Abstract
Context
Hypertension affects one third of the U.S. adult population. Although cost-effectiveness analyses of antihypertensive medicines have been published, a comprehensive systematic review across medicine classes is not available.
Evidence acquisition
PubMed, Embase, Cochrane Library, and Health Technology Assessment were searched to identify original cost-effectiveness analyses published from 1990 through August 2016. Results were summarized by medicine class: angiotensin-converting enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARBs), calcium channel blockers (CCBs), thiazide-type diuretics, β-blockers, and others. Incremental cost-effectiveness ratios (ICERs) were adjusted to 2015 U.S. dollars.
Evidence synthesis
Among 76 studies reviewed, 14 compared medicines with no treatment, 16 compared medicines with conventional therapy, 29 compared between medicine classes, 13 compared within medicine class, and 11 compared combination therapies. All antihypertensives were cost effective compared with no treatment (ICER/quality-adjusted life year [QALY]=dominant–$19,945). ARBs were more cost effective than CCBs (ICER/QALY=dominant–$13,016) in nine comparisons, whereas CCBs were more cost effective than ARBs (ICER/QALY=dominant) in two comparisons. ARBs were more cost effective than ACEIs (ICER/QALY=dominant–$34,244) and β-blockers (ICER/QALY=$1,498–$18,137) in all eight comparisons.
Conclusions
All antihypertensives were cost effective compared with no treatment. ARBs appeared to be more cost effective than CCBs, ACEIs, and β-blockers. However, these latter findings should be interpreted with caution because these findings are not robust due to the substantial variability across the studies, including study settings and analytic models, changes in the cost of generic medicines, and publication bias.
CONTEXT
Hypertension is associated with a high economic burden at the individual and population levels. It is one of the most common primary diagnoses in the U.S., affecting one third of the adult population.1 In the U.S., the annual estimated direct and indirect costs of hypertension were $47.3 billion and $3.9 billion, respectively (annual average 2012–2013).2 The annual costs for patients treated for hypertension averaged $733 per adult in 2010.3 In addition, hypertension is an independent risk factor for other costly diseases. Antihypertensive therapy reduces the incidence of stroke (35%–40%), myocardial infarction (20%–25%), and heart failure (>50%).4 Prescription medicine costs account for about half of the total medical costs for the treatment of hypertension.3,5,6
Many pharmacologic treatment options are available for the management of hypertension. The following medicine classes are commonly used7: Angiotensin-converting enzyme inhibitors (ACEIs) inhibit the formation of angiotensin II, which is a vasoconstrictor. Angiotensin II receptor blockers (ARBs) block the binding of angiotensin II to receptors on blood vessels, leading to vasodilation. Calcium channel blockers (CCBs) decrease vascular resistance by vascular smooth muscle relaxation. Diuretics are divided into three groups: thiazide-type or thiazide-like diuretics (TDs), loop diuretics, and potassium-sparing diuretics. TDs are the most commonly used diuretics,8 and work by blocking sodium chloride reabsorption at the distal convoluted tubule cells in the kidneys. β-blockers inhibit activation by directly suppressing renin release and also block the effects of circulating catecholamines and reduce heart rate and cardiac output.
The 2014 evidence-based guideline for the management of high blood pressure in adults9 recommends several possible medicine classes for initial treatment of hypertension. TD, CCB, ACEI, or ARB classes are recommended as the initial choice of antihypertensive medicines for non–African-American patients and for patients with diabetes. For African-American patients TDs and CCBs are recommended, and for patients with chronic kidney disease, ACEIs and ARBs are recommended. Prescribers may consider adding another medicine from TD, CCB, ACEI, or ARB classes for the second step, and then β-blockers, aldosterone antagonists, or others for the third step. Similarly, several medicine classes are recommended for first-line therapy in the National Institute for Health and Care Excellence guideline for hypertension.10 The recommended initial treatment option is ACEIs or low-cost ARBs for patients aged <55 years, CCBs for those aged ≥55 years or African American, and TD if CCBs are not suitable; β-blockers are not a preferred initial therapy. The second-line therapy is dual therapy of ACEs or ARBs with a CCB for most patients. The third-line therapy is the use of three medicines, including ACE or ARB with a CCB, and a TD, if required. Because several pharmacologic treatment options can be used for the first-line therapy, it is important to evaluate which medicines are more cost effective among those options.
The evidence from pharmacoeconomic evaluations can provide valuable information for decision makers in setting public health priorities. Many pharmacoeconomic studies of antihypertensive medicines conducted in recent years have found control of hypertension to be cost effective. Several systematic reviews of these studies also have been published, but their focus has been on a specific medicine, such as irbesartan,11 or medicine class, such as ACEIs or ARBs.12 Thus, no comprehensive review has been conducted for studies across all anti-hypertensive medicine classes. The objectives are to systematically review all pharmacoeconomic evaluations of antihypertensive medicines and summarize the cost effectiveness of these medicines.
EVIDENCE ACQUISITION
Search Strategy
A systematic review was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.13 The authors performed a literature search using PubMed, Embase, Cochrane Library, and Health Technology Assessment from January 1, 1990 through August 31, 2016. The search terms were a combination of study type (health economics OR pharmacoeconomics OR economic burden OR cost analysis OR economic analysis OR cost-effectiveness OR cost-utility OR cost-benefit) and study intervention or outcomes (hypertension OR high blood pressure OR antihypertensive). All references were manually checked for the review articles retrieved.
Eligibility Criteria
Pharmacoeconomic studies were included if: (1) the study population was being treated for hypertension; (2) antihypertensive medicines were used to treat hypertension; (3) both costs and outcomes were assessed; (4) outcomes were reported as natural unit (e.g., life year [LY], blood pressure reduction, cardiovascular event avoided), utility unit (quality-adjusted life year [QALY]), or monetary unit; and (5) full-text articles were published in English. Studies were excluded if they did not describe specific antihypertensive medicines and compared medicine adherence, medicine price, single-pill fixed-dose combination therapy, and administration times.
