SUMMARY
Aging in men is associated with loss of bone mass, impaired physical function and altered body composition. The objective of this proof-of-concept randomized, double-blind, placebo-controlled, parallel-group, single-center trial was to determine the relative effects of testosterone (T) and estradiol (E2) on bone mineral density, body composition, and physical performance in older men. The primary outcome was lumbar spine bone mineral density (BMD), and secondary outcomes were body composition, muscle strength, gait speed, and sex hormone concentrations. Forty three men (age range, 65–82 years; mean age 71 years) with low total T levels <350 ng/dL were randomized to one of three groups: 5 g transdermal testosterone gel (TT) (N = 16), anastrozole (AI) 1 mg (N = 14) or placebo daily (N = 13) for 12 months. Outcomes were assessed at baseline, 3, 6, and 12 months. Both TT and AI increased serum TT levels (>500 ng/dL, p < 0.05) compared to baseline; T values remained stable throughout the duration of the trial. At 12 months, TT improved the primary outcome of lumbar spine BMD (p < 0.01). Both interventions improved knee strength at 12 months compared to baseline (p < 0.05) while lean body mass significantly increased only in the AI group at 6 and 12 months (1.49 ± 0.38 kg, p < 0.01; 1.24 ± 0.39 kg, p < 0.05, respectively) compared to baseline. Interestingly, TT improved fast gait speed at 3 and 12 months (p < 0.01, p < 0.05, respectively). In summary, this proof-of-concept study confirms that aromatization of T is required for maintaining BMD in older men with low-T levels. The trial also uncovered the novel finding that aromatization of T is required for improvement in fast gait speed, an observation that needs to be verified in future studies.
Keywords: aging, aromatase inhibition, bone, gait speed, muscle strength, testosterone
INTRODUCTION
Testosterone (T) levels in men decline with age (Harman et al., 2001) and this decline is associated with loss of lean body mass (LBM), muscle strength, and bone mineral density (BMD) observed in most individuals as they age (Jackson et al., 1992; Katznelson et al., 1996; Behre et al., 1997). Studies of testosterone replacement therapy (TRT) in older men have shown inconsistent benefits (Spitzer et al., 2013). In spite of this, T prescriptions written for middle-age and older men in the absence of pituitary and testicular disease have risen exponentially (Basaria, 2013) despite recent concerns regarding adverse effects of TRT (Bhasin et al., 2011; Finkle et al., 2014).
Hence, increasing endogenous T by using aromatase inhibitors (AI) has recently drawn some interest. As estrogen (E) is a potent inhibitor of the gonadal axis, inhibition of E synthesis by AI results in an increase in gonadotropins, which, in turn, stimulate the testes, resulting in an increase in circulating T levels. The main focus of this work was to evaluate the effect of both testosterone and estradiol in male skeletal health as male hypogonadism is associated with loss of BMD. Although previous studies have investigated the role of AI on skeletal effects in older men with low T (Burnett-Bowie et al., 2009), however; they did not directly compare AI with TRT to determine what effects of T replacement are mediated via its aromatization. Furthermore, none of the studies evaluated the effects of AI on muscle strength or gait speed.
In this proof-of-concept study, we evaluated differential effects of transdermal testosterone (TT), AI, and placebo on body composition, bone mass, muscle strength and gait speed over 1 year in older men with age-related low serum T levels.
MATERIALS AND METHODS
Study subjects, recruitment, and eligibility
Community dwelling men aged 65 years and older with total circulating T levels <350 ng/dL on a fasting morning samples obtained between 7 and 10 am were enrolled. The trial was conducted at the National Institute on Aging (NIA)/NIH Intramural Research Program and was approved by MedStar Harbor Hospital IRB/NIH repository 11-AG-N079; ClinicalTrials.gov Identifier: NCT00104572. Subjects were required to have normal circulating levels of gonadotropins, prolactin, parathyroid hormone, and prostate-specific antigen levels ≤4.0 ng/dL. Men with hematocrit <36%, mini-mental status examination score <24, polycythemia, osteoporosis, history of stroke, history of diabetes, uncontrolled high blood pressure, severe benign prostatic hypertrophy, recent acute coronary syndrome, severe arthritis, history of knee, or hip joint replacement were excluded. They were also excluded if using bisphosphonate, selective estrogen receptor modulator or any anabolic agents. Subjects were requested to refrain from drinking more than 30 g of alcohol daily rousing any tobacco product for the study duration. All participants provided written informed consent.
Study protocol
The study was a double-blind, randomized, placebo-controlled trial of 12 months duration. Thirty seven subjects were randomized to three groups: transdermal T gel (Androgen) 5 g/day and placebo tablet (TT-group) (n = 16); Anastrozole (Arimidex) 1 mg/day and placebo gel (AI-group) (n = 14); placebo tablet and placebo gel daily (placebo) (n = 13) in a 1:1:1 ratio fashion. Randomization was generated by computer. Study outcomes were assessed at baseline, 3, 6 and 12 months. Dose adjustments were performed by the unblinded research pharmacist who also assessed compliance. The target range for serum TT was between 500 and 1000 ng/dL. A single dose adjustment was performed 6-weeks after randomization. If the TT dose was <500 ng/dL in any subject, the dose was increased by 2.5 g daily and reciprocal ‘sham increase’ with the placebo gel was performed in either the AI-group or the placebo group to maintain the blind. Seven participants in the TT-group had an increase in dose (to reach a cumulative dose of 7.5 g daily). Safety monitoring included measurements of hematocrit, hemoglobin, prostate-specific antigen, digital prostate examination, transrectal prostate ultrasound, and assessment of adverse events. Compliance was determined by counts of used and unused tablets and gel packets. All enrolled subjects were also provided calcium (1500 mg) and vitamin D (600 IU) daily for the duration of the study.
