Abstract
Background
An increased rate of cell death by apoptosis has been implicated in both diabetes and atherosclerosis. Apoptosis can be induced through activation of the death receptors TNF receptor 1 (TNFR-1), TRAIL receptor 2 (TRAILR-2) and Fas. Soluble forms of these receptors are found in plasma. The objective of this study was to determine if soluble death receptors are markers of receptor-activated apoptosis and predict risk for development of diabetes and cardiovascular events.
Methods
Fas ligand was used to induce apoptosis in peripheral blood mononuclear cells and INS-1 pancreatic β-cells and release of TNFR-1, TRAILR-2 and Fas measured by ELISA. Proximity Extension Assay was used to analyze plasma levels of TNFR-1, TRAILR-2 and Fas in baseline samples of 4742 subjects in the Malmö Diet and Cancer Study and related to development of diabetes and cardiovascular events during 19.2 years of follow-up.
Results
Activation of apoptosis by Fas ligand was associated with release of soluble Fas, TNFR-1 and TRAILR-2 in both cell types. Circulating levels of all three receptors were higher in subjects with diabetes and correlated with markers of impaired glucose metabolism in non-diabetic subjects. Among the latter, those in the highest tertile of soluble Fas, TNFR-1 and TRAILR-2 had increased risk for development of diabetes and cardiovascular events. These associations became weaker when adjusting for cardiovascular risk factors in Cox regression models, but remained significant for TRAILR-2 with incident diabetes, cardiovascular mortality, myocardial infarction and ischemic stroke, and for TNFR-1 with myocardial infarction.
Conclusion
The present study demonstrates an association between several cardiovascular risk factors and elevated levels of circulating markers of apoptotic cell death. It also shows that subjects with high levels of these biomarkers have increased risk of diabetes and CVD. This implies that soluble death receptors are markers of β-cell and vascular injury and potentially could be used as surrogate markers of therapeutic efficiency in risk factor interventions.
Abbreviations: (TNFR-1), TNF receptor 1; (TRAILR-2), TNF-related apoptosis-inducing ligand receptor 2; (MDC), Malmö Diet and Cancer study; (PBMC), peripheral blood mononuclear cells; (MI), myocardial infarction
Keywords: Apoptosis, Diabetes mellitus, Myocardial infarction, Ischemic stroke
Graphic Abstract
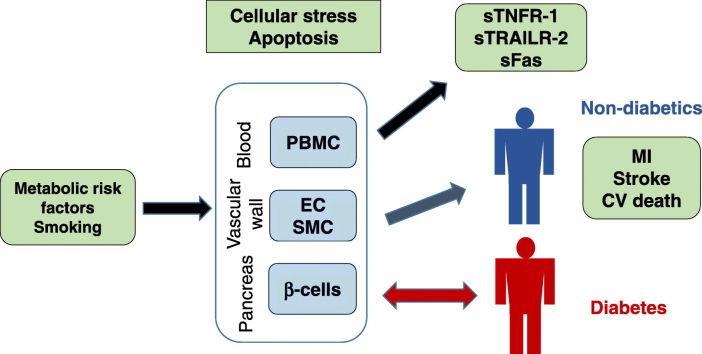
Associations between metabolic stress, release of soluble death receptors and development of diabetes and cardiovascular disease.
Highlights
-
•
Receptor-activated apoptosis is associated with release of soluble death receptors that act as biomarkers of apoptosis
-
•
Several cardiovascular risk factors including markers of impaired glucose metabolism associate with elevated plasma levels of death receptors
-
•
Subjects with high plasma levels of death receptors have an increased risk of diabetes and cardiovascular disease
Atherosclerosis has been proposed to develop in response to chronic arterial injury caused by cardiovascular risk factors. The present study provides clinical evidence for this hypothesis by demonstrating an association between several cardiovascular risk factors and elevated levels of circulating markers of apoptotic cell death and that subjects with high levels of these biomarkers have increased risk of cardiovascular mortality, MI and stroke. These observations point to the possibility that the plasma level of soluble death receptors can be used as surrogate markers of arterial injury and atherosclerotic disease activity in cardiovascular interventions. Finally, our findings imply that soluble death receptors also may serve as biomarkers of the damage caused by metabolic stress to β-cells and risk for development of type 2 diabetes.
1. Introduction
Apoptosis is a genetically determined cell death program required to maintain cell homeostasis in the body but it has also been implicated in a number of different diseases including diabetes and cardiovascular disease (Hetts, 1998, Van Vre et al., 2012, Butler et al., 2003, Allen et al., 2005, Anuradha et al., 2014, Donath and Halban, 2004, Kolodgie et al., 1999, Crisby et al., 1997). Apoptosis can be triggered by intrinsic pathways induced by cellular stress and DNA damage or by extrinsic signals activating cell-surface death receptors. The death receptors are part of the tumor necrosis factor (TNF) receptor gene super family and include TNF receptor 1 (TNFR-1), TNF-related apoptosis-inducing ligand receptor 2 (TRAILR-2) and Fas (Ashkenazi and Dixit, 1998). These receptors have a common cytoplasmic sequence termed the “death domain” that transmits activation of caspase-8, which in turn induces apoptosis through activation of the effector caspases 3, 6 and 7. Several metabolic factors associated with diabetes have been shown to induce β-cell apoptosis including hyperglycemia and saturated fatty acids, whereas HDL has been attributed a protective effect (Federici et al., 2001, Maedler et al., 2001, Shimabukuro et al., 1998, Roehrich et al., 2003). One mechanism through which these factors enhance β cell apoptosis is through upregulation of Fas (Anuradha et al., 2014, Maedler et al., 2001). Metabolic stress is considered to be of key importance also in the vascular injury that drives development of atherosclerosis (Ross, 1999, Hansson, 2005). However, to what extent the higher incidence of cardiovascular disease (CVD) in diabetes is related to increased death receptor-activated apoptosis in the cardiovascular system in response to metabolic stress has not been extensively studied. The lack of circulating biomarkers has made it difficult to assess the clinical importance of death receptor-activated apoptosis for cardiovascular risk. We demonstrate here that induction of apoptosis through activation of Fas is associated with release of soluble TNFR-1, TRAILR-2 and Fas from cells suggesting that the circulating levels of these receptors can be used as biomarkers of apoptosis induced by activation of cell death receptors. Based on this observation we analyzed baseline plasma levels of soluble TNFR-1, TRAILR-2 and Fas in 4742 subjects participating the cardiovascular sub-cohort of the Malmö Diet and Cancer (MDC) study to investigate (Hetts, 1998) if metabolic changes characteristic for an impaired glucose metabolism are associated with elevated plasma levels of soluble TNFR-1, TRAILR-2 Fas and (Van Vre et al., 2012) if these markers of receptor-activated apoptosis predict risk for development of diabetes and cardiovascular events.
