Abstract
Background
To evaluate the structural changes associated with visual acuity (VA) in patients with idiopathic macular telangiectasia (MT) type 1 using multimodal imaging modalities.
Methods
A retrospective study of 14 patients with MT type 1 and of 10 eyes from 10 healthy individuals as age-matched controls was conducted. The medical records of patients who had undergone colour fundus photography, spectral domain optical coherence tomography (OCT), fluorescein angiography and OCT angiography were reviewed. Central macular thickness (CMT), the areas of macular oedema and ellipsoid zone (EZ) disruption, EZ length, disorganization of the retinal inner layers (DRIL) and external limiting membrane (ELM) disruption, as measured by spectral domain OCT; and vascular density and the foveal avascular zones (FAZ) of the superficial capillary plexus (SCP) and deep capillary plexus (DCP), as measured by OCT angiography, were assessed in MT type 1 eyes and correlated with VA.
Results
The mean baseline best-corrected VA of MT type 1 eyes was 0.45 ± 0.28. The mean CMT was 385.19 ± 75.21 μm in MT type 1 eyes and 252.43 ± 15.77 μm in contralateral eyes (Z = − 4.113, p < 0.001). The mean vessel density of the DCP was lower in MT type 1 eyes (47.25 ± 4.69%) than in contralateral eyes (53.93 ± 2.94%) and normal eyes (59.37 ± 2.50%) (Z = − 3.492, − 4.099; p < 0.001, < 0.001). The baseline logMAR VA was correlated with CMT (r = 0.682, p = 0.007), SCP density (r = − 0.652, p = 0.012), DCP density (r = − 0.700, p = 0.005), total area of EZ disruption (r = 0.649, p = 0.012); and total lengths of EZ (r = 0.681, p = 0.007), ELM (r = 0.699, p = 0.005) and DRIL (r = 0.707, p = 0.005) disruption in the 1-mm-diameter foveal region in MT type 1 eyes.
Conclusions
Decreased DCP density and the presence of DRIL may be predictive biomarkers of VA in MT type 1. CMT, SCP density, total area of EZ disruption, and lengths of EZ and ELM disruption within the 1-mm-diameter central region were strongly associated with VA.
Keywords: Macular telangiectasia type 1, Disorganization of the retinal inner layers, Optical coherence tomography angiography, Ellipsoid zone disruption
Background
Idiopathic macular telangiectasia (MT) is characterized by abnormally dilated and tortuous capillaries around the fovea for no known reason. MT was originally termed idiopathic juxtafoveolar retinal telangiectasis, and was classified by Gass and Oyakawa into three categories: exudation, non-exudation and combination with or without nervous system vasculopathy [1]. Based on recent advances in imaging that have led to better characterization of the disease, Yannuzzi et al. have recently defined two distinct forms of the disease, MT type 1 (aneurysmal telangiectasia) and MT type 2 (perifoveal telangiectasia) [2].
MT type 1 is typically a unilateral disease that is found more often in men and is clinically associated with loss of retinal transparency, salient extensive ectasia, lipid exudates, arteriolar aneurysms, deeply right-angled venules, and intra-retinal cystoid degeneration mainly on the temporal side of the macula [2]. Previous studies of patients with MT type 1 have assessed the correlations of visual acuity (VA) with photoreceptor inner segment–outer segment (IS/OS) disruption and the cystoid space using spectral domain optical coherence tomography (SD-OCT), and that with the microvascular density of the superficial capillary plexus (SCP) and deep capillary plexus (DCP) using OCT angiography (OCTA) [3, 4]. However, few studies have explored the associations of SD-OCT- and OCTA-derived parameters with VA.
Although SD-OCT can be effective for evaluating the thickness of vascularized layers by automatic segmentation [5, 6], OCTA provides more detailed information of the vascular networks. OCTA is a new technology used as an auxiliary examination to provide high-resolution imaging of retinal morphology and sensitive identification of the vascular layers [7], and has become a critical method for evaluating retinal vascular disorders such as diabetic retinopathy [8, 9], retinal vein occlusion [10, 11] and MT types 1 [4, 12, 13] and 2 [14, 15].
The aims of our study were to evaluate the OCTA and SD-OCT features of MT type 1, to compare SCP and DCP microvascular densities between normal and contralateral eyes, and to correlate VA with central macular thickness (CMT), areas of macular oedema and ellipsoid zone (EZ) disruption, lengths of the EZ, disorganization of the retinal inner layers (DRIL) and external limiting membrane (ELM) disruption, as measured by OCT; and with vascular density and the foveal avascular zone (FAZ) areas of the SCP and DCP, as measured by OCTA.