Primary endpoint
Bone mineral density and body composition
Femoral neck and lumbar spine BMD, lean body mass (LBM), and fat mass (FM) were measured, using dual-energy X-ray absorptiometry (DEXA) (Lunar Prodigy Advance, GE Healthcare, Madisson, WI). All DEXA scans were read by a single operator and analyzed using Encore 2006 software version 10.51.006 (GE Healthcare, Madisson, WI) for body composition analysis.
Secondary endpoints
Strength assessments
Grip strength was measured in both hands using a Jamar Hand dynamometer (Sammons Preston, Inc., Warrenville, IL). The hand dynamometer was calibrated to known weights and three trials were conducted for each hand. The highest value of the six measures was recorded.
Concentric knee flexion and extension strength were measured using Kin-Com Kinetic Communicator (Chattecx, Chattanooga, TN) and the Biodex Medical Systems (Shirley, NY). The highest value from three maximal efforts which were separated by 30-second rest intervals was used as the maximum concentric knee flexion and extension strength (Simonsick et al., 2001; Walsh et al., 2008). Measurements from the Kin-Com were standardized to those of the Biodex with the calibration equation, which was calculated from data collected in 100 subjects evaluated with both methods.
Gait speed
Gait speed was measured according to a previously validated standard protocol (Simonsick et al., 2001; Schrack et al., 2012) and was analyzed in meters per second. Participants walked in a straight line in an uncarpeted corridor between cones placed 15 m apart in one direction at their fastest speed without running (fast gait speed) for the return trip.
Laboratory methods
Both T and E2 levels were measured by liquid chromatography tandem mass spectroscopy as previously described (Thienpont et al., 2008). Bioavailable T was measured using ammonium sulfate precipitation method (Manni et al., 1985). Sex hormone-binding globulin (SHBG) was measured using the electrochemiluminescence method (Loric et al., 1988). T detection limit was 2.5 ng/dL and intra-assay coefficient of variation (CV) was 3.5%; inter-assay CV 5.3%; bioavailable T detection limit was 4.7 mg/dL; intra-assay CV 2.0%; inter-assay CV 2.3%; E2 detection limit was 1 pg/mL; intra-assay CV 7.1%; inter-assay CV 9.2%; SHBG detection limit was 10 nm; intra-assay CV 2.3%; inter-assay CV 1.8%. LH (luteinizing hormone), and FSH (follicle-stimulating hormone) were measured by ELISA with magnetic beads from Millipore (Chicago, IL): minimum detectable concentration of FSH and LH 0.01 ± 0.02 mL U/mL; intra-assay CV <10%; inter-assay CV <15%.
STATISTICAL ANALYSIS
Sample size adequacy
The comparison of placebo (n = 13) to TT (n = 16) and AI (n = 14) had 80% power to detect mean differences in changes in BMD from baseline to 12 months of 1.03 SD and 1.05 SD, respectively, assuming an intraclass correlation of 0.50 and using a chi-square test (with type I error of 0.05) derived from linear mixed-effects regression models. These effect sizes are considered large (Cohen, 1988) as is consistent with the pilot studies. We anticipated an SD of 0.140 g/cm2 for BMD (Kenny et al., 2001). Therefore, we have adequate power to detect differences in changes of BMD of 0.144 g/cm2 between placebo and TT, and of 0.147 g/cm2 between placebo and AI.
Analysis
Baseline characteristics were compared across the three study groups, using anova (Tables 1 and 2). Assessment of the normality of the data was done by creating histogram and frequency distribution and by performing the Shapiro–Wilk test. For each participant and each endpoint, changes from baseline to each follow-up time point were computed. These changes were regressed on the baseline value of the endpoint, treatment group, time, and treatment group-by-time interactions, using linear mixed-effects models for repeated measures with random intercepts. Mean changes from baseline, and differences in changes between groups, were calculated using model estimates. The primary analysis of all endpoints (primary and secondary endpoints) involved assessing differences in changes over time between study groups using the likelihood ratio chi-square tests of interaction terms derived from the models. The secondary analysis of all endpoints involved assessment of differences in changes between groups at each individual time point (Tables 2 and 3 and Figs 2 and 3). All analyses were unadjusted for multiple comparisons owing to the objective of this trial as a pilot proof-of-concept study and the clear hierarchy of primary and secondary endpoints. All reported p-values are two-sided. The p-value (p) <0.05 was considered statistically significant. Data are presented as means ± SEM (standard error of the mean).
Table 1.