2. Methods
2.1. Receptor-Activated Release of Soluble Death Receptor and Apoptosis in Cultured Peripheral Blood Mononuclear Cells
Peripheral blood mononuclear cells were isolated from leukocyte concentrate from healthy donors using Ficoll Paque Plus (GE Healthcare). Cells were seeded at a density of 0.5 × 106 cells/well in complete RPMI (10 U/ml Penicillin/streptomycin, 1% l-glutamine, 1% sodium pyruvate, 1% Hepes and 0,1% mercaptoethanol) with 2% human serum (Sigma Aldrich). The cells were exposed to IL-1β (10 ng/ml), soluble Fas ligand (0.5–5.0 μg/ml) and TNF-α (10–100 ng/ml) for 24 h in 37 °C with 5% CO2 and the cell medium was subsequently analyzed by a multiplex assay detecting TNFR-1, TRAIL-R2 and Fas (R&D Systems, Minneapolis, MN, USA) and Luminex (Bio-Rad). The intra-assay and inter-assay CV for Luminex assays are < 20% and < 25%, respectively according to the manufacturer. The analytical range for the TNFR-1, TRAIL-R2 and Fas Luminex assays are 3–4803 pg/ml, 14–35,453 pg/ml and 25–53,758 pg/ml, respectively. For analyses of apoptosis, the cells were stained with Annexin V-PE and the viability marker 7-amino-actinomycin (7-AAD), and analyzed by flow cytometry according to the manufacturer's protocol (Biolegend). Early apoptotic cells were defined as AnnexinV+/7-AAD− and late apoptotic cells as AnnexinV+/7-AAD+.
INS-1837/13 cells (kindly provided by Dr. Pontus Dunér, Lund University), were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum, 50 IU/ml penicillin, 50 mg/L streptomycin, 10 mM HEPES, 2 mM l-glutamine, 1 mM sodium pyruvate, and 50 μM beta-mercaptoethanol. They were then stimulated with IL-1β, soluble Fas ligand and TNF-α and the release of TNFR-1, TRAIL-R2 and Fas analyzed as described above.
2.2. Study Population
The Malmö Diet and Cancer (MDC) study is a prospective population-based cohort (n = 28,449) study examining the association between diet and cancer (Berglund et al., 1993). Subjects born between 1926 and 1945 and living in Malmö were eligible for inclusion in the study. Between October 1991 and February 1994, every other participant was also invited to take part in a sub-study focusing on the epidemiology of carotid artery disease (MDC study cardiovascular cohort, n = 6103) (Hedblad et al., 2000). Out of these, 5405 came to a second baseline examination where fasting plasma samples were collected. We excluded 545 of these subjects from the present study due to incomplete clinical data and 118 subjects were further excluded because the analysis of their plasma samples did not pass the internal quality control for the biomarker analyses. The remaining 4742 subjects were followed from baseline examination until first event of cardiovascular disease (CVD), emigration from Sweden or death, up until December 31 st, 2013. Ascertainment of cases and validity of the registries used (the Swedish Discharge Registry, the Stroke Register of Malmö and the Cause of Death Registry of Sweden) have been proven to be high. A coronary event was defined as a fatal or non-fatal myocardial infarction on the basis of the International Classification of Diseases 9th and 10th revisions (ICD-9 and ICD-10) codes 410 and I21, respectively. Death due to ischemic heart disease was defined based on codes 412 and 414 (ICD-9) or I22, I23 and I25 (ICD-10). Cases with a first ischemic stroke were identified from the Malmö stroke registry, which has continuously monitored and searched for stroke cases in both inpatient and outpatient care since 1989 (Zia et al., 2007). Stroke diagnoses registered before December 31 st, 2010 were validated by review of the medical records. Strokes registered after this date were not included in the analysis due to lack of validation. Ischemic stroke was defined as ICD-9 codes 434 or 436 or ICD-10 codes I63 or I64 and hemorrhagic stroke as ICD-9 codes 430 or 431 or ICD-10 codes I60 or I61. Incident diabetes was identified by the Malmö HbA1c register, the Swedish National Diabetes Register, the Swedish inpatient register, the Swedish outpatient register, the nationwide Swedish drug prescription register, and the regional Diabetes 2000 register of the Skåne region as previously described (Muhammad et al., 2016). Diabetes at baseline was defined as a history of diabetes or an over-night fasting whole blood glucose of ≥ 6.1 mmol/l according to the WHO criteria (Alberti and Zimmet, 1998). Hypertension was defined as blood pressure ≥ 140/90 mm Hg or blood pressure lowering medication, hypercholesterolemia as > 5 mmol/l, smoking as current smoking. Blood pressure, smoking habits and, hsCRP and circulating lipoprotein lipid levels were determined as previously described (Hedblad et al., 2000). The study was approved by the Regional Ethical Review Board in Lund (LU 51–90) and was conducted in accordance with the Helsinki Declaration. All subjects gave written consent. The reporting of this cohort study is in accordance with the STROBE guidelines.
2.3. Analysis of Soluble Death Receptor in Plasma
Plasma levels of TNFR-1, TRAIL-R2 and Fas were analyzed by the Proximity Extension Assay technique using the Proseek Multiplex CVD96x96 reagents kit (Olink Bioscience, Uppsala, Sweden) at the Clinical Biomarkers Facility, Science for Life Laboratory, Uppsala. Oligonucleotide-labeled antibody probe pairs were allowed to bind to their respective targets present in the plasma sample and addition of a DNA polymerase led to an extension and joining of the two oligonucleotides and formation of a PCR template. Universal primers were used to pre-amplify the DNA templates in parallel. Finally, the individual DNA sequences were detected and quantified using specific primers by microfluidic real-time quantitative PCR chip (96.96, Dynamic Array IFC, Fluidigm Biomark). The chip was run with a Biomark HD instrument. The CV for intra-assay variation (within-run) and inter-assay variation (between-run) for TNFR-1, TRAIL-R2 and Fas are 8% and 12%, 10% and 5%, 8% and 12%, respectively and the analytical ranges 7.1–31,250 ng/ml, 0.2–7812 ng/ml and 1.0–15,625, respectively. Data analysis was performed by a preprocessing normalization procedure using Olink Wizard for GenEx (Multid Analyses, Sweden). All data are presented as arbitrary units (AU). General calibrator curves to calculate the approximate concentrations as well as technical information about the assays are available on the Olink homepage (http://www.olink.com).