Methods
This was a retrospective study that enrolled consecutive patients with MT type 1 diagnosed from December 2014 to September 2017 at the outpatient clinic of the Eye & ENT Hospital of Fudan University, Shanghai, China. This study was approved by the Institutional Review Board of the Eye and ENT Hospital of Fudan University. All procedures were performed according to the principles of the Declaration of Helsinki. Written informed consent was obtained from all participants or their guardians.
Patients underwent comprehensive ocular examinations in both eyes, including best-corrected VA (BCVA) evaluation, intraocular pressure reading, slit-lamp biomicroscopic ophthalmoscopic exam, dilated fundoscopy, fundus photography (Topcon TRC50LX; Topcon, Tokyo, Japan), OCTA (Optovue RTVue XR 100 Avanti, Fremont, CA, USA), SD-OCT (Heidelberg Engineering, Heidelberg, Germany) and fluorescein angiography (FA, Topcon TRC501X; Topcon). None of the patients in this study had undergone previous ocular treatment. As the control group, 10 right eyes imaged by SD-OCT and OCTA from 10 healthy individuals without any ocular diseases who were of similar age and sex as the MT type 1 cases were evaluated.
The clinical criteria used for diagnosing MT type 1 were confirmed by FA and included the following: (1) FA detection of unilateral, visible telangiectasis, aneurysms during the early stage and fluorescein leakage during the late stage of MT type 1, (2) OCTA detection of telangiectasis, aneurysms and decreased vascular densities of the SCP and DCP and (3) SD-OCT detection of intra-retinal oedema and hard exudates. Patients with neovascular maculopathies (e.g., age-related macular degeneration, polypoidal choroidal vasculopathy, retinal angiomatous proliferation, angioid streaks and other causes of secondary MT, including Coats’ disease, Leber disease, retinal vein occlusion and radiation retinopathy) were excluded. Also excluded were patients with diabetes, hypertension, ischemic heart disease, a history of vitreoretinal surgery, ophthalmic disorders excluding mild refractive errors and mild cataracts, or an oral history of anti-oestrogen tamoxifen for breast cancer.
SD-OCT was performed with 6-mm line scans (vertical and horizontal) across the centre of the fovea. A 19 line scans that covered a 20 × 15° (5.8 × 4.3 mm) area centred on the fovea was obtained in all eyes, with 20 automated real-time means per scan on high-resolution mode. The CMT in the 1-mm-diameter central region of the macula according to the Early Treatment of Diabetic Retinopathy Study thickness map was measured using Spectralis software. The ELM is formed by the junctions between the inner segments of photoreceptors and Müller cells [16]. The EZ is formed by the reflectivity generated from the high mitochondrial density of the outermost portion of the inner photoreceptor segments [17]. To calculate the area of EZ disruption, the intensity of the EZ band and border of the EZ disruption were measured using the plot profile function in ImageJ software (National Institutes of Health, Bethesda, MD, USA) and the grayscale image obtained by SD-OCT. The border of the disrupted EZ area was defined as a decrease in EZ reflectivity of 2 SD compared with an EZ band in the normal retina [18]. DRIL was defined as the inability to identify and differentiate any of the boundaries of the ganglion cell layer–inner plexiform layer (IPL) complex, inner nuclear layer, and outer plexiform layer (OPL) [19]. All images were obtained by well-trained operators (GJL, TWY). All data were measured within a 1-mm-diameter region encompassing the foveal centre and also on three B-scans performed immediately above and below.
Two independent observers (GJL, TWY) assessed the OCTA data using the best-quality 3 × 3-mm scan and controlled the corrected segmentation for the 14 patients before reporting the data. Vascular retinal layers were divided into the SCP, DCP, outer retina and choroidal layers by OCTA. The FAZ and density of the macula were assessed using flow density map software Angio-Analytics (Optovue RTVue XR 100 Avanti). The signal strength index of all images was greater than 60. Whole-image data were used to measure the microvascular densities in the SCP and DCP layers.
Statistical analysis
BCVA values were converted into logarithm of the minimum angle of resolution values (logMAR) for statistical evaluation. Quantitative data (mean BCVA, CMT, vascular density, FAZ areas of the SCP and DCP) were compared among MT type 1, contralateral and normal eyes by the Mann–Whitney test and Wilcoxon test using IBM SPSS Statistics v19 (SPSS Inc., Chicago, IL, USA). Significance was defined as P < 0.05. Pearson’s correlation coefficient analyses were used to identify the factors associated with VA. The correlation was defined as none/very weak at r < 0.1, weak at 0.1 < r < 0.3, moderate at 0.3 < r < 0.6, strong at 0.6 < r < 0.8, or very strong at 0.8 < r < 1 [19].
