Abstract
Although metabolomics are desirable to understand the pathophysiology of gestational diabetes mellitus (GDM), comprehensive metabolomic studies of GDM are rare. We aimed to offer a holistic view of metabolites alteration in GDM patients and investigate the possible multimarker models for GDM diagnosis. Biochemical parameters and perinatal data of 131 GDM cases and 138 controls were collected. Fasting serum samples at 75 g oral glucose tolerance test were used for metabolites by ultra performance liquid chromatography-quadrupole-time of flight-mass spectrometry, ultra performance liquid chromatography-triple triple-quadrupole-mass spectrometry and gas chromatography- time-of- flight mass spectrometry platforms. Significant changes were observed in free fatty acids, bile acids, branched chain amino acids, organic acids, lipids and organooxygen compounds between two groups. In receiver operating characteristic (ROC) analysis, different combinations of candidate biomarkers and metabolites in multimarker models achieved satisfactory discriminative abilities for GDM, with the values of area under the curve (AUC) ranging from 0.721 to 0.751. Model consisting of body mass index (BMI), retinol binding protein 4 (RBP4), n-acetylaspartic acid and C16:1 (cis-7) manifested the best discrimination [AUC 0.751 (95% CI: 0.693–0.809), p < 0.001], followed by model consisting of BMI, Cystatin C, acetylaspartic acid and 6,7-diketoLCA [AUC 0.749 (95% CI: 0.691–0.808), p < 0.001]. Metabolites alteration reflected disorders of glucose metabolism, lipid metabolism, amino acid metabolism, bile acid metabolism as well as intestinal flora metabolism in GDM state. Multivariate models combining clinical markers and metabolites have the potential to differentiate GDM subjects from healthy controls.
As a consequence of growing obesity prevalence and advancing maternal age, the incidence of gestational diabetes mellitus (GDM)1 is gradually increasing worldwide, ranging from 5.8% to 12.9% (1). A large epidemiological survey in Tianjin, China, shows that the incidence of GDM has risen from 6.9% in 2008 to 9.9% in 2010 (2). Previous studies have indicated that GDM women were at a higher risk of adverse perinatal outcomes, including preeclampsia, infection, premature delivery, increased caesarean rates and premature rupture of membrane (PROM) 3–5). Hyperglycemia during pregnancy also leads to harmful impact on neonates such as fetal malformations, macrosomia, neonatal asphyxia and hypoglycemia as well (6). In addition, both women with GDM and their children are more prone to develop type 2 diabetes (T2DM) later in life (7, 8).
At present, oral glucose tolerance test (OGTT) is considered as the gold standard for the diagnosis of GDM. However, this screening test is complex and time-consuming. There is a strong need for researches on potential ways that can be used to discriminate women of normal pregnancies and pregnancies complicated by GDM. Although adiponectin levels have been repeatedly reported to be lower in GDM women and closely associated with plasma glucose concentrations of OGTT, the area under the receiver-operator characteristic (ROC) curve of adiponectin was relatively low (9–11). Alanbay et al. found that in the GDM group, Gamma Glutamyl Transpeptidase (γ-GT) were significantly higher and determined to be an independent risk factor, but the specificity of γ-GT for indicating GDM was merely 37% (12). Only a handful of studies on some metabolites of GDM have been conducted. With respect to GDM, higher levels of homocysteine have been found by Guven et al. (13). In another research, such significant difference of homocysteine levels was not observed (14). Tarim et al. (15) reported triacylglycerols were higher in women with GDM. However, Seghieri et al. (16) found no difference in triacylglycerols levels among women with GDM and controls. In addition to the inconsistent results from these studies, the sensitivity and specificity required for clinical use were also lacking.
In the current study, comprehensive metabolite profiles of 269 participants were constructed by liquid chromatography-mass spectrometry (LC-MS) and gas chromatography-mass spectrometry (GC-MS). Furthermore, using different combinations of metabolites and clinical variables, we developed a number of multivariate models obtained by ROC analysis for distinguishing women with GDM from those without.
EXPERIMENTAL PROCEDURES
Participants
This was a nested case-control study of pregnant women enrolled in a prospective cohort study. Briefly, from January 2013 to August 2016, a cohort of pregnant women who received prenatal care at the Department of Obstetrics and Gynecology of Shanghai Jiao-Tong University Affiliated Sixth People's Hospital was set up. Their socio-demographic and clinical profiles were recorded from antenatal visit to delivery. All subjects in the current study met the following inclusion criteria: (a) no alcohol consumption, (b) no preconceptional diabetes, (c) no chronic or serious acute infections, (d) no cardiovascular hematological diseases, (e) normal liver or kidney function, (f) negative results for hepatitis C antibodies or HIV. During 24–28th weeks of gestation, diagnostic 75-g, 3-hour oral glucose tolerance test (OGTT) was conducted. The diagnosis of GDM was according to the International Association of Diabetes and Pregnancy Study Group (IADPSG) criteria, with one or more plasma glucose values being equal or greater than the following plasma glucose values: fasting, 5.1 mmol/L, 1 h, 10.0 mmol/L, and 2 h, 8.5 mmol/L (17). The study was approved by the Ethics Committee of the Shanghai Jiao-Tong University Affiliated Sixth People's Hospital. The informed consents were obtained from all participants. All methods performed in this study adhered to the tenets of the Declaration of Helsinki.
Measurement of Clinical and Biochemical Characteristics
General background information including medical and family history, reproductive history, alcohol consumption and smoking status were collected. BMI was calculated as BMI = body weight (in kg)/height (in m2). Blood samples were drawn in the morning after an overnight fast at about 12 weeks of gestation. All biochemical parameters were assayed in the same serum sample. Alanine aminotransferase (ALT), aspartate aminotransferase (AST), γ-GT, cholinesterase (ChE), blood urea nitrogen (BUN), creatinine (Cr), and uric acid (UA) were assessed on an automatic analyzer (7600–020 biochemistry automatic analyzer, Hitachi, Tokyo, Japan). Serum lipids including total triglyceride (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) were measured by enzymatic method. Macrosomia was defined as birth weight ≥ 4000 × g. Hypertension and proteinuria occurred after 20 weeks of gestation were diagnosed as preeclampsia. The diagnosis of PROM was established when membranes ruptured before the onset of labor.
Sample Preparation
Fasting serum specimens were collected at OGTT and stored at −80 °C until analyzed. Metabolome was tested using three instrumental platforms, ultra performance liquid chromatography-quadrupole-time of flight-mass spectrometry (UPLC-QTOFMS), ultraperformance liquid chromatography-triple triple-quadrupole-mass spectrometry (UPLC-TQMS) and gas chromatography- time-of-flight mass spectrometry (GC-TOFMS).
For quantification of free fatty acids (FFAs), 40 μl of sample was mixed with 10 μl of isotope labeled internal standard (5 μg/ml C19:0-d37) and 500 μl of isopropyl/hexane (v/v = 4/1) with 2% phosphate (2 m). Then 400 μl of hexane and 300 μl of water were added. After vortexing, the mixture was centrifuged for 10 min at 12,000 g. The supernatant (400 μl) was transferred to a new tube. The remaining mixture was extracted with 400 μl of hexane. After centrifugation for 10 min, 500 μl of supernatant was mixed with the first supernatant and dried under vacuum. The dried analyte was redissolved with 80 μl of methanol, and then filtered with 0.22-μm membrane (EMD Millipore, Billerica, MA) for analysis.
The internal standard of bile acids (BAs) included cholic acid-D4, ursodeoxycholic acid-D4, lithocholic acid, glycocholic acid-D4, and glycodeoxycholic acid-D4. Each 100 μl of serum was mixed with 10 μl of internal standard and dried under vacuum. The residue was reconstituted with 25 μl of acetonitrile and methanol (v/v = 19/1) with 0.1% formic acid and 25 μl of water with 0.1% formic acid. After centrifugation, the supernatant was retained for analysis.