Data Extraction
Studies that met eligibility criteria were categorized by five comparison types: (1) medicines versus placebo or no treatment; (2) medicines versus conventional therapy or standard of care that was defined as a situation where patients used any medicines that they had used before clinical trials except intervention medicines, but authors did not describe the names of medicines; (3) medicines between different medicine classes; (4) medicines within the same medicine class; and (5) different combination therapies. The cost-effectiveness evidence of medicines was summarized by medicine class: ACEIs, ARBs, CCBs, TDs, β-blockers, and others. The following information was summarized for each study: country where the study was conducted, medical conditions of study population, kind of economic evaluation methods, perspective, study framework, time horizon, sensitivity analyses, treatment type, outcomes, funding source, and cost-effectiveness evidence (e.g., incremental cost-effectiveness ratio [ICER]). Two reviewers screened studies according to the eligibility criteria, and differences were resolved by consensus between the reviewers. The cost estimates of the studies were adjusted to 2015 U.S. dollars using the Personal Consumption Expenditures by Health Function.14 For studies reporting costs in other currencies, the Purchasing Power Parity Index was used to convert the estimates to U.S. dollars in the same year and then adjusted for inflation.15
Quality Assessment
The quality of the pharmacoeconomic studies was assessed by the 100-point Quality of Health Economic Studies scale with all 16 items.16
EVIDENCE SYNTHESIS
Study Characteristics
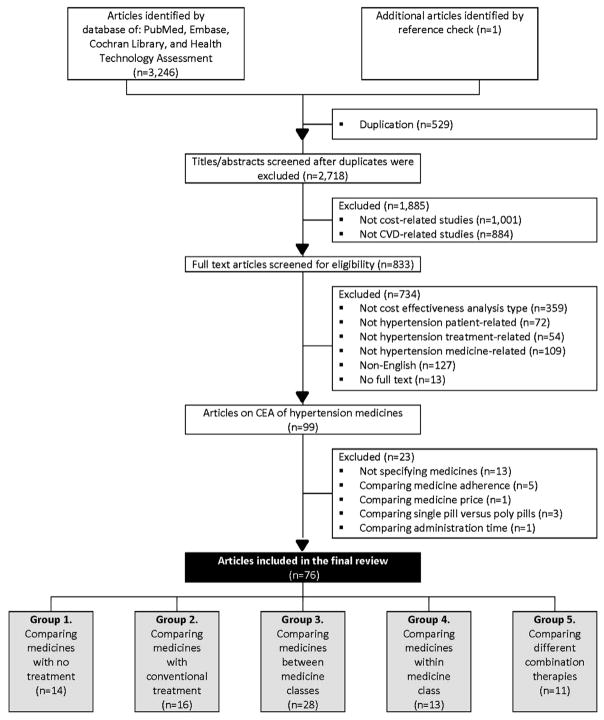
Figure 1 is a flow diagram of the review process based on the PRISMA guideline. A total of 3,247 potentially relevant articles were identified. After excluding duplication, the abstracts of 2,718 articles were screened and 1,885 were excluded. Then the full text of 833 articles was screened to assess eligibility based on the inclusion criteria. Through this process, 99 articles were identified as pharmacoeconomic studies of antihypertensive medicines. An additional 23 studies were excluded for not meeting the inclusion criteria, and the remaining 76 studies were grouped into (1) studies that compared antihypertensive medicines with no treatment (n=14), (2) studies that compared antihypertensive medicines with conventional therapy (n=16), (3) studies that compared medicines between medicine classes (n=28), (4) studies that compared medicines within medicine class (n=13), and (5) studies that compared different combination therapies (n=11). Six studies were included in two groups.
Figure 1.
Article identification and selection process of CEAs of antihypertensive medicines published in 1990–2016.
Note: The sum of the number of articles in the five groups was > 76 because six studies were included in multiple groups.
CEA, cost-effectiveness analysis; CVD, cardiovascular disease.
Table 1 shows the characteristics of pharmacoeconomic studies of antihypertensive medicines. Most studies were conducted in Europe (n=41), followed by North America (n=16). About half of the study populations were patients with hypertension alone (n=39), and another half were patients with hypertension and comorbidities (n=37). Cost-effectiveness analysis and cost-utility analysis were used frequently (n=56 and n=31, respectively). The perspectives of health care and third-party payer were used frequently. Among 64 studies that reported funding source for research, 80% (n=51) received partial or full support from private industry.
Table 1.
Characteristics and Quality of Cost-Effectiveness Analyses of Antihypertensive Medicines Published in 1990–2016 (n=76)
| Characteristics | Number of studies |
|---|---|
| Location | |
| Europe | 41 |
| North America (U.S. and Canada) | 16 |
| Others | 19 |
| Medical conditions of study population | |
| HTN | 39 |
| HTN+diabetes+renal disease | 12 |
| HTN+diabetes | 9 |
| HTN+renal failure | 4 |
| HTN+cardiovascular disease | 9 |
| HTN+cerebrovascular disease | 3 |
| Economic evaluation type | |
| Cost-minimization analysis | 1 |
| Cost-effectiveness analysis | 42 |
| Cost-utility analysis | 17 |
| Cost-benefit analysis | 2 |
| Cost-effectiveness and utility analysis | 14 |
| Perspectivea | |
| Health care | 30 |
| Third-party payer | 19 |
| Not specified payer | 8 |
| Societal | 7 |
| Others | 4 |
| Not reported | 10 |
| Study framework | |
| Trial-based | 28 |
| Model-based | 24 |
| Trial- and model-based | 24 |
| Time horizonb | |
| ≤1 year | 13 |
| >1 year and ≤10 years | 38 |
| >10 years | 35 |
| Not reported | 3 |
| Sensitivity analysis | |
| Conducted | 64 |
| Not conducted | 12 |
| Treatment type | |
| Monotherapy | 35 |
| Combination therapy | 14 |
| Monotherapy and/or combination therapy | 27 |
| Outcomesc | |
| QALY | 31 |
| LY | 37 |
| Blood pressure reduction | 8 |
| Cardiovascular disease-related | 4 |
| Renal disease-related | 7 |
| Monetary | 2 |
| Others | 6 |
| Funding source | |
| Private industry | 46 |
| Nonprofit organization | 8 |
| Private industry+nonprofit organization | 5 |
| None | 5 |
| Not reported | 12 |
| Quality assessment scored | |
| >90 | 21 |
| 81–90 | 33 |
| 71–80 | 12 |
| ≤70 | 10 |
Two studies took two perspectives.