Results
A total of 14 eyes in 14 patients (8 men and 6 women) were included in the study. The mean ± SD age was 55.07 ± 12.74 years (range, 34–75 years). The BCVA values (mean ± SD) in the MT type 1, contralateral and control eyes at baseline (logMAR) were 0.45 ± 0.28 (range, 20/20 to 20/200), 0.07 ± 0.07 (range, 20/20 to 20/32) and 0.02 ± 0.04 (range, 20/20 to 20/25), respectively. The CMT values (mean ± SD) in the MT type 1, contralateral and normal eyes were 385.19 ± 75.21 (range, 224–487) μm, 252.43 ± 15.77 (range, 225–280) μm and 230.30 ± 7.04 (221–241) μm, respectively (Fig. 1). The fundus photography revealed oedematous maculae in 13 eyes, in which macular oedema appeared as hypo-reflective areas, and hard exudates and a few aneurysms appeared as hyper-reflective areas on SD-OCT at the first visit. In the remaining patient, SD-OCT displayed multiple disorganized hyper-reflective points from the IPL to the OPL (Fig. 2). The CMT values were higher in the 14 MT type 1 eyes than in the contralateral and normal eyes (Z = − 3.296, Z = − 4.101; p < 0.001, p < 0.001). An interrupted EZ structure was found in 13 patients. On FA, abnormally dilated and tortuous capillaries and aneurysms were observed during the early phase and intense hyperfluorescence and parafoveal leakage during the late phase, mainly in the temporal hemisphere. The demographic characteristics of the patients are listed in Table 1.
Fig. 1.
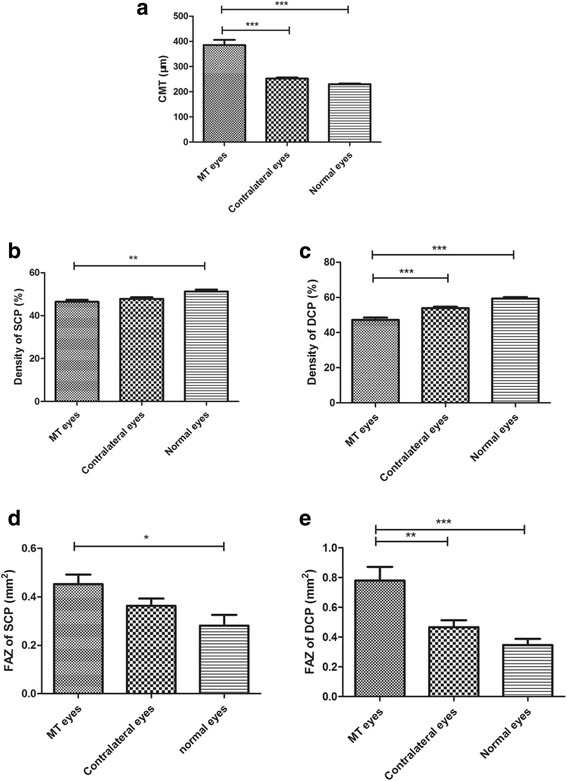
Comparisons among idiopathic macular telangiectasia (MT) type 1 eyes, contralateral eyes and normal eyes. (a) Central macular thickness (CMT). (b) Density of the superficial capillary plexus (SCP). (c) Density of the deep capillary plexus (DCP). (d) Foveal avascular zone (FAZ) area of the SCP. (e) FAZ area of the DCP. *p < 0.05, ** p < 0.01, *** p < 0.001
Fig. 2.
