Abstract
Despite advances in understanding the pathophysiology of Huntington’s disease (HD), there are currently no effective pharmacological agents available to treat core symptoms or to stop or prevent the progression of this hereditary neurodegenerative disorder. Pridopidine, a novel small molecule compound, has demonstrated potential for both symptomatic treatment and disease modifying effects in HD. While pridopidine failed to achieve its primary efficacy outcomes (Modified motor score) in two trials (MermaiHD and HART) there were consistent effects on secondary outcomes (TMS). In the most recent study (PrideHD) pridiopidine did not differ from placebo on TMS, possibly due to a large enduring placebo effect.
This review describes the process, based on in vivo systems response profiling, by which pridopidine was discovered and discusses its pharmacological profile, aiming to provide a model for the system-level effects, and a rationale for the use of pridopidine in patients affected by HD. Considering the effects on brain neurochemistry, gene expression and behaviour in vivo, pridopidine displays a unique effect profile. A hallmark feature in the behavioural pharmacology of pridopidine is its state-dependent inhibition or activation of dopamine-dependent psychomotor functions. Such effects are paralleled by strengthening of synaptic connectivity in cortico-striatal pathways suggesting pridopidine has potential to modify phenotypic expression as well as progression of HD. The preclinical pharmacological profile is discussed with respect to the clinical results for pridopidine, and proposals are made for further investigation, including preclinical and clinical studies addressing disease progression and effects at different stages of HD.
Keywords: Huntington’s disease, pridopidine, prefrontal cortex, striatum, motor control, dopamine, stabilizer, indirect pathway, dopamine D2 receptors
INTRODUCTION
Huntington’s disease (HD) is a rare neurodegenerative disorder of the central nervous system (CNS) characterized by progressive deterioration of motor and cognitive functions, as well as behavioral and psychiatric disturbances [1]. The disease has an autosomal dominant inheritance and is caused by an expanded CAG repeat in the huntingtin (HTT) gene on chromosome 4, encoding the mutant protein huntingtin [2, 3]. The hallmark neuropathological feature of HD is degeneration of medium spiny neurons (MSNs) in the striatum [4, 5] and such atrophy is evident some years before a formal clinical diagnosis can be made [6, 7]. The onset of clinical symptoms is usually in the fourth or fifth decade of life, but may occur at any time from childhood until old age. A diagnosis of HD is made following unequivocal signs of motor impairment, and may also be confirmed by genetic testing. Following disease onset, motor and cognitive functions steadily decline, ultimately leading to a state of immobility and severe dementia, and to premature death [8]. Pridopidine is a small molecule in clinical development for the treatment of motor symptoms in HD. This article provides an overview of the published pre-clinical pharmacology of pridopidine. The pharmacology is discussed in terms of major neuronal pathways disrupted in HD, aiming to provide a mechanistic rationale for the use of pridopidine in HD.
MOTOR SYMPTOMS IN HUNTINGTON’S DISEASE
The motor phenotype in HD consists of a number of symptoms, including involuntary choreatic movements and a loss of voluntary motor functions such as a progressive decline in fine and gross motor skills, motor impersistence, speech and swallowing difficulties, gait disorder and postural dysfunction. While chorea is considered a hallmark symptom of HD, the severity of disease and disability is more precisely defined by the progressive impairment in voluntary motor function [9, 10]. Also, health economic investigations suggest that voluntary motor impairment is a major determinant of burden of disease in HD [11]. The neuronal mechanisms underlying many of these symptoms are hypothesized to be linked to dysfunctions in cortico-striatal circuits [12–15] and a recent study observed strong correlations between motor symptoms and levels of degeneration in the motor cortico-striatal pathway [16]. Post-mortem neuroanatomical studies have shown that the motor impairment is strongly correlated to the degree of atrophy and cell loss in the striatum [17–19].
The dopamine system in Huntington’s disease
Dopamine is a monoamine neurotransmitter modulating several aspects of brain function, including motor control [20], and disrupted dopaminergic signaling has been implicated in a number of neurological and psychiatric conditions [21–24]. Motor control is exerted by dopamine released from the nigrostriatal pathway, modulating the activity of MSNs involved in the facilitation of movement, and inhibition of unwanted movement [25]. MSNs are GABAergic neurons, expressing high densities of dopamine receptors, and a progressive decline in striatal dopamine receptor density is one of the earliest findings in patients with HD [26, 27]. Such changes have been well described in post mortem studies, and corroborated in vivo by positron emission tomographic (PET) studies [28–31]. In comparison to these post-synaptic changes, the integrity of the pre-synaptic dopaminergic system in HD has been less extensively studied. While the dopamine neuron population in the substantia nigra appears preserved [32], a loss of dopamine terminals has been reported [33]. The latter finding has been confirmed in a PET study including a smaller number of patients with HD [28]. Studies in transgenic animal models suggest that a change in dopaminergic function, such as compromised dopamine release, is an early sign of neuropathology in HD [34–36]. Clinical studies have demonstrated that HD is characterized by pre-synaptic as well as post-synaptic dopamine-related dysfunctions with reduction in striatal dopamine synthesis, dopamine storage, dopamine transporter binding, and both dopamine D1 and D2 receptor binding [37]. Loss of both pre- and post-synaptic markers of dopamine neurotransmission is positively correlated with cognitive performance in both asymptomatic and symptomatic HD patients [38], but the integrity of extrastriatal dopamine D2 receptors has been reported to appear relatively well preserved in patients with HD [39]. A recent review on dopamine changes in Huntington’s disease outlined an integrated view, in essence suggesting a continuous decline in dopamine receptors over the patient’s life-span, and biphasic changes in striatal dopamine levels, initially increased but thereafter decreasing, in parallel with the transition from hyper- to hypokinetic motor disturbances [40].
It is well established that pharmacological treatments that modify dopaminergic function impact on the motor symptoms of HD. Levodopa challenges were used to provoke chorea as a clinical diagnostic test more than a decade before genetic testing was available [41]. Conversely, tetrabenazine, a monoamine-depleting drug, and dopamine D2 receptor antagonist drugs (antipsychotics) are used to alleviate chorea [42, 43]. On the other hand, Parkinsonian symptoms such as bradykinesia and hypokinesia in HD are hypothesized to be linked to dopaminergic impairment as these symptoms are aggravated by the use of antipsychotic medication [44, 45]. Furthermore, HD patients treated with antidopaminergic drugs have been reported to display a more rapid disease progression [46] and a more severe phenotype [47]. Thus, motor symptoms in HD are sensitive to drugs that alter dopaminergic transmission, where enhancement of dopaminergic activity is associated with increased chorea, and attenuation is conceivably associated with worsening of negative motor symptoms such as bradykinesia.
THE DIRECT AND INDIRECT PATHWAY IN HUNTINGTON’S DISEASE
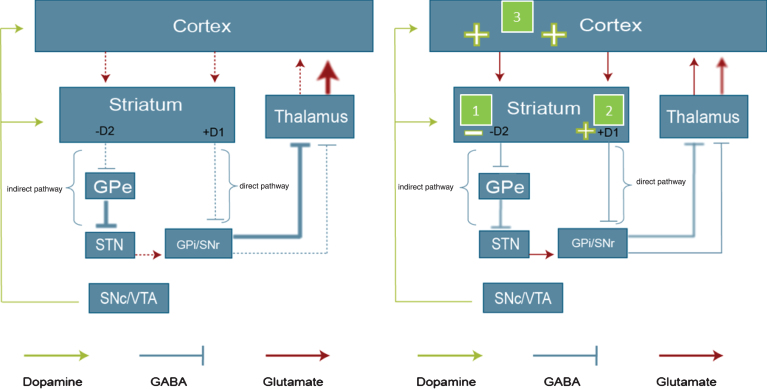
Two major components of the cortico-striatal network are the so-called indirect and direct pathways, each forming part of one closed, cortico-basal ganglia-thalamo-cortical feedback circuit [12, 48–50]. The indirect pathway projects from MSNs co-expressing dopamine D2 receptors and enkephalin, and involves relays in the external segment of the external globus pallidus and the subthalamic nucleus, before reaching the internal globus pallidus (GPi) and the substantia nigra pars reticulata (SNr). Output from GPi/SNr targets the thalamus, projecting further to glutamatergic neurons of the cortex which project back onto the striatum. The indirect pathway forms part of a negative feedback loop, involved in suppression of movement. Striatal neurons that co-express dopamine D1 receptors, substance P and dynorphin give rise to the direct pathway. In essence, the direct pathway connects GABAergic MSNs expressing excitatory dopamine D1 receptors, substance P and dynorphin, with the GPi/SNr via a single neuron pathway. Further projections from GPi via the thalamus reach the cortex, closing the circuit by a glutamatergic corticostriatal connection back to the MSNs. This circuit, functioning as a positive feedback loop, is involved in the selection and facilitation of voluntary movements. In healthy conditions, the direct and indirect pathways act in balance leading to adequate control of voluntary movement and suppression of involuntary movements [12, 48]. In HD a number of changes that affect striato-thalamic output arise as a consequence of the pathologic process occurring in the disease. A progressive degeneration of striatal MSNs leads to weakened output in both pathways (Fig. 2). Cortico-striatal and nigrostriatal inputs are progressively weakened, leading to decreased striatal glutamate and dopamine release. In manifest HD, a progressive metabolic decline is seen in the thalamus, an observation likely to reflect a net loss of pallidothalamic output [51].
Fig.2.