Eleven studies used more than two time horizons.
Seventeen studies assessed more than two outcomes.
The mean Quality of Health Economic Studies score was 82.5 (SD=13.8).
HTN, hypertension; LY, life year; QALY, quality-adjusted life year.
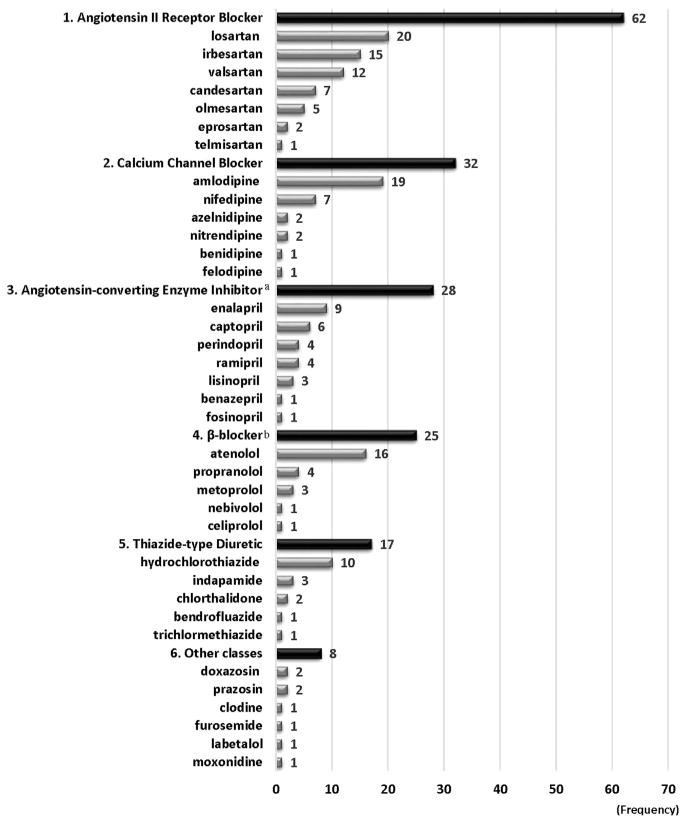
Figure 2 shows the frequencies of antihypertensive medicines analyzed in the literature. Thirty-six medicines were assessed. ARBs were the most frequently evaluated and were assessed 62 times as either interventions or comparators in 42 studies. The most frequently included ARBs were losartan (n=20) and irbesartan (n=15). CCBs were assessed 32 times (n=31) and amlodipine was the most commonly used medicine in this class (n=19). Cost effectiveness of ACEIs was assessed 28 times (n=28). β-blockers were included 25 times (n=23) and atenolol was the most common medicine in this class (n=16). Cost effectiveness of TDs was evaluated 17 times (n=17) and hydrochlorothiazide (HCTZ) was the most frequently used medicine in this class (n=10). The mean Quality of Health Economic Studies score was 82.5 points (SD=13.8 points). Appendix Table 1 (available online) describes the results of quality assessment in the literature based on the Quality of Health Economic Studies instrument.
Figure 2.
Frequencies of antihypertensive medicines by medicine classes in cost-effectiveness analyses published in 1990–2016 (n=76).
Note: Medicines were excluded for counting if they were used as adjunctive therapy for both intervention and control groups or as needed. All calcium channel blockers were dihydropyridine calcium channel blockers. All β-blockers were β1-selective β-blockers except propranolol, which is non-selective.
Cost-Effectiveness Evidence
Table 2A shows the summary of cost effectiveness of antihypertensive medicines compared with no treatment and conventional treatment. In 14 studies, all types of medicines were cost effective compared with placebo/no treatment (25 comparisons). Of these 14 studies, hypertension was defined by systolic blood pressure in five studies: ≥160 mmHg for two studies, ≥150 mmHg for one study, and ≥140 mmHg for two studies. ACEIs and TDs were frequently evaluated medicines (ten comparisons for both) and ARBs and β-blockers were also assessed (four comparisons and one comparison, respectively). The ICER ranges were from dominant to $19,945 for QALY and from dominant to $13,856 for LY. The least cost-effective scenario for QALY was the mono-therapy of HCTZ, and that for LY was the combination therapy of perindopril and indapamide. Appendix Table 2 (available online) summarizes each article on the comparisons between antihypertensive medicines and no treatment.
Table 2A.
Summary of Cost Effectiveness of Antihypertensive Medicines From the Literature Published in 1990–2016: Intervention Treatment Versus No Treatment and Intervention Treatment Versus Conventional Treatment (n=30)
| Interventions | Controls | |
|---|---|---|
| No treatment | Conventional treatment | |
| ARB preferred | ||
| Comparison, n | 4 | 13 |
| ICER | QALY: dominant–$10,976 LY: $7,594 |
QALY: dominant–$29,331 LY: dominant |
| Medicines assessed | Valsartan, irbesartan, losartan | Irbesartan, losartan, candesartan |
| CCB preferreda | ||
| Comparison, n | 0 | 1 |
| ICER | NR | |
| Medicines assessed | Amlodipine | |
| ACEI preferred | ||
| Comparison, n | 10 | 2 |
| ICER | QALY: dominant–$17,851 LY: dominant–$13,856 |
NR |
| Medicines assessed | Lisinopril, perindopril, ramipril, benazepril, enalapril, captopril, perindopril+indapamide | Ramipril, captopril |
| β-blocker preferred | ||
| Comparison, n | 1 | 0 |
| ICER | QALY: dominant | |
| Medicines assessed | Labetalol | |
| TD preferred | ||
| Comparison, n | 10 | 0 |
| ICER | QALY: $4,987–$19,945 LY: dominant–$13,856 |
|
| Medicines assessed | HCTZ, indapamide, perindopril+indapamide | |
In one comparison, amlodipine is less cost-effective than conventional therapy.