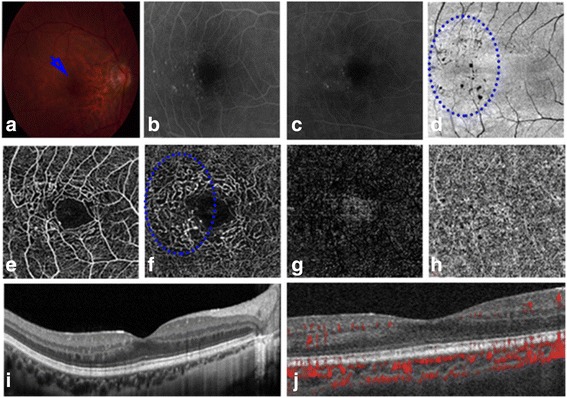
Imaging of the right eye of Patient 4. Capillary variations are visible from the inner to the outer plexiform layers on the temporal side of the macula. (a) Blue arrow shows sparse micro-aneurysms. (b) Fluorescein angiography (FA) reveals hyperfluorescence from micro-aneurysms on the temporal side of the macula during the early stage of MT type 1. (c) FA showing leakage of fluorescein due to vascular endothelial cell injury during the late stage of MT type 1. (d) No lesion is visible in the inner and outer segment junction (IS/OS) and blue-dotted oval shows sparse micro-aneurysms on en-face OCT. (e) OCT angiography (OCTA) reveals decreased capillary density in the superficial capillary plexus. (f) OCTA image showing the transformational foveal avascular zone (FAZ), aneurysms, and telangiectasia in the deep capillary plexus (DCP) on the temporal side of the macula. (g and h) OCTA images show no changes in the outer or choroidal layers, respectively. (i) SD-OCT shows areas of hyper-reflectance from the inner to the outer plexiform layers on the temporal side. (j) OCTA showing blood flow
Table 1.
Characteristics of idiopathic macular telangiectasia type 1 patients and normal patients
| MT eyes | Fellow eyes | Normal eyes | |
|---|---|---|---|
| Number of eyes | 14 | 14 | 10 |
| Age (years) | 55.07 ± 12.74 (34-75) | 55.07 ± 12.74 (34-75) | 48.80 ± 9.32(36-60) |
| Sex (men/women) | 8/6 | 8/6 | 6/4 |
| BCVA(LogMAR) | 0.45 ± 0.28 | 0.07 ± 0.07 | 0.02 ± 0.04 |
| BCVA (Snellen equivalent) | 20/44 (20/20-20/200) | 20/23 (20/20-20/32) | 20/21 (20/20-20/25) |
| Microvascular density of SCP(%) | 46.47 ± 3.42 (38.25-51.90) | 47.78 ± 2.89 (42.52-52.33) | 51.30 ± 2.81 (46.46-54.50) |
| Microvascular density of DCP(%) | 47.25 ± 4.69 (39.90-54.60) | 53.93 ± 2.94 (48.36-57.83) | 59.37 ± 2.50 (54.62-61.96) |
| FAZ of SCP (mm2) | 0.45 ± 0.15 (0.22-0.71) | 0.36 ± 0.11 (0.21-0.59) | 0.28 ± 0.14 (0.07-0.55) |
| FAZ of DCP (mm2) | 0.78 ± 0.35 (0.25-1.45) | 0.47 ± 0.17 (0.33-0.94) | 0.35 ± 0.13 (0.11-0.57) |
| CMT (μm) | 385.19 ± 75.21 (224-487) | 252.43 ± 15.77 (225-280) | 230.30 ± 7.04(221-241) |
| Mean areas with photoreceptor damage (mm2) | 3.22 ± 2.02 (0-5.81) |
BCVA best-corrected visual acuity, SCP superficial plexus, DCP deep plexus, FAZ foveal avascular zone, CMT centre macular thickness, MT idiopathic macular telangiectasia
In the OCTA examination, decreased vessel density and telangiectasis in the SCP and DCP were observed in all of the MT type 1 eyes, and these observations were much clearer compared with those on FA or OCT when observing telangiectasis variations in real time. The high resolution of OCTA enabled the clearest view of the vascular structure of the retina. The mean vessel density value of the DCP (47.25 ± 4.69%) was lower in MT type 1 eyes than in contralateral eyes (53.93 ± 2.94%) and normal eyes (59.37 ± 2.50%) (Z = − 3.492, Z = − 4.099; p < 0.001, p < 0.001). The FAZ areas of the SCP and DCP were larger in MT type 1 eyes (0.45 ± 0.15 mm2 and 0.78 ± 0.35 mm2, respectively) than in normal eyes (0.28 ± 0.14 mm2 and 0.35 ± 0.13 mm2, respectively) (Z = − 2.372, Z = − 3.455; p = 0.018, p = 0.001; Fig. 1).
The baseline logMAR VA was correlated with the CMT (r = 0.682, p = 0.007), densities of the SCP (r = − 0.652, p = 0.012) and DCP (r = − 0.700, p = 0.005), total area of EZ disruption (r = 0.649, p = 0.012); and the lengths of EZ (r = 0.681, p = 0.007), ELM (r = 0.699, p = 0.005) and DRIL (r = 0.707, p = 0.005) disruption in the 1-mm-diameter foveal region in MT type 1 eyes. The associations of baseline SD-OCT and OCTA parameters with baseline logMAR VA are shown in Table 2.