Proposed in vivo mode of action of pridopidine in manifest HD. A schematic overview of the organization of the basal ganglia, involving the direct and indirect pathway, and the proposed in vivo effects of pridopidine. The left panel shows the direct and indirect pathway in the state of manifest HD. Dashed lines represent reduced transmission, thick lines increased transmission. In manifest HD, output in both striatal pathways is attenuated, and cortico-striatal connectivity is impaired [53, 55]. The right panel illustrates the suggested mode of action for pridopidine: (1) Pridopidine normalizes the aberrant function in the indirect pathway, by blocking DA D2 receptors, which results in attenuation of involuntary movements. (2) Pridopidine improves voluntary movements by stimulating the direct pathway via activation of the DA D1 receptor. (3) Pridopidine strengthens the prefrontal cortex, which indirectly stimulates both the direct and indirect pathways. The schemes shown represent the changes in late stage, symptomatic HD, and the tentative impact of pridopidine. At earlier stages of HD an increase in cortico-striatal glutamate transmission has been suggested (not shown). D1, dopamine D1 receptors; D2, dopamine D2 receptors; GPe, globus pallidus pars externa; SNc, substantia nigra pars compacta; VTA, ventral tegmental area; GPi, globus pallidus pars interna.
A decreased output from the MSNs in the indirect pathway results in reduced inhibition of unwanted movements. In patients with HD this is hypothesized to underlie the presence of involuntary movements, such as chorea and dystonia [52]. This would explain why dopamine D2 antagonists, or dopamine depleters, could suppress chorea, as blockade of the inhibitory influence of dopamine on the indirect pathway would strengthen the GABAergic output from the MSNs expressing dopamine D2 receptors, thereby facilitating the suppression of involuntary movements [12]. Decreased activity in the direct pathway, due to cellular degeneration and loss of connectivity in D1-receptor-expressing MSNs, is hypothesized to lead to impaired ability to perform voluntary motor functions in patients with HD [12, 53, 54]. In addition to the striatal degeneration, deterioration of cortical function and cortico-striatal connectivity is observed in HD [53, 55]. Animal studies exploiting regionally specific expression of mutant huntingtin suggest that cortical expression of htt is required for the complete HD phenotype to develop. Furthermore, abnormalities specifically affecting neuronal activity and morphology in the cortex, are being increasingly recognized as determinants contributing to the HD phenotype [56]. Such aberrations are likely to contribute to the impaired motor control as well as psychiatric disturbances and cognitive impairments in patients with HD [52, 57, 58].
PHARMACOLOGICAL TREATMENT OF MOTOR SYMPTOMS IN HUNTINGTON’S DISEASE
Numerous medications from different classes are prescribed off-label to ameliorate the motor symptoms associated with HD. These medications include, e.g., antidopaminergic drugs, energy metabolites, benzodiazepines, and glutamate-modifying drugs (riluzole and amantadine) [43, 59]. However, the evidence for the effectiveness of such treatments is poor [60]. Among the motor symptoms of HD, chorea is the most frequently treated symptom, and a vast majority of medications prescribed for this indication are based on the principle of reducing dopaminergic tone. Thus, antipsychotics drugs, i.e., dopamine D2 receptor antagonists, and tetrabenazine, a vesicular monoamine transporter (VMAT) inhibitor, are regarded as “first line” treatment of chorea [43]. In the US, tetrabenazine is approved for the treatment of chorea in HD [42], but no beneficial effects on the more functionally determining voluntary motor function have been demonstrated [60]. Thus, there are no approved or established treatments for general improvement of the multifaceted motor symptoms. Hence, there is a significant unmet medical need to ameliorate both positive and negative motor symptoms of HD [42, 60] and to slow or halt the progression [61].
PRIDOPIDINE IN HUNTINGTON’S DISEASE
Pridopidine (Teva Pharmaceutical Industries Ltd, Israel) belongs to a novel class of compounds characterized by their psychomotor stabilizing properties, i.e., the ability to restore normal levels of psychomotor activity in both hypo- and hyperactive states, in pre-clinical in vivo behavioral models. The efficacy of pridopidine in HD was first investigated in a small-scale study, which suggested that pridopidine could improve voluntary motor functions in patients with HD [62]. Subsequently, two large clinical trials in patients with manifest HD indicated that pridopidine can improve motor function [63, 64]. The Unified Huntington’s Disease Rating Scale (UHDRS) total motor score (TMS) was improved by around 3 points versus placebo in both these trials. One point on this scale represents an approximately 10% reduced likelihood of being able to work, manage finances, drive a car or supervise children [65]. Further analysis of subscales of the TMS indicated that pridopidine significantly improved voluntary movements (balance and gait), including hand movements, in both trials. There were also signals of effects on eye movements and involuntary movements. Furthermore, both studies concluded that pridopidine displayed a good safety and tolerability profile [63, 64], consistent with available reports from the recently concluded PRIDE-HD study, a placebo-controlled, dose-ranging study with 52 weeks treatment duration [66]. Long-term safety and tolerability over 36 months was assessed in the recently published OPEN-HART study, showing similar results [67]. 29.7% of the safety population (n = 118) had adverse events (AEs) considered to be related to study drug, the most common of which were falls, decreased weight, anxiety, insomnia, and depression. Two subjects were reported to have seizures, one of which was considered possibly treatment related.
A meta-analysis of pooled data sets from these studies supports the conclusions drawn from each individual trial, indicating significant effects on overall motor function (measured by TMS) as well as on voluntary movements (hand movements, and gait and balance), eye movements and involuntary movements (dystonia) [68]. Pridopidine is, therefore, the first compound showing promise in large-scale clinical trials in treating the core motor symptoms of HD without worsening other symptom domains.
Pridopidine – discovery and overview of pharmacology
Pridopidine was discovered using an in vivo systems pharmacology approach, focusing on measuring biomarkers of neurotransmission in neuronal circuits known for their involvement in the control of psychomotor functions. The theory is that systematic in vivo profiling of CNS compounds, using a comprehensive range of neurochemical, gene expression and behavioral biomarkers collected in both healthy and diseased states, would give a reliable handle on preclinical properties and allow for predictions of clinical features of the novel compounds tested in the system. Data on a wide variety of known therapeutic agents including antipsychotics, antidepressants, anxiolytics and psychostimulants, as well as experimental reference compounds, are collected, and then used to define a multidimensional compound “map” serving as a guide towards the sought after in vivo profile [69]. This methodology was developed to enable a rational and predictive drug discovery process in a field where target-based discovery approaches have hitherto shown to be largely unsatisfactory in terms of determining clinically promising molecules and molecular action mechanisms [70]. In the specific program that led to the discovery and subsequent development of pridopidine in HD, the vital impact of central monoaminergic and specifically dopaminergic pathways in the cortico-subcortical circuitry regulating motor function was considered. The complex, progressive symptomatology in HD, combining hypo- and hyperkinetic motor disturbances, cognitive and psychiatric symptoms, suggest any pharmacological intervention should be delicately balanced not to disturb remaining functions or worsen aspects of the heterogeneous symptoms. The combined basic research efforts and considerations described above led to the formulation of a preclinical target profile. In short, a compound of interest should: 1) show no interference with spontaneous locomotor patterns over a wide dose range; 2) have the ability to normalize states of hypoactivity; 3) have the ability to normalize states of hyperactivity; and 4) act primarily through the dopamine system. The initial screening was performed in vivo as described in the following (see section Overall in vivo phenotypic response profile).
From the medicinal chemistry perspective, the fourth criterion above suggested the conceptual starting point of the program to focus on design and synthesis of compounds with dopamine D2 receptor agonist-like chemical motifs. The key chemical strategy in the program was to modify the agonist motif so that the pharmacophore elements essential for intrinsic activity were lost, to obtain compounds that primarily interact with the dopamine D2 receptor in a similar way as agonists, but without the ability to stabilize the active and catalytic conformation(s) of the receptor G-protein complex. The working hypothesis was that this should result in a portfolio of compounds with agonist-like receptor interactions but with antagonist-like pharmacological features. Further, the hydrophilic characteristics of the agonist motif were to be retained to ensure similar binding mode and adherence to druggability features important for favorable drug metabolism and pharmacokinetics, and reduced safety and toxicological risks.
Starting from a series of compounds with partial dopamine D2 agonist properties reported by Mewshaw et al. [71] including 3-(1-benzylpiperidin-4-yl)phenol, the key elements for its intrinsic activity were identified to be the phenol group, the anilinic nitrogen and the large N-alkyl group. A series of modifications of these elements led to a range of phenylpiperidines, including 4-[3-(methylsulfonyl)phenyl]-1-propylpiperidine (ACR16, pridopidine) [72], displaying the desired profile.
Overall in vivo phenotypic response profile
The initial in vivo pharmacological assessment of pridopidine was performed by acute dose response studies in intact rats, collecting behavioral data and ex vivo monoaminergic neurochemical indices according to a systematic, standardized model, followed by multivariate analysis of the response profile, including comparisons to a wide range of CNS compounds and a tentative therapeutic classification [69, 73]. This “screening” suggested that three major criteria were met; i.e., no behavioral inhibition, subtle behavioral activation in the hypoactive phase, and significant effects on dopaminergic indices reflecting dopamine D2 receptor modulation. Furthermore, the multivariate comparison to reference compounds indicated clear similarities with the cluster of anti-psychotic compounds tested, as well as some effects in cortical brain areas overlapping with effects of pro-cognitive compounds. This prompted further preclinical investigations in specific models of dopaminergic hyperactivity as well as glutamatergic hypoactivity, and assays of cortical neurotransmission.