ACEI, angiotensin-converting-enzyme inhibitor; ARB, angiotensin receptor blocker; CCB, calcium channel blocker; HCTZ, hydrochlorothiazide; ICER, incremental cost-effectiveness ratio; LY, life year; NR, not reported; QALY, quality-adjusted life year; TD, thiazide-type diuretic.
For 16 studies, the control group was treated with conventional therapy, which was mostly defined as using control hypertensive medicines (e.g., β-blockers, TDs, and other classes) except intervention hypertensive medicines (e.g., ACEIs, ARBs, and CCBs) without the specification of medicine name. All types of medicines were more cost effective than conventional treatment (16 comparisons), except one comparison that amlodipine was less cost effective. The most frequent evaluations were ARBs (13 comparisons) and ACEIs and CCBs were also assessed (two comparisons for each). Except for amlodipine, the ICER ranged from dominant to $29,331 for QALY, and the least cost-effective scenario for QALY was the use of irbesartan. Intervention medicines were a dominant option for LY compared with conventional treatment. Appendix Table 3 (available online) provides the summary of each article on the comparisons between intervention antihypertensive medicines and conventional treatment used in this review.
Table 2B summarizes the cost effectiveness of antihypertensive medicines from different medicine classes. The cost effectiveness of ARBs was most frequently assessed. First, nine of 11 comparisons between ARBs and CCBs concluded that ARBs were more cost effective than CCBs. Eprosartan, irbesartan, losartan, and valsartan were cost effective compared with amlodipine, and eprosartan was cost effective compared with nitrendipine (ICER/QALY=dominant–$10,016, ICER/LY=dominant); whereas CCBs (amlodipine) were more cost effective than ARBs (valsartan) in two comparisons. Second, all studies concluded that ARBs were more cost effective than ACEIs and β-blockers. Regarding ACEIs, eprosartan was more cost effective than enalapril and perindopril, and losartan was more cost effective than fosinopril (three comparisons; ICER/QALY=dominant–$34,244, ICER/LY=dominant). Regarding β-blockers, losartan was more cost effective than atenolol (five comparisons; ICER/QALY=$1,498–$18,137, ICER/LY=dominant–$13,603). Third, only one comparison between ARBs and TDs was evaluated, which found that chlorthalidone was more cost effective than losartan (ICER not reported).
Table 2B.
Summary of Cost Effectiveness of Antihypertensive Medicines From the Literature Published in 1990–2016: Comparison Between Medicine Classes (n=28)
| Interventions | Controls | ||||
|---|---|---|---|---|---|
| ARB | CCB | ACEI | β-blocker | TD | |
| ARB preferred | |||||
| Comparison, n | — | 9 | 3 | 5 | 0 |
| ICER | QALY: dominant–$13,016 LY: dominant |
QALY: dominant–$34,244 LY: dominant |
QALY: $1,498–$18,137 LY: dominant–$13,603 |
||
| Medicines assessed | Eprosartan vs amlodipine; eprosartan vs nitrendipine; irbesartan vs amlodipine; losartan vs amlodipine; valsartan vs amlodipine | Eprosartan vs enalpril; eprosartan vs perindopril; losartan vs fosinopril | Losartan vs atenolol | ||
| CCB preferred | |||||
| Comparison, n | 2 | — | 3 | 1 | 1 |
| ICER | QALY: dominant | NR | NR | QALY: $53,594 LY: $62,202 |
|
| Medicines assessed | Amlodipine vs valsartan | Nifedipine vs lisinopril; amlodipine vs enalapril; nifedipine vs captopril | Nifedipine vs propronolol | Amlodipine vs CTD | |
| ACEI preferred | |||||
| Comparison, n | 0 | 0 | — | 1 | 1 |
| ICER | NR | QALY: $19,457 | |||
| Medicines assessed | Amlodipine vs atenolol | Enalapril vs HCTZ | |||
| β-blocker preferred | |||||
| Comparison, n | 0 | 1 | 1 | — | 2 |
| ICER | NR | NR | LY: $4,748 | ||
| Medicines assessed | Propranolol vs nifedipine | Atenolol vs enalpril | Metoprolol vs HCTZ; Propranolol vs HCTZ | ||
| TD preferred | |||||
| Comparison, n | 1 | 2 | 5 | 2 | — |
| ICER | NR | NR | QALY: dominant LY: dominant |
NR | |
| Medicines assessed | CTD vs losartan | HCTZ vs nifedipine; CTD vs amlodipine | HCTZ vs lisinopril; CTD vs lisinopril; CTD vs enalapril; HCTZ vs enalapril; HCTZ vs captopril | HCTZ vs propranolol; CTD vs propranolol | |
ACEI, angiotensin-converting-enzyme inhibitor; ARB, angiotensin receptor blocker; CCB, calcium channel blocker; CTD, chlorthalidone; HCTZ, hydrochlorothiazide; ICER, incremental cost-effectiveness ratio; LY, life year; NR, not reported; QALY, quality-adjusted life year; TD, thiazide-type diuretic.
In three comparisons between CCBs and ACEIs, CCBs were the more cost-effective option. Nifedipine was more cost effective than lisinopril or captopril, and amlodipine was more cost effective than enalapril (ICER not reported). When comparing CCBs with β-blockers, the results were inconsistent. Nifedipine was more cost effective than propranolol in one comparison (ICER not reported), whereas propranolol was more cost effective than nifedipine in one comparison (ICER not reported). In the comparison between CCBs and TDs, amlodipine was more cost effective than chlorthalidone (one comparison; ICER/QALY=$53,594, ICER/LY=$62,202), whereas HCTZ and chlorthalidone were more cost effective than nifedipine and amlodipine (two comparisons; ICER not reported). TDs were more cost effective than ACEIs in five comparisons (ICER/QALY=dominant, ICER/LY=dominant, HCTZ vs lisinoril, enalapril, or captopril, and chlorthalidone vs lisinoril and enalapril), whereas ACEIs were more cost effective than TDs in only one study (ICER/QALY=$19,474, enalapril vs HCTZ).