2.4. Genotyping, Quality Control, Genome Wide Association Analyses (GWAS)
Genotyping was performed using Illumina HumanOmniExpress BeadChip v. 1 at Broad Institute, Cambridge, MA USA. During the quality control procedures (QC) we removed individuals having a call rate of < 0.95, an inbreeding coefficient of > 3 SD away from mean, discordance between inferred and reported gender, duplicate samples, unexpected high proportion of IBD sharing, first and second degree relatives or deviating from the common population structure in the MDCS-CC (exceeding 8 sigma on first two principal components). Single nucleotide polymorphisms (SNPs) were filtered out if they were monomorphic or had a call rate of < 0.95, an extreme deviation from HWE (P < 1 × 10− 07), were missing in either cases or controls (P < 1 × 10− 07 and MAF > 0.01) or plate assignment (P < 1 × 10− 08 and MAF > 0.01). After QC 5453 individuals and a total number of 850,658 SNPs were eligible in the dataset. The GWA analysis on TNFR-1, TRAIL-R2 and Fas concentration were performed 4233 individuals with measured fasting plasma levels of the proteins.
2.5. Statistics
Measures of skewness and kurtosis were used to test for normality. Differences between means of normally distributed continuous variables were assessed with independent sample t-tests and between skewed variables with the Mann-Whitney U test. χ2 test was used for categorical variables. Soluble death receptor values were normalized by log 2 transformation before being used in statistical analysis. Correlation coefficients between continuous variables were calculated using the Spearman Rank test. The relation between soluble death receptor tertiles and incidence of diabetes and a first cardiovascular event during follow-up was assessed by Kaplan Meier survival curves and quantified by Log rank test. Cox proportional hazards regression models were used to assess the hazard ratio (HR), and 95% confidence interval (CI) of first event in relation to soluble death receptor tertiles and per standard deviation. The time variables used in Kaplan Meier curves and the Cox regressions models were based on time from the baseline investigation to first clinical event or end of follow up which was 2013-12-31 for coronary events and 2010-12-31 for stroke. Linear regression models were used to determine the association between gene variants and soluble death receptor in plasma. The genetic association analyses were carried out assuming an additive genetic model adjusted for age and gender. Genotypes were coded as 0, 1 or 2 with regard to the number of minor alleles. We analyzed in total 850,361 SNPs for associations to soluble death receptors. Out of the 4742 individuals with measurements on soluble death receptor levels, 4233 were included in the association study. The discrepancies were due to bad or insufficient amount of DNA, call rate below 95%, excess heterozygosity and/or gender mismatches when comparing gender calculated from the genotypes compared to that reported in the MDC study database. Logistic regression models were used to calculate associations between genotypes and the risk for development of diabetes and cardiovascular events. IBM SPSS Statistics 22 was used for statistical analysis.
3. Results
3.1. Activation of Apoptosis through Fas is Associated with Release of Soluble Death Receptors
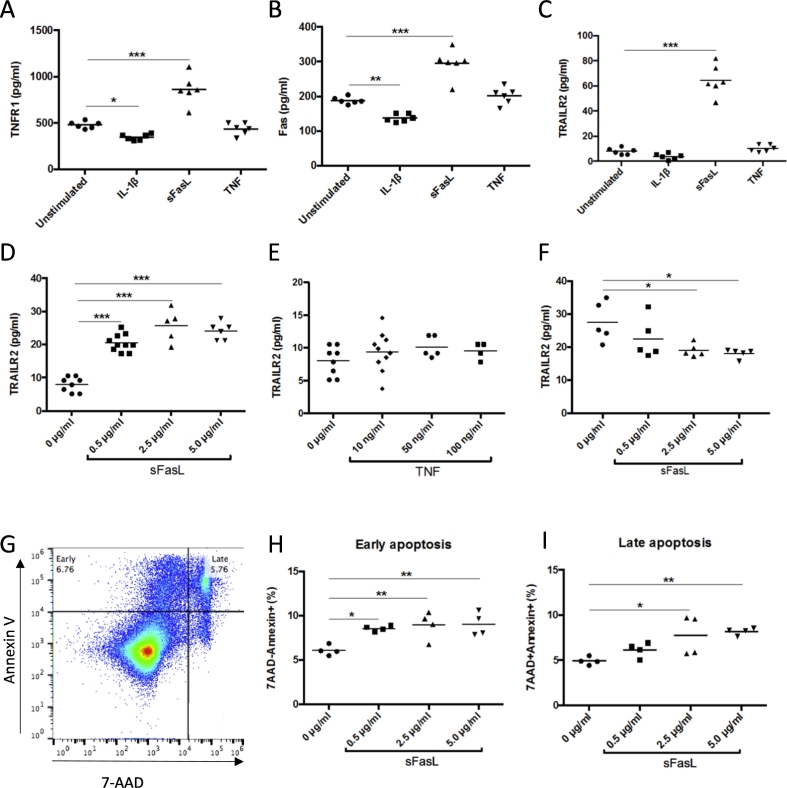
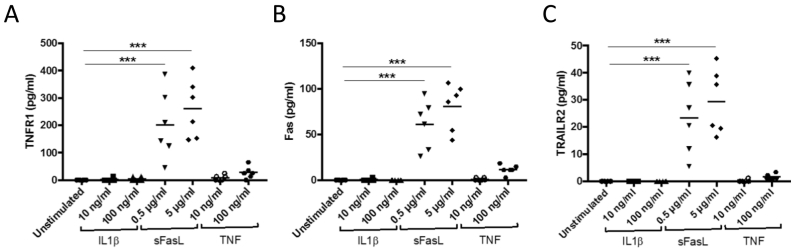
It has previously been shown that death receptors can be detected in the circulation in a soluble form (Blanco-Colio et al., 2007, Mattey et al., 2007). To characterize the processes leading to release of soluble death receptors we exposed cultured human peripheral blood mononuclear cells to Fas ligand (FasL), interleukin (IL)-1β and tumor necrosis factor (TNF)-α. Exposure of the cells to FasL resulted in an increased release of TNFR-1, TRAILR-2 and Fas into the culture medium, whereas no effect was seen in cells exposed to TNF-α (Fig. 1A–E). A decreased release of TNFR-1 and Fas was observed in cells exposed to IL-1β (Fig. 1A and B). The release of TRAILR-2 from cells stimulated with 5 μg/ml of FasL was associated with an about 30% reduction of cellular TRAILR-2 levels (Fig. 1F). To determine if FasL activated apoptosis at the same concentrations that it induced the release of soluble death receptors we stained cells exposed to different concentrations of FasL for Annexin V and 7-AAD. Cells in early stages of apoptosis stain only for Annexin V while cells in late apoptotic state stain positive for both Annexin V and 7-AAD (Fig. 1G). The results demonstrated that FasL activates apoptosis at the same concentrations as it stimulates the release of soluble death receptors (Fig. 1H and I). We next exposed cultured pancreatic INS-1 β-cells to Il-β, FasL and TNF-α and found that only FasL stimulated the release of TNFR-1, TRAILR-2 and Fas from the cells (Fig. 2). These findings show that activation of apoptosis through the FasL/Fas signal pathway is associated with a cellular release of the soluble form of all three types of death receptors from both PBMC and β-cells suggesting that the circulating level of these receptors reflects the level of death receptor-activated apoptosis in the body.