For semi-quantification of other metabolites, 100 μl of serum was mixed with two internal standard solutions (10 μl of l-2-chlorophenylalanine in water, 0.3 mg/ml; 10 μl of heptadecanoic acid in methanol, 1 mg/ml) and 300 μl of methanol/chloroform (v/v = 3/1). The mixture was vibrating for 30 s and kept for 10 min at −20 °C. After centrifugation at 10,000 rpm for 10 min, 300 μl supernatant was vacuum-dried. The residue was dissolved in 80 μl of methoxyamine with pyridine (15 mg/ml) and kept for 90 min at 30 °C. Then the mixture was silylated with 80 μl of BSTFA (1%TMCS) for 60 min at 70 °C. After keeping for one hour at room temperature, the sample was ready for analysis.
Metabolites Analysis
All samples were injected randomly, a sample for quality control (QC) was run after every ten serum samples to monitor the stability of the instrument.
FFAs measurement was performed by UPLC/QTOFMS (Xevo G2, Waters Corp., MA, USA). A 2.1 mm × 100 mm, 1.7 μm BEH C18 chromatographic column (Agilent J&W Scientific, CA, USA) was used for separation and the column temperature was set at 40 °C. The elution solvents were water (A) and acetonitrile/isopropyl (v/v = 4/1, B) with a flow rate of 0.4 ml/min. The elution procedure for the column was 70% B for 2 min; 70–75% B over 2–5 min; 75–80% B over 5–10 min; 80–90% B over 10–13 min; 90–99% B over 13–16 min and kept at 99% B. The MS was operated at a positive electrospray ionization mode. The capillary voltage was set to 2.5 kV. The sample cone and the extraction cone were set at 55 V and 4 V, respectively. The source and desolvation temperature was set at 150 °C and 450 °C, respectively.
BAs were analyzed using UPLC/TQMS platform (Xevo G2, Waters Corp., Milford, MA). The elution solvents were water with 0.01% formic acid (A) and acetonitrile with 0.01% formic acid (B). The initial gradient was 20% B and maintained for 2 min, increased to 25% B over 1 min and kept for 3 min, increased to 35% B over 2 min and kept for 3.5 min, increased to 99% over 6.5 min kept for 2 min before switching back to the initial condition. The MS was operated at a negative electrospray ionization mode. The capillary voltage was set to 3.0 kV. The extraction cone was set at 4 V. The source and desolvation temperature was set at 120 °C and 350 °C, respectively. The desolvation gas flow rate was 650 L nitrogen per hour.
Other metabolites were selected for GC/TOFMS analysis (Pegasus HT, Leco Corp., St. Joseph, MI). An aliquot of 1 μl sample was injected into a DB-5 ms capillary column (30 m × 250 μm, 0.25 μm; Agilent J&W Scientific, Folsom, CA). The temperature of injection, transfer interface, and ion source was set to 270, 260, and 200 °C, respectively. Ultra-pure helium (99.9996%) was used as the carrier gas. Its flow rate was set at 1.0 ml/min. The initial GC oven temperature was 80 °C and maintained for 2 min, then increased to 180 °C with a rate of 10 °C/min, increased to 240 °C with a rate of 5 °C/min, increased to 290 °C with a rate of 25 °C/min, and kept at 290 °C for 9 min. The analyses were performed with electron impact ionization (70 eV) in the full scan mode (m/z 30–600).
Statistical Analysis
The raw data from UPLC/QTOFMS and UPLC/TQMS was targeted and initially processed by TargetLynx applications manager (version 4.1, Waters Corp.) to detect peak signals, obtain calibration equations, and calculate the concentration of each FFA and BA. The acquired data from GC/TOFMS was exported in NetCDF format by ChromaTOF software (v3.30, Leco Co., CA, USA). CDF files were extracted using custom scripts in the MATLAB 7.0 (The Math Works, Inc.) for data pretreatments such as denoising, baseline correction, time-window splitting, etc (18). Missing values (less than 10%) were supplemented by mean values of corresponding groups.
Variables were expressed as mean ± standard deviation (S.D.) or median (25% quartile, 75% quartile) for continuous variables and percentages (%) for categorical variables. Student's t test or Mann-Whitney U test were performed to evaluated the difference among groups for continuous variables. For categorical variables, Chi-square test or Fisher's exact test were conducted to compare the difference. Binary logistic regression analysis was used to assess the correlations of clinical data and metabolites with the risk of GDM. The Receiver Operator Characteristics (ROC) analysis was performed and area under the curve (AUC) was used to evaluate their diagnostic capabilities. All the statistical analyses above were performed by SPSS 21.0 (SPSS Inc., Chicago, IL). A two-sided p value less than 0.05 was considered statistically significant. The test performances of the multimarker models were assessed using sensitivity, specificity, positive predictive values (PPVs) and negative predictive values (NPVs) at different estimated GDM prevalence. A supervised multivariate model named orthogonal partial least square discriminant analysis (OPLS-DA) was carried out in Simca-p + (Umetrix, Sweden, V12.0). Benjamini-Hochberg procedure was used for false discovery rate correction.
RESULTS
Baseline Characteristics of the Subjects
In this nested case-control study, a total of 269 pregnant women were enrolled. The clinical data of control and GDM patients are shown in Table I. Women with GDM were significantly older than control women. The incidence of diabetes family history, abortion history and multiparity history before pregnancy showed no remarkable difference. Pre-pregnancy BMI was higher in GDM participants, as were ChE, retinol binding protein 4 (RBP4), Cystatin C (Cys C) and TG (all p < 0.05). All subjects were followed up until delivery. The incidence of caesarean section was significantly higher in women with GDM than healthy pregnant women (caesarean section, 12.3% versus 22.9%, p = 0.025). Other adverse pregnant outcomes such as preeclampsia and PROM and newborn characteristics showed no marked difference.
Table I. Maternal and offspring characteristics of GDM and healthy pregnant women. GDM, gestational diabetes mellitus; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; GA, glycated albumin; HbA1c, glycosylated hemoglobin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; γ-GT, γ-glutamyl transferase; ChE, cholinesterase; BUN, blood urea nitrogen; Cr, creatinine; UA, uric acid; RBP4, retinol binding protein 4; Cys C, Cystatin C; TC, total cholesterol; TG, total triglyceride; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; BPD, biparietal diameter; PROM, premature rupture of membrane; AFI, amniotic fluid index. Data represent means ± S.D., median (25% quantile, 75% quantile) or percentage (%).
| Group | Control | GDM | p value |
|---|---|---|---|
| N | 138 | 131 | |
| Clinical and biochemical variables | |||
| Age (years) | 30.4 ± 3.8 | 31.4 ± 3.8 | 0.030a |
| Prepregnancy BMI (kg/m2) | 20.9 ± 2.9 | 22.3 ± 2.9 | <0.001a |
| SBP (mm Hg) | 112.4 ± 13.2 | 114.4 ± 12.7 | 0.198a |
| DBP (mm Hg) | 67.7 ± 10.0 | 69.6 ± 10.6 | 0.142a |
| Family history of diabetes (n) | 0 | 1 | – |
| Nulliparous (%) | 73.2 | 66.4 | 0.141b |
| Abortion history (%) | 45.7 | 48.9 | 0.343b |
| ALT (U/L) | 13.0 (10.0–21.0) | 13.0 (9.5–20.5) | 0.475c |
| AST (U/L) | 18.0 (15.0–22.0) | 17.0 (14.0–20.0) | 0.266c |
| γ-GT (U/L) | 14.0 (11.0–19.0) | 14.0 (11.0–19.5) | 0.997c |
| ChE (U/L) | 270.6 ± 51.7 | 293.8 ± 50.7 | <0.001a |
| BUN (mmol/L) | 2.7 (2.3–3.1) | 2.6 (2.3–3.1) | 0.749c |
| Cr (μmol/L) | 43.5 (40.0–48.0) | 43.0 (39.0–47.0) | 0.240c |
| UA (μmol/L) | 202.5 (178.0–226.0) | 204.0 (181.0–236.0) | 0.401c |
| RBP4 (mg/L) | 34.0 (28.0, 39.0) | 37.0 (30.0, 45.0) | 0.003c |
| Cys C (mg/L) | 0.5 (0.4, 0.5) | 0.5 (0.5, 0.6) | 0.001c |
| TC (mmol/L) | 4.8 ± 1.0 | 4.8 ± 0.9 | 0.506a |
| TG (mmol/L) | 1.3 (1.1–1.7) | 1.5 (1.2–1.9) | 0.021c |
| HDL-C (mmol/L) | 1.9 ± 0.4 | 1.8 ± 0.4 | 0.070a |
| LDL-C (mmol/L) | 2.3 (1.9–2.7) | 2.4 (2.0–2.8) | 0.383c |
| Pregnancy outcomes | |||
| Gestational age at delivery (weeks) | 39.0 (38.0–40.0) | 39.0 (38.0–40.0) | 0.451c |
| Caesarean section (%) | 12.3 | 22.9 | 0.025b |
| PROM (%) | 15.2 | 12.2 | 0.486b |
| Preeclampsia (%) | 2.2 | 1.5 | 0.525b |
| Amount of postpartum hemorrhage (ml) | 310.0 (280.0–340.0) | 300.0 (275.0–350.0) | 0.599c |
| AFI (cm) | 11.4 (9.8–13.9) | 12.1 (10.2–14.4) | 0.184c |
| Offspring | |||
| BPD (mm) | 94.0 (91.0–96.0) | 93.0 (90.0–96.0) | 0.462c |
| Weight (g) | 3279.3 ± 420.5 | 3377.0 ± 450.7 | 0.067a |
| Macrosomia (%) | 4.3 | 9.2 | 0.145b |
| Apgar score | 10.0 (10.0–10.0) | 10.0 (10.0–10.0) | 0.305c |
a Derived from Student's t-test.