ARBs were more cost effective than CCBs in hypertensive patients with diabetes or renal disease, or both, in all comparisons. Specifically, irbesartan and valsartan were more cost effective than amlodipine in patients with diabetes mellitus (five comparisons and one comparison, respectively; ICER/QALY=dominant, ICER/LY=dominant) and renal disease (six comparisons; ICER/QALY=dominant, ICER/LY=dominant). ARBs were more cost effective than β-blockers in hypertensive patients with left ventricular hypertrophy in all comparisons. Specifically, losartan was more cost effective than atenolol (four comparisons; ICER/QALY=$1,498–$18,137, ICER/LY=dominant–$13,603). Appendix Table 4 (available online) summarizes the articles on the comparisons of antihypertensive medicines between different medicine classes.
Table 2C summarizes 13 studies about the cost effectiveness of antihypertensive medicines within the same medicine class for ARBs, β-blockers, and CCBs. ARBs were the most frequently evaluated class; ten studies made 19 comparisons. Overall, olmesartan, irbesartan, candesartan, and telmisartan were more cost effective than losartan and valsartan. Olmesartan, irbesartan, candesartan, telmisartan, and valsartan were more cost effective than losartan (eight comparisons; ICER/QALY=dominant–$33,567, ICER/LY=dominant–$28,326), whereas losartan was more cost effective than candesartan in one comparison. Olmesartan, irbesartan, candesartan, and telmisartan were more cost effective than valsartan (six comparisons; ICER/QALY=dominant–$34,678, ICER/LY=$10,275–$27,006). Appendix Table 5 (available online) provides the summary of each study on the comparisons of antihypertensive medicines within the medicine classes.
Table 2C.
Summary of Cost Effectiveness of Antihypertensive Medicines From the Literature Published in 1990–2016: Comparison Within Medicine Class (n=13).
| Interventions | Controls | |||
|---|---|---|---|---|
| ARBa | ||||
| Olmesartan preferred | Candesartan | Irbesartan | Valsartan | Losartan |
| Comparison, n | 1 | 2 | 2 | 2 |
| ICER | NR | QALY: dominant | QALY: dominant | QALY: dominant |
| Candesartan preferred | Candesartan | Irbesartan | Valsartan | Losartan |
| Comparison, n | — | 1 | 1 | 2 |
| ICER | — | NR | NR | QALY: dominant |
| Telmisartan preferred | Candesartan | Irbesartan | Valsartan | Losartan |
| Comparison, n | 0 | 0 | 1 | 1 |
| ICER | — | — | QALY: $6,450–$34,678 LY: $10,275–$27,006 |
QALY: $4,029–14,569 LY: $2,369–$9,457 |
| Irbesartan preferred | Candesartan | Irbesartan | Valsartan | Losartan |
| Comparison, n | 0 | — | 2 | 2 |
| ICER | — | — | QALY: dominant | QALY: dominant |
| Valsartan preferred | Candesartan | Irbesartan | Valsartan | Losartan |
| Comparison, n | 0 | 0 | — | 1 |
| ICER | — | — | — | QALY: $31,341–$33,567 LY: $22,448–$28,326 |
| Losartan preferred | Candesartan | Irbesartan | Valsartan | Losartan |
| Comparison, n | 1 | 0 | 0 | — |
| ICER | QALY: over threshold | — | — | — |
| CCB | ||||
| Nifedipine preferred | Amlodipine | |||
| Comparison, n | 1 | |||
| ICER | NR | |||
| β-blocker | ||||
| Nebivolol preferred | Metoprolol | |||
| Comparison, n | 1 | |||
| ICER | NR | |||
| Celiprolol preferred | Altenolol | |||
| Comparison, n | 1 | |||
| ICER | BP reduction: dominant | |||
Note: ICER for QALY and LY were summarized.
Among ARBs, no study used olmesartan and telmisartan as a control group.
ACEI, angiotensin-converting-enzyme inhibitor; ARB, angiotensin receptor blocker; BP, blood pressure; CCB, calcium channel blocker; CTD, chlorthalidone; HCTZ, hydrochlorothiazide; ICER, incremental cost-effectiveness ratio; LY, life year; NR, not reported; TD, thiazide-type diuretic; QALY, quality-adjusted life year.
Eleven studies compared the cost effectiveness of different types of combination therapies. In two studies, amlodipine-based treatment with perindopril as an adjunct treatment was more cost effective than atenolol-based treatment with bendroflumethiazide as an adjunct treatment (ICER/QALY=$5,649–$31,975, ICER/LY=$20,495–$31,165). Dual therapies were more cost effective than monotherapy and triple therapy. A dual therapy of azelnidipine and olmesartan was more cost effective than a monotherapy with either azelnidipine or olmesartan (ICER/QALY=dominant). A dual therapy of candesartan (low dose) and nifedipine was also more cost effective than a monotherapy with candesartan or nifedipine (ICER/blood pressure reduction=dominant). However, a triple therapy of bendrofluazide, atenolol, and enalapril was less cost effective than a dual therapy of bendrofluazide and atenolol (ICER=not reported). Appendix Table 6 (available online) summarizes the articles on the comparisons among different types of combination therapies.
DISCUSSION
Cost-Effectiveness Evidence
As expected, this review found that treating hypertension with medicines was consistently more cost effective than not treating it, thanks to the remarkable progress in the treatment of hypertension in recent decades. ARBs were found to be more cost effective than CCBs in nine comparisons, whereas CCBs appeared to be more cost effective than ARBs in two comparisons. ARBs were more cost effective than ACEIs and β-blockers in all eight comparisons. Within ARBs, losartan was a less cost effective medicine in eight comparisons. These findings are less robust because of the heterogeneity of study setting and analytic methods and changes in the cost of generic medications.