Behavioral pharmacology
Pridopidine reduces both hyperactivity and the behavioral abnormalities pharmacologically induced in animal models of elevated dopamine or decreased glutamate neurotransmission, while the locomotor activity of intact animals is unaffected over the same dose range. Hence, pridopidine counteracts hyperactivity induced by psychotomimetics including d-amphetamine and N-methyl-d-aspartate (NMDA) antagonist MK-801 [72, 74]. In addition, pridopidine enhances locomotor activity in animals with a low baseline psychomotor activity, as seen in animals habituated to their environment [74], as well as in rats co-treated with the VMAT inhibitor tetrabenazine [75]. Pridopidine is unable to induce profound hypoactivity or catalepsy, indicating that it has a low likelihood of displaying the adverse neurological effects associated with classical dopamine D2 receptor antagonist antipsychotics [74, 76]. Pridopidine does not induce catalepsy, even at doses producing D2 receptor occupancy reaching 80% or above [76]. Considering qualitative aspects of the behavioral effects of pridopidine, it has been shown to restore social interactions in rats treated with MK-801 [77], and ameliorate the behavioral primitivization induced by MK-801 in mice [78]. Both findings are proposed to imply beneficial effects on cognitive symptoms. Furthermore, pridopidine is efficacious in the conditioned avoidance response model of antipsychotic activity [76]. Pridopidine has also been shown to display potent and efficacious antidepressant activity in the mouse tail suspension test [74]. A chronic study in a rodent model of L-DOPA induced motor complications (sensitization to repeated L-DOPA upon unilateral 6-OH-dopamine lesion) demonstrated that pridopidine reduced L-DOPA induced rotational behavior while not impairing forward locomotion [79]. In behavioral studies using the R6/2 mouse model of HD, Squitieri et al., recently reported data indicating that pridopidine reduces and/or prevents the expression of the HD motor phenotype on the horizontal ladder task and in open-field locomotor measurements during 6 weeks’ treatment, starting in the pre-symptomatic stage. It also prolonged the life-span in R6/2 mice. Wild-type behaviors in the same assays were not affected by pridopidine [80].
Neurochemical effects
Pridopidine increases synthesis, release and metabolism of dopamine in sub-cortical areas [74], mimicking the effects of dopamine D2 antagonists in general [22]. The increases in dopamine turnover and transmission biomarkers produced by dopamine D2 antagonists in sub-cortical areas are due to inhibition of dopamine-D2-receptor-dependent negative feedback [22, 81]. These results indicate that pridopidine acts as an antagonist at dopamine D2 receptors in vivo. Furthermore, in an in vivo study designed to detect agonist properties, Natesan et al., tested pridopidine’s ability to reverse DOPA accumulation induced by reserpine (an inhibitor of the vesicular monoamine transporter) [76]. They found that pridopidine had no effect in this sensitive agonist assay. Pridopidine increases plasma levels of prolactin in rats, suggesting antagonistic effects at hypothalamic dopamine D2 receptors in vivo [82]. The interaction of pridopidine at dopamine D2 receptors is further demonstrated by in vivo binding experiments showing that pridopidine dose-dependently displaces the dopamine D2 antagonist raclopride from dopamine D2 receptors [76]. Taken together, the in vivo dopaminergic neurochemistry and in vivo binding data on pridopidine consistently indicate that pridopidine acts as a full dopamine D2 antagonist. In vivo microdialysis studies in conscious rats have demonstrated that pridopidine dose-dependently increases the efflux of dopamine and noradrenaline in the cortical and subcortical projection areas of the ascending dopamine projections [74]. At doses efficacious with respect to the key behavioral effects, prefrontal cortex and striatal levels of both dopamine and noradrenaline are increased.
Analysis of gene expression profiles has demonstrated that pridopidine increases the expression of activity-regulated cytoskeleton-associated protein [83] (Arc) mRNA in the frontal cortex and the striatum [84]. Furthermore, pridopidine has been shown to reverse the reduction in striatal Arc levels induced by MK-801 [85]. The increase of Arc mRNA expression suggests an increase in synaptic activity [86], possibly related to facilitation of NMDA receptor activation [87, 88]. In addition to the ex vivo biomarker studies demonstrating cortical effects of pridopidine, a recent study shows that pridopidine increases firing in prefrontal pyramidal cells [79]. The pridopidine-driven increase in pyramidal cell firing was antagonized by the dopamine D1 antagonist SCH23390, suggesting that the pyramidal cells are indirectly activated by pridopidine through increased levels of dopamine binding with D1 receptors. The cortical effects of pridopidine, as reflected, by increased dopamine release in the frontal cortex, increased Arc gene expression, and increased cortical neuronal activity, may contribute to its potency in behavioral assays. This is reported to be higher than the potency in increasing striatal 3,4-dihydroxyphenylacetic acid, essentially reflecting D2 antagonism in vivo [74]. It has been shown that addition of a compound that increases cortical dopamine release improves the potency of D2 antagonists in behavioral models of antipsychotic activity [89, 90]. Also, dopamine D1 agonists per se have been shown to exert anti-psychotic like behavioral effects [91].
In vitro pharmacology
In vitro binding studies demonstrate affinity in the micromolar range at dopamine D2 receptors. [72]. Assessments on functional responses in different settings in vitro show that pridopidine displays competitive antagonism with a fast dissociation rate from the dopamine D2 receptor [92], and that pridopidine, just as in the in vivo assays, lacks intrinsic activity at dopamine D2 receptors [92–94].
The affinity of pridopidine measured at dopamine D2 receptors is slightly higher using agonist vs antagonist counter ligands, ki antagonist/ki agonist = 2.3 [72]. Dopamine D2 receptors exists in two states (i) the resting and low-affinity state (D2RLow) and (ii) the active, catalytic, high-affinity state (D2RHigh). Dopamine D2 receptors agonists bind with preference to receptors in D2RHigh and induce a full catalytic reaction, i.e. they have affinity and intrinsic activity. Pridopidine has been proposed to preferentially bind to the high-affinity state, but without intrinsic activity [72, 95, 96]. This would differentiate pridopidine from classical D2 receptor antagonists, which, in contrast, stabilize the D2RLow state and do not show preference for either receptor state. In summary, in vitro as well as in vivo studies addressing dopamine receptor interactions consistently indicate that pridopidine acts as a competitive, low-affinity dopamine D2 antagonist with fast-off receptor-dissociation kinetics and with a slight preference for the agonist binding site.
Pridopidine has also been reported to display in vitro affinity in the micromolar range at adrenergic alpha 2A/C receptors (ki 20/2.3 μM), serotonergic 5HT1A (ki 3.1 μM) and 5HT2A (ki 15 μM) receptors, and histamine H3 (ki 7.2 μM) receptors [79]. Interactions at these receptors, each of which has been shown to modulate extracellular levels of monoamines [97–100], as well as glutamatergic transmission in the cortex [101–104], may contribute to the in vivo effects of pridopidine. Apart from the monoaminergic receptors, pridopidine has been reported to display moderate affinity at the sigma-1 receptor in vitro [105]. The significance of this is unclear, as sigma-receptor binding is displayed by a wide range of CNS-active compounds from different therapeutic classes, but it has been proposed to contribute to neuroprotective properties of pridopidine [80]. In support of this, a recent ex vivo study on striatal gene expression in rats showed upregulation of several neuroprotective pathways by pridopidine, including brain derived neurotrophic factor (BDNF), glucocorticoid receptor, and AKT/phosphoinositide 3-kinase (PI3K), and dopamine D1 pathways, and further suggested that sigma1-receptor activation is necessary for the effect of pridopidine on the effect on the BDNF pathway, based on assessment of in vitro BDNF secretion in neuroblastoma cells, which was antagonized by co-incubation with a sigma-1 antagonist [106]. Recently published additional gene expression analyses from the same study demonstrated upregulation of calcium regulating proteins including calbindin and homer1a upon chronic treatment with pridopidine. Furthermore, it was shown that pridopidine normalized calcium homeostasis and prevented spine loss in aging corticostriatal co-cultures from YAC128 mice. Spine rescue and normalized Ca2 + levels were prevented by deleting sigma-1 receptors, suggesting pridopidine exerts synapto-protective effects, through sigma-1 receptors and effects on calcium signaling [107].
Summary of preclinical pharmacology
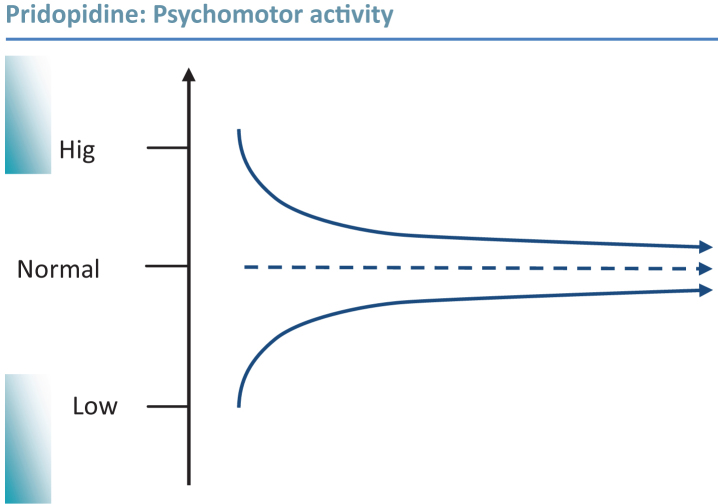
The in vivo pharmacological profile of pridopidine can be summarized as state-dependent inhibition or activation of dopamine-dependent psychomotor functions (Fig. 1). This is achieved through low-affinity/fast-off antagonism of dopamine D2 receptors, combined with an increased cortical and sub-cortical monoamine release resulting in enhanced activity of prefrontal cortex neurons. It thus appears as if pridopidine exerts its behavioral stabilizing effects by acting on several key nodes of the cortico-striatal network regulating psychomotor functions. In addition to these acute in vivo effects as described in terms of major neuronal pathways, emerging data suggest sigma-1 receptor mediated effects, putatively involving regulation of intraneuronal calcium signaling, which may be of relevance in particular with regard to neuroprotective effects of pridopidine.
Fig.1.
Pridopidine is able to enhance or inhibit dopamine-dependent functions. Graphic illustration of psycho-motor stabilization: In vivo pharmacological studies have consistently demonstrated state dependent behavioral effects of pridopidine; reducing psychomotor activity in hyperactive states, and enhancing activity in hypoactive states. This is proposed to translate to stabilization of both hyper- and hypokinetic motor disturbances in HD; to some extent overlapping with observations of disturbed dopamine transmission over the course of the disease [40].