Baseline characteristics of the study participants
| Characteristic | Placebo (n = 9) | TT-group (n = 13) | AI-group (n = 13) | p value |
|---|---|---|---|---|
| Age (years) | 72 ± 1 | 72 ± 1 | 70 ± 1 | 0.61 |
| Systolic blood pressure (mmHg) | 133.67 ± 3.72 | 137.85 ± 2.61 | 134.46 ± 4.02 | 0.67 |
| Diastolic blood pressure (mmHg) | 73.11 ± 3.45 | 73.30 ± 1.48 | 72.38 ± 2.62 | 0.96 |
| BMI (kg/m2) | 27.62 ± 1.15 | 30.12 ± 1.11 | 27.55 ± 1.21 | 0.27 |
| Total testosterone (ng/dL) | 303.78 ± 16.56 | 300.05 ± 13.44 | 271.58 ± 12.75 | 0.40 |
| Bioavailable-Testosterone (ng/dL) | 91.22 ± 10.56 | 114.41 ± 10.74 | 114.83 ± 10.47 | 0.26 |
| Estradiol (pg/mL) | 16.0 ± 2.0 | 20.0 ± 2.0 | 15.0 ± 2.0 | 0.16 |
| Sex hormone binding globulin (nm) | 58.56 ± 7.10 | 43.39 ± 6.15 | 39.91 ± 5.59 | 0.13 |
| LH (mL U/mL) | 12.23 ± 3.36 | 11.42 ± 2.28 | 6.38 ± 0.82 | 0.17 |
| FSH (mL U/mL) | 8.22 ± 3.56 | 8.02 ± 1.77 | 6.53 ± 1.61 | 0.85 |
| Bone mineral density femoral neck (g/cm2) | 0.88 ± 0.02 | 0.97 ± 0.05 | 0.99 ± 0.05 | 0.29 |
| Bone mineral density lumbar spine (g/cm2) | 1.23 ± 0.04 | 1.40 ± 0.07 | 1.26 ± 0.07 | 0.13 |
| Lean body mass (kg) | 55.98 ± 1.26 | 56.04 ± 1.95 | 54.74 ± 2.12 | 0.86 |
| Fat mass (kg) | 28.52 ± 2.71 | 31.59 ± 2.88 | 26.91 ± 2.72 | 0.45 |
| Hand grip (kg) | 39.33 ± 2.40 | 41.08 ± 1.99 | 39.15 ± 2.73 | 0.82 |
| Knee extension (N–m) | 155.39 ± 13.97 | 133.82 ± 14.05 | 138.73 ± 13.39 | 0.39 |
| Knee flexion (N–m) | 77.60 ± 5.96 | 67.78 ± 5.25 | 64.75 ± 8.00 | 0.21 |
Data are expressed as mean ± SEM, groups were compared with anova.
Table 2.
Changes over time in bone mineral density and body composition
| Placebo (n = 9) | TT-group (n = 13)a |
AI-group (n = 13)a |
p value Placebo vs. TT |
p value Placebo vs. AI |
p value TT vs. AI |
|
|---|---|---|---|---|---|---|
| Lumbar spine (g/cm2) | ||||||
| Δ12 months | 0.047 ± 0.013††† | 0.042 ± 0.008†† | 0.008 ± 0.012†† | 0.77 | 0.025 | 0.047 |
| Femoral neck (g/cm2) | ||||||
| Δ12 months | 0.006 ± 0.010 | 0.0005 ± 0.008 | −0.002 ± 0.011 | 0.93 | 0.90 | 0.84 |
| Lean body mass (kg) | ||||||
| Δ3 months | −0.42 ± 0.35 | 0.80 ± 0.31 | 0.20 ± 0.71 | 0.36 | 0.23 | 0.32 |
| Δ6 months | −0.13 ± 0.29 | 0.93 ± 0.49 | 1.49 ± 0.38†† | |||
| Δ12 months | 0.26 ± 0.64 | 0.55 ± 0.66 | 1.24 ± 0.39† | |||
| Fat mass (kg) | ||||||
| Δ3 months | −0.32 ± 0.28 | 0.01 ± 0.48 | −0.59 ± 0.51 | 0.57 | 0.53 | 0.19 |
| Δ6 months | −0.62 ± 0.72 | −0.11 ± 0.48 | −1.23 ± 0.63† | |||
| Δ12 months | −0.64 ± 0.61 | −0.89 ± 0.76 | −1.76 ± 0.60†† | |||
Groups were compared by linear mixed-effects model for repeated measures and anova. Average change over time (mean ± SEM). Bold values are statistically significant.
p ≤ 0.05 and
p ≤ 0.01,
= p ≤ 0.001 compared to baseline value.
TT-group and AI-group N = 13 except for femoral neck and lumbar spine N = 9.
Table 3.
Changes over time in hand grip and lower extremity strength
| Placebo (n = 9) | TT-group (n = 12) |
AI-group (n = 12) |
p value Placebo vs. TT |
p value Placebo vs. AI |
p value TT vs. AI |
|
|---|---|---|---|---|---|---|
| Hand grip (kg) | ||||||
| Δ3 months | −1.29 ± 1.21 | −2.36 ± 0.71 | 0.17 ± 1.42 | 0.60 | 0.33 | 0.34 |
| Δ6 months | 1.25 ± 1.41 | −0.80 ± 0.90 | −1.60 ± 1.03 | |||
| Δ12 months | −2.67 ± 2.29 | −1.70 ± 1.41 | −2.10 ± 1.68 | |||
| Knee extensor strength (N–m) | ||||||
| Δ3 months | −1.61 ± 5.35 | 3.31 ± 4.26 | 18.71 ± 8.98†† | 0.90 | 0.13 | 0.17 |
| Δ6 months | −0.83 ± 5.91 | 10.78 ± 6.46 | 22.83 ± 7.31†† | |||
| Δ12 months | 2.22 ± 6.59 | 16.59 ± 8.43† | 14.64 ± 9.21† | |||
| Knee flexor strength (N–m) | ||||||
| Δ3 months | −1.53 ± 2.08 | 5.73 ± 7.41 | 10.66 ± 4.48† | 0.32 | 0.16 | 0.44 |
| Δ6 months | −2.06 ± 3.65 | 11.79 ± 10.32† | 12.74 ± 5.55† | |||
| Δ12 months | 0.97 ± 4.49 | 18.62 ± 10.30††† | 9.61 ± 3.45 | |||
Groups were compared by linear mixed effects model for repeated measures. Average change over time (mean ± SEM). Bold values are statistically significant.
p ≤ 0.05,
p ≤ 0.01 and
p ≤ 0.001 compared to baseline value.