b Derived from Chi-square test or Fisher's Exact Test.
c Derived from Mann-Whitney U-test.
Metabolite Identification and Quantitation
UPLC-QTOFMS, UPLC-TQMS and GC-TOFMS platforms were used for metabolite measurement. We identified 131 metabolites based on our in-house standard library and online available libraries, FFAs and BAs were quantified. The metabolites included FFAs, BAs, amino acids (AAs), organic acids, lipids, organooxygen compounds, pyridines and others. FFAs were further separated into saturated fatty acids, monounsaturated fatty acids, and polyunsaturated fatty acids. BAs were separated into primary bile acids (PBA) and secondary bile acids (SBA), AAs were separated into aromatic amino acids (AAAs), branched chain amino acids (BCAAs) and other amino acids. The numbers and proportions of the metabolite types and subtypes are shown in Fig. 1A. Detailed list of metabolites was displayed in supplemental Table S1.
Fig. 1.
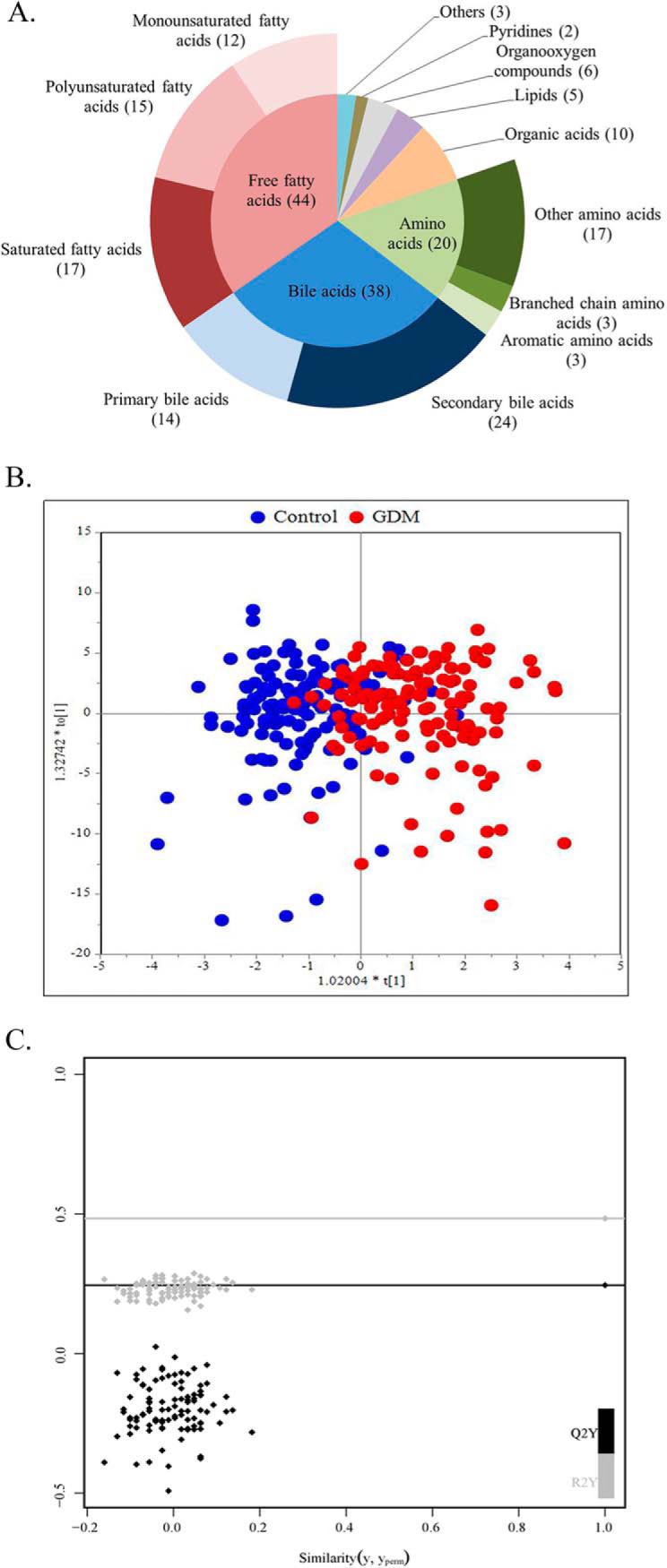
Metabolite types and the scores plot of the OPLS-DA model. A, Pie chart displays 6 metabolite types and subtypes measured in our research. Free fatty acids (red) include12 monounsaturated fatty acids, 15 polyunsaturated fatty acids and 17 saturated fatty acids; bile acids (blue) include 14 primary bile acids and 24 secondary bile acids; amino acids (green) include 3 branched chain amino acids, 3 aromatic amino acids and other 17 amino acids. B, The OPLS-DA scores plot shows the groupings of control (blue), and GDM (red) subjects based on all metabolite profiles. R2X = 0.314, R2Y = 0.543, Q2 = 0.254. C, The permutation test for the OPLS-DA model. Permutated R2 = 0.01, permutated Q2 = 0.01.
Changes of Metabolic Profile in GDM Status
Metabolome alteration between the two groups was evaluated by the score values of multivariate statistics orthogonal partial least square discriminant analysis (OPLS-DA) model (Fig. 1B). The permutation test was performed for the OPLS-DA model (Fig. 1C). It was clear from the OPLS-DA scores scatter plot that individuals with GDM were separated from those without based on all metabolites assayed. In the FFA subgroup, twenty FFAs were significantly elevated in women who developed GDM (Fig. 2A). In the BA subgroup, four BAs were significantly increased and three BAs were significantly reduced in women with GDM (Fig. 2B). In the AA subgroup, BCAAs and other three AAs were significantly higher in GDM patients. Eight organic acids, two lipids and four organooxygen compounds showed marked difference between GDM cases and controls (Fig. 2C). After false discovery rate correction by the Benjamini-Hochberg procedure, eleven FFAs, two BAs, five AAs, six organic acids, one lipids and two organooxygen compounds remained significantly different (p < 0.05). Concentrations of differential metabolites between the two groups were shown in supplemental Table S2.
Fig. 2.