PRIDOPIDINE: MODE OF ACTION AT THE INTEGRATED CIRCUITRY LEVEL
The pharmacological effects of pridopidine as outlined above, at the levels of receptor interactions, neurochemistry, gene expression firing activity and behavior, can be brought together in a tentative, integrated and testable model of the system-level mode of action of pridopidine based on three main core features (Fig. 2). The discussion is focused on the relief of motor symptoms in manifest HD, as the primary clinical indication for pridopidine at present. First, pridopidine is a low-affinity/fast-off dopamine D2 receptor antagonist, thus modifying output in the indirect striato-thalamic output pathway, tentatively leading to improved control of involuntary movements in HD. Secondly, pridopidine induces dopamine release in the basal ganglia and the frontal cortex. This, in combination with the D2 inhibiting properties, leads to a shift in balance towards D1 receptor signaling, strengthening the direct striato-thalamic output pathway, which would enhance voluntary motor functions. Thirdly, pridopidine enhances dopamine transmission and neuronal activity in the frontal cortex, leading to strengthened cortico-striatal signaling. These three core features of pridopidine are proposed to act in synergy to reduce the complex mixture of negative and positive motor symptoms associated with cortical and striatal degeneration in HD.
Pridopidine strengthens the indirect pathway via antagonism of dopamine D2 receptors
The mechanism of action for pridopidine involves dopamine D2 receptor antagonism [72, 92, 95, 96]. MSNs projecting to the indirect pathway are negatively modulated by dopamine through dopamine D2 receptors. Hence, dopamine attenuates the GABAergic output from these neurons. Diminished activity in this pathway results in a reduced capacity to suppress involuntary movements. By blocking the dopamine D2 receptors on the MSNs of the indirect pathway, the inhibitory influence of dopamine is reduced, and the output via the indirect pathway is strengthened. Therefore, by antagonizing dopamine D2 receptors in the striatum, pridopidine normalizes the aberrant function in the indirect pathway, which results in attenuation of involuntary movements (Fig. 2, right panel). This is supported by clinical results suggesting reduced involuntary motor symptoms in HD patients treated with pridopidine, reported from large randomized clinical trials [63, 64].
Pridopidine strengthens the direct pathway by stimulating dopamine D1 receptors
MSNs projecting to the direct pathway are positively modulated by dopamine through dopamine D1 receptors. Hence, dopamine enhances the GABAergic output from these neurons. Administration of pridopidine increases the release of striatal dopamine [74]. This implies that pridopidine, by increasing synaptic availability of dopamine, thus indirectly stimulating the dopamine D1 receptors, could strengthen the striatal output in the direct pathway (Fig. 2). This would result in improvements of voluntary movement control in HD. In our view, this hypothesis seems to be supported by clinical outcomes of the MermaiHD (Multinational European Multicentre ACR16 study in Huntington’s Disease) and HART (Huntington’s disease ACR16 Randomized Trial) trials indicating improvement in voluntary motor control such as oculomotor and postural function, and hand movements [63, 64].
Consistent with the notion that pridopidine activates striatal D1 receptors, and antagonizes D2 receptors, pridopidine has been demonstrated to dose-dependently increase expression of striatal Arc mRNA [84]. To the best of our knowledge, pridopidine displays no affinity or direct activity at any glutamate receptors investigated. Therefore, such NMDA receptor activation is not likely to occur as a direct effect. Rather, given the functional association between D1 and NMDA receptors in MSNs [108] the increase in striatal Arc mRNA levels could arise indirectly as a consequence of synaptic NMDA receptor modulation, related to activation of dopamine D1 receptors. This is in line with the findings that dopamine D1 receptor agonists increase, and dopamine D1 antagonists decrease striatal Arc expression [109, 110]. Activation of DA D1 receptor mediated pathways by pridopidine was corroborated in a recent gene profiling study indicating upregulation of D1 mediated gene expression upon repeated administration of pridopidine [106]. The effect of pridopidine on striatal Arc levels may also be associated with the direct antagonism of striatal D2 receptors, leading to reduced inhibitory tone on cortico-striatal glutamate release, and therefore to increased glutamate transmission. Induction of striatal Arc gene expression has been reported for several D2 receptor antagonists [111, 112]. Further studies, e.g. investigating the localization of Arc induced in D1 vs D2 receptor expressing MSNs, would be needed to determine more precisely how MSNs of the direct and indirect pathways are affected by pridopidine. Furthermore, animal studies have suggested a biphasic pattern of changes in cortico-striatal communication in HD, with increased signaling in early stages, before reaching the late stage represented in Fig. 2 [53, 55]. Thus, it would be of interest to assess the impact of pridopidine on cortico-striatal communication at different stages of the disease.
Pridopidine strengthens cortical activity
In manifest HD, progressive thinning of the cortex is observed [113], and preclinical studies suggest decreased communication in the cortico-striatal glutamatergic projections, in addition to the degeneration of striatal MSNs [53]. These alterations are associated with cognitive impairments [114] and are proposed to result in reduced activation of the direct and indirect pathway [53] (Fig. 2, left panel), hampering motor control [53]. The pathogenetic impact of disrupted cortico-striatal connectivity for the HD phenotype has further been demonstrated in pre-clinical HD models, showing that expression of mutant htt in both the cortex and the striatum is required to develop the full pathological phenotype [115].
Pridopidine has been demonstrated to dose-dependently increase dopamine in the prefrontal cortex [74]. The strengthening of frontal cortex dopamine transmission is further hypothesized to drive down-stream effects in the cortico-striatal circuitry, regulating motor functions.
As a more direct read-out of neuronal activity in the frontal cortex, administration of pridopidine has been demonstrated to dose-dependently increase Arc gene expression in rat frontal cortex, interpreted as increased activation of synaptic NMDA receptors [84]. Preliminary observations that the extra-synaptically acting NMDA receptor antagonists memantine and ifenprodil display similar effects as pridopidine, with respect to frontal cortex Arc gene expression, provide indirect support for the notion that pridopidine shifts the balance towards synaptic NMDA receptor signaling [85]. Given the synergistic interaction between dopamine D1 and NMDA receptor signaling in cortical pyramidal cells, it is proposed that such enhancement of synaptic NMDA receptor signaling by pridopidine arises indirectly due to increased cortical dopamine transmission followed by activation of dopamine D1 receptors. This is supported by recently recorded observations that pridopidine increases the firing frequency of rat pyramidal neurons in the frontal cortex, and that this effect could be blocked by administration of the D1 antagonist SCH23390 [79]. Increased activity of dopamine D1-expressing glutamatergic cells in the cortex would promote cortico-striatal communication, and indirectly drive the indirect and direct pathways (Fig. 2). The effects of pridopidine to reduce hypoactivity in partially monoamine-depleted rats, concurring with increased frontal cortex Arc gene expression, provide some support for a cortically driven improvement of voluntary motor function by pridopidine.
Pridopidine displays clear-cut effects on cortical dopamine transmission, which are likely to contribute to effects such as those on Arc gene expression and pyramidal cell firing activity, and to the overall behavioral profile. However, pridopidine may also influence cortical neurons by other mechanisms. In vivo microdialysis studies have demonstrated increased levels of not only dopamine, but also noradrenaline, which modulates cortical activity through alpha 1 and alpha 2 receptors [74, 79]. α2-adrenoceptor blockade has been shown to induce cortical Arc gene expression, likely by increased NA release [116]. Furthermore, the affinity of pridopidine at adrenergic alpha2c, 5HT1a and histamine H3 receptors [79], as well as sigma-1 receptors [105], may be of relevance. Regardless of the exact mechanism for the Arc upregulation in vivo, it provides evidence of unique cortical effects of the dopidines, as compared to other DA modulating compounds in general, and antipsychotics in particular [84]. Such effects could contribute to the characteristic behavioral pharmacology of the dopidines, and further support the notion of potential cognitive enhancing properties.
The proposed in vivo mode of action of pridopidine is based on the state of manifest HD, a symptomatic stage in which impairment of both the indirect and direct pathways has developed (Fig. 2). This would suggest the use of pridopidine for motor symptom alleviation in advanced HD, targeting both voluntary and nonvoluntary motor impairment. However, emerging evidence suggesting potential beneficial effects on disease progression could motivate earlier interventions.
THE CLINICAL POTENTIAL OF PRIDOPIDINE
Results from multicenter trials MermaiHD and HART were recently published [63, 64]. While the primary endpoint, defined as reduction in a modified motor score, was not met, a 3-point reduction in the UHDRS total motor score, compared to placebo, was observed in both studies. Exploratory analysis of these data indicated that negative motor symptoms such as gait and balance, hand movements and oculomotor function improved. There were also improvements on involuntary motor features. Of note, the clinical results indicate that the dopamine-enhancing properties of the compound are not translated into an increase in involuntary movements, such as those seen after as example L-dopa treatment in patients with HD. Furthermore, in contrast to classical D2 receptor blocking antipsychotics or dopamine depleters like tetrabenazine, pridopidine does not give rise to the bradykinesia and rigidity associated with such treatments. Rather, the data reported so far suggest that pridopidine reduces negative motor symptoms.
There is also a possibility that pridopidine, via the aforementioned pharmacological profile, including modulation of dopaminergic transmission as well as through sigma-1 receptor interactions, may modify disease progression itself in HD. Neurodegeneration in HD is strikingly selective where striatal MSNs are most vulnerable to the pathological process. The underlying causes for this selectivity are not completely known. Striatal MSNs receive massive glutamatergic input from the cortex and a longstanding hypothesis is that changes in NMDA-receptor-dependent plasticity and transmission are a major factor contributing to this selective vulnerability. It was more recently proposed that the balance between synaptic and extrasynaptic NMDA receptors determines whether resulting signaling is beneficial or detrimental. Synaptic activation promotes a number of pro-survival pathways whereas extrasynaptic signaling opposes these and triggers pro-death pathways [117]. Pridopidine increases Arc mRNA expression, and increases pyramidal cell firing in the frontal cortex, both effects likely driven by dopamine release and D1 receptor stimulation leading to enhanced NMDA receptor activity. Thus, pridopidine may indirectly enhance synaptic NMDA receptor signaling in the frontal cortex. In support of this interpretation, memantine, which has been shown to preferentially antagonize extrasynaptic NMDA receptors, and hence shifts the balance in NMDA-receptor-mediated transmission from extrasynaptic to synaptic sites, displays similar effects as pridopidine on cortical Arc mRNA expression [85]. Memantine has been shown to display neuroprotective effects in vivo. Recently, pridopidine was reported to promote brain cell survival, activate pro-survival pathways and improve motor phenotype in R6/2 mice, providing support for a protective potential in HD [80]. A tentative mechanism for such an effect could be indirect facilitation of synaptic NMDA receptor transmission. There are also studies suggesting neuroprotective effects in vitro and in vivo mediated by sigma-1 receptor interactions [105–107, 120]. Mechanisms directly related to altered dopaminergic neurotransmission could also be involved. Of note, given the proposed biphasic changes in cortico-striatal glutamate transmission, it could be argued that indirect enhancement of DA D1, as well as NMDA receptor mediated transmission, in particular in the striatum, could be unfavorable in early HD, potentially aggravating chorea, and accelerating disease progression, e.g., by triggering apoptotic pathways. The studies in R6/2 mice speaks against such concerns; rather supporting the notion of neuroprotective effects, tentatively arising as a net system level effect of dopamine modulation at multiple sites as outlined above, combined with sigma-1 receptor interactions. Also, the phenotype improved. Furthermore, there have been no indications of worsened chorea in pridopidine treated patients, to the contrary, involuntary motor features tend to improve.