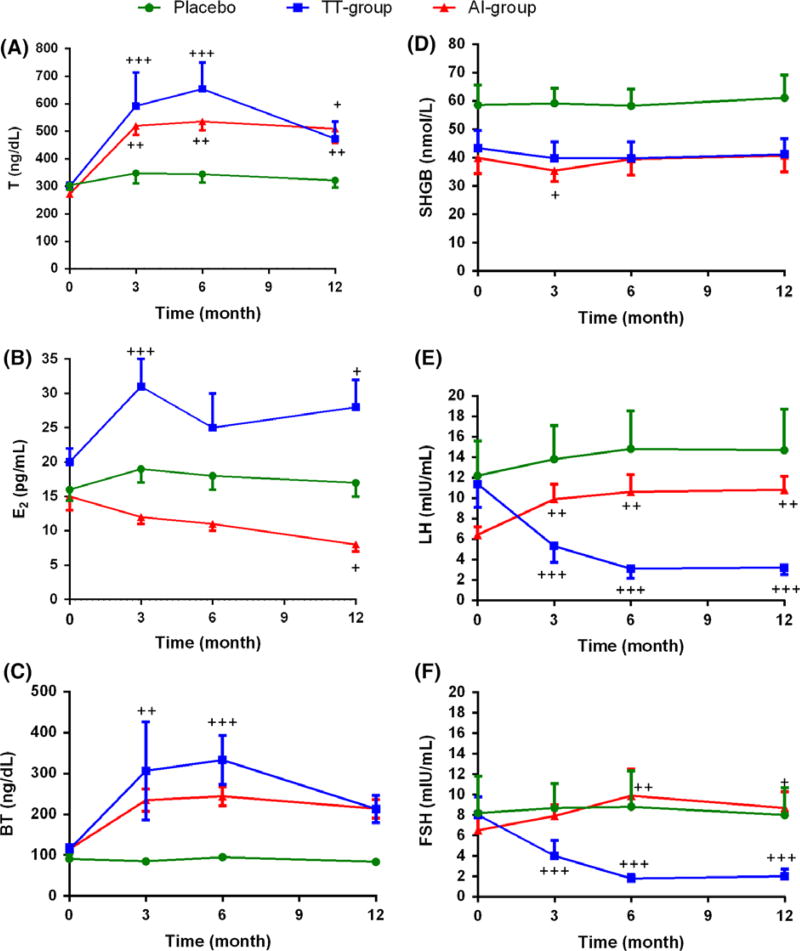
Figure 2.
Effects of Aromatase inhibitor and transdermal testosterone on circulating sex hormone and gonadotropin levels in older men with low testosterone. (A) Total T, (B) Estradiol, (C) Bioavailable-T, (D) Sex hormone binding globulin (SHBG), (E) LH (Luteinizing hormone), and, (F) FSH (Follicle-stimulating hormone). Data are mean ± SEM obtained from baseline values subtracted from values at each time point. Statistical comparisons were done by using linear mixed-effects models with random intercepts. +p ≤ 0.05; ++p ≤ 0.01; +++p ≤ 0.001 compared to baseline values (+). Statically significant p-value of group comparison for (A) were Placebo vs. TT-group p = 0.04; for (B) were TT-group vs. AI-group p = 0.02; for (E2) were Placebo vs. TT-group p < 0.0001, TT-group vs. AI-group p < 0.0001; for (F) were Placebo vs. TT-group p < 0.0001, TT-group vs. AI-group p < 0.0001. The comparison includes baseline values as a covariate. Placebo N = 9, TT-group N = 13, AI-group N = 13.
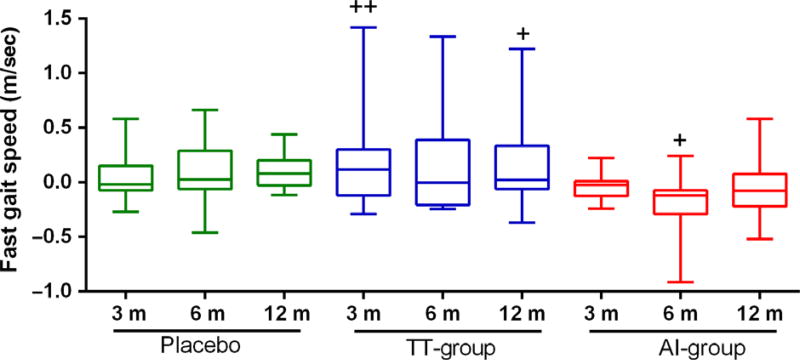
Figure 3.
Effects of Aromatase inhibitor and transdermal testosterone on gait speed in older men with low testosterone. Fast gait. Data are mean ± SEM obtained from baseline values subtracted from values at each time point. Statistical comparisons were done by using linear mixed-effects models with random intercepts. +p ≤ 0.05; ++p ≤ 0.01 compared to baseline values (+). Statistically significant p-value of group comparison was TT-group vs. AI-group p = 0.04. The comparison includes baseline values as a covariate. Placebo N = 9, TT-group N = 13, AI-group N = 13.
RESULTS
Baseline characteristics and study completion
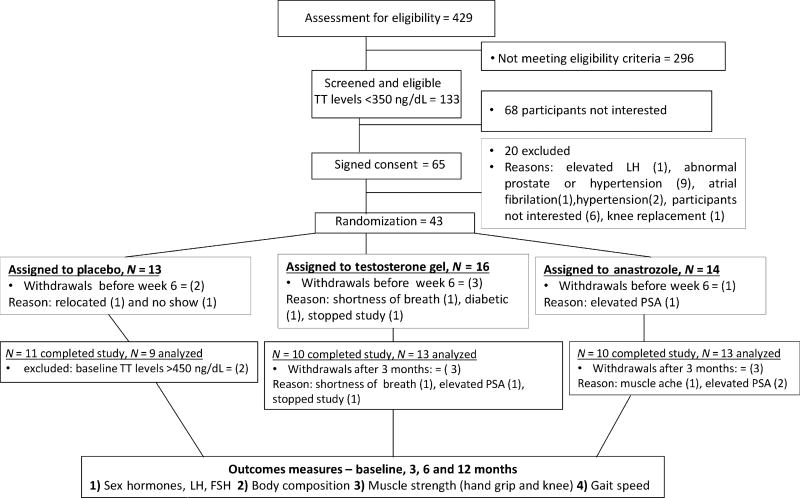
We assessed 429 men for eligibility of which 133 men had T levels < 350 ng/dL. We randomized 43 men. Baseline characteristics of the participants are summarized in Table 1. The groups were well-matched at baseline and none of the outcome parameters were statistically different. The majority of the men were overweight. Some participants withdrew before week 6 of the study, two men in the placebo, three in the TT-group and one in the AI-group. The study was completed by 37 participants. The study flow is summarized in the CONSORT diagram (Fig. 1).