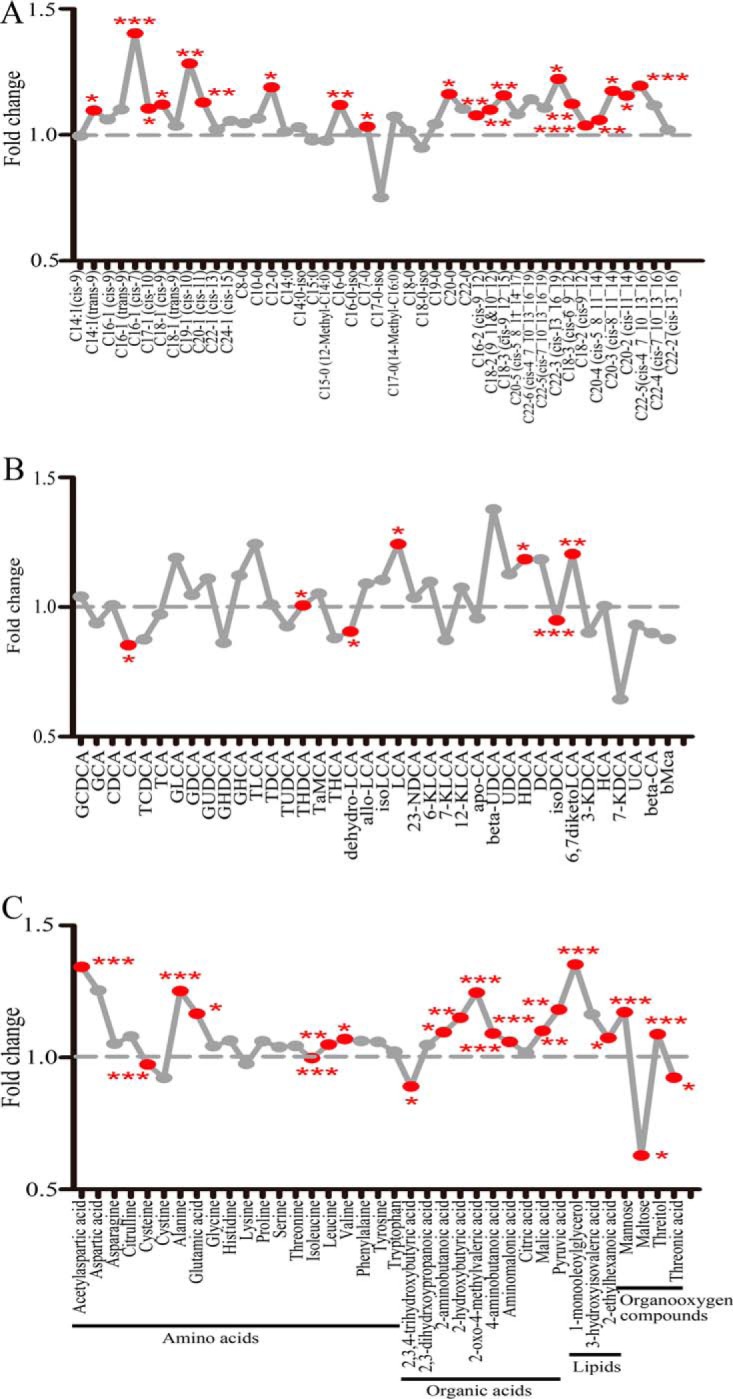
Fold change plot of metabolites (GDM/control). Fold change plot of (A) free fatty acids, (B) bile acids and (C) other metabolites. Fold changes are ratios of mean values in GDM over control group. Metabolites labeled with red dots were significantly different between two groups. Symbol * indicates statistical significance from Student's t test or Mann-Whitney U test (*p < 0.05, **p < 0.01, ***p < 0.001). GCDCA, glycochenodeoxycholic acid; GCA, glycocholic acid; TCDCA, taurochenodeoxycholic acid; TCA, taurocholic acid; CDCA, chenodeoxycholic acid; CA, cholic acid; HCA, hyocholic acid; GHCA, glycohy-ocholic acid; TaMCA, tauro-αmuricholic acid; THCA, taurohyocholic acid; UCA, ursocholic acid; bMCA, β-muricholic acid; GLCA, glycollithoc-holic acid; GDCA, glycodeoxycholic acid; GUDCA, glycoursodeoxycholic acid; GHDCA, glycohyodeoxycholic acid; TLCA, taurolithocholicacid; TDCA, taurodeoxycholic acid; TUDCA, tauroursodeoxycholic acid; THDCA, taurohyodeoxycholic acid; LCA, lithocholic acid; 23_NDCA, 23-nordeoxycholic acid; 6-KLCA, 6-ketolithocholic acid; beta-UDCA, β-ursodeoxycholic acid; HDCA, hyodeoxycholic acid; DCA, deoxycholic acid; 7-KDCA,7-ketodeoxycholic acid.
Univariate Analysis of the Association of Clinical Characteristics with Metabolites and GDM
For all clinical variables and metabolites that initially exhibited significant differences between cases and controls, ROC analyses were constructed and AUC values evaluated (Table II). Of these clinical parameters, BMI showed the best predictive performance (AUC 0.651 (95% CI: 0.586–0.716), p < 0.001). Of these metabolites, 2-Oxo-4-methylvaleric acid and 4-Aminobutanoic acid showed the largest AUC values (AUC 0.687 (95% CI: 0.618–0.756), p < 0.001; AUC 0.687 (95% CI: 0.618–0.756), p < 0.001; respectively).
Table II. Clinical parameters and metabolites and their statistical significance in discriminating GDM and healthy individuals. ROC, receiver operating characteristic; AUC, area under the curve; FC, fold change; GDM, gestational diabetes mellitus; BMI, body mass index; ChE, cholinesterase; TG, total triglycerides; RBP4, retinol binding protein 4; Cys C, Cystatin C; C16:1 (cis-7), cis-7-hexadecenoate; C16:0, n-hexadecanoic acid; C18:3 (cis-6_9_12), cis-6,9,12-octadecatrienoic acid; C18:2 (cis-9_12), cis-9,12-octadecadienoic acid; C20:1 (cis-11), cis-11-eicosenoic acid; C20:3 (cis-8_11_14), cis-8,11,14-eicosatrienoic acid; C20:2 (cis-11_14), cis-11,14-eicosadienoic acid; C22:5 (cis-4_7_10_13_16), cis-4,7,10,13,16-docosapentaenoic acid; C20:3 (cis-8_11_14), cis-8,11,14-eicosatrienoic acid; 6,7-diketoLCA, 6,7-diketocholic acid; isoDCA, isodeoxycholic acid.