Table 2.
Associations of baseline spectral-domain optical coherence tomography and optical coherence tomography angiography parameters with baseline logMAR visual acuity
| Parameters | MT type 1 eyes | r | p value |
|---|---|---|---|
| FAZ of SCP (mm2) | 0.45 ± 0.15 | 0.451 | 0.106 |
| FAZ of DCP (mm2) | 0.78 ± 0.35 | 0.521 | 0.056 |
| Microvascular density of SCP (%) | 46.47 ± 3.42 | −0.652 | 0.012 |
| Microvascular density of DCP (%) | 47.25 ± 4.69 | − 0.700 | 0.005 |
| CMT (μm) | 385.19 ± 75.21 | 0.682 | 0.007 |
| Total area of macular oedema (mm2) | 0.08 ± 0.10 | 0.443 | 0.112 |
| Largest area of macular edema (mm2) | 0.08 ± 0.09 | 0.512 | 0.061 |
| Length of EZ disruption (μm) | 642.26 ± 349.66 | 0.681 | 0.007 |
| Area of EZ disruption (mm2) | 0.93 ± 1.22 | 0.27 | 0.351 |
| Total area of EZ disruption (mm2) | 3.22 ± 2.02 | 0.649 | 0.012 |
| Length of ELM disruption (μm) | 372.57 ± 279.16 | 0.699 | 0.005 |
| Length of DRIL disruption (μm) | 480.79 ± 340.73 | 0.707 | 0.005 |
p-values indicate the relationships between logMAR VA and spectral domain OCT and OCTA parameters
OCT spectral domain optical coherence tomography, OCTA optical coherence tomography angiography, logMAR logarithm of the minimal angle of resolution, VA visual acuity, MT idiopathic macular telangiectasia, FAZ foveal avascular zone, SCP superficial capillary plexus, DCP deep capillary plexus, CMT central macular thickness, EZ ellipsoid zone, ELM external limiting membrane, DRIL disorganization of retinal inner layers, r Pearson’s correlation coefficient
Early-stage MT type 1 was observed in Patient 4 (Fig. 2). In this patient, central macular oedema (CME) was not detected by SD-OCT (Fig. 2g), but the patient had blurred vision and abnormal microvascular variations in the IPL and OPL. Aneurysms were subsequently seen on FA (Fig. 2b) and OCTA. The telangiectasis of SCP and DCP was clearly revealed by OCTA (Fig. 2c). The vascular changes were barely evident in the SCP but prominent in the DCP.
In addition, the DCP density was decreased in MT type 1 eyes compared with contralateral and normal eyes. The FAZ areas in the SCP and DCP were larger in MT type 1 eyes than in contralateral and normal eyes.
Discussion
Few studies have evaluated the correlations between anatomic factors and baseline VA in MT type 1 [3, 4]. Takayama et al. reported strong associations between intra-retinal cystoid spaces and IS/OS alterations and retinal sensitivity [3]. Matet et al. found that logMAR VA was inversely correlated with SCP and DCP densities [4]. The current study demonstrated strong correlations of baseline logMAR VA with DRIL and microvascular DCP density, and associations were also found between baseline logMAR VA and CMT, SCP density, lengths of EZ and ELM disruption within the 1-mm-diameter central region of the macula, and the total area of EZ disruption. Because these SD-OCT- and OCTA-derived anatomic parameters can provide further information on VA, they could be useful in determining therapeutic regimens and for developing patient counselling strategies.
In the present study, a strong correlation was found between baseline logMAR VA and DRIL. The inner retinal layers play crucial roles in neural transmission from the photoreceptors to the retinal ganglion cells. The inability to distinguish the retinal layer boundaries likely represents partial destruction of the inner retinal cells, thus interrupting transmission between the photoreceptors and the ganglion cells [20]. Sun et al. were the first to report that DRIL is correlated with worse VA in eyes with diabetic macular oedema [21]. All of the MT type 1 eyes in the present study had DRIL, which was positively correlated with baseline logMAR VA. Furthermore, the reduced viable tissue in DRIL may further decrease the demand for a blood supply, resulting in loss of the deep vascular plexus [22]. This view was confirmed by our findings that the DCP density was decreased in all patients with MT type 1 and was correlated with logMAR VA. The ELM, which exhibits several different junctional complexes between Muller and rod/cone photoreceptor cells, may be an indispensable factor for the survival of photoreceptor cells. The integrity of the ELM and EZ may be regarded as predictors of a better VA prognosis [10]. In our study, the lengths of EZ and ELM disruption within the 1-mm-diameter central region and the total area of EZ disruption were positively correlated with the baseline logMAR VA.