No clinical studies primarily assessing disease-modifying aspects of pridopidine have been performed. In a recently concluded phase 2 study, Pride-HD, disease progression as represented by total functional capacity (tfc) score was measured as an exploratory end-point. The primary endpoint, the change in total motor score at 26 weeks, was not met in this study which was complicated by a large, enduring placebo response [66]. Considering tfc, pridopidine 45 mg b.i.d. treated subjects displayed a slower decline at 52 weeks, compared to placebo. Recently published data from Open-HART, an extension of the HART study reporting outcomes upon up to 36 months of treatment with pridopidine, suggested a numerical reduction in clinical progression in terms of tfc as compared to the expected natural course of progression, however not statistically significant based on a post-hoc analysis comparing a limited sample of matched placebo control subjects from two other studies in HD (2CARE and CARE-HD) with pridopidine treated subjects [67], when adjusting for multiple comparisons. In Table 1, rates of progression in terms of tfc decline over 1 year or more for observational and randomized studies in HD are listed. Rate of progression is related to baseline tfc, with faster rates observed in early stages of HD (Marder). The rate of functional decline observed in observational cohorts, or with placebo or other treatments are consistently faster than the rates observed upon long term treatment with pridopidine.
Table 1.
Progression rates in terms of TFC decline in randomized and observational studies. Shown are mean values of annual TFC change estimated for each study listed; negative values indicate worsening on this scale. For Pride-HD, exact baseline data were not available; however, the study population was similar to, e.g., the HART study population, i.e. ambulatory patients, a large proportion in TFC stage I or II. The delay of TFC decline observed in pridopidine treated subjects was particularly evident in the early-stage population. CoQ: Coenzyme Q. HSG: Huntington study Group
| Study | Baseline TFC | Treatment | Annual TFC progression | Follow-up time |
| HSG [121] | 7.5 | Observational | –0.72 | 18 (median) |
| HSG [121] | Stage 2 (tfc 7–10) | Observational | –0.84 | 18 mo |
| REGISTRY [122] | 8 (M), 7.4 (F) | Observational | –0.74(M), 0.94 (F) | 21 mo |
| CARE-HD [123] | 10.3 | Placebo | –1.096 | 30 mo |
| CARE-HD [123] | 10.1 | CoQ | –0.96 | 30 mo |
| CARE-HD [123] | 10.0 | Remacemide | –1.08 | 30 mo |
| 2CARE [124] | 11.0 | Placebo | –0.952 | 60 mo |
| 2CARE [124] | 10.8 | CoQ | –0.906 | 60 mo |
| CREST-E [125] | 10.2 | Placebo | –0.7 | 18–48 mo |
| CREST-E [125] | 10.2 | Creatine | –0.82 | 18–48 mo |
| Tetra-HD continuation [126] | 7.6 | Tetrabenazine | –1.3 | 18 mo |
| Open-HART [67] | 9.1 (cohort completing 36 mo) | Pridopidine | –0.57 | 36 mo |
| Open-HART [67] | 8.4 (cohort completing 12 mo) | Pridopidine | –0.6 | 12 mo |
| Pride-HD [66] | – | Pridopidine | –0.04 | 12 mo |
| Pride-HD [66] | – | Placebo | –0.83 | 12 mo |
CONCLUSION
HD is a devastating hereditary neurodegenerative disorder causing brain atrophy and rapidly progressive disability. Clinical and preclinical data in HD patients and transgenic rodent models have provided evidence for multiple neuronal and neurotransmitter dysfunctions in the cortico-basal ganglia systems. The neuronal degeneration and dysfunction in HD explains the motor symptoms seen in this disorder, and the involvement of both the direct and indirect output pathways from the striatum, underlies the complex mixed negative and positive motor symptoms presented. Preclinical pharmacology data on pridopidine demonstrate balancing effects on motor function through the dopamine system, and indirect enhancement of cortico-striatal synaptic activity. Hence, negative motor features such as impairment of fine motor skills, bradykinesia and gross motor coordination difficulties, may be improved by pridopidine through the activation of cortical dopamine transmission and downstream cortico-striatal synaptic activation, strengthening both the direct and the indirect pathways. The involuntary motor symptoms, dystonia and chorea, may be further alleviated by antagonism of striatal dopamine D2 receptors. In addition to effects directly and indirectly related to modulation of dopamine signaling, emerging evidence points towards a role for sigma-1 receptor interactions and regulation of calcium signaling contributing to potential disease modifying effects of pridopidine. Mechanistic studies specifically testing these hypotheses could be performed in animal models of HD. In addition, further human studies are warranted to investigate whether pridopidine may modify HD progression rate or prolong the time to phenoconversion in HD gene carriers.
CONFLICT OF INTEREST
CS, NW, SW, and JT are co-inventors on a composition of matter patent covering pridopidine, held by Teva Pharmaceutical Industries Ltd, Israel.
REFERENCES
- [1]. Martin JB, Gusella JF. Huntington’s disease. Pathogenesis and management. N Engl J Med. 1986;315(20):1267–76. [DOI] [PubMed] [Google Scholar]
- [2]. HDCRG. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. The Huntington’s Disease Collaborative Research Group. Cell. 1993;72(6):971–83. [DOI] [PubMed] [Google Scholar]
- [3]. Krobitsch S, Kazantsev AG. Huntington’s disease: From molecular basis to therapeutic advances. Int J Biochem Cell Biol. 2011;43(1):20–4. [DOI] [PubMed] [Google Scholar]
- [4]. Goto S, Hirano A, Rojas-Corona RR. An immunohistochemical investigation of the human neostriatum in Huntington’s disease. Ann Neurol. 1989;25(3):298–304. [DOI] [PubMed] [Google Scholar]
- [5]. Graveland GA, Williams RS, DiFiglia M. Evidence for degenerative and regenerative changes in neostriatal spiny neurons in Huntington’s disease. Science. 1985;227(4688):770–3. [DOI] [PubMed] [Google Scholar]
- [6]. Aylward EH, Sparks BF, Field KM, Yallapragada V, Shpritz BD, Rosenblatt A, et al. Onset and rate of striatal atrophy in preclinical Huntington disease. Neurology. 2004;63(1):66–72. [DOI] [PubMed] [Google Scholar]
- [7]. Paulsen JS, Langbehn DR, Stout JC, Aylward E, Ross CA, Nance M, et al. Detection of Huntington’s disease decades before diagnosis: The Predict-HD study. J Neurol Neurosurg Psychiatry. 2008;79(8):874–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8]. Hersch SM, Rosas HD. Neuroprotection for Huntington’s disease: Ready, set, slow. Neurotherapeutics. 2008;5(2):226–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9]. Mahant N, McCusker EA, Byth K, Graham S. Huntington’s disease: Clinical correlates of disability and progression. Neurology. 2003;61(8):1085–92. [DOI] [PubMed] [Google Scholar]
- [10]. Reedeker N, Van Der Mast RC, Giltay EJ, Van Duijn E, Roos RA. Hypokinesia in Huntington’s disease co-occurs with cognitive and global dysfunctioning. Mov Disord. 2010;25(11):1612–8. [DOI] [PubMed] [Google Scholar]
- [11]. Dorey J, Tedroff J, Squitieri F, Nicola ND, Urbinati D, Lamure M, et al. European Huntington’s disease burden study (Euro-HDB) - preliminary results for Italy and France Poster presented at the European HD Network Plenary Meeting, Prague, Czech Republic 2010; 3-5 September.