A previous study found that approximately one half of U.S. adults with hypertension did not have their hypertension controlled. Of those who were uncontrolled: 33.1% were unaware; 20.3% were aware, but uncontrolled; and 46.6% were aware, treated, and uncontrolled.17 When considering the economic benefit of treating hypertension, more effort should be required to identify people who are not aware of their hypertension and to treat those who are not receiving treatment despite being aware of their hypertension.
Pharmacoeconomic evaluations of medicines are usually conducted for newly marketed medicines. ARBs are the newest class of antihypertensive medicine. In 1995, the first ARB, losartan, was approved by the U.S. Food and Drug Administration, followed by valsartan in 1996. Irbesartan, eprosartan, candesartan, telmisartan, and olmesartan were approved between 1997 and 2002.18 Thus, ARBs were the most frequently evaluated—in 42 of 76 studies in this review, mostly published between 2000 and 2009. The studies reviewed found that ARBs were a more cost-effective option than medicines in the CCB, ACEI, or β-blocker classes. Furthermore, losartan and valsartan, the first U.S. Food and Drug Administration –approved ARBs, were less favorable options among ARBs. Thus, more frequent evaluations of ARBs may lead to better cost-effectiveness results for this medicine class, especially for more recently marketed ARBs than losartan and valsartan.
This review found that ARBs were more cost effective than CCBs in hypertensive patients with diabetes and renal disease (irbesartan and valsartan versus amlodipine) and β-blockers in patients with left ventricular hypertrophy (losartan versus atenolol). According to the new evidence-based guideline for the management of high blood pressure,9 TDs, CCBs, ACEIs, and ARBs are recommended as the initial choice for patients with diabetes, and ACEIs and ARBs are recommended for patients with chronic kidney disease. The findings of this review could be useful for choosing appropriate anti-hypertensive medicines for patients with diabetes, renal disease, or left ventricular hypertrophy.
Study Design and Quality
When assessing study quality, study perspective, the source of input parameters, time horizons, and outcome measures must be considered. Societal perspective is the gold standard of pharmacoeconomic studies because it incorporates all costs and health outcomes, although other perspectives may be better for some decision-making situations.19,20 However, only a few studies in this review used a societal perspective. The majority stated that they considered a payer perspective (e.g., third-party payer perspective).
Second, efficacy refers to outcomes under ideal circumstances, whereas effectiveness refers to outcomes under real-world settings.21,22 Although efficacy and effectiveness lie on a continuum, the source of input parameters of pharmacoeconomic studies from clinical trials have higher internal validity, whereas those from observation studies have higher external validity. About one third of the studies combined efficacy data from clinical trials using a Markov model, and another third evaluated efficacy data from clinical trials by using regression-type analyses. Thus, the source of input parameters came from clinical trials in the majority of the included studies.
Third, more than half of the studies adopted a time horizon of less than 10 years, even though hypertension is a chronic disease. Although medicine dosages might be able to be reduced after patients achieve normal blood pressure and maximize healthy lifestyle behaviors beneficial for hypertension control (maintaining a normal weight, being physically active, and consuming a low sodium diet) and maintain it for a year or more, the use of antihypertensive medicines is often not able to be stopped. Treatment frequently should be continued over a lifetime.23 When considering the natural course of hypertension, a long time horizon is preferred for pharmacoeconomic studies.
Finally, a number of outcomes were evaluated in the studies. Primary clinical outcomes were commonly used. The most common outcome was LYs, followed by QALYs. Other intermediate clinical outcomes were blood pressure reduction and avoiding cardiovascular disease events, end-stage renal disease, or dialysis. QALYs has been considered a more important measure of effectiveness in pharmacoeconomic evaluation than LYs.24,25 However, only about 40% of the included studies used QALYs as an effectiveness measure in this review.
This study has several strengths. First, this review is the first comprehensive synthesis of the evidence of cost effectiveness for antihypertensive medicines to the authors’ knowledge, and it presents detailed cost-effectiveness information. Second, this review included the majority of the published cost-effectiveness studies of antihypertensive medicines and adjusted all cost-related values from different time and settings to 2015 U.S. dollars for better comparison. Finally, this review also assessed quality of the literature and pointed out the weaknesses of the literature. Because the findings from the literature were similar across low- and high-quality literature, this review included all 76 studies. In addition, identifying the weaknesses of the current literature will help future cost-effectiveness analyses of antihypertensive medicines.
Limitations
This study also has several limitations. First, only English language peer-reviewed publications were included. Thus, other important pharmacoeconomic studies of antihypertensive medicines published in non-English language peer-reviewed journals may have been missed. Second, the availability of generic medicines changes over time. Although it is well known that substituting generic medicines for brand-name medicines lowers costs,26 it is important to acknowledge the impact of including pharmacoeconomic studies that do not specify the cost—based on generic or brand formulations or both —on a cost-effectiveness analysis. For example, since losartan became available as a generic in 2010,27 the results of studies evaluating the cost effectiveness of losartan prior to 2010 cannot be generalized with the results of studies conducted after 2010. The same is true for all other ARBs cost-effectiveness studies where the cost of the formulation (i.e., generic versus brand) was not specified. In this analysis, most studies did not clarify if the cost of medicines were based on the brand or generic formulation. For this reason, the cost-effectiveness calculations reported in this report may be imperfect, and the usefulness of the results limited. Finally, cost-effectiveness evidence may depend on the blood pressure level, race/ethnicity, age, or comorbidity status of the patients. However, many studies in the literature did not provide this specific information; thus, cost-effectiveness evidence for specific population groups could not be derived.