With the rapid development of imaging techniques, OCTA has become a convenient and noninvasive method for evaluating ocular variations in retinal vascular diseases (particularly variations in the deep capillary) that can be used at every visit to the clinic [23]. SD-OCT and FA enable gross evaluation of changes in the vascular network. Because of the limited visualization of FA due to dye leakage, we were unable to observe the deep vascular plexus in more detail using this method. However, OCTA has the particular advantage of visualizing real-time dynamic changes in various retinal layers, and enables gross quantification of capillary density in several retinal layers simultaneously [23]. Accordingly, we can use OCTA to assess the reductions in capillary density in both the SCP and DCP in comparison with those of the contralateral healthy eye, despite the lack of an accurate measuring technique. Moreover, the alterations in DCP density and FAZ area are specific parameters for analysis at follow-up. Two unique characteristics of MT type 1 eyes were revealed in the present patients by OCTA. First, there was a negative correlation between DCP density in the lesion and logMAR VA in our cases. Second, decreased capillary density variation in the deep layer was a predictor of very-early-stage MT type 1. Birol et al. confirmed in an animal model that the deep capillary bed is conducive to the oxygen requirements of the photoreceptor layer [24]. In their study, the primary oxygen supply to photoreceptor layers was derived from the choroidal circulation, but 10%–15% was derived from the retinal circulation. As oxygenation of the fovea is somewhat different from that of the perifoveal retina, it would be useful to discuss this and any other potentially relevant correlations regarding the foveal and perifoveal areas. We speculate that the vascular variation in the DCP and DRIL may predict hypoxidosis in the photoreceptor layer. Of the 13 patients in the study of Birol et al., 12 had characteristics of macular oedema rather than retinal atrophy. However, among our cases, patient 4, who had obviously distorted capillaries and aneurysms during the early stage, and leakage during the late stage according to FA, had slightly blurred vision without CME, but the DCP density and FAZ area already showed obvious transformations. One possible explanation for this finding is that the abnormal morphology and structure from the IPL to OPL on the temporal side of the macula are also related to telangiectasia. Gass and Oyakawa [1] suggested that disruption of the deeper capillary plexus predominantly causes variation in the retinal vasculature and that the late diffuse fluorescence is derived from the outer retina. It has been reported that in MT type 1, changes occur earlier in the deep capillary bed than in the superficial capillary bed [25], which was confirmed in the present series.
The limitations of our study included its retrospective design and the small sample size due to the low prevalence of MT type 1. The captured images inevitably have artefacts despite being obtained by two experienced observers (GJL and TWY).
Conclusion
The greater the scale of EZ disruption and/or lengths of DRIL and ELM disruption in the lesion, the worse the vision. Decreased DCP density is potentially an earlier indicator of MT type 1 than are macular oedema and EZ layer disruption. We speculate that variation in structure from the IPL to the OPL on OCT and morphological changes on OCTA are manifestations of very-early-stage MT type 1.
Acknowledgements
We would like to thank all of the participants and staff for their valuable contributions to this research.
Funding
This study was supported by grant SHDC12016116 from the Science and Technology Commission of Shanghai Municipality.
Availability of data and materials
Transcripts from this study are available for sharing upon request; however, all identifying and confidential information of the participants will be removed.
Abbreviations
- BCVA
best-corrected visual acuity
- CMT
central macular thickness
- DCP
deep capillary plexus
- DRIL
disorganization of the retinal inner layers
- ELM
external limiting membrane
- EZ
ellipsoid zone
- FA
fluorescein angiography
- FAZ
foveal avascular zone
- MT type 1
idiopathic macular telangiectasia type 1
- OCTA
optical coherence tomography angiography
- SCP
superficial capillary plexus
- SD-OCT
spectral-domain optical coherence tomography
- VA
visual acuity
Authors’ contributions
GJL: substantial contributions to the study conception and design and data acquisition, analysis and interpretation; TWY: substantial contributions to data analysis and interpretation; YXF: acquisition of data and research design; WHX: acquisition of data and research design; XGZ: acquisition of data and research design. LW: final approval of the version to be published, research design and manuscript preparation. ZYJ: final approval of the version to be published, research design, data analysis and manuscript preparation. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
This study was approved by the Institutional Review Board of the Eye and ENT Hospital of Fudan University. All procedures were approved by the principles of the Declaration of Helsinki. Written informed consent was obtained from all participants or their guardians.