Figure 1.
Recruitment of participants and study completion. The schema is showing participants invited for screening and assessed for eligibility, common reasons for exclusion, numbers of eligible participants randomized to placebo, transdermal testosterone and aromatase inhibitor, reasons for withdrawal from the study, and final numbers of participants in each group. LH, luteinizing hormone, PSA, prostate-specific antigen.
Serum hormones
Changes in sex hormones, gonadotropins, and SHBG levels in the three groups at 3, 6, and 12 month visits are shown in Fig. 2. In both the TT and AI groups, T levels significantly increased from baseline and were maintained in the a priori determined range throughout the course of the study. As expected, bioavailable T levels increased in both treatment groups. E2 levels significantly increased in the TT-group and decreased in the AI-group. SHBG levels did not change during intervention in any of the groups. Suppression of gonadotropins was seen in the TT group compared to placebo (p < 0.0001) whereas an increase was seen in the AI-group.
Bone mineral density (BMD)
At the 12-month time point, the increase in lumbar spine BMD in the TT-group (0.042 ± 0.008 g/cm2; p < 0.01) and placebo group(0.047 ± 0.013 g/cm2; p < 0.0001) was greater than in the AI group (0.008 ± 0.012). The average femoral neck BMD was similar between the three groups at baseline and did not change significantly over the course of the study (Table 2).
Body composition
Although LBM increased in both of the intervention groups, it only reached statistical significance in the AI group in which LBM increased by (1.2 ± 0.6 kg) at 12 months. Similarly, the reduction in FM was only significant in men on AI (1.8 ± 0.6 kg) at 12 months. However, across groups, these changes were not statistically significant (Table 2).
Muscle strength
The knee extensor strength increased significantly in both the AI and TT-groups; however, the changes between the groups were not statistically different. Compared to baseline, the knee flexor strength also increased in both the treatment groups but not in the placebo group. However, changes over time in knee flexor strength were also not significantly different among the three groups. Hand grip strength did not change in any group (Table 3).
Gait speed
At 12 months, the fast gait speed in the TT group was significantly increased by 0.18 ± 0.08 m/when compared to baseline. The fast gait speed remained stable in the placebo group, while it declined in the AI group. Fast gait speed was significantly different between the treatment groups (p = 0.042), but no statistical difference was observed between the placebo vs. TT-group (p = 0.57) or between the placebo vs. AI-group (p = 0.25) (Fig. 3).
DISCUSSION
This is the first proof-of-concept randomized-controlled trial investigating the effect of TT, AI, and placebo over 12 months on a range of outcomes in older men with low T levels. Additionally, to our knowledge, this is the first intervention study that has examined the effects of an aromatase inhibitor on muscle mass, muscle strength, and physical performance (gait speed) in older men. The findings of this study are made all more convincing by its strengths of design, including blinding, placebo control, concealed randomization, and the parallel-group design. Randomization effectively generated three groups that were similar in baseline characteristics. Screening and on-treatment T levels were measured using LC-MS/MS, the current standard of measurement. At baseline, mean total and free T levels were well below the lower limits of established norms in community-based samples and validated against outcomes in epidemiologic studies (Bhasin et al., 2011). T dose was adjusted and was effective in increasing T levels into the target range. Lastly, validated tools were used to evaluate outcome measures. Although the study was small, its strengths and findings provide ample motivation for a future confirmatory trial in a larger sample.
After 12 months, we found AI treatment was associated with lower lumbar spine BMD compared to TT and placebo group. Our results confirm previous reports that aromatization of T to E is important for the male skeleton. For example, a previous 12-month study in older hypogonadal men treated with AI also showed lower lumbar spine BMD compared to placebo (Burnett-Bowie et al., 2009). Although previous studies of older men have shown that T treatment increases lumbar spine BMD (Amory et al., 2004; Leder, 2007), we were unable to find a significant difference between the TT and placebo groups, perhaps because small sample size. We speculate that the increase in lumbar spine BMD in placebo could be attributed to oral calcium and vitamin D intake. Indeed, daily intake of 500 mg calcium and 700 IU vitamin D for 1 year by men >65 years increased BMD in lumbar spine (Dawson-Hughes et al., 1997). Our findings support the notion that sufficient E levels in older men is an important contributor to bone density (Falahati-Nini et al., 2000; Gennari et al., 2003; Amory et al., 2004; Rodriguez-Tolra et al., 2013; Argoud et al., 2014).
LBM increased in both intervention arms but was only statistically significant in the AI group. Our findings confirm previous observations that increase in LBM is primarily an androgen-dependent process (Katznelson et al., 1996; Srinivas-Shankar et al., 2010; Finkelstein et al., 2013) and does not require aromatization to E2. Men in the AI group lost 1.8 kg of FM. These findings are in contrast to those reported in a recent study by Finkelstein et al. showing that estradiol is obligatory to achieve loss of body fat (Finkelstein et al., 2013). These differences, at least partially, can be explained because of differences in age and baseline body composition of the participants in the two trials. In fact, T treatment is known to decrease FM in several studies. Contrary to other studies (Isidori et al., 2005; Hamilton et al., 2011; Frederiksen et al., 2012), the reduction in FM was not statistically significant in our trial; this could be because of the small sample size of our proof-of-concept study.