- [12]. Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12(10):366–75. [DOI] [PubMed] [Google Scholar]
- [13]. Kloppel S, Draganski B, Golding CV, Chu C, Nagy Z, Cook PA, et al. White matter connections reflect changes in voluntary-guided saccades in pre-symptomatic Huntington’s disease. Brain. 2008;131(Pt 1):196–204. [DOI] [PubMed] [Google Scholar]
- [14]. Rosas HD, Tuch DS, Hevelone ND, Zaleta AK, Vangel M, Hersch SM, et al. Diffusion tensor imaging in presymptomatic and early Huntington’s disease: Selective white matter pathology and its relationship to clinical measures. Mov Disord. 2006;21(9):1317–25. [DOI] [PubMed] [Google Scholar]
- [15]. Tabrizi SJ, Scahill RI, Durr A, Roos RA, Leavitt BR, Jones R, et al. Biological and clinical changes in premanifest and early stage Huntington’s disease in the TRACK-HD study: The 12-month longitudinal analysis. Lancet Neurol. 2011;10(1):31–42. [DOI] [PubMed] [Google Scholar]
- [16]. Bohanna I, Georgiou-Karistianis N, Egan GF. Connectivity-based segmentation of the striatum in Huntington’s disease: Vulnerability of motor pathways. Neurobiol Dis. 2011;42(3):475–81. [DOI] [PubMed] [Google Scholar]
- [17]. Vonsattel JP, Myers RH, Stevens TJ, Ferrante RJ, Bird ED, Richardson EP Jr.. Neuropathological classification of Huntington’s disease. J Neuropathol Exp Neurol. 1985;44(6):559–77. [DOI] [PubMed] [Google Scholar]
- [18]. Guo N, Yao W, Wang SR, Zhu J, Huang D, Zuo PL, et al. Nicotine dynamically modulates dopamine clearance in rat striatum in vivo. Neurochem Int. 2012;60(4):355–9. [DOI] [PubMed] [Google Scholar]
- [19]. Rosenblatt A, Liang KY, Zhou H, Abbott MH, Gourley LM, Margolis RL, et al. The association of CAG repeat length with clinical progression in Huntington disease. Neurology. 2006;66(7):1016–20. [DOI] [PubMed] [Google Scholar]
- [20]. Nieoullon A, Coquerel A. Dopamine: A key regulator to adapt action, emotion, motivation and cognition. Curr Opin Neurol. 2003;16(Suppl 2):S3–9. [PubMed] [Google Scholar]
- [21]. Carlsson A. The occurrence, distribution and physiological role of catecholamines in the nervous system. Pharmacol Rev. 1959;11(2, Part 2):490–3. [PubMed] [Google Scholar]
- [22]. Carlsson A, Lindqvist M. Effect of Chlorpromazine or Haloperidol on Formation of 3methoxytyramine and Normetanephrine in Mouse Brain. Acta Pharmacol Toxicol. 1963;20:140–4. [DOI] [PubMed] [Google Scholar]
- [23]. Dunlop BW, Nemeroff CB. The role of dopamine in the pathophysiology of depression. Arch Gen Psychiatry. 2007;64(3):327–37. [DOI] [PubMed] [Google Scholar]
- [24]. Engel JA, Fahlke C, Hard E, Johannessen K, Svensson L, Soderpalm B. Serotonergic and dopaminergic involvement in ethanol intake. Clin Neuropharmacol. 1992;15(Suppl 1 Pt A):64A–5A. [DOI] [PubMed] [Google Scholar]
- [25]. Crossman AR. Functional anatomy of movement disorders. J Anat. 2000;196(Pt 4):519–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26]. Filloux F, Wagster MV, Folstein S, Price DL, Hedreen JC, Dawson TM, et al. Nigral dopamine type-1 receptors are reduced in Huntington’s disease: A postmortem autoradiographic study using [3H]SCH 0 and correlation with [3H]forskolin binding. Exp Neurol. 1990;110(2):219–27. [DOI] [PubMed] [Google Scholar]
- [27]. Joyce JN, Lexow N, Bird E, Winokur A. Organization of dopamine D1 and D2 receptors in human striatum: Receptor autoradiographic studies in Huntington’s disease and schizophrenia. Synapse. 1988;2(5):546–57. [DOI] [PubMed] [Google Scholar]
- [28]. Ginovart N, Lundin A, Farde L, Halldin C, Backman L, Swahn CG, et al. PET study of the pre- and post-synaptic dopaminergic markers for the neurodegenerative process in Huntington’s disease. Brain. 1997;120(Pt 3):503–14. [DOI] [PubMed] [Google Scholar]
- [29]. Sedvall G, Karlsson P, Lundin A, Anvret M, Suhara T, Halldin C, et al. Dopamine D1 receptor number–a sensitive PET marker for early brain degeneration in Huntington’s disease. Eur Arch Psychiatry Clin Neurosci. 1994;243(5):249–55. [DOI] [PubMed] [Google Scholar]
- [30]. Turjanski N, Weeks R, Dolan R, Harding AE, Brooks DJ. Striatal D1 and D2 receptor binding in patients with Huntington’s disease and other choreas. A PET study. Brain. 1995;118(Pt 3):689–96. [DOI] [PubMed] [Google Scholar]
- [31]. Pavese N, Andrews TC, Brooks DJ, Ho AK, Rosser AE, Barker RA, et al. Progressive striatal and cortical dopamine receptor dysfunction in Huntington’s disease: A PET study. Brain. 2003;126(Pt 5):1127–35. [DOI] [PubMed] [Google Scholar]
- [32]. Waters CM, Peck R, Rossor M, Reynolds GP, Hunt SP. Immunocytochemical studies on the basal ganglia and substantia nigra in Parkinson’s disease and Huntington’s chorea. Neuroscience. 1988;25(2):419–38. [DOI] [PubMed] [Google Scholar]
- [33]. Ferrante RJ, Kowall NW. Tyrosine hydroxylase-like immunoreactivity is distributed in the matrix compartment of normal human and Huntington’s disease striatum. Brain Res. 1987;416(1):141–6. [DOI] [PubMed] [Google Scholar]
- [34]. Bibb JA, Yan Z, Svenningsson P, Snyder GL, Pieribone VA, Horiuchi A, et al. Severe deficiencies in dopamine signaling in presymptomatic Huntington’s disease mice. Proc Natl Acad Sci U S A. 2000;97(12):6809–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35]. Johnson MA, Rajan V, Miller CE, Wightman RM. Dopamine release is severely compromised in the R6/2 mouse model of Huntington’s disease. J Neurochem. 2006;97(3):737–46. [DOI] [PubMed] [Google Scholar]
- [36]. Ortiz AN, Osterhaus GL, Lauderdale K, Mahoney L, Fowler SC, von Horsten S, et al. Motor function and dopamine release measurements in transgenic Huntington’s disease model rats. Brain Res. 1450)148–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37]. Nikolaus S, Antke C, Muller HW. In vivo imaging of synaptic function in the central nervous system: I. Movement disorders and dementia. Behav Brain Res. 2009;204(1):1–31. [DOI] [PubMed] [Google Scholar]
- [38]. Backman L, Farde L. Dopamine and cognitive functioning: Brain imaging findings in Huntington’s disease and normal aging. Scand J Psychol. 2001;42(3):287–96. [DOI] [PubMed] [Google Scholar]
- [39]. Esmaeilzadeh M, Farde L, Karlsson P, Varrone A, Halldin C, Waters S, et al. Extrastriatal dopamine D(2) receptor binding in Huntington’s disease. Hum Brain Mapp. 2011;32(10):1626–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40]. Schwab LC, Garas SN, Drouin-Ouellet J, Mason SL, Stott SR, Barker RA. Dopamine and Huntington’s disease. Expert Rev Neurother. 2015;15(4):445–58. [DOI] [PubMed] [Google Scholar]
- [41]. Klawans HL, Goetz CG, Paulson GW, Barbeau A. Levodopa and presymptomatic detection of Huntington’s disease–eight-year follow-up. N Engl J Med. 1980;302(19):1090. [DOI] [PubMed] [Google Scholar]
- [42]. Frank S. Tetrabenazine: The first approved drug for the treatment of chorea in US patients with Huntington disease. Neuropsychiatr Dis Treat. 2010;6:657–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43]. Burgunder JM, Guttman M, Perlman S, Goodman N, van Kammen DP, Goodman L. An international survey-based algorithm for the pharmacologic treatment of chorea in Huntington’s disease. PLoS Curr. 2011;3:RRN1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44]. Shoulson I. Huntington disease: Functional capacities in patients treated with neuroleptic and antidepressant drugs. Neurology. 1981;31(10):1333–5. [DOI] [PubMed] [Google Scholar]
- [45]. van Vugt JP, Siesling S, Vergeer M, van der Velde EA, Roos RA. Clozapine versus placebo in Huntington’s disease: A double blind randomised comparative study. J Neurol Neurosurg Psychiatry. 1997;63(1):35–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46]. Tedroff J, Waters S, Barker RA, Roos R, Squitieri F, Group ERS. Antidopaminergic medication is associated with more rapidly progressive Huntington’s disease. J Huntingtons Dis. 2015;4(2):131–40. [DOI] [PubMed] [Google Scholar]
- [47]. Orth M, Handley OJ, Schwenke C, Dunnett S, Wild EJ, Tabrizi SJ, et al. Observing Huntington’s disease: The European Huntington’s Disease Network’s REGISTRY. J Neurol Neurosurg Psychiatry. 2011;82(12):1409–12. [DOI] [PubMed] [Google Scholar]
- [48]. DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 1990;13(7):281–5. [DOI] [PubMed] [Google Scholar]
- [49]. DeLong M, Wichmann T. Update on models of basal ganglia function and dysfunction. Parkinsonism Relat Disord. 2009;15(Supplement 3):S237–S40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50]. Gerfen CR, Surmeier DJ. Modulation of striatal projection systems by dopamine. Ann Rev Neurosci. 2011;34:441–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51]. Eidelberg D, Moeller JR, Kazumata K, Antonini A, Sterio D, Dhawan V, et al. Metabolic correlates of pallidal neuronal activity in Parkinson’s disease. Brain. 1997;120(Pt 8):1315–24. [DOI] [PubMed] [Google Scholar]
