insights Reprod Health. 2013;7:71–7. doi: 10.4137/CMRH.S10850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wright JD, Ananth CV, Lewin SN, Burke WM, Lu YS, Neugut AI, Herzog TJ, Hershman DL. Robotically assisted vs laparoscopic hysterectomy among women with benign gynecologic disease. JAMA. 2013;309:689–98. doi: 10.1001/jama.2013.186. [DOI] [PubMed] [Google Scholar]
- 4.Cardenas-Goicoechea J, Soto E, Chuang L, Gretz H, Randall TC. Integration of robotics into two established programs of minimally invasive surgery for endometrial cancer appears to decrease surgical complications. J Gynecol Oncol. 2013;24:21–8. doi: 10.3802/jgo.2013.24.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Backes FJ, Fowler JM. Hysterectomy for the treatment of gynecologic malignancy. Clin Obstet Gynecol. 2014;57:115–27. doi: 10.1097/GRF.0000000000000006. [DOI] [PubMed] [Google Scholar]
- 6.Danic MJ, Chow M, Alexander G, Bhandari A, Menon M, Brown M. Anesthesia considerations for robotic-assisted laparoscopic prostatectomy: A review of 1,500 cases. J Robotic Surg. 2007;1:119–23. doi: 10.1007/s11701-007-0024-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosero EB, Kho KA, Joshi GP, Giesecke M, Schaffer JI. Comparison of robotic and laparoscopic hysterectomy for benign gynecologic disease. Obstet Gynecol. 2013;122:778–86. doi: 10.1097/AOG.0b013e3182a4ee4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cela V, Freschi L, Simi G, Ruggiero M, Tana R, Pluchino N. Robotic single-site hysterectomy: Feasibility, learning curve and surgical outcome. Surg Endosc. 2013;27:2638–43. doi: 10.1007/s00464-012-2780-8. [DOI] [PubMed] [Google Scholar]
- 9.Shen Y, Drum M, Roth S. The prevalence of perioperative visual loss in the United States: A 10-year study from 1996 to 2005 of spinal, orthopedic, cardiac, and general surgery. Anesth Analg. 2009;109:1534–45. doi: 10.1213/ane.0b013e3181b0500b. [DOI] [PubMed] [Google Scholar]
- 10.Martin DP, Weingarten TN, Gunn PW, Lee K, Mahr MA, Schroeder DR, Sprung J. Performance improvement system and postoperative corneal injuries: Incidence and risk factors. Anesthesiology. 2009;111:320–6. doi: 10.1097/ALN.0b013e3181ae63f0. [DOI] [PubMed] [Google Scholar]
- 11.Roth S, Thisted RA, Erickson JP, Black S, Schreider BD. Eye injuries after nonocular surgery: A study of 60,965 anesthetics from 1988 to 1992. Anesthesiology. 1996;85:1020–7. doi: 10.1097/00000542-199611000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Batra YK, Bali IM. Corneal abrasions during general anesthesia. Anesth Analg. 1977;56:363–5. doi: 10.1213/00000539-197705000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Vetter TR, Ali NM, Boudreaux AM. A case-control study of an intraoperative corneal abrasion prevention program: Holding the gains made with a continuous quality improvement effort. Jt Comm J Qual Patient Saf. 2012;38:490–6. doi: 10.1016/s1553-7250(12)38065-3. [DOI] [PubMed] [Google Scholar]
- 14.Awad H, Santilli S, Ohr M, Roth A, Yan W, Fernandez S, Roth S, Patel V. The effects of steep Trendelenburg positioning on intraocular pressure during robotic radical prostatectomy. Anesth Analg. 2009;109:473–8. doi: 10.1213/ane.0b013e3181a9098f. [DOI] [PubMed] [Google Scholar]
- 15.Ghomi A, Kramer C, Askari R, Chavan NR, Einarsson JI. Trendelenburg position in gynecologic robotic-assisted surgery. J Minim Invasive Gynecol. 2012;19:485–9. doi: 10.1016/j.jmig.2012.03.019. [DOI] [PubMed] [Google Scholar]
- 16.Lestar M, Gunnarsson L, Lagerstrand L, Wiklund P, Odeberg-Wernerman S. Hemodynamic perturbations during robot-assisted laparoscopic radical prostatectomy in 45 degrees Trendelenburg position. Anesth Analg. 2011;113:1069–75. doi: 10.1213/ANE.0b013e3182075d1f. [DOI] [PubMed] [Google Scholar]
- 17.Cucchiara RF, Black S. Corneal abrasion during anesthesia and surgery. Anesthesiology. 1988;69:978–9. doi: 10.1097/00000542-198812000-00034. [DOI] [PubMed] [Google Scholar]
- 18.Trinh Q-D, Sammon J, Sun M, Ravi P, Ghani KR, Bianchi M, Jeong W, Shariat SF, Hansen J, Schmitges J, Jeldres C, Rogers CG, Peabody JO, Montorsi F, Menon M, Karakiewicz PI. Perioperative outcomes of robot-assisted radical prostatectomy compared with open radical prostatectomy: Results from the Nationwide Inpatient Sample. Eur Urol. 2012;61:679–85. doi: 10.1016/j.eururo.2011.12.027. [DOI] [PubMed] [Google Scholar]
- 19.Gordon HS, Harper DL, Rosenthal GE. Racial variation in predicted and observed in-hospital death: A regional analysis. JAMA. 1996;276:1639–44. [PubMed] [Google Scholar]
- 20.Xian Y, Holloway RG, Noyes K, Shah MN, Friedman B. Racial differences in mortality among patients with acute ischemic stroke: An observational study. Ann Intern Med. 2011;154:152–9. doi: 10.1059/0003-4819-154-3-201102010-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Volpp KG, Strone R, Lave JR, Jha AK, Pauly M, Klusaritz H, Chen H, Cen L, Brucker N, Polsky D. Is thirty-day hospital mortality really lower for black veterans compared with white veterans? Health Serv Res. 2007;42:1613–31. doi: 10.1111/j.1475-6773.2006.00688.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Polsky D, Lave J, Klusaritz H, Jha A, Pauly MV, Cen L, Xie H, Stone R, Chen Z, Volpp K. Is lower 30-day mortality posthospital admission among blacks unique to the Veterans Affairs health care system? Med Care. 2007;45:1083–9. doi: 10.1097/MLR.0b013e3180ca960e. [DOI] [PubMed] [Google Scholar]
- 23.Tsui C, Klein R, Garabrant M. Minimally invasive surgery: National trends in adoption and future directions for hospital strategy. Surg Endosc. 2013;27:2253–7. doi: 10.1007/s00464-013-2973-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.