We did not observe a significant improvement in hand grip strength. Interestingly, in women, treatment with AI has been associated with loss of grip strength (Crew et al., 2007; Lintermans et al., 2011). Other studies in elderly men have demonstrated that T treatment improves hand grip strength (Sih et al., 1997; Page et al., 2005). Our sample included healthy older men and the lack of improvement in hand grip strength might be as a result of the low-ceiling effect. However, both TT and AI treatment were associated with increased muscle strength. These data suggest that aromatization of T is not required in mediating its effects on muscle strength, as reported by another recent dose–response study in men aged 20–50 years (Finkelstein et al., 2013). The increase in muscle strength of >5 N-min in our study has been associated with improved physical function (Kenny et al., 2010; Srinivas-Shankar et al., 2010; O’Connell et al., 2011).
Slow gait speed has been associated with increasing mortality in older individuals (Studenski et al., 2011), illustrating the importance of preserving walking speed. The observed gait speed in healthy individuals ranges from 0.5 to 1.9 m/s with an average of 1.1 ± 0.2 m/s (Schrack et al., 2012). In this study, participants had no mobility difficulties and yet a significant improvement in fast gait speed was observed in the TT group within subjects. Fast gait speed improved in the TT group but decreased at 6 months in the AI group. At 3 and 6 months, the difference in fast gait speed between the two treatment groups was statistically and clinically relevant as meaningful changes in gait speed in older individuals is considered to be 0.05 m/sec (Perera et al., 2014). Interestingly, the reduction in gait speed in the AI group did not persist at 12 months. The gait speed is a function of neuromuscular unit as a whole (not just skeletal muscle). It is conceivable that decreased production of estradiol has an initial impact on the performance of the neuromuscular unit, which later adapts, leading to recovery. Not all observational studies in elderly individuals are in agreement in respect to the association between T or E levels, and physical performance including walking. Some studies found total T levels positively associated with walking speed (Araujo et al., 2008) but not with E (Araujo et al., 2008; Schaap et al., 2008; LeBlanc et al., 2011) while other studies are contradictory (Schaap et al., 2008). Our findings suggest that reduced levels of E2 are unfavorable for walking speed, however; these findings should be confirmed in future interventional studies.
This study has some limitations. First, this study has a small sample size. Nonetheless; we were able to find statistically and clinically significant changes in the major outcomes of this trial. Second, circulating E2 levels may not reflect levels in the tissues. Third, we administered vitamin D and calcium to prevent exaggerated bone loss to all participants. We recommend larger mechanistic studies to validate our findings.
In summary, the primary contribution of this trial is contrasting the effects of TT and AI on physiological functions in older men. These data highlight the novel finding regarding the importance of E2 in walking speed. We also confirmed that serum E2 is essential in the maintenance of bone mineral density. Once these findings are confirmed in large prospective trials, the modalities of raising serum testosterone levels in older androgen-deficient men can be individualized to achieve benefit on the desired organ system.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Institute on Aging. Authors are most grateful to Dr. Jeffrey Metter for his advice and to the NIH Fellows Editorial Board.
CONFLICT OF INTEREST
Dr. Basaria has previously received investigator-initiated research grants from Abbvie Pharmaceuticals (previously Solvay Pharmaceuticals) and is a consultant for Eli Lilly, Inc.
Footnotes
AUTHORS CONTRIBUTIONS
JPD collected the data, conducted blood sample analysis and statistical analysis, and wrote the manuscript; DM assigned the nurse and clinical coordinator of the study; EMS wrote the manuscript; OC conducted blood sample analysis, MDS reviewed statistical analysis and wrote the manuscript; LF reviewed study findings and wrote the manuscript; CWC contributed to clinical aspect of the trial and reviewed manuscript; SB designed the study, wrote and reviewed the manuscript; JME designed the study, wrote and reviewed the manuscript.
References
- Amory JK, Watts NB, Easley KA, Sutton PR, Anawalt BD, Matsumoto AM, Bremner WJ, Tenover JL. Exogenous testosterone or testosterone with finasteride increases bone mineral density in older men with low serum testosterone. J Clin Endocrinol Metab. 2004;89:503–510. doi: 10.1210/jc.2003-031110. [DOI] [PubMed] [Google Scholar]
- Araujo AB, Travison TG, Bhasin S, Esche GR, Williams RE, Clark RV, McKinlay JB. Association between testosterone and estradiol and age-related decline in physical function in a diverse sample of men. J Am Geriatr Soc. 2008;56:2000–2008. doi: 10.1111/j.1532-5415.2008.01965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argoud T, Boutroy S, Claustrat B, Chapurlat R, Szulc P. Association between sex steroid levels and bone microarchitecture in men - the STRAMBO study. J Clin Endocrinol Metab. 2014;99:1400–10. doi: 10.1210/jc.2013-3233. [DOI] [PubMed] [Google Scholar]