Fig. 1.
Activation of Fas results in release of soluble death receptors. Peripheral blood mononuclear cells (PBMC) were exposed to 10 ng/ml IL-1β, 2.5 μg/ml Fas ligand (sFasL) and 10 ng/ml TNF-α for 24 h in cell culture and the amount of (A) TNFR-1 (B) Fas and (C) TRAILR-2 in the medium analyzed by ELISA. Dose-response effects of (D) sFasL and (E) TNF-α on the release of TRAILR-2 from cultured PBMC. (F) TRAILR-2 remaining in PBMC cell lysates after exposure to sFasL for 24 h. Activation of apoptosis was determined by analyzing staining for 7-AAD and AnnexinV using flow cytometry. Cells in early apoptosis were defined as 7AAD−/AnnexinV+ and cells in late apoptosis as 7AAD+/AnnexinV+. Effect of 24 h of FasL exposure on activation of early (g) and late (h) apoptosis in cultured PBMC. P values where calculated using one-way ANOVA comparing treated cells with untreated controls. *p < 0.05. **p < 0.01 and ***p < 0.001.
Fig. 2.
Fas ligand (FasL) activates the release of soluble death receptors from pancreatic INS-1 β-cells. INS-1 β-cells were exposed to 10–100 ng/ml IL-1β, 0.5–5.0 mg/ml Fas ligand and 10–100 ng/ml TNF-α for 24 h in cell culture and the amount of (A) TNFR-1 (B) Fas and (C) TRAILR-2 in the cell supernatant analyzed by multiplex. P values where calculated using one-way ANOVA comparing stimulated cells with unstimulated controls. ***p < 0.001.
3.2. Association between Risk Factors and Plasma Levels of Soluble Death Receptors
To determine if circulating levels of these markers of death receptor-activated apoptosis correlate with traditional risk factors we analyzed baseline plasma levels of soluble TNFR-1, TRAILR-2 and Fas in 4742 subjects participating in cardiovascular cohort of the MDC study. These analyses were done using the Proximity Extension Assay technology and values expressed as arbitrary units (AU). Significant associations with age, BMI, triglycerides, fasting glucose, insulin, systolic blood pressure, hsCRP, as well as inverse associations with HDL were observed for the plasma levels of all three receptors (Table 1). There were also associations with LDL but these were generally weaker. Associations were similar in subjects with (n = 363) and without (n = 4379) manifest diabetes (data not shown). Subjects with manifest diabetes had higher levels of TNFR-1 (5763 ± 1834 versus 5000 ± 1389 AU, p < 0.0001), TRAILR-2 (2.85 ± 0.90 versus 2.52 ± 0.75 AU, p < 0.0001) and Fas (186 ± 75 versus 167 ± 90 AU, p < 0.0001) than those without diabetes. Also current smokers (n = 951) had higher levels of TNFR-1 (5126 ± 1523 versus 4965 ± 1347 AU, p = 0.003), TRAILR-2 (2.86 ± 0.93 versus 2.46 ± 0.69 AU, p < 0.0001) and Fas (176 ± 117 versus 166 ± 79 AU, p = 0.01) than those that did not smoke.
Table 1.
Correlations between markers of death receptor-activated apoptosis and cardiovascular risk factors.
| TNFR1 | p | FAS | p | TRAILR2 | p | |
|---|---|---|---|---|---|---|
| Age | 0.22 | < 0.001 | 0.20 | < 0.001 | 0.23 | < 0.001 |
| Glucose | 0.13 | < 0.001 | 0.14 | < 0.001 | 0.12 | < 0.001 |
| Insulin | 0.19 | < 0.001 | 0.19 | < 0.001 | 0.17 | < 0.001 |
| BMI | 0.20 | < 0.001 | 0.17 | < 0.001 | 0.13 | < 0.001 |
| Triglycerides | 0.19 | < 0.001 | 0.19 | < 0.001 | 0.20 | < 0.001 |
| HDL | - 0.21 | < 0.001 | − 0.17 | < 0.001 | − 0.18 | < 0.001 |
| LDL | 0.06 | 0.001 | 0.10 | < 0.001 | 0.08 | < 0.001 |
| Systolic BP | 0.15 | < 0.001 | 0.13 | < 0.001 | 0.13 | < 0.001 |
| hsCRP | 0.23 | < 0.001 | 0.09 | < 0.001 | 0.22 | < 0.001 |
Correlation coefficients were calculated using Spearman Rank test. BP; blood pressure.