Consent for publication
Not Applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jingli Guo, Email: 18553522773@163.com.
WenYi Tang, Email: drtangwy@163.com.
Xiaofeng Ye, Email: newnewye@163.com.
Haixiang Wu, Email: whx577@163.com.
Gezhi Xu, Email: drxugezhi@163.com.
Wei Liu, Email: bfgf14@aliyun.com.
Yongjin Zhang, Email: yongjinzhang@yahoo.com.
References
- 1.Gass JD, Oyakawa RT. Idiopathic juxtafoveolar retinal telangiectasis. Arch Ophthalmol. 1982;100:769–780. doi: 10.1001/archopht.1982.01030030773010. [DOI] [PubMed] [Google Scholar]
- 2.Yannuzzi LA, Bardal AM, Freund KB, Chen KJ, Eandi CM, Blodi B. Idiopathic macular telangiectasia. Arch Ophthalmol. 2006;124:450–460. doi: 10.1001/archopht.124.4.450. [DOI] [PubMed] [Google Scholar]
- 3.Takayama K, Ooto S, Tamura H, Yamashiro K, Otani A, Tsujikawa A, et al. Retinal structural alterations and macular sensitivity in idiopathic macular telangiectasia type 1. Retina. 2012;32:1973–1980. doi: 10.1097/IAE.0b013e318251a38b. [DOI] [PubMed] [Google Scholar]
- 4.Matet A, Daruich A, Dirani A, Ambresin A, Behar-Cohen F. Macular telangiectasia type 1: capillary density and microvascular abnormalities assessed by optical coherence tomography angiography. Am J Ophthalmol. 2016;167:18–30. doi: 10.1016/j.ajo.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 5.Demirkaya N, van Dijk HW, van Schuppen SM, Abramoff MD, Garvin MK, Sonka M, et al. Effect of age on individual retinal layer thickness in normal eyes as measured with spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2013;54(7):4934–4940. doi: 10.1167/iovs.13-11913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abdolrahimzadeh S, Parisi F, Scavella V, Recupero SM. Optical coherence tomography evidence on the correlation of choroidal thickness and age with vascularized retinal layers in normal eyes. Retina. 2016;36(12):2329–2338. doi: 10.1097/IAE.0000000000001097. [DOI] [PubMed] [Google Scholar]
- 7.Wylegala A, Teper S, Dobrowolski D, Wylegala E. Optical coherence angiography: a review. Medicine (Baltimore) 2016;95(41):e4907. doi: 10.1097/MD.0000000000004907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ishibazawa A, Nagaoka T, Yokota H, Takahashi A, Omae T, Song YS, et al. Characteristics of retinal neovascularization in proliferative diabetic retinopathy imaged by optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2016;57(14):6247–6255. doi: 10.1167/iovs.16-20210. [DOI] [PubMed] [Google Scholar]
- 9.Soares M, Neves C, Marques IP, Pires I, Schwartz C, Costa MA, et al. Comparison of diabetic retinopathy classification using fluorescein angiography and optical coherence tomography angiography. Br J Ophthalmol. 2017;101(1):62–68. doi: 10.1136/bjophthalmol-2016-309424. [DOI] [PubMed] [Google Scholar]
- 10.Kang JW, Yoo R, Jo YH, Kim HC. Correlation of microvascular structures on optical coherence tomography angiography with visual acuity in retinal vein occlusion. Retina. 2017;37(9):1700–1709. doi: 10.1097/IAE.0000000000001403. [DOI] [PubMed] [Google Scholar]
- 11.Balaratnasingam C, Inoue M, Ahn S, McCann J, Dhrami-Gavazi E, Yannuzzi LA, et al. Visual acuity is correlated with the area of the foveal avascular zone in diabetic retinopathy and retinal vein occlusion. Ophthalmology. 2016;123(11):2352–2367. doi: 10.1016/j.ophtha.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 12.Yannuzzi NA, Gregori NZ, Roisman L, Gupta N, Goldhagen BE, Goldhardt R. Fluorescein angiography versus optical coherence tomography angiography in macular telangiectasia type I treated with bevacizumab therapy. Ophthalmic Surg Lasers Imaging Retina. 2017;48(3):263–266. doi: 10.3928/23258160-20170301-12. [DOI] [PubMed] [Google Scholar]