- Basaria S. Testosterone therapy in older men with late-onset hypogonadism: a counter-rationale. Endocr Pract. 2013;19:853–863. doi: 10.4158/EP13318.RA. [DOI] [PubMed] [Google Scholar]
- Behre HM, Kliesch S, Puhse G, Reissmann T, Nieschlag E. High loading and low maintenance doses of a gonadotropin-releasing hormone antagonist effectively suppress serum luteinizing hormone, follicle-stimulating hormone, and testosterone in normal men. J Clin Endocrinol Metab. 1997;82:1403–1408. doi: 10.1210/jcem.82.5.3959. [DOI] [PubMed] [Google Scholar]
- Bhasin S, Pencina M, Jasuja GK, Travison TG, Coviello A, Orwoll E, Wang PY, Nielson C, Wu F, Tajar A, Labrie F, Vesper H, Zhang A, Ulloor J, Singh R, D’Agostino R, Vasan RS. Reference ranges for testosterone in men generated using liquid chromatography tandem mass spectrometry in a community-based sample of healthy nonobese young men in the Framingham Heart Study and applied to three geographically distinct cohorts. J Clin Endocrinol Metab. 2011;96:2430. doi: 10.1210/jc.2010-3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett-Bowie SA, McKay EA, Lee H, Leder BZ. Effects of aromatase inhibition on bone mineral density and bone turnover in older men with low testosterone levels. J Clin Endocrinol Metab. 2009;94:4785–4792. doi: 10.1210/jc.2009-0739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2. Lawrence Earlbaum Associates; Hillsdale, NJ: 1988. [Google Scholar]
- Crew KD, Greenlee H, Capodice J, Raptis G, Brafman L, Fuentes D, Sierra A, Hershman DL. Prevalence of joint symptoms in postmenopausal women taking aromatase inhibitors for early-stage breast cancer. J Clin Oncol. 2007;25:3877–3883. doi: 10.1200/JCO.2007.10.7573. [DOI] [PubMed] [Google Scholar]
- Dawson-Hughes B, Harris SS, Krall EA, Dallal GE. Effect of calcium and vitamin D supplementation on bone density in men and women 65 years of age or older. N Engl J Med. 1997;337:670–676. doi: 10.1056/NEJM199709043371003. [DOI] [PubMed] [Google Scholar]
- Falahati-Nini A, Riggs BL, Atkinson EJ, O’Fallon WM, Eastell R, Khosla S. Relative contributions of testosterone and estrogen in regulating bone resorption and formation in normal elderly men. J Clin Invest. 2000;106:1553–1560. doi: 10.1172/JCI10942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein JS, Lee H, Burnett-Bowie SA, Pallais JC, Yu EW, Borges LF, Jones BF, Barry CV, Wulczyn KE, Thomas BJ, Leder BZ. Gonadal steroids and body composition, strength, and sexual function in men. N Engl J Med. 2013;369:1011–1022. doi: 10.1056/NEJMoa1206168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkle WD, Greenland S, Ridgeway GK, Adams JL, Frasco MA, Cook MB, Fraumeni J, Jr, Hoover RN. Increased risk of non-fatal myocardial infarction following testosterone therapy prescription in men. PLoS ONE. 2014;9:e85805. doi: 10.1371/journal.pone.0085805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederiksen L, Hojlund K, Hougaard DM, Brixen K, Andersen M. Testosterone therapy increased muscle mass and lipid oxidation in aging men. Age (Dordr) 2012;34:145–156. doi: 10.1007/s11357-011-9213-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gennari L, Merlotti D, Martini G, Gonnelli S, Franci B, Campagna S, Lucani B, Dal Canto N, Valenti R, Gennari C, Nuti R. Longitudinal association between sex hormone levels, bone loss, and bone turnover in elderly men. J Clin Endocrinol Metab. 2003;88:5327–5333. doi: 10.1210/jc.2003-030736. [DOI] [PubMed] [Google Scholar]
- Hamilton EJ, Gianatti E, Strauss BJ, Wentworth J, Lim-Joon D, Bolton D, Zajac JD, Grossmann M. Increase in visceral and subcutaneous abdominal fat in men with prostate cancer treated with androgen deprivation therapy. Clin Endocrinol (Oxf) 2011;74:377–383. doi: 10.1111/j.1365-2265.2010.03942.x. [DOI] [PubMed] [Google Scholar]
- Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab. 2001;86:724–731. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- Isidori AM, Giannetta E, Greco EA, Gianfrilli D, Bonifacio V, Isidori A, Lenzi A, Fabbri A. Effects of testosterone on body composition, bone metabolism and serum lipid profile in middle-aged men: a meta-analysis. Clin Endocrinol (Oxf) 2005;63:280–293. doi: 10.1111/j.1365-2265.2005.02339.x. [DOI] [PubMed] [Google Scholar]
- Jackson JA, Riggs MW, Spiekerman AM. Testosterone deficiency as a risk factor for hip fractures in men: a case-control study. Am J Med Sci. 1992;304:4–8. doi: 10.1097/00000441-199207000-00003. [DOI] [PubMed] [Google Scholar]
- Katznelson L, Finkelstein JS, Schoenfeld DA, Rosenthal DI, Anderson EJ, Klibanski A. Increase in bone density and lean body mass during testosterone administration in men with acquired hypogonadism. J Clin Endocrinol Metab. 1996;81:4358–4365. doi: 10.1210/jcem.81.12.8954042. [DOI] [PubMed] [Google Scholar]
- Kenny AM, Prestwood KM, Gruman CA, Marcello KM, Raisz LG. Effects of trandermal testosterone on bone and muscle in older men with low bioavailable testosterone levels. J Gerontol A Biol Sci Med Sci. 2001;56:M266–M272. doi: 10.1093/gerona/56.5.m266. [DOI] [PubMed] [Google Scholar]