- [52]. Andre VM, Cepeda C, Levine MS. Dopamine and glutamate in Huntington’s disease: A balancing act. CNS Neurosci Thera. 2010;16(3):163–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53]. Raymond LA, Andre VM, Cepeda C, Gladding CM, Milnerwood AJ, Levine MS. Pathophysiology of Huntington’s disease: Time-dependent alterations in synaptic and receptor function. Neuroscience. 2011;198:252–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54]. Andre VM, Cepeda C, Fisher YE, Huynh M, Bardakjian N, Singh S, et al. Differential electrophysiological changes in striatal output neurons in Huntington’s disease. J Neurosci. 2011;31(4):1170–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55]. Plotkin JL, Surmeier DJ. Corticostriatal synaptic adaptations in Huntington’s disease. Curr Opin Neurobiol. 2015;33:53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56]. Estrada-Sanchez AM, Rebec GV. Role of cerebral cortex in the neuropathology of Huntington’s disease. Front Neural Circuits. 2013;7:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57]. Cepeda C, Wu N, Andre VM, Cummings DM, Levine MS. The corticostriatal pathway in Huntington’s disease. Prog Neurobiol. 2007;81(5-6):253–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58]. Lawrence AD, Sahakian BJ, Robbins TW. Cognitive functions and corticostriatal circuits: Insights from Huntington’s disease. Trends Cogn Sci. 1998;2(10):379–88. [DOI] [PubMed] [Google Scholar]
- [59]. Armstrong MJ, Miyasaki JM. Evidence-based guideline: Pharmacologic treatment of chorea in Huntington disease: Report of the guideline development subcommittee of the American Academy of Neurology. Neurology. 2012;79(6):597–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60]. Mestre T, Ferreira J, Coelho MM, Rosa M, Sampaio C. Therapeutic interventions for symptomatic treatment in Huntington’s disease. Cochrane Database Syst Rev (Online). 2009(3):CD006456. [DOI] [PubMed] [Google Scholar]
- [61]. Mestre T, Ferreira J, Coelho MM, Rosa M, Sampaio C. Therapeutic interventions for disease progression in Huntington’s disease. Cochrane Database Syst Rev (Online). 2009(3):CD006455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62]. Lundin A, Dietrichs E, Haghighi S, Goller ML, Heiberg A, Loutfi G, et al. Efficacy and safety of the dopaminergic stabilizer Pridopidine (ACR16) in patients with Huntington’s disease. Clin Neuropharmacol. 2010;33(5):260–4. [DOI] [PubMed] [Google Scholar]
- [63]. de Yebenes JG, Landwehrmeyer B, Squitieri F, Reilmann R, Rosser A, Barker RA, et al. Pridopidine for the treatment of motor function in patients with Huntington’s disease (MermaiHD): A phase 3, randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2011;10(12):1049–57. [DOI] [PubMed] [Google Scholar]
- [64]. Huntington Study Group HI. A randomized, double-blind, placebo-controlled trial of pridopidine in Huntington’s disease. Mov Disord. 2013;28(10):1407–15. [DOI] [PubMed] [Google Scholar]
- [65]. Beglinger LJ, O’Rourke JJ, Wang C, Langbehn DR, Duff K, Paulsen JS. Earliest functional declines in Huntington disease. Psychiatry Res. 2010;178(2):414–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66]. Kieburtz K, Landwehrmeyer GB, Reilmann R, Savola J, Eyal E, Grachev I, et al. Efficacy, safety, and tolerability of pridopidine in Huntington disease (HD): Results from the phase II, double-blind, placebo-controlled, dose-ranging study, Pride-HD (P2.005). . Neurology. 2017;88(16 Supplement). [Google Scholar]
- [67]. McGarry A, Kieburtz K, Abler V, Grachev ID, Gandhi S, Auinger P, et al. Safety and exploratory efficacy at 36 months in Open-HART, an open-label extension study of pridopidine in Huntington’s disease. J Huntingtons Dis. 2017;6(3):189–199. [DOI] [PubMed] [Google Scholar]
- [68]. Landwehrmeyer B, Marder K, Biilmann Ronn B, Haglund M. Effects of the dopaminergic stabilizer pridopidine on motor symptoms in Huntington’s disease: A meta-analysis. Clin Genet. 2011;80(Suppl 1):14–69. [Google Scholar]
- [69]. Waters S, Svensson P, Kullingsjo J, Ponten H, Andreasson T, Sunesson Y, et al. In vivo systems response profiling and multivariate classification of CNS active compounds: A structured tool for CNS drug discovery. ACS Chem Neurosci. 2017;8(4):785–97. [DOI] [PubMed] [Google Scholar]
- [70]. Swinney DC, Anthony J. How were new medicines discovered? Nat Rev Drug Discov 2011;10(7):507–19. [DOI] [PubMed] [Google Scholar]
- [71]. Mewshaw RE, Kavanagh J, Stack G, Marquis KL, Shi X, Kagan MZ, et al. New generation dopaminergic agents. 1. Discovery of a novel scaffold which embraces the D2 agonist pharmacophore. Structure-activity relationships of a series of 2-(aminomethyl)chromans. J Med Chem. 1997;40(26):4235–56. [DOI] [PubMed] [Google Scholar]
- [72]. Pettersson F, Ponten H, Waters N, Waters S, Sonesson C. Synthesis and evaluation of a set of 4-phenylpiperidines and 4-phenylpiperazines as D2 receptor ligands and the discovery of the dopaminergic stabilizer 4-[3-(methylsulfonyl)phenyl]-1-propylpiperidine (huntexil, pridopidine, ACR16). J Med Chem. 2010;53(6):2510–20. [DOI] [PubMed] [Google Scholar]
- [73]. Holm Waters S. Göteborgs universitet. Comparative in vivo pharmacology of dopidines: A novel class of compounds discovered by phenotypic screening [Diss Göteborg Göteborgs universitet, 2015]. Göteborg: Department of Department of Pharmacology, Institute of Neuroscience and Physiology, Sahlgrenska Academy at University of Gothenburg, 2015.
- [74]. Ponten H, Kullingsjo J, Lagerkvist S, Martin P, Pettersson F, Sonesson C, et al. In vivo pharmacology of the dopaminergic stabilizer pridopidine. Eur J Pharmacol. 2010;644(1-3):88–95. [DOI] [PubMed] [Google Scholar]
- [75]. Waters S, Ponten H, Klamer D, Waters N. Co-administration of the dopaminergic stabilizer pridopidine and tetrabenazine in rats. J Huntingtons Dis. 2014;3(3):285–98. [DOI] [PubMed] [Google Scholar]
- [76]. Natesan S, Svensson KA, Reckless GE, Nobrega JN, Barlow KB, Johansson AM, et al. The dopamine stabilizers (S)-(-)-(3-methanesulfonyl-phenyl)-1-propyl-piperidine [(-)-OSU] and 4-(3-methanesulfonylphenyl)-1-propyl-piperidine (ACR16) show high in vivo D2 receptor occupancy, antipsychotic-like efficacy, and low potential for motor side effects in the rat. J Pharmacol Exp Thera. 2006;318(2):810–8. [DOI] [PubMed] [Google Scholar]
- [77]. Rung JP, Carlsson A, Markinhuhta KR, Carlsson ML. The dopaminergic stabilizers (-)-OSUand ACR16 reverse (+)-MK-801-induced social withdrawal in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29(5):833–9. [DOI] [PubMed] [Google Scholar]
- [78]. Nilsson M, Carlsson A, Markinhuhta KR, Sonesson C, Pettersson F, Gullme M, et al. The dopaminergic stabiliser ACR16 counteracts the behavioural primitivization induced by the NMDA receptor antagonist MK-801 in mice: Implications for cognition. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28(4):677–85. [DOI] [PubMed] [Google Scholar]
- [79]. Gronier B, Waters S, Ponten H. The dopaminergic stabilizer pridopidine increases neuronal activity of pyramidal neurons in the prefrontal cortex. J Neural Trans. 2013;120(9):1281–94. [DOI] [PubMed] [Google Scholar]
- [80]. Squitieri F, Di Pardo A, Favellato M, Amico E, Maglione V, Frati L. Pridopidine, a dopamine stabilizer, improves motor performance and shows neuroprotective effects in Huntington disease R6/2 mouse model. J Cell Mol Med. 2015;19(11):2540–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81]. Westerink BHC, Justice JB Jr. Chapter 2 - Microdialysis compared with other in vivo release models In: Robinson TE, Justice JB, editors. Techniques in the Behavioral and Neural Sciences. Volume 7: Elsevier; 1991. pp. 23–43. [Google Scholar]
- [82]. Rung JP, Rung E, Johansson AM, Svensson K, Carlsson A, Carlsson ML. Effects of the dopamine stabilizers (S)-(-)-OSUand ACR16 on prolactin secretion in drug-naive and monoamine-depleted rats. Naunyn Schmiedebergs Arch Pharmacol. 2011;384(1):39–45. [DOI] [PubMed] [Google Scholar]
- [83]. Link W, Konietzko U, Kauselmann G, Krug M, Schwanke B, Frey U, et al. Somatodendritic expression of an immediate early gene is regulated by synaptic activity. Proc Natl Acad Sci U S A. 1995;92(12):5734–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84]. Waters S, Ponten H, Edling M, Svanberg B, Klamer D, Waters N. The dopaminergic stabilizers pridopidine and ordopidine enhance cortico-striatal Arc gene expression. J Neural Transm. 2014;121(11):1337–47. [DOI] [PubMed] [Google Scholar]
- [85]. Waters S, Tedroff J, Edling M, Svanberg B, Klamer D, Pontén H, et al. editors. Pridopidine: Mechanism of action - focus on glutamatergic transmission 2011; Palm Springs, CA, US. [Google Scholar]
- [86]. Bramham CR, Alme MN, Bittins M, Kuipers SD, Nair RR, Pai B, et al. The Arc of synaptic memory. Exp Brain Res. 2010;200(2):125–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87]. Martin KC. Arc mRNA dynamics: Return to sender–the NMDA receptor provides the targeting address for Arc mRNA. Trends Neurosci. 2001;24(11):621–3. [DOI] [PubMed] [Google Scholar]
- [88]. Steward O, Worley PF. Selective targeting of newly synthesized Arc mRNA to active synapses requires NMDA receptor activation. Neuron. 2001;30(1):227–40. [DOI] [PubMed] [Google Scholar]