| Clinical parameters and metabolites | ROC analysis |
FC | Biochemical pathway | |
|---|---|---|---|---|
| AUC (95% CI) | p value | |||
| Age | 0.583 (0.514–0.651) | 0.019 | ||
| BMI | 0.651 (0.586–0.716) | <0.001 | ||
| ChE | 0.629 (0.563–0.695) | <0.001 | ||
| TG | 0.581 (0.513–0.650) | 0.021 | ||
| RBP4 | 0.606 (0.539–0.674) | 0.003 | ||
| Cys C | 0.611 (0.544–0.679) | 0.002 | ||
| C16:1 (cis-7) | 0.655 (0.590–0.721) | <0.001 | 1.40 | Fatty acid metabolism |
| C16:0 | 0.619 (0.552–0.687) | 0.001 | 1.12 | Fatty acid metabolism, glycerolipid metabolism |
| C18:3 (cis-6_9_12) | 0.600 (0.532–0.667) | 0.005 | 1.12 | Alpha linolenic acid and linoleic acid metabolism |
| C18:2 (cis-9_12) | 0.653 (0.587–0.719) | <0.001 | 1.04 | Alpha linolenic acid and linoleic acid metabolism |
| C20:1 (cis-11) | 0.614 (0.547–0.681) | 0.001 | 1.13 | Fatty acid metabolism |
| C20:2 (cis-11_14) | 0.641 (0.575–0.707) | <0.001 | 1.16 | Fatty acid metabolism |
| C20:3 (cis-8_11_14) | 0.602 (0.534–0.670) | 0.004 | 1.17 | Fatty acid metabolism |
| 6,7-diketoLCA | 0.623 (0.553–0.692) | 0.001 | 1.20 | Bile acid biosynthesis |
| isoDCA | 0.686 (0.614–0.758) | <0.001 | 0.95 | Bile acid biosynthesis |
| Alanine | 0.649 (0.580–0.719) | <0.001 | 1.25 | Glucose-alanine cycle, glutamate metabolism, glycine and serine metabolism, et al |
| N-acetylaspartic acid | 0.669 (0.603–0.736) | <0.001 | 1.34 | Aspartate metabolism |
| 2-Oxo-4-methylvaleric acid | 0.687 (0.618–0.756) | <0.001 | 1.25 | Valine, leucine and isoleucine degradation |
| 4-Aminobutanoic acid | 0.687 (0.618–0.756) | <0.001 | 1.09 | Glutamate metabolism |
| Pyruvic acid | 0.613 (0.544–0.683) | 0.001 | 1.18 | Gluconeogenesis, glycolysis, citric acid cycle, glucose-alanine cycle, et al |
| Aminomalonic acid | 0.676 (0.605–0.746) | <0.001 | 1.06 | – |
| 1-Monooleoylglycerol | 0.624 (0.557–0.707) | <0.001 | 1.35 | – |
| Mannose | 0.658 (0.593–0.723) | <0.001 | 1.17 | Galactose metabolism |
| Threitol | 0.661 (0.593–0.728) | <0.001 | 1.09 | – |
The Development of Multivariate Models to Indicate GDM
To investigate more sensitive potential indicator from the multiple biomarker models for discriminating the risk of GDM, binominal logistic regression analysis and ROC analysis were constructed using different combinations of variables. Setting the AUC value for GDM at 0.6 and fold change between the two groups at 1.2 to identify variables for testing in the multivariate models, we selected six metabolites such as C16:1 (cis-7), 6,7-diketoLCA, alanine, n-acetylaspartic acid, 2-oxo-4-methylvaleric acid and 1-monooleoylglycerol. BMI, ChE, RBP4 and Cys C were chosen as candidate clinical markers by setting the AUC value for GDM at 0.6 (bold in Table II). Three modeling strategies were used in this study: (1) metabolites only, (2) BMI plus metabolites, (3) BMI plus other candidate clinical markers plus metabolites. No more than four biomarkers were permitted for each model in order to avoid overfitting of the data. The top six models with the largest AUC values of three modeling strategies were listed in Table III. Strategy 3 performed better than the other two strategies in distinguishing GDM patients and controls. Of all models listed, Model 13 consisting of BMI, RBP4, n-acetylaspartic acid and C16:1 (cis-7) manifested the best discrimination (AUC 0.751 (95% CI: 0.693–0.809), p < 0.001), followed by Model 14 consisting of BMI, Cys C, n-acetylaspartic acid and 6,7-diketoLCA (AUC 0.749 (95% CI: 0.691–0.808), p < 0.001).
Table III. ROC analysis of multimarker models to indicate GDM. GDM, gestational diabetes mellitus; ROC, receiver operating characteristic; AUC, area under the curve; BMI, body mass index; ChE, cholinesterase; RBP4, retinol binding protein 4; Cys C, Cystatin C; C16:1 (cis-7), cis-7-hexadecenoate; 6,7-diketoLCA, 6,7-diketocholic acid.
| Model | Variables included in the model | AUC (95% CI) | p value |
|---|---|---|---|
| Metabolites | |||
| 1 | N-acetylaspartic acid, 2-Oxo-4-methylvaleric acid, C16:1 (cis-7), 6,7-diketoLCA | 0.740 (0.680–0.799) | <0.001 |
| 2 | N-acetylaspartic acid, alanine, C16:1 (cis-7), 6,7-diketoLCA | 0.740 (0.681–0.800) | <0.001 |
| 3 | N-acetylaspartic acid, 1-monooleoyl glycerol, C16:1 (cis-7), 6,7-diketoLCA | 0.738 (0.679–0.798) | <0.001 |
| 4 | N-acetylaspartic acid, C16:1 (cis-7), 6,7-diketoLCA | 0.732 (0.672–0.792) | <0.001 |
| 5 | N-acetylaspartic acid, 1-monooleoyl glycerol, 6,7-diketoLCA, 2-oxo-4-methylvaleric acid | 0.722 (0.661–0.783) | <0.001 |
| 6 | N-acetylaspartic acid, 1-monooleoyl glycerol, C16:1 (cis-7), 2-oxo-4-methylvaleric acid | 0.721 (0.661–0.781) | <0.001 |
| BMI and metabolites | |||
| 7 | BMI, 2-oxo-4-methylvaleric acid, n-acetylaspartic acid, 6,7-diketoLCA | 0.744 (0.685–0.803) | <0.001 |
| 8 | BMI, 2-oxo-4-methylvaleric acid, n-acetylaspartic acid, C16:1 (cis-7) | 0.743 (0.685–0.802) | <0.001 |
| 9 | BMI, 2-oxo-4-methylvaleric acid, C16:1 (cis-7), 6,7-diketoLCA | 0.740 (0.681–0.800) | <0.001 |
| 10 | BMI, n-acetylaspartic acid, alanine, C16:1 (cis-7) | 0.740 (0.681–0.798) | <0.001 |
| 11 | BMI, n-acetylaspartic acid, 1-monooleoyl glycerol, C16:1 (cis-7) | 0.740 (0.681–0.799) | <0.001 |
| 12 | BMI, n-acetylaspartic acid, C16:1 (cis-7) | 0.732 (0.672–0.791) | <0.001 |
| BMI, biochemical markers and metabolites | |||
| 13 | BMI, RBP4, n-acetylaspartic acid, C16:1 (cis-7) | 0.751 (0.693–0.809) | <0.001 |
| 14 | BMI, Cys C, n-acetylaspartic acid, 6,7-diketoLCA | 0.749 (0.691–0.808) | <0.001 |
| 15 | BMI, ChE, n-acetylaspartic acid, C16:1 (cis-7) | 0.748 (0.690–0.806) | <0.001 |
| 16 | BMI, Cys C, n-acetylaspartic acid, C16:1 (cis-7) | 0.747 (0.689–0.805) | <0.001 |
| 17 | BMI, Cys C, RBP 4, n-acetylaspartic acid | 0.743 (0.683–0.803) | <0.001 |
| 18 | BMI, Cys C, n-acetylaspartic acid | 0.731 (0.671–0.792) | <0.001 |
***p < 0.001 from ROC analysis.
The ROC curves of the top two models of each modeling strategy e.g. Model 1, Model 2, Model 7, Model 8, Model 13 and Model 14 were displayed in Fig. 3. Corresponding sensitivity and specificity at the optimal cutoff points were calculated (Table IV). A recent review of data published over the past decade indicated that the median prevalence of GDM varied worldwide, ranging from 5.8% to 12.9% (1). Thus, positive and negative predictive values were calculated using different cohort prevalence of GDM (5 and 10%). As shown in Table IV, both sensitivity and specificity of these six models were ∼70%. In cases which were diagnosed positive in these ROC models, only 10%∼20% (PPVs) had GDM. On the other hand, in cases which were diagnosed negative, almost 100% (NPVs) could be ruled out GDM. These models' values in real clinics were much more appropriate for exclusion screening than inclusion screening.
Fig. 3.