- Kenny AM, Kleppinger A, Annis K, Rathier M, Browner B, Judge JO, McGee D. Effects of transdermal testosterone on bone and muscle in older men with low bioavailable testosterone levels, low bone mass, and physical frailty. J Am Geriatr Soc. 2010;58:1134–1143. doi: 10.1111/j.1532-5415.2010.02865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc ES, Wang PY, Lee CG, Barrett-Connor E, Cauley JA, Hoffman AR, Laughlin GA, Marshall LM, Orwoll ES. Higher testosterone levels are associated with less loss of lean body mass in older men. J Clin Endocrinol Metab. 2011;96:3855–3863. doi: 10.1210/jc.2011-0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leder BZ. Testosterone, estradiol and aromatase inhibitor therapy in elderly men. J Steroid Biochem Mol Biol. 2007;106:162–167. doi: 10.1016/j.jsbmb.2007.05.018. [DOI] [PubMed] [Google Scholar]
- Lintermans A, Van CB, Van HM, Pans S, Verhaeghe J, Westhovens R, Henry NL, Wildiers H, Paridaens R, Dieudonne AS, Leunen K, Morales L, Verschueren K, Timmerman D, De Smet L, Vergote I, Christiaens MR, Neven P. Aromatase inhibitor-induced loss of grip strength is body mass index dependent: hypothesis-generating findings for its pathogenesis. Ann Oncol. 2011;22:1763–1769. doi: 10.1093/annonc/mdq699. [DOI] [PubMed] [Google Scholar]
- Loric S, Guechot J, Duron F, Aubert P, Giboudeau J. Determination of testosterone in serum not bound by sex-hormone-binding globulin: diagnostic value in hirsute women. Clin Chem. 1988;34:1826–1829. [PubMed] [Google Scholar]
- Manni A, Pardridge WM, Cefalu W, Nisula BC, Bardin CW, Santner SJ, Santen RJ. Bioavailability of albumin-bound testosterone. J Clin Endocril Metab. 1985;61:705–710. doi: 10.1210/jcem-61-4-705. [DOI] [PubMed] [Google Scholar]
- O’Connell MD, Roberts SA, Srinivas-Shankar U, Tajar A, Connolly MJ, Adams JE, Oldham JA, Wu FC. Do the effects of testosterone on muscle strength, physical function, body composition, and quality of life persist six months after treatment in intermediate-frail and frail elderly men? J Clin Endocrinol Metab. 2011;96:454–458. doi: 10.1210/jc.2010-1167. [DOI] [PubMed] [Google Scholar]
- Page ST, Amory JK, Bowman FD, Anawalt BD, Matsumoto AM, Bremner WJ, Tenover JL. Exogenous testosterone (T) alone or with finasteride increases physical performance, grip strength, and lean body mass in older men with low serum T. J Clin Endocrinol Metab. 2005;90:1502–1510. doi: 10.1210/jc.2004-1933. [DOI] [PubMed] [Google Scholar]
- Perera S, Studenski S, Newman A, Simonsick E, Harris T, Schwartz A, Visser M. Are estimates of meaningful decline in mobility performance consistent among clinically important subgroups? (Health ABC Study) J Gerontol A Biol Sci Med Sci. 2014;69:576–583. doi: 10.1093/gerona/glu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Tolra J, Torremade J, di Gregorio S, Del RL, Franco E. Effects of testosterone treatment on bone mineral density in men with testosterone deficiency syndrome. Andrology. 2013;1:570–575. doi: 10.1111/j.2047-2927.2013.00090.x. [DOI] [PubMed] [Google Scholar]
- Schaap LA, Pluijm SM, Deeg DJ, Penninx BW, Nicklas BJ, Lips P, Harris TB, Newman AB, Kritchevsky SB, Cauley JA, Goodpaster BH, Tylavsky FA, Yaffe K, Visser M. Low testosterone levels and decline in physical performance and muscle strength in older men: findings from two prospective cohort studies. Clin Endocrinol (Oxf) 2008;68:42–50. doi: 10.1111/j.1365-2265.2007.02997.x. [DOI] [PubMed] [Google Scholar]
- Schrack JA, Simonsick EM, Chaves PH, Ferrucci L. The role of energetic cost in the age-related slowing of gait speed. J Am Geriatr Soc. 2012;60:1811–1816. doi: 10.1111/j.1532-5415.2012.04153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sih R, Morley JE, Kaiser FE, Perry HM, III, Patrick P, Ross C. Testosterone replacement in older hypogonadal men: a 12-month randomized controlled trial. J Clin Endocrinol Metab. 1997;82:1661–1667. doi: 10.1210/jcem.82.6.3988. [DOI] [PubMed] [Google Scholar]
- Simonsick EM, Newman AB, Nevitt MC, Kritchevsky SB, Ferrucci L, Guralnik JM, Harris T. Measuring higher level physical function in well-functioning older adults: expanding familiar approaches in the Health ABC study. J Gerontol A Biol Sci Med Sci. 2001;56:M644–M649. doi: 10.1093/gerona/56.10.m644. [DOI] [PubMed] [Google Scholar]
- Spitzer M, Bhasin S, Travison TG, Davda MN, Stroh H, Basaria S. Sildenafil increases serum testosterone levels by a direct action on the testes. Andrology. 2013;1:913–918. doi: 10.1111/j.2047-2927.2013.00131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas-Shankar U, Roberts SA, Connolly MJ, O’Connell MD, Adams JE, Oldham JA, Wu FC. Effects of testosterone on muscle strength, physical function, body composition, and quality of life in intermediate-frail and frail elderly men: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2010;95:639–650. doi: 10.1210/jc.2009-1251. [DOI] [PubMed] [Google Scholar]
- Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, Brach J, Chandler J, Cawthon P, Connor EB, Nevitt M, Visser M, Kritchevsky S, Badinelli S, Harris T, Newman AB, Cauley J, Ferrucci L, Guralnik J. Gait speed and survival in older adults. JAMA. 2011;305:50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thienpont LM, Van UK, Blincko S, Ramsay CS, Xie H, Doss RC, Keevil BG, Owen LJ, Rockwood AL, Kushnir MM, Chun KY, Chandler DW, Field HP, Sluss PM. State-of-the-art of serum testosterone measurement by isotope dilution-liquid chromatography-tandem mass spectrometry. Clin Chem. 2008;54:1290–1297. doi: 10.1373/clinchem.2008.105841. [DOI] [PubMed] [Google Scholar]
- Walsh S, Liu D, Metter EJ, Ferrucci L, Roth SM. ACTN3 genotype is associated with muscle phenotypes in women across the adult age span. J Appl Physiol. 2008;105:1486–1491. doi: 10.1152/japplphysiol.90856.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]