3.3. High Plasma Levels of Soluble Death Receptors are Associated with Risk for Development of Diabetes
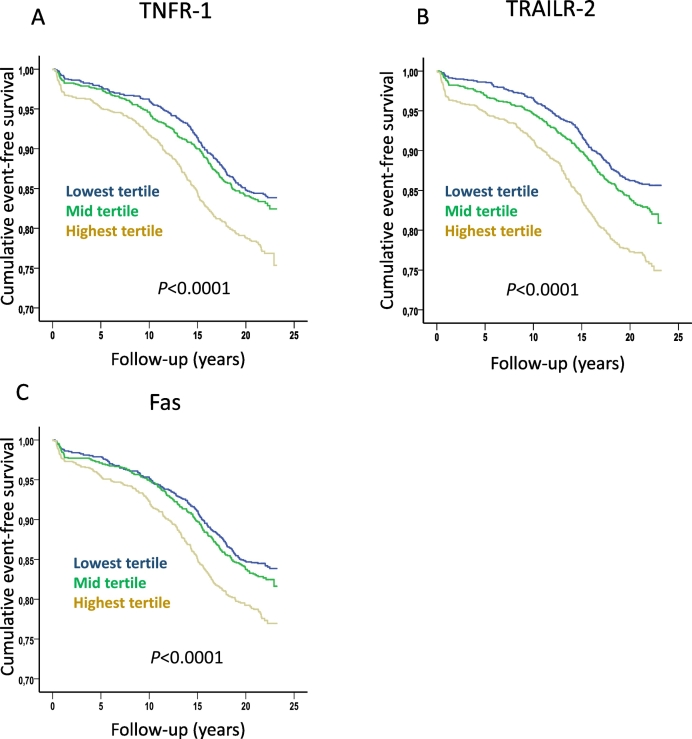
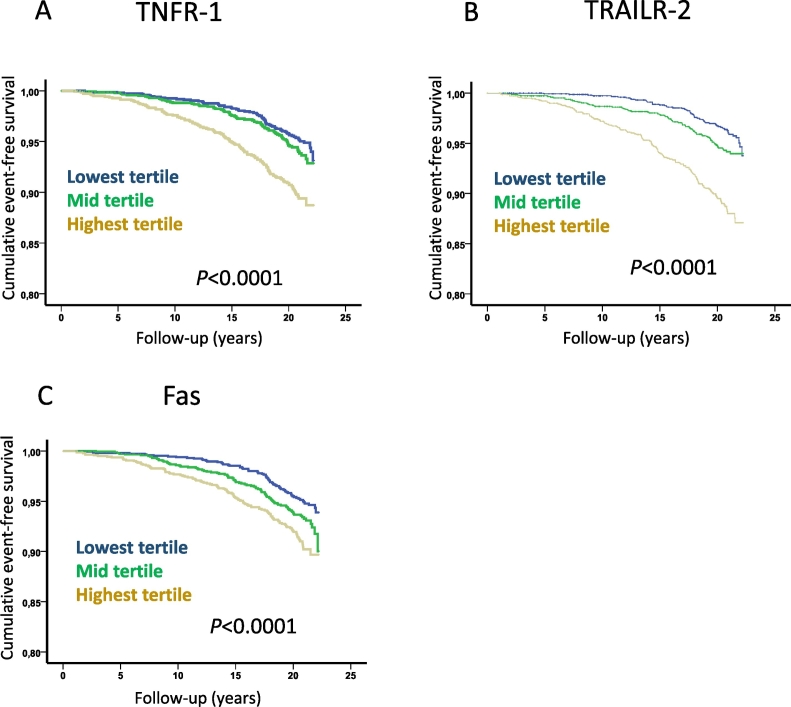
A total of 667 cases of incident diabetes were registered during follow up and also these subjects had higher baseline levels of TNFR-1, TRAILR-2 and Fas than those that remained free of diabetes (5255 ± 1501 versus 4980 ± 1377 AU, p < 0.0001, 2.66 ± 0.84 versus 2.50 ± 0.73 AU, p < 0.0001 and 173 ± 52 versus 166 ± 90 AU, p < 0.0001, respectively). The increased risk of incident diabetes was primarily observed for those in the highest tertile of TNFR-1, TRAILR-2 and Fas (Fig. 3) with hazard ratios (HR) of 1.43 (95% CI 1.19–1.73, p = 0.0002), 1.75 (95% CI 1.45–2.12, p < 0.0001) and 1.36 (95% CI 1.13–1.65, p = 0.001), respectively adjusting for age and sex in Cox proportional hazard models. When glucose, triglycerides, HDL and hsCRP also were included in the models only the highest tertile of TRAILR-2 remained significantly associated with incident diabetes HR 1.13 (95% CI 1.05–1.22, p = 0.002). These observations show that markers of death receptor-activated apoptosis correlate with factors associated with impaired glucose metabolism and are elevated in both subjects with diabetes and in subjects that later will develop diabetes.
Fig. 3.
Soluble death receptor tertiles and risk for development of diabetes. Kaplan-Meier plots demonstrating the association between tertiles of soluble (A) TNFR-1, (B) TRAILR-2 and (C) Fas, and the development of diabetes. P values were calculated using log rank test.
3.4. Genetic Influence on the Circulating Level of Death Receptors
The data presented above suggest an effect of classical risk factors on the circulating levels of death receptors. To investigate if there also exists an influence of genetic factors we performed a genome wide association study. With a significant association threshold of P < 5E-8 for genome wide significance we identified 6 single nucleotide polymorphisms (SNPs) associated with soluble TNFR-1, 13 associated with soluble TRAILR-2 and 7 SNPs associated with soluble Fas levels in plasma (Table 2). Both Fas and TRAILR-2 levels were associated with SNPs in their respective gene regions. There were also significant associations between TRAILR-2 levels and SNPs in the gene regions encoding Rho related BTB domain containing 2 (RHOPTB2, also referred to as “deleted in breast cancer 2” (DBC2)), a protein known to induce apoptosis in cancer cells (Mao et al., 2011). Interestingly, three SNPs demonstrated associations with the circulating level of all three death receptors. These were located in the gene regions encoding Zinc Finger Protein 333 (ZNF333), transmembrane protein 44 (TMEM44) and ADAM metallopeptidase with thrombospondin type 1 motif 14 (ADAMTS14). Adjusting for age, glucose, sex, triglycerides, HDL, BMI and hsCRP had no or only minor influence on the associations between the SNPs and circulating levels of soluble death receptors (Table 2).
Table 2.
SNPs reaching genome wide association significance for plasma levels of soluble death receptors levels.