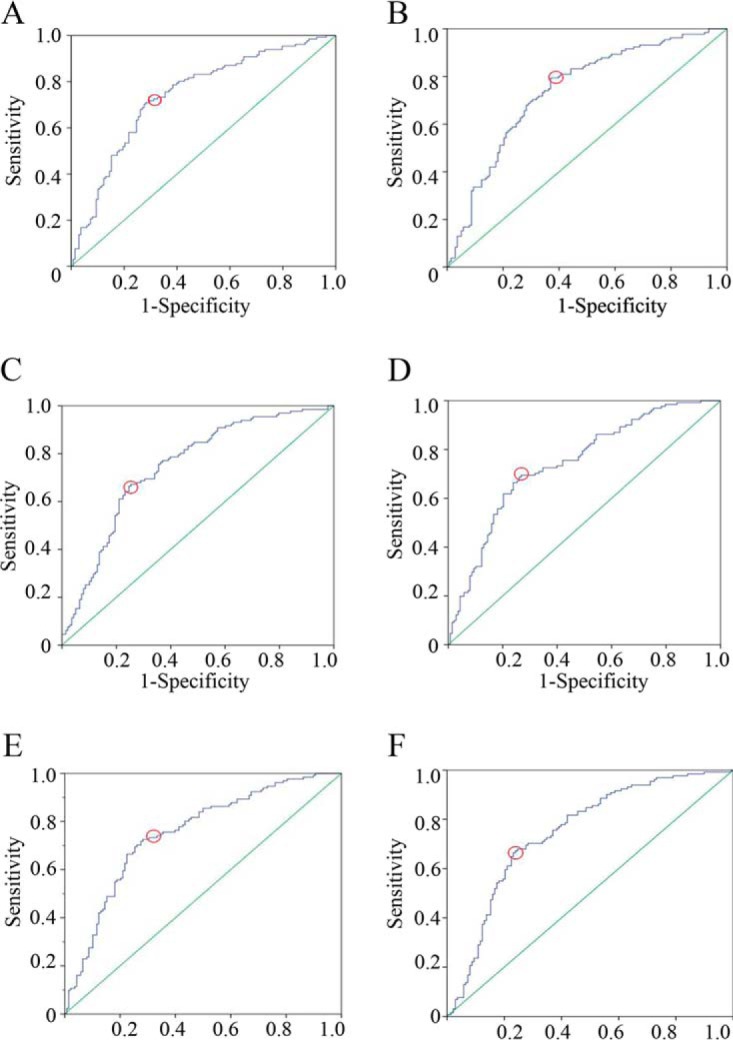
ROC analysis of multimarker models. Multivariate analysis of (A) Model 1, (B) Model 2, (C) Model 7, (D) Model 8, (E) Model 13, and (F) Model 14. Red circles, the optimal cut-off points.
Table IV. PPV and NPV calculations of multimarker models. PPV, positive predictive value; NPV, negative predictive value.
| Model | Sensitivity (%) | Specificity (%) | 5% prevalence |
10% prevalence |
||
|---|---|---|---|---|---|---|
| PPV (%) | NPV (%) | PPV (%) | NPV (%) | |||
| 1 | 71.0 | 71.7 | 11.7 | 97.9 | 21.8 | 95.7 |
| 2 | 79.4 | 63.0 | 10.1 | 98.3 | 19.3 | 96.5 |
| 7 | 66.4 | 75.4 | 12.5 | 97.7 | 23.1 | 95.3 |
| 8 | 69.5 | 73.2 | 12.0 | 97.9 | 22.4 | 95.6 |
| 13 | 72.5 | 71.7 | 11.9 | 98.0 | 22.2 | 95.9 |
| 14 | 67.2 | 76.1 | 12.9 | 97.8 | 23.8 | 95.4 |
DISCUSSION
In the present study, three high-resolution and high-sensitivity mass spectrometry (MS) platforms were combined to detect a panel of 131 well-annotated metabolites, with 81 accurately quantitated. OPLS-DA scores scatter plot revealed that the metabolite profiling of women with GDM were well separated from controls. Significant alteration in FFAs, BAs, AAs, organic acids, lipids and organooxygen compounds were observed. Moreover, we established a cluster of multimarker models that differentiated among individuals with GDM and without by diverse combinations of metabolites and clinical indexes. Among them, the model with the best discriminative performance composed of BMI, RBP4, n-acetylaspartic acid and C16:1 (cis-7) (AUC = 0.751; 95% CI, 0.693–0.809; sensitivity, 72.5%; specificity, 71.7%; p < 0.001).
In recent years, metabolomics has gained immense popularity based on its application in identification of novel pathways and specific biomarker for insulin resistance and type 2 diabetes mellitus (T2DM) (19–21). Metabolomic analyses are typically carried out by liquid chromatography mass spectrometry (LC-MS), gas chromatography-mass spectrometry (GC-MS) and nuclear magnetic resonance (NMR) spectroscopy (19). The first two metabolomic techniques used in the present study, were adequately sensitive to detect subtle differences in serum protein levels. MS allows the separation of ions within an analyte according to their mass-to-charge ratio through using an electromagnetic field. When MS is coupled with gas or liquid chromatography, specific metabolite classes can be detected. Our data offered a holistic view of the changes in serum metabolites of relatively large population. One PBA named CA and six SBA including THDCA, HDCA, isoDCA, dehydro_LCA, LCA and 6_7_diketoLCA showed significant alteration in GDM participants. As we know, PBAs are transformed into SBAs by gut microbiome and both of them are closely related with obesity, insulin resistance and T2DM (22, 23). The alteration of BA metabolome may indicate intestinal flora imbalance and glucose metabolic disorders in GDM state. Another BA metabolomics studied in Chinese subjects reported that THDCA levels were similarly higher in GDM patients, but the changes in CA, HDCA and LCA levels were not found (24). UDCA has been frequently reported to be closely associated with lipid and glucose metabolism (25, 26), but UDCA concentrations were comparable between the two groups. Although fasting UDCA levels were significantly increased in T2DM patients versus normal glucose tolerance (NGT) subjects in Sonne's report, the number of cases enrolled in each group was only 15 (27). Some studies have shown that elevated FFAs contributed to hyperglycemia by inhibiting muscular insulin signaling, pancreatic insulin secretion and hepatic endogenous glucose production (28, 29). In our study, almost half of FFAs were elevated in GDM patients, in accordance with previous research findings (30–32). Alanine transfer its amino group by the action of alanine aminotransferase to α-ketoglutarate, forming pyruvate and glutamate, pyruvate regenerated forms glucose through gluconeogenesis (33). Elevation of alanine, glutamic acid, pyruvic acid in our GDM cases may indicate enhanced gluconeogenetic process. BCAAs and AAAs were associated with risk factors for diabetes, including insulin resistance and obesity (34). BCAAs are involved in several pathways of insulin resistance, including fatty acid oxidation, mTOR, JNK and IRS1 pathways (35, 36). Nevertheless, the trend of these AAs alteration in GDM women reported from earlier studies varied (32, 37). In the current study, BCAAs were increased in GDM patients, whereas AAAs showed no significant change. Rahimi and colleagues found GDM mothers had higher plasma concentrations of arginine, glycine and methionine (38), however, these glucogenic AAs showed no significant differences between the two groups in our research. Cysteine, precursor to glutathione, has antioxidant properties (39). The levels of cysteine in GDM cases were lower than controls, but in the study from Butte's group, cysteine showed the opposite change (37). Dissimilarities among findings from the studies could be caused by differences in GDM diagnostic criteria, metabolites profiling platforms, timing of metabolome profiling, specimen prepared for test, ethnic origin and size of the study populations. Future investigations that follow the same strict guidelines are needed to improve replication of findings.
We identified some metabolites that were not measured in GDM patients before, such as n-acetylaspartic acid, 2,3,4-trihydroxybutyric acid, 2-aminobutanoic acid, 2-oxo-4-methylvaleric acid, 4-aminobutanoic acid, aminomalonic acid, 1-monooleoylglycerol, 2-ethylhexanoic acid, mannose, maltose, threitol, threonic acid. 2-aminobutanoic acid is a key intermediate in the biosynthesis of ophthalmic acid, which was used as a biomarker in oxidative stress (40). The observation of diminished cysteine levels and increased 2-aminobutanoic acid levels could imply disturbance of redox homeostasis in GDM cases. Gamma-aminobutanoic acid (GABA) is an inhibitory neurotransmitter found in the nervous systems. GABA concentrations were higher in T2DM participants. Similar phenomenon was observed in GDM cases. Our results along with previous research confirmed the association between diabetes and accelerated cognitive decline. In summary, changes in these metabolites reflected disorders in glucose metabolism, lipid metabolism, amino acid metabolism, bile acid metabolism as well as intestinal flora metabolism in pregnancies complicated by GDM.
A strength of our study is the development of different multivariate models that discriminated GDM women from controls. Firstly, we combined only three or four metabolites for modeling, the AUC values of the top six models were ranging from 0.721 to 0.740. BMI was a well-known risk factor of GDM and was very convenient to measure (41). In our study, prepregnancy BMI was significantly higher in GDM participants. Furthermore, the AUC values were slightly improved by addition of BMI to metabolites in models, ranging from 0.732 to 0.744.