These findings should be interpreted with caution for several reasons. First, potential conflicts of interest for authors of industry-sponsored studies may have influenced the way they develop models and gathered input information for analytic models. A systematic review on bias in published cost-effectiveness studies demonstrated that studies sponsored by industry were associated with a favorable cost-effectiveness ratio compared with studies sponsored by non-industry sources, although there was no difference in the quality of the research.28,29 In this review, 64 studies reported a funding source for research, 51 (80%) of them financially supported by industry. These studies provided positive evidence for the companies that sponsored them. As such, there is a possibility of publication bias of the results in this review. Another reason is a substantial variability across studies. For example, study settings varied across countries because of differences in financial and healthcare systems,30,31 and drug prices often differ widely because of bargaining power.32 Many studies were conducted in Europe. This may be due to the fact that European governments often emphasize cost-effectiveness evidence in their healthcare system. In addition, the definitions and measurements of outcomes and analytical approaches in each study also varied across study settings and methodologies. Although studies were categorized into five groups, there were still substantial variabilities across studies within groups. As such, the Appendix Tables 1–6 (available online) are provided to summarize each study. Finally, although cost effectiveness of antihypertensive medicines could be an aid for clinical decisions, the availability of generic medicines might make the published cost-effectiveness information less valid.
Several research gaps were identified in the literature. First, no study assessed the cost effectiveness of medicines according to race/ethnicity in the U.S. Because race/ethnicity is a key factor when determining appropriate treatment options, future studies could evaluate the cost effectiveness of antihypertensive medicines by race/ethnicity. Second, more research is needed on the cost effectiveness of antihypertensive medicines by patient blood pressure level, because using hypertensive medicines may be less cost effective in patients with mild hypertension compared with moderate or severe hypertension. Third, more evaluations should be conducted on different types of combination therapies. This review found 11 studies that compared different types of combination therapies. However, considering many hypertensive adults take multiple medications concurrently, research should be conducted to identify optimal number of medications or types of combination by their characteristics. Finally, developing a standard to measure effectiveness in hypertension should be considered, especially in adults with other comorbidities, such as diabetes and renal disease.
CONCLUSIONS
The treatment of hypertension using antihypertensive medicines is cost effective compared with no treatment. Among medicine classes, ARBs appear to be more cost effective than CCB, ACEI, and β-blocker classes. However, these results should be interpreted with caution because of potential publication bias (i.e., funding bias) and the heterogeneity of study setting.
Supplementary Material
Acknowledgments
Publication of this article was supported by the U.S. Centers for Disease Control and Prevention (CDC), an Agency of the U.S. Department of Health and Human Services, and the Association for Prevention Teaching and Research (APTR) Cooperative Agreement No. 1U36 OE000005.
The findings and conclusions in this publication are those of the authors and do not necessarily represent the official position of the CDC.
No financial disclosures were reported by the authors of this paper.
Footnotes
Supplemental material associated with this article can be found in the online version at https://doi.org/10.1016/j.amepre.2017.06.020.
References
- 1.CDC. Vital signs: prevalence, treatment, and control of hypertension—United States, 1999–2002 and 2005–2008. MMWR Morb Mortal Wkly Rep. 2011;60(4):103–108. [PubMed] [Google Scholar]
- 2.Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics–2017 update: a report from the American Heart Association. Circulation. 2017;135(10):e146–e603. doi: 10.1161/CIR.0000000000000485. https://doi.org/10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davis K. Expenditures for Hypertension among Adults Age 18 and Older, 2010: Estimates for the U.S. Civilian Noninstitutionalized Population. Statistical Brief #404. Rockville, MD: Agency for Healthcare Research and Quality; Apr, 2013. [PubMed] [Google Scholar]
- 4.Neal B, MacMahon S, Chapman N Blood Pressure Lowering Treatment Trialists’ Collaboration. Effects of ACE inhibitors, calcium antagonists, and other blood-pressure-lowering drugs: results of prospectively designed overviews of randomised trials. Blood Pressure Lowering Treatment Trialists’ Collaboration. Lancet. 2000;356(9246):1955–1964. doi: 10.1016/s0140-6736(00)03307-9. https://doi.org/10.1016/S0140-6736(00)03307-9. [DOI] [PubMed] [Google Scholar]
- 5.Balu S, Thomas J., 3rd Incremental expenditure of treating hypertension in the United States. Am J Hypertens. 2006;19(8):810–816. doi: 10.1016/j.amjhyper.2005.12.013. https://doi.org/10.1016/j.amjhyper.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 6.Trogdon JG, Finkelstein EA, Nwaise IA, Tangka FK, Orenstein D. The economic burden of chronic cardiovascular disease for major insurers. Health Promot Pract. 2007;8(3):234–242. doi: 10.1177/1524839907303794. https://doi.org/10.1177/1524839907303794. [DOI] [PubMed] [Google Scholar]
- 7.Kalra S, Kalra B, Agrawal N. Combination therapy in hypertension: an update. Diabetol Metab Syndr. 2010;2(1):44. doi: 10.1186/1758-5996-2-44. https://doi.org/10.1186/1758-5996-2-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carter BL, Ernst ME, Cohen JD. Hydrochlorothiazide versus chlorthalidone: evidence supporting their interchangeability. Hypertension. 2004;43(1):4–9. doi: 10.1161/01.HYP.0000103632.19915.0E. https://doi.org/10.1161/01.HYP.0000103632.19915.0E. [DOI] [PubMed] [Google Scholar]
- 9.James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311(5):507–520. doi: 10.1001/jama.2013.284427. https://doi.org/10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 10.Krause T, Lovibond K, Caulfield M, McCormack T, Williams B Guideline Development Group. Management of hypertension: summary of NICE guidance. BMJ. 2011;343:d4891. doi: 10.1136/bmj.d4891. https://doi.org/10.1136/bmj.d4891. [DOI] [PubMed] [Google Scholar]
- 11.Gialama F, Maniadakis N. Comprehensive overview: efficacy, tolerability, and cost-effectiveness of irbesartan. Vasc Health Risk Manag. 2013;9:575–592. doi: 10.2147/VHRM.S50831. https://doi.org/10.2147/VHRM.S50831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang Y, Zhou Q, Haaijer-Ruskamp FM, Postma MJ. Economic evaluations of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers in type 2 diabetic nephropathy: a systematic review. BMC Nephrol. 2014;15:15. doi: 10.1186/1471-2369-15-15. https://doi.org/10.1186/1471-2369-15-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336–341. doi: 10.1016/j.ijsu.2010.02.007. https://doi.org/10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 14.U.S. Bureau of Economic Analysis (BEA) [Accessed February 20, 2017];National Data. Table 2.5.5. Personal Consumption Expenditures by Function. 2016 Aug 3; www.bea.gov/iTable/iTable.cfm?reqid=9&step=1&acrdn=2#reqid=9&step=3&isuri=1&903=74.