| SNP | CHR | Position (bp) | Gene | MAF | MA | RA | Basic model |
Extended model |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BETA | SE | P | BETA | SE | P | |||||||
| TNFR1 | ||||||||||||
| exm1435650 | 19 | 14,805,885 | ZNF333 | 0.01 | A | G | − 0.35 | 0.04 | < 1E-15 | − 0.31 | 0.04 | < 1E-15 |
| exm1594730 | 22 | 24,982,011 | GGT1 | 0.01 | T | C | − 0.39 | 0.05 | < 1E-15 | − 035 | 0.04 | 1E-14 |
| exm373967 | 3 | 194,353,889 | TMEM44 | 0.02 | A | C | − 0.29 | 0.03 | < 1E-15 | − 0.27 | 0.03 | < 1E-15 |
| exm831645 | 10 | 72,511,968 | ADAMTS14 | 0.02 | T | C | − 0.32 | 0.03 | < 1E-15 | − 0.29 | 0.03 | < 1E-15 |
| exm646262 | 7 | 101,921,289 | CUX1 | 0.04 | G | A | − 0.15 | 0.02 | 2.5E-13 | − 0.14 | 0.02 | 24.4E-11 |
| exm1369095 | 17 | 80,040,534 | FASN | 0.01 | A | G | − 0.39 | 0.06 | 6E-11 | − 0.37 | 0.06 | 3.2E-10 |
| Fas | ||||||||||||
| rs7911226 | 10 | 90,768,965 | FAS | 0.31 | G | A | − 0.09 | 0.01 | < 1E-15 | − 0.08 | 0.01 | 1.21E-15 |
| exm831645 | 10 | 72,511,968 | ADAMTS14 | 0.02 | T | C | − 0.26 | 0.03 | 5.1E-14 | − 0.25 | 0.04 | 1.6E-11 |
| rs10887883 | 10 | 90,782,973 | NA | 0.38 | A | G | 0.07 | 0.01 | 5.1E-14 | 0.07 | 0.01 | 3.3E-12 |
| rs9658786 | 10 | 90,776,349 | NA | 0.17 | T | C | − 0.09 | 0.01 | 2E-13 | − 0.08 | 0.01 | 3.0E-10 |
| rs1977389 | 10 | 90,773,494 | FAS | 0.37 | G | T | 0.06 | 0.01 | 2.6E-12 | 0.07 | 0.01 | 3.0E-11 |
| exm373967 | 3 | 194,353,889 | TMEM44 | 0.02 | A | C | − 0.22 | 0.03 | 5.3E-11 | − 0.22 | 0.04 | 1.3E-10 |
| exm1435650 | 19 | 14,805,885 | ZNF333 | 0.01 | A | G | − 0.26 | 0.04 | 1.7E-10 | − 0.24 | 0.04 | 2.1E-9 |
| TRAILR2 | ||||||||||||
| exm689164 | 8 | 22,886,020 | TRAILR2 | 0.09 | A | G | − 0.16 | 0.01 | < 1E-15 | − 0.16 | 0.01 | < 1E-15 |
| kgp3559045 | 8 | 22,875,610 | RHOBTB2 | 0.08 | A | G | − 0.12 | 0.01 | < 1E-15 | − 0.12 | 0.02 | 1.31E-15 |
| rs2241260 | 8 | 22,875,985 | RHOBTB2 | 0.32 | G | A | 0.09 | 0.01 | < 1E-15 | 0.09 | 0.01 | < 1E-15 |
| rs2430807 | 8 | 22,859,307 | RHOBTB2 | 0.38 | G | A | − 0.07 | 0.01 | < 1E-15 | − 0.07 | 0.01 | 1.0E-15 |
| rs11785599 | 8 | 22,892,274 | TRAILR2 | 0.47 | T | C | − 0.06 | 0.01 | 9.1E-12 | − 0.06 | 0.01 | 1.2E-13 |
| rs13251475 | 8 | 22,846,335 | RHOBTB2 | 0–31 | A | G | 0.06 | 0.01 | 1.4E-10 | 0.06 | 0.01 | 1.4E-12 |
| exm831645 | 10 | 72,511,968 | ADAMTS14 | 0.02 | T | C | − 0.21 | 0.03 | 4.5E-10 | − 0.17 | 0.03 | 5.9E-8 |
| exm1435650 | 19 | 14,805,885 | ZNF333 | 0.01 | A | G | − 0.23 | 0.03 | 6.3E-10 | − 0.18 | 0.03 | 9.4E-8 |
| rs2430813 | 8 | 22,863,247 | RHOBTB2 | 0.23 | C | T | − 0.06 | 0.01 | 1.8E-9 | − 0.06 | 0.01 | 1.1E-10 |
| exm373967 | 3 | 194,353,889 | TMEM44 | 0.02 | A | C | − 0.19 | 0.03 | 2.4E-9 | − 0.16 | 0.03 | 4.7E-8 |
| rs7826882 | 8 | 22,849,422 | RHOBTB2 | 0.36 | G | T | − 0.05 | 0.01 | 3E-9 | − 0.06 | 0.01 | 8.7E-12 |
| rs17088859 | 8 | 22,832,115 | NA | 0.31 | T | C | 0.06 | 0.01 | 5.7E-9 | 0.06 | 0.01 | 4.5E-10 |
| rs2466178 | 8 | 22,864,622 | RHOBTB2 | 0.16 | T | C | − 0.07 | 0.01 | 9.3E-9 | − 0.07 | 0.01 | 1.9E-9 |
CHR; chromosome, bp; basepair, MAF; minor allele frequency, MA; minor allele, RA; reference allele, BETA; standardized correlation coefficients. Genomic positions (bp) are according to NCBI Build 37 and allele coding is expressed relative to the forward strand. Associations between SNP and plasma levels of soluble death receptors were calculated using linear regression models. The basic model was unadjusted and the extended model adjusted for age, sex, glucose, triglycerides, HDL, BMI and hsCRP. ZNF333; gene for Zinc Finger Protein 333, GGT1; gene for gamma-glutamyltransferase 1, TMEM44; gene for transmembrane protein 44, ADAMTS14; gene for ADAM metallopeptidase with thrombospondin type 1 motif 14, NA, no gene annotation, CUX1; gene for cut like homeobox 1, FASN; gene for fatty acid synthase and RHOBTB2; gene for Rho related BTB domain containing 2.
Several of the SNPs demonstrated strong co-variation indicating linkage disequilibrium (data not shown). When all SNPs associated with plasma level of TNFR-1 were entered into a linear regression model the SNPs in the ADAMTS14 (p = 0.005), TMEM44 (p < 0.05) and GGT1 (p < 0.05) genes regions remained significant. For circulating Fas, the two SNPs in the FAS gene region (p = 7.6E-8 and 0.002, respectively) and the SNP in the ADAMTS14 gene regions(p = 0.001) demonstrated independent associations in the corresponding regression models. Finally, we identified independent associations between circulating TRAILR-2 and the SNP in the ADAMTS14 (p < 0.05) gene region, as well as one SNP each in the gene regions forTRAILR-2 (exm689164) and RHOBTB2 (rs2241260, p = 1.3E-9 and 0.0003, respectively).
Controlling for age and sex in logistic regressions models there were no significant associations between SNPs demonstrating independent association with soluble death receptors and diabetes (prevalent and incident combined) in this cohort.