Though UA were found positively related with insulin resistance (42), the early pregnancy UA concentrations between GDM and control case showed no significant difference, in consistence with previous research (43). RBP4 and Cys C have been described as latent biomarkers of GDM in numerous publications (44–47). Here, these two serum parameters were confirmed as the potential clinical serum markers for GDM. With respect to GDM, ChE concentrations were significantly decreased from Khosrowbeygi's report (48) but remained unchanged from Cocelli' results (49). In this Chinese population, ChE levels were elevated in GDM cases and considered as potential biomarker. We further added these first-trimester candidate biomarkers to BMI and metabolites in models, and this modeling strategy performed better than the other two strategies mentioned above. All these results suggested that the discriminative abilities of diverse combinations of variables are much better than single variable, offering an appropriate and convenient screening for GDM.
Some limitations of our study deserved mention. As a nested case-control study, we only conducted serum metabolomics analysis of women at late pregnancy, therefore the evolutionary process of metabolites throughout pregnancy could not be assessed. Secondly, complete maternal history data were not obtained from all participants. For example, we did not obtain the information of diet and physical activity, failing to evaluate the impact of these confounding factors on metabolites profiling. Large cohorts, dynamic monitoring of metabolites during pregnancy, analyses of various specimen types can improve our understanding of metabolites alteration and verify the validity of multimarker models of GDM.
In conclusion, our data provided a comprehensive overview of metabolites alteration from a relatively large participant population, which offered deeper insights into the pathogenesis of GDM. What is more, multivariate models using different combinations including metabolites, anthropometry data and candidate biomarkers improved the diagnostic ability over single marker. The performance of the multimaker models can be a feasible and simple tool for distinguishing women with GDM from those without.
Supplementary Material
Footnotes
* This work was supported by the grants from National Science Foundation of China (81770802, 81270397 for Fang Liu) and Shanghai Science & Technology Pillar Program in the Field of Medicine and Agriculture (15411953100 to Fang Liu) and Shanghai Municipal Education Commission—Gaofeng Clinical Medicine Grant Support (20152232 for Fang Liu).
 This article contains supplemental material.
This article contains supplemental material.
1 The abbreviations used are:
- GDM
- gestational diabetes millitus
- PROM
- premature rupture of membrane
- T2DM
- Type 2 diabetes millitus
- OGTT
- oral glucose tolerance test
- ROC
- receiver-operator characteristics
- AUC
- area under the curve.
REFERENCES
- 1. Zhu Y., and Zhang C. (2016) Prevalence of gestational diabetes and risk of progression to type 2 diabetes: a global perspective. Curr. Diab. Rep. 16, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chang Y., Chen X., Cui H., Zhang Z., and Cheng L. (2014) Follow-up of postpartum women with gestational diabetes mellitus (GDM). Diabetes Res. Clin. Pract. 106, 236–240 [DOI] [PubMed] [Google Scholar]
- 3. Metzger B. E., Lowe L. P., Dyer A. R., Trimble E. R., Chaovarindr U., Coustan D. R., Hadden D. R., McCance D. R., Hod M., McIntyre H. D., Oats J. J., Persson B., Rogers M. S., and Sacks D. A. (2008) Hyperglycemia and adverse pregnancy outcomes. N. Engl. J. Med. 358, 1991–2002 [DOI] [PubMed] [Google Scholar]
- 4. Crowther C. A., Hiller J. E., Moss J. R., McPhee A. J., Jeffries W. S., and Robinson J. S. (2005) Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N. Engl. J. Med. 352, 2477–2486 [DOI] [PubMed] [Google Scholar]
- 5. Catalano P. M., McIntyre H. D., Cruickshank J. K., McCance D. R., Dyer A. R., Metzger B. E., Lowe L. P., Trimble E. R., Coustan D. R., Hadden D. R., Persson B., Hod M., and Oats J. J. (2012) The hyperglycemia and adverse pregnancy outcome study: associations of GDM and obesity with pregnancy outcomes. Diabetes Care 35, 780–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Buchanan T. A., Xiang A., Kjos S. L., and Watanabe R. (2007) What is gestational diabetes? Diabetes Care 30, S105–S111 [DOI] [PubMed] [Google Scholar]
- 7. Kim C., Newton K. M., and Knopp R. H. (2002) Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care 25, 1862–1868 [DOI] [PubMed] [Google Scholar]
- 8. Fetita L. S., Sobngwi E., Serradas P., Calvo F., and Gautier J. F. (2006) Consequences of fetal exposure to maternal diabetes in offspring. J. Clin. Endocrinol. Metab. 91, 3718–3724 [DOI] [PubMed] [Google Scholar]
- 9. Worda C., Leipold H., Gruber C., Kautzky-Willer A., Knofler M., and Bancher-Todesca D. (2004) Decreased plasma adiponectin concentrations in women with gestational diabetes mellitus. Am. J. Obstet. Gynecol. 191, 2120–2124 [DOI] [PubMed] [Google Scholar]
- 10. Retnakaran R., Hanley A. J., Raif N., Connelly P. W., Sermer M., and Zinman B. (2004) Reduced adiponectin concentration in women with gestational diabetes: a potential factor in progression to type 2 diabetes. Diabetes Care 27, 799–800 [DOI] [PubMed] [Google Scholar]
- 11. Weerakiet S., Lertnarkorn K., Panburana P., Pitakitronakorn S., Vesathada K., and Wansumrith S. (2006) Can adiponectin predict gestational diabetes? Gynecol. Endocrinol. 22, 362–368 [DOI] [PubMed] [Google Scholar]
- 12. Alanbay I., Coksuer H., Ercan M., Keskin U., Karasahin E., Ozturk M., Tapan S., Ozturk O., Kurt I., and Ergun A. (2012) Can serum gamma-glutamyltransferase levels be useful at diagnosing gestational diabetes mellitus? Gynecol. Endocrinol. 28, 208–211 [DOI] [PubMed] [Google Scholar]
- 13. Guven M. A., Kilinc M., Batukan C., Ekerbicer H. C., and Aksu T. (2006) Elevated second trimester serum homocysteine levels in women with gestational diabetes mellitus. Arch. Gynecol. Obstet. 274, 333–337 [DOI] [PubMed] [Google Scholar]
- 14. Idzior-Walus B., Cyganek K., Sztefko K., Seghieri G., Breschi M. C., Walus-Miarka M., Kawalec E., Seretny M., and Sieradzki J. (2008) Total plasma homocysteine correlates in women with gestational diabetes. Arch. Gynecol. Obstet. 278, 309–313 [DOI] [PubMed] [Google Scholar]
- 15. Tarim E., Bagis T., Kilicdag E., Erkanli S., Aslan E., Sezgin N., and Kuscu E. (2004) Elevated plasma homocysteine levels in gestational diabetes mellitus. Acta Obstet. Gynecol. Scand. 83, 543–547 [DOI] [PubMed] [Google Scholar]
- 16. Seghieri G., Breschi M. C., Anichini R., De Bellis A., Alviggi L., Maida I., and Franconi F. (2003) Serum homocysteine levels are increased in women with gestational diabetes mellitus. Metabolism 52, 720–723 [DOI] [PubMed] [Google Scholar]
- 17. (2013) Standards of medical care in diabetes–2013. Diabetes Care 36, S11–S66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jonsson P., Johansson A. I., Gullberg J., Trygg J., Grung A. J. B., Marklund S., Sjostrom M., Antti H., and Moritz T. (2005) High-throughput data analysis for detecting and identifying differences between samples in GC/MS-based metabolomic analyses. Anal. Chem. 77, 5635–5642 [DOI] [PubMed] [Google Scholar]
- 19. Friedrich N. (2012) Metabolomics in diabetes research. J. Endocrinol. 215, 29–42 [DOI] [PubMed] [Google Scholar]
- 20. Roberts L. D., Koulman A., and Griffin J. L. (2014) Towards metabolic biomarkers of insulin resistance and type 2 diabetes: progress from the metabolome. Lancet Diabetes Endocrinol. 2, 65–75 [DOI] [PubMed] [Google Scholar]