- 15.Organisation for Economic Co-operation and Development (OECD) OECD data. [Accessed February 20, 2017];Purchasing power parities (PPP) https://data.oecd.org/conversion/purchasing-power-parities-ppp.htm#indicator-chart.
- 16.Ofman JJ, Sullivan SD, Neumann PJ, et al. Examining the value and quality of health economic analyses: implications of utilizing the QHES. J Manag Care Pharm. 2003;9(1):53–61. doi: 10.18553/jmcp.2003.9.1.53. https://doi.org/10.18553/jmcp.2003.9.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Merai R, Siegel C, Rakotz M, et al. CDC grand rounds: a public health approach to detect and control hypertension. MMWR Morb Mortal Wkly Rep. 2016;65:1261–1264. doi: 10.15585/mmwr.mm6545a3. https://doi.org/10.15585/mmwr.mm6545a3. [DOI] [PubMed] [Google Scholar]
- 18.Sanders G, Coeytaux R, Dolor R, et al. Angiotensin-converting Enzyme Inhibitors (ACEIs), Angiotensin II Receptor Antagonists (ARBs), and Direct Renin Inhibitors for Treating Essential Hypertension: An Update. Comparative Effectiveness Review No. 34. AHRQ Publication No. 11-EHC063-EF. Rockville, MD: Agency for Healthcare Research and Quality; 2011. www.ncbi.nlm.nih.gov/books/NBK61789/ [PubMed] [Google Scholar]
- 19.Weinstein MC. Principles of cost-effective resource allocation in health care organizations. Int J Technol Assess Health Care. 1990;6(1):93–103. doi: 10.1017/s0266462300008953. https://doi.org/10.1017/S0266462300008953. [DOI] [PubMed] [Google Scholar]
- 20.Sanders GD, Neumann PJ, Basu A, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA. 2016;316(10):1093–1103. doi: 10.1001/jama.2016.12195. https://doi.org/10.1001/jama.2016.12195. [DOI] [PubMed] [Google Scholar]
- 21.Godwin M, Ruhland L, Casson I, et al. Pragmatic controlled clinical trials in primary care: the struggle between external and internal validity. BMC Med Res Methodol. 2003;3:28. doi: 10.1186/1471-2288-3-28. https://doi.org/10.1186/1471-2288-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gartlehner G, Hansen RA, Nissman D, Lohr KN, Carey TS. Criteria for Distinguishing Effectiveness from Efficacy Trials in Systematic Reviews. Rockville, MD: Agency for Healthcare Research and Quality; 2006. [PubMed] [Google Scholar]
- 23.American Heart Association. [Accessed January 24, 2017];Managing high blood pressure medications. www.heart.org/HEARTORG/Conditions/HighBloodPressure/MakeChangesThatMatter/Managing-High-Blood-Pressure-Medications_UCM_303246_Article.jsp#.WUKV701dBZs. Published 2016.
- 24.Rasanen P, Roine E, Sintonen H, Semberg-Konttinen V, Ryynanen OP, Roine R. Use of quality-adjusted life years for the estimation of effectiveness of health care: a systematic literature review. Int J Technol Assess Health Care. 2006;22(2):235–241. doi: 10.1017/S0266462306051051. https://doi.org/10.1017/S0266462306051051. [DOI] [PubMed] [Google Scholar]
- 25.Whitehead SJ, Ali S. Health outcomes in economic evaluation: the QALY and utilities. Br Med Bull. 2010;96:5–21. doi: 10.1093/bmb/ldq033. https://doi.org/10.1093/bmb/ldq033. [DOI] [PubMed] [Google Scholar]
- 26.Haas JS, Phillips KA, Gerstenberger EP, Seger AC. Potential savings from substituting generic drugs for brand-name drugs: medical expenditure panel survey, 1997–2000. Ann Intern Med. 2005;142(11):891–897. doi: 10.7326/0003-4819-142-11-200506070-00006. https://doi.org/10.7326/0003-4819-142-11-200506070-00006. [DOI] [PubMed] [Google Scholar]
- 27.U.S. Food and Drug Administration. [Accessed January 24, 2017];Generic drug roundup. 2010 Dec; www.fda.gov/ForConsumers/ConsumerUpdates/ucm236544.htm#LosartanPotassiumTablets. Published 2010.
- 28.Bell CM, Urbach DR, Ray JG, et al. Bias in published cost effectiveness studies: systematic review. BMJ. 2006;332(7543):699–703. doi: 10.1136/bmj.38737.607558.80. https://doi.org/10.1136/bmj.38737.607558.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lexchin J, Bero LA, Djulbegovic B, Clark O. Pharmaceutical industry sponsorship and research outcome and quality: systematic review. BMJ. 2003;326(7400):1167–1170. doi: 10.1136/bmj.326.7400.1167. https://doi.org/10.1136/bmj.326.7400.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frogner BK, Frech HE, 3rd, Parente ST. Comparing efficiency of health systems across industrialized countries: a panel analysis. BMC Health Serv Res. 2015;15:415. doi: 10.1186/s12913-015-1084-9. https://doi.org/10.1186/s12913-015-1084-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keynejad R, Semrau M, Toynbee M, et al. Building the capacity of policy-makers and planners to strengthen mental health systems in low- and middle-income countries: a systematic review. BMC Health Serv Res. 2016;16(1):601. doi: 10.1186/s12913-016-1853-0. https://doi.org/10.1186/s12913-016-1853-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Danzon PM, Furukawa MF. International prices and availability of pharmaceuticals in 2005. Health Aff (Millwood) 2008;27(1):221–233. doi: 10.1377/hlthaff.27.1.221. https://doi.org/10.1377/hlthaff.27.1.221. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.