3.5. Increased Circulating Levels of Soluble Death Receptors are Associated with Increased Risk of CV Events
The incidences of cardiovascular events (non-fatal and fatal myocardial infarction and stroke) were more than two-fold higher in subjects with prevalent diabetes than in subjects without diabetes (17.1% versus 6.8% p < 0.0001 and 26.2% versus 12.1%, p < 0.0001, respectively as determined by chi square test). Those in the diabetes group that suffered a cardiovascular event were older, had higher fasting glucose, HbA1c, systolic blood pressure and lower HDL than those remained free of CVD (Table 3). Notably, there was no difference in baseline BMI, LDL or hsCRP between those with and without incident CVD in the diabetes group. All risk factors were significantly more common among those that suffered from a cardiovascular event during follow up in the non-diabetes group (Table 3).
Table 3.
Baseline clinical characteristics of diabetic and non-diabetic subjects with and without incident CVD.
| Diabetes |
Non diabetes |
|||||
|---|---|---|---|---|---|---|
| Non CVD |
CVD |
p | Non CVD |
CVD |
p | |
| n = 268 | n = 95 | n = 3847 | n = 532 | |||
| Age | 58.57 ± 5.79 | 61 ± 4.77 | < 0.0001 | 56.95 ± 5.9 | 60.27 ± 5.30 | < 0.0001 |
| Sex (M/F) | 147/121 | 58/37 | n.s | 1399/2448 | 291/241 | < 0.0001 |
| BMI (kg/m2) | 28.8 ± 4.81 | 29.0 ± 4.32 | n.s | 25.3 ± 3.69 | 26.1 ± 3.84 | < 0.0001 |
| Current smoker | 50 (18.7%) | 19 (20.2%) | n.s | 801 (20.8%) | 150 (28.4%) | < 0.0001 |
| Glucose (mmol/l) | 7.95 ± 2.99 | 8.69 ± 3.14 | 0.041 | 4.87 ± 0.45 | 4.95 ± 0.45 | 0.0004 |
| Insulin | 11.5 (7.0–18.0) | 11.0 (7.0–16-0) | ns. | 6.0 (4.0–8.0) | 7.0 (4.0–11.0) | 0.001 |
| Blood pressure lowering (%) | 82 (30.6%) | 42 (44.2%) | 0.016 | 503 (13.1%) | 135 (25.4%) | < 0.0001 |
| Lipid lowering (%) | 11 (4.1%) | 6 (6.3%) | n.s | 69 (1.8%) | 29 (5.5%) | < 0.0001 |
| Triglycerides (mmol/l) | 1.60 (1.11–2.31) | 1.92 (1.27–2.68) | n.s | 1.11 (0.84–1.51) | 1.25 (0.94–1.68) | < 0.0001 |
| LDL (mmol/l) | 4.22 ± 0.96 | 4.07 ± 0.99 | n.s | 4.14 ± 0.98 | 4.3 ± 0.98 | 0.0002 |
| HDL (mmol/l) | 1.24 ± 0.34 | 1.15 ± 0.37 | 0.019 | 1.42 ± 0.37 | 1.29 ± 0.34 | < 0.0001 |
| Systolic BP (mmHg) | 148 ± 20 | 155 ± 20 | 0.008 | 139 ± 18 | 148 ± 19 | < 0.0001 |
| Diastolic BP (mm Hg) | 90 ± 9 | 91 ± 10 | n.s | 86 ± 9 | 90 ± 9 | < 0.0001 |
| HbA1c (%) | 6.06 ± 1.50 | 6.70 ± 1.75 | 0.001 | 4.77 ± 0.42 | 4.84 ± 0.45 | < 0.0001 |
| hsCRP (mg/l) | 4.02 ± 5.72 | 4.10 ± 5.13 | n.s | 2.3 ± 3.96 | 3.28 ± 5.15 | < 0.0001 |
| TNFR-1 (AU) | 5732 ± 1758 | 5850 ± 2040 | n.s | 4945 ± 1380 | 5400 ± 1758 | < 0.0001 |
| Fas (AU) | 185 ± 79 | 189 ± 63 | n.s. | 166 ± 93 | 177 ± 67 | < 0.0001 |
| TRAILR-2 (AU) | 2.81 ± 0.85 | 2.94 ± 1.04 | n.s. | 2.49 ± 0.74 | 2.76 ± 0.75 | < 0.0001 |
Data presented as number and percent for categorical data, mean ± SD for continuous variables and median (inter quartile range) for non-normally distributed variables. t-test was used for normally distributed data, Mann-Whitney for non-normally distributed data, and Chi-2 test for categorical data. BMI; body mass index, LDL; low-density lipoprotein, HDL; high-density lipoprotein, SBP; systolic blood pressure, hsCRP; high sensitive C-reactive protein, n.s.; not significant. All values are derived from the baseline investigation and current smokers refer to those being smokers at that time point.