- [89]. Marcus M, Wiker C, Frånberg O, Konradsson-Geuken Å, Langlois X, Jardemark K, et al. Adjunctive a2-adrenoceptor blockade enhances the antipsychotic-like effect of risperidone and facilitates cortical dopaminergic and glutamatergic, NMDA receptor-mediated transmission. Int J Neuropsychopharmacol. 2010;13(7 August 2010):891–903. [DOI] [PubMed] [Google Scholar]
- [90]. Wadenberg M-L, Wiker C, Svensson TH. Enhanced efficacy of both typical and atypical antipsychotic drugs by adjunctive a2 adrenoceptor blockade: Experimental evidence. Int J Neuropsychopharmacol. 2007;10:191–202. [DOI] [PubMed] [Google Scholar]
- [91]. Isacson R, Kull B, Wahlestedt C, Salmi P. A 0 and dihydrexidine inhibit locomotor activity and d-amphetamine induced hyperactivity in rats: A role of inhibitory dopamine D1/5 receptors in the prefrontal cortex? Neuroscience. 2004;124:33–42. [DOI] [PubMed] [Google Scholar]
- [92]. Dyhring T, Nielsen EO, Sonesson C, Pettersson F, Karlsson J, Svensson P, et al. The dopaminergic stabilizers pridopidine (ACR16) and (-)-OSUdisplay dopamine D(2) receptor antagonism and fast receptor dissociation properties. Eur J Pharmacol. 2010;628(1-3):19–26. [DOI] [PubMed] [Google Scholar]
- [93]. Tadori Y, Kitagawa H, Forbes RA, McQuade RD, Stark A, Kikuchi T. Differences in agonist/antagonist properties at human dopamine D(2) receptors between aripiprazole, bifeprunox and SDZ 208-912. Eur J Pharmacol. 2007;574(2-3):103–11. [DOI] [PubMed] [Google Scholar]
- [94]. Kara E, Lin H, Strange PG. Co-operativity in agonist binding at the D2 dopamine receptor: Evidence from agonist dissociation kinetics. J Neurochem. 2010;112(6):1442–53. [DOI] [PubMed] [Google Scholar]
- [95]. Seeman P, Guan HC. Dopamine partial agonist action of (-)OSUis consistent with dopamine hyperactivity in psychosis. Eur J Pharmacol. 2007;557(2-3):151–3. [DOI] [PubMed] [Google Scholar]
- [96]. Seeman P, Tokita K, Matsumoto M, Matsuo A, Sasamata M, Miyata K. The dopaminergic stabilizer ASP/ACR16 selectively interacts with D2(High) receptors. Synapse. 2009;63(10):930–4. [DOI] [PubMed] [Google Scholar]
- [97]. Devoto P, Flore G, Pira L, Longu G, Gessa GL. Alpha2-adrenoceptor mediated co-release of dopamine and noradrenaline from noradrenergic neurons in the cerebral cortex. J Neurochem. 2004;88(4):1003–9. [DOI] [PubMed] [Google Scholar]
- [98]. Esbenshade TA, Browman KE, Miller TR, Krueger KM, Komater-Roderwald V, Zhang M, et al. Pharmacological properties and procognitive effects of ABT-288, a potent and selective histamine H3 receptor antagonist. J Pharmacol Exp Therap. 2012;343(1):233–45. [DOI] [PubMed] [Google Scholar]
- [99]. Esposito E. Serotonin-dopamine interaction as a focus of novel antidepressant drugs. Curr Drug Targets. 2006;7(2):177–85. [DOI] [PubMed] [Google Scholar]
- [100]. Rollema H, Lu Y, Schmidt AW, Sprouse JS, Zorn SH. 5-HT(1A) receptor activation contributes to ziprasidone-induced dopamine release in the rat prefrontal cortex. Biol Psychiatry. 2000;48(3):229–37. [DOI] [PubMed] [Google Scholar]
- [101]. Aghajanian GK, Marek GJ. Serotonin model of schizophrenia: Emerging role of glutamate mechanisms. Brain research Brain Res Rev. 2000;31(2-3):302–12. [DOI] [PubMed] [Google Scholar]
- [102]. Brown RE, Haas HL. On the mechanism of histaminergic inhibition of glutamate release in the rat dentate gyrus. J Physiol. 1999;515(Pt 3):777–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103]. Carr DB, Andrews GD, Glen WB, Lavin A. alpha2-Noradrenergic receptors activation enhances excitability and synaptic integration in rat prefrontal cortex pyramidal neurons via inhibition of HCN currents. J Physiol. 2007;584(Pt 2):437–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104]. Celada P, Puig MV, Artigas F. Serotonin modulation of cortical neurons and networks. Front Integr Neurosci. 2013;7:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105]. Sahlholm K, Arhem P, Fuxe K, Marcellino D. The dopamine stabilizers ACR16 and (-)-OSUdisplay nanomolar affinities at the [sigma]-1 receptor. Mol Psychiatry. 2013;18(1):12–4. [DOI] [PubMed] [Google Scholar]
- [106]. Geva M, Kusko R, Soares H, Fowler K, Birnberg T, Barash S, et al. Pridopidine activates neuroprotective pathways impaired in Huntington disease. Hum Mol Genet. 2016;25(18):3975–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107]. Ryskamp D, Wu J, Geva M, Kusko R, Grossman I, Hayden M, et al. The sigma-1 receptor mediates the beneficial effects of pridopidine in a mouse model of Huntington disease. Neurobiol Dis. 2017;97(Pt A):46–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108]. Wang M, Wong AH, Liu F. Interactions between NMDA and dopamine receptors: A potential therapeutic target. Brain Res. 2012(1476):154–63. [DOI] [PubMed] [Google Scholar]
- [109]. Waters S, Pettersson F, Dyhring T, Sonesson C, Tedroff J, Waters N, et al. Pharmacology of the dopaminergic stabilizer pridopidine (ACR16). Neurotherapeutics. 2010;7(1):145. [Google Scholar]
- [110]. Yamagata K, Suzuki K, Sugiura H, Kawashima N, Okuyama S. Activation of an effector immediate-early gene arc by methamphetamine. Ann N Y Acad Sci. 2000;914:22–32. [DOI] [PubMed] [Google Scholar]
- [111]. Bruins Slot LA, Lestienne F, Grevoz-Barret C, Newman-Tancredi A, Cussac D. F3, a potential antipsychotic with dopamine D(2)/D(3) receptor antagonist and 5-HT(1A) receptor agonist properties: Influence on immediate-early gene expression in rat prefrontal cortex and striatum. Eur J Pharmacol. 2009;620(1-3):27–35. [DOI] [PubMed] [Google Scholar]
- [112]. Fumagalli F, Frasca A, Racagni G, Riva MA. Antipsychotic drugs modulate Arc expression in the rat brain. Eur Neuropsychopharmacol. 2009;19(2):109–15. [DOI] [PubMed] [Google Scholar]
- [113]. Rosas HD, Salat DH, Lee SY, Zaleta AK, Pappu V, Fischl B, et al. Cerebral cortex and the clinical expression of Huntington’s disease: Complexity and heterogeneity. Brain. 2008;131(Pt 4):1057–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114]. Kuwert T, Lange HW, Langen KJ, Herzog H, Aulich A, Feinendegen LE. Cortical and subcortical glucose consumption measured by PET in patients with Huntington’s disease. Brain. 1990;113(Pt 5):1405–23. [DOI] [PubMed] [Google Scholar]
- [115]. Gu X, Andre VM, Cepeda C, Li SH, Li XJ, Levine MS, et al. Pathological cell-cell interactions are necessary for striatal pathogenesis in a conditional mouse model of Huntington’s disease. Mol Neurodegener. 2007;2:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116]. Serres F, Rodriguez M, Rivet JM, Galizzi JP, Lockhart B, Sharp T, et al. Blockade of alpha2-adrenoceptors induces Arc gene expression in rat brain in a glutamate receptor-dependent manner: A combined qPCR, in situ hybridisation and immunocytochemistry study. Neuropharmacology. 2012;63(6):992–1001. [DOI] [PubMed] [Google Scholar]
- [117]. Milnerwood AJ, Kaufman AM, Sepers MD, Gladding CM, Zhang L, Wang L, et al. Mitigation of augmented extrasynaptic NMDAR signaling and apoptosis in cortico-striatal co-cultures from Huntington’s disease mice. Neurobiol Dis. 2012;48(1):40–51. [DOI] [PubMed] [Google Scholar]
- [118]. Hardingham GE, Bading H. Synaptic versus extrasynaptic NMDA receptor signalling: Implications for neurodegenerative disorders. Nat Rev. 2010;11(10):682–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119]. Okamoto S, Pouladi MA, Talantova M, Yao D, Xia P, Ehrnhoefer DE, et al. Balance between synaptic versus extrasynaptic NMDA receptor activity influences inclusions and neurotoxicity of mutant huntingtin. Nat Med. 2009;15(12):1407–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120]. Nguyen L, Lucke-Wold BP, Mookerjee SA, Cavendish JZ, Robson MJ, Scandinaro AL, et al. Role of sigma-1 receptors in neurodegenerative diseases. J Pharmacol Sci. 2015;127(1):17–29. [DOI] [PubMed] [Google Scholar]
- [121]. Marder K, Zhao H, Myers RH, Cudkowicz M, Kayson E, Kieburtz K, et al. Rate of functional decline in Huntington’s disease. Huntington Study Group. Neurology. 2000;54(2):452–8. [DOI] [PubMed] [Google Scholar]
- [122]. Zielonka D, Marinus J, Roos RA, De Michele G, Di Donato S, Putter H, et al. The influence of gender on phenotype and disease progression in patients with Huntington’s disease. Parkinsonism Relat Disord. 2013;19(2):192–7. [DOI] [PubMed] [Google Scholar]
- [123]. Huntington Study G. A randomized, placebo-controlled trial of coenzyme Q10 and remacemide in Huntington’s disease. Neurology. 2001;57(3):397–404. [DOI] [PubMed] [Google Scholar]
- [124]. McGarry A, McDermott M, Kieburtz K, de Blieck EA, Beal F, Marder K, et al. A randomized, double-blind, placebo-controlled trial of coenzyme Q10 in Huntington disease. Neurology. 2017;88(2):152–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125]. Hersch SM, Schifitto G, Oakes D, Bredlau AL, Meyers CM, Nahin R, et al. The CREST-E study of creatine for Huntington disease: A randomized controlled trial. Neurology. 2017;89(6):594–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126]. Frank S. Tetrabenazine as anti-chorea therapy in Huntington disease: An open-label continuation study. Huntington Study Group/TETRA-HD Investigators. BMC Neurol. 2009;9:62. [DOI] [PMC free article] [PubMed] [Google Scholar]