- 21. Pallares-Mendez R., Aguilar-Salinas C. A., Cruz-Bautista I., and Del Bosque-Plata L. (2016) Metabolomics in diabetes, a review. Ann. Med. 48, 89–102 [DOI] [PubMed] [Google Scholar]
- 22. Catoi A. F., Parvu A., Muresan A., and Busetto L. (2015) Metabolic mechanisms in obesity and type 2 diabetes: insights from bariatric/metabolic surgery. Obes. Facts 8, 350–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Staels B., and Fonseca V. A. (2009) Bile acids and metabolic regulation: mechanisms and clinical responses to bile acid sequestration. Diabetes Care 32, S237–S245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gao J., Xu B., Zhang X., Cui Y., Deng L., Shi Z., Shao Y., and Ding M. (2016) Association between serum bile acid profiles and gestational diabetes mellitus: A targeted metabolomics study. Clin. Chim. Acta 459, 63–72 [DOI] [PubMed] [Google Scholar]
- 25. Chung Y. R., Choi J. A., Koh J. Y., and Yoon Y. H. (2017) Ursodeoxycholic acid attenuates endoplasmic reticulum stress-related retinal pericyte loss in streptozotocin-induced diabetic mice. J. Diabetes Res. 2017, 1763292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tsuchida T., Shiraishi M., Ohta T., Sakai K., and Ishii S. (2012) Ursodeoxycholic acid improves insulin sensitivity and hepatic steatosis by inducing the excretion of hepatic lipids in high-fat diet-fed KK-Ay mice. Metabolism 61, 944–953 [DOI] [PubMed] [Google Scholar]
- 27. Sonne D. P., van Nierop F. S., Kulik W., Soeters M. R., Vilsboll T., and Knop F. K. (2016) Postprandial plasma concentrations of individual bile acids and FGF-19 in patients with type 2 diabetes. J. Clin. Endocrinol. Metab. 101, 3002–3009 [DOI] [PubMed] [Google Scholar]
- 28. Boden G., and Shulman G. I. (2002) Free fatty acids in obesity and type 2 diabetes: defining their role in the development of insulin resistance and beta-cell dysfunction. Eur. J. Clin. Invest. 32, 14–23 [DOI] [PubMed] [Google Scholar]
- 29. Boden G. (2003) Effects of free fatty acids (FFA) on glucose metabolism: significance for insulin resistance and type 2 diabetes. Exp. Clin. Endocrinol. Diabetes 111, 121–124 [DOI] [PubMed] [Google Scholar]
- 30. Metzger B. E., Phelps R. L., Freinkel N., and Navickas I. A. (1980) Effects of gestational diabetes on diurnal profiles of plasma glucose, lipids, and individual amino acids. Diabetes Care 3, 402–409 [DOI] [PubMed] [Google Scholar]
- 31. Chen X., Scholl T. O., Leskiw M., Savaille J., and Stein T. P. (2010) Differences in maternal circulating fatty acid composition and dietary fat intake in women with gestational diabetes mellitus or mild gestational hyperglycemia. Diabetes Care 33, 2049–2054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pappa K. I., Vlachos G., Theodora M., Roubelaki M., Angelidou K., and Antsaklis A. (2007) Intermediate metabolism in association with the amino acid profile during the third trimester of normal pregnancy and diet-controlled gestational diabetes. Am. J. Obstet. Gynecol. 196, 65.e61–e65 [DOI] [PubMed] [Google Scholar]
- 33. Karmen A., Wroblewski F., and Ladue J. S. (1955) Transaminase activity in human blood. J. Clin. Invest. 34, 126–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang T. J., Larson M. G., Vasan R. S., Cheng S., Rhee E. P., McCabe E., Lewis G. D., Fox C. S., Jacques P. F., Fernandez C., O'Donnell C. J., Carr S. A., Mootha V. K., Florez J. C., Souza A., Melander O., Clish C. B., and Gerszten R. E. (2011) Metabolite profiles and the risk of developing diabetes. Nat. Med. 17, 448–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Copps K. D., and White M. F. (2012) Regulation of insulin sensitivity by serine/threonine phosphorylation of insulin receptor substrate proteins IRS1 and IRS2. Diabetologia 55, 2565–2582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Newgard C. B. (2012) Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab. 15, 606–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Butte N. F., Hsu H. W., Thotathuchery M., Wong W. W., Khoury J., and Reeds P. (1999) Protein metabolism in insulin-treated gestational diabetes. Diabetes Care 22, 806–811 [DOI] [PubMed] [Google Scholar]
- 38. Rahimi N., Razi F., Nasli-Esfahani E., Qorbani M., Shirzad N., and Larijani B. (2017) Amino acid profiling in the gestational diabetes mellitus. J. Diabetes Metab. Disord. 16, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Singh K., Ali V., Pratap Singh K., Gupta P., Suman S. S., Ghosh A. K., Bimal S., Pandey K., and Das P. (2017) Deciphering the interplay between cysteine synthase and thiol cascade proteins in modulating Amphotericin B resistance and survival of Leishmania donovani under oxidative stress. Redox Biol. 12, 350–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Soga T., Baran R., Suematsu M., Ueno Y., Ikeda S., Sakurakawa T., Kakazu Y., Ishikawa T., Robert M., Nishioka T., and Tomita M. (2006) Differential metabolomics reveals ophthalmic acid as an oxidative stress biomarker indicating hepatic glutathione consumption. J. Biol. Chem. 281, 16768–16776 [DOI] [PubMed] [Google Scholar]
- 41. van Leeuwen M., Opmeer B. C., Zweers E. J., van Ballegooie E., ter Brugge H. G., de Valk H. W., Visser G. H., and Mol B. W. (2010) Estimating the risk of gestational diabetes mellitus: a clinical prediction model based on patient characteristics and medical history. Bjog 117, 69–75 [DOI] [PubMed] [Google Scholar]
- 42. Sharaf El Din U. A. A., Salem M. M., and Abdulazim D. O. (2017) Uric acid in the pathogenesis of metabolic, renal, and cardiovascular diseases: A review. J. Adv. Res. 8, 537–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Maged A. M., Moety G. A., Mostafa W. A., and Hamed D. A. (2014) Comparative study between different biomarkers for early prediction of gestational diabetes mellitus. J. Matern. Fetal Neonatal. Med. 27, 1108–1112 [DOI] [PubMed] [Google Scholar]
- 44. Su Y. X., Hong J., Yan Q., Xu C., Gu W. Q., Zhang Y. F., Shen C. F., Chi Z. N., Dai M., Xu M., Zhang Y. W., Liu Q. R., Li X. Y., Ning G., and Wang W. Q. (2010) Increased serum retinol-binding protein-4 levels in pregnant women with and without gestational diabetes mellitus. Diabetes Metab. 36, 470–475 [DOI] [PubMed] [Google Scholar]
- 45. Tepper B. J., Kim Y. K., Shete V., Shabrova E., and Quadro L. (2010) Serum retinol-binding protein 4 (RBP4) and retinol in a cohort of borderline obese women with and without gestational diabetes. Clin. Biochem. 43, 320–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhao W., Pan J., Li H., Huang Y., Liu F., Tao M., and Jia W. (2016) Relationship between high serum cystatin C levels and the risk of gestational diabetes mellitus. PLoS ONE 11, e0147277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yousefzadeh G., Pezeshki S., Gholamhosseinian A., Nazemzadeh M., and Shokoohi M. (2014) Plasma cystatin-C and risk of developing gestational diabetes mellitus. Diabetes Metab. Syndr. 8, 33–35 [DOI] [PubMed] [Google Scholar]
- 48. Khosrowbeygi A., Shiamizadeh N., and Taghizadeh N. (2016) Maternal circulating levels of some metabolic syndrome biomarkers in gestational diabetes mellitus. Endocrine 51, 245–255 [DOI] [PubMed] [Google Scholar]
- 49. Cocelli L. P., Dikensoy E., Cicek H., Ibar Y., Kul S., and Balat O. (2012) Pseudocholinesterase in gestational diabetes: positive correlation with LDL and negative correlation with triglyceride. Arch. Gynecol. Obstet. 286, 43–49 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