- 13.Pappuru RR, Peguda HK, Dave VP. Optical coherence tomographic angiography in type 1 idiopathic macular telangiectasia. Clin Exp Optom. 2018;101(1):143–144. doi: 10.1111/cxo.12536. [DOI] [PubMed] [Google Scholar]
- 14.Mao L, Weng SS, Gong YY, Yu SQ. Optical coherence tomography angiography of macular telangiectasia type 1: comparison with mild diabetic macular edema. Lasers Surg Med. 2017;49(3):225–232. doi: 10.1002/lsm.22645. [DOI] [PubMed] [Google Scholar]
- 15.Chidambara L, Gadde SG, Yadav NK, Jayadev C, Bhanushali D, Appaji AM, et al. Characteristics and quantification of vascular changes in macular telangiectasia type 2 on optical coherence tomography angiography. Br J Ophthalmol. 2016;100(11):1482–1488. doi: 10.1136/bjophthalmol-2015-307941. [DOI] [PubMed] [Google Scholar]
- 16.Toto L, Di Antonio L, Mastropasqua R, Mattei PA, Carpineto P, Borrelli E, et al. Multimodal imaging of macular telangiectasia type 2: focus on vascular changes using optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2016;57(9):T268–T276. doi: 10.1167/iovs.15-18872. [DOI] [PubMed] [Google Scholar]
- 17.Drexler W, Sattmann H, Hermann B, Ko TH, Stur M, Unterhuber A, et al. Enhanced visualization of macular pathology with the use of ultrahigh-resolution optical coherence tomography. Arch Ophthalmol. 2003;121(5):695–706. doi: 10.1001/archopht.121.5.695. [DOI] [PubMed] [Google Scholar]
- 18.Staurenghi G, Sadda S, Chakravarthy U, Spaide RF. Proposed lexicon for anatomic landmarks in normal posterior segment spectral domain optical coherence tomography. The IN•OCT consensus. Ophthalmology. 2014;121(8):1572–1578. doi: 10.1016/j.ophtha.2014.02.023. [DOI] [PubMed] [Google Scholar]
- 19.Wakazono T, Ooto S, Hangai M, Yoshimura N. Photoreceptor outer segment abnormalities and retinal sensitivity in acute zonal occult outer retinopathy. Retina. 2013;33(3):642–648. doi: 10.1097/IAE.0b013e3182671104. [DOI] [PubMed] [Google Scholar]
- 20.Grewal DS, O'Sullivan ML, Kron M, Jaffe GJ. Association of disorganization of retinal inner layers with visual acuity in eyes with uveitic cystoid macular edema. Am J Ophthalmol. 2017;177:116–125. doi: 10.1016/j.ajo.2017.02.017. [DOI] [PubMed] [Google Scholar]
- 21.Sun JK, Lin MM, Lammer J, Prager S, Sarangi R, Silva PS, et al. Disorganization of the retinal inner layers as a predictor of visual acuity in eyes with center-involved diabetic macular edema. JAMA Ophthalmol. 2014;132(11):1309–1316. doi: 10.1001/jamaophthalmol.2014.2350. [DOI] [PubMed] [Google Scholar]
- 22.Spaide RF. Volume-rendered optical coherence tomography of diabetic retinopathy pilot study. Am J Ophthalmol. 2015;160(6):1200–1210. doi: 10.1016/j.ajo.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 23.Abreu-Gonzalez R, Diaz-Rodriguez R, Rubio-Rodriguez G, Gil-Hernandez MA, Abreu-Reyes P. Macular vascular flow area and vascular density in healthy population using optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2016;57(15):6713. doi: 10.1167/iovs.16-20359. [DOI] [PubMed] [Google Scholar]
- 24.Birol G, Wang S, Budzynski E, Wangsa-Wirawan ND, Linsenmeier RA. Oxygen distribution and consumption in the macaque retina. Am J Physiol Heart Circ Physiol. 2007;293(3):H1696–H1704. doi: 10.1152/ajpheart.00221.2007. [DOI] [PubMed] [Google Scholar]
- 25.Sugiura Y, Okamoto F, Okamoto Y, Hiraoka T, Oshika T. Visual function in patients with idiopathic macular telangiectasia type 1. Acta Ophthalmol. 2016;94(7):e672–e673. doi: 10.1111/aos.13127. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Transcripts from this study are available for sharing upon request; however, all identifying and confidential information of the participants will be removed.