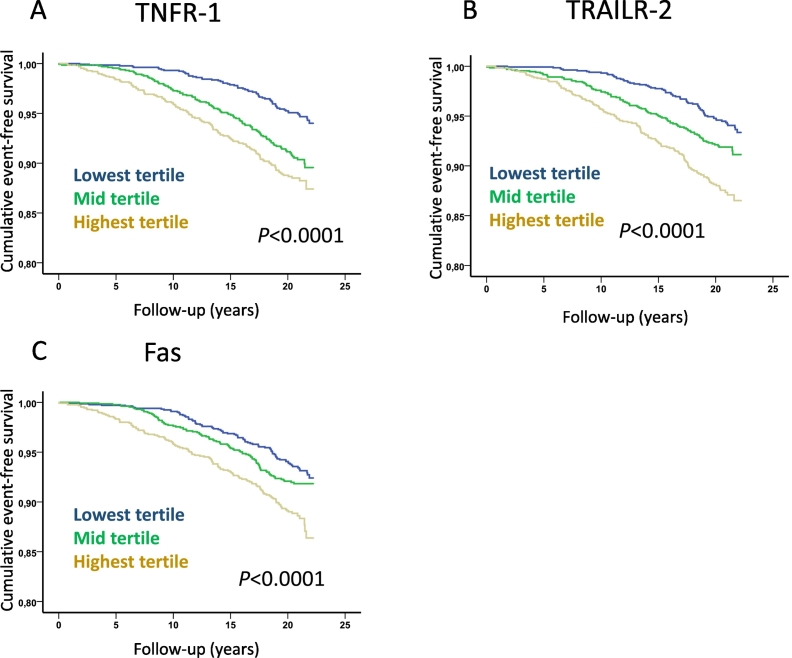
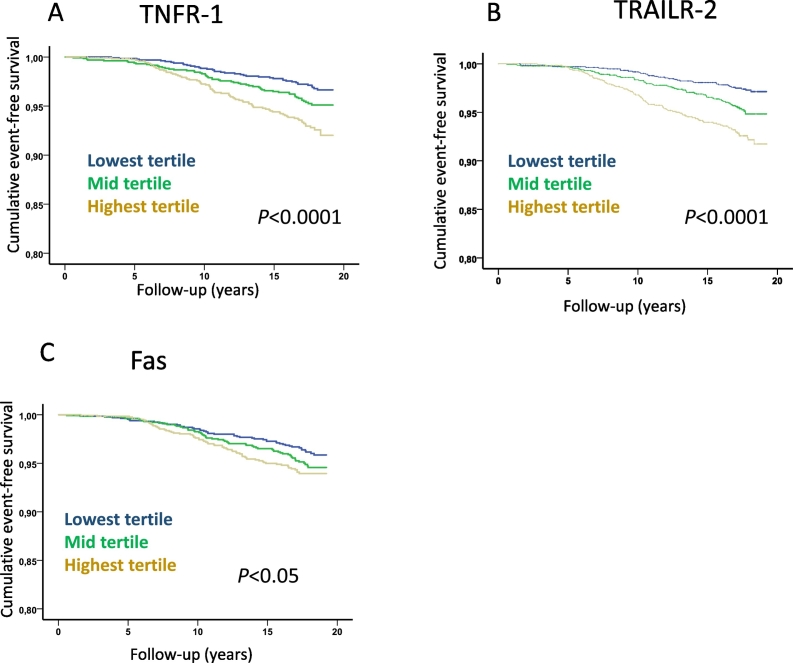
While non-diabetic subjects with incident CVD had significantly higher baseline levels of TNFR-1, TRAILR-2 and Fas than those that remained free of CV events no significant differences were found between cases and CVD-free subjects with diabetes at baseline (Table 3). Further analyses of non-diabetic subjects demonstrated that those in the highest tertiles of TNFR-1, TRAILR-2 and Fas had increased risk for cardiovascular death, MI and ischemic stroke (Fig. 4, Fig. 5, Fig. 6 and Table 4). Subjects with hemorrhagic stroke were excluded from the latter analysis due to the more heterogenic etiology. After controlling for age, sex, BMI, smoking, fasting glucose, HDL, LDL, triglycerides, hsCRP and systolic blood pressure in Cox proportional hazard regression models the highest tertile of TRAILR-2 remained significantly associated with cardiovascular death (hazard ratio (HR) and 95% confidence interval; 1.54 (1.10–2.11), p = 0.01), incident MI (HR 1.60 (1.22–2.11), p = 0.001) and stroke (1.60 (1.11–2.32), p = 0.014). Adjusting for the same factors the highest tertile of TNFR-1 remained significantly associated with incident MI (HR 1.42 (1.07–1.87), p = 0.012). Similar results were obtained when hazard ratios for TNFR-1, TRAILR-2 and Fas were calculated based on increase of one standard deviation (Table 4).
Fig. 4.
Soluble death receptor tertiles and risk for cardiovascular mortality. Kaplan-Meier plots demonstrating the association between tertiles of soluble (A) TNFR-1, (B) TRAILR-2 and (C) Fas, and cardiovascular mortality. P values were calculated using log rank test.
Fig. 5.
Soluble death receptor tertiles and risk for development of acute myocardial infarction. Kaplan-Meier plots demonstrating the association between tertiles of soluble (A) TNFR-1, (B) TRAILR-2 and (C) Fas, and the development of acute myocardial infarction. P values were calculated using log rank test.
Fig. 6.
Soluble death receptor tertiles and risk for development of ischemic stroke. Kaplan-Meier plots demonstrating the association between tertiles of soluble (A) TNFR-1, (B) TRAILR-2 and (C) Fas, and the development of ischemic stroke. P values were calculated using log rank test.
Table 4.
Soluble death receptor tertiles and risk for cardiovascular mortality, MI and ischemic stroke.
| HR by tertiles | HR by SD | ||||
|---|---|---|---|---|---|
| Cardiovascular mortality (n = 278) | |||||
| TNFR-1 | 1s | ||||
| Model 1 | 1 | 1.00 (0.73–1.38) | 1.42.(1.05–1.92) | n.s. | 1.66 (1.25–2.20) |
| Model 2 | 1 | 0.98 (0.71–1.35) | 1.32 (0.97–1.78) | (0.07) | 1.47 (1.10–1.95) |
| Model 3 | 1 | 0.96 (0.69–1.33) | 1.12 (0.81–1.53) | n.s. | 1.12 (0.83–1.52) |
| TRAILR-2 | |||||
| Model 1 | 1 | 1.21 (0.85–1.69) | 1.97 (1.44.2.69) | < 0.0001 | 1.43 (1.25–1.64) |
| Model 2 | 1 | 1.12 (0.80–1.58) | 1.65 (1.20–2.26) | 0.002 | 1.36 (1.16–1.60) |
| Model 3 | 1 | 1.06 (0.74–1.50) | 1.52 (1.10–2.11) | 0.01 | 1.27 (1.04–1.56) |
| Fas | |||||
| Model 1 | 1 | 1.12 (0.82–1.53) | 1.22 (0.90–1.65) | n.s. | 1.27 (1.01–1.62) |
| Model 2 | 1 | 1.07 (0.77–1.46) | 1.12 (0.83–1.46) | n.s. | 1.18 (0.93–1.59) |
| Model 3 | 1 | 0.97 (0.70–1.34) | 1.03 (0.75–1.40) | n.s. | 1.05 (0.82–1.36) |
| Myocardial infarction (n = 312) | |||||
| TNFR-1 | 1s | 2nd | 3rd | P for trend | |
| Model 1 | 1 | 1.68 (1.24–2.86) | 1.82.(1.35–2.47) | 0.0003 | 1.64 (1.29–1.59) |
| Model 2 | 1 | 1.64 (1.21–2.23) | 1.74 (1.28–2.36) | 0.001 | 1.51 (1.18–1.93) |
| Model 3 | 1 | 1.47 (1.12–1.94) | 1.42 (1.07–1.87) | 0.015 | 1.23 (0.95–1.58) |
| TRAILR-2 | |||||
| Model 1 | 1 | 1.31 (0.97–1.77) | 1.78 (1.33.2.38) | 0.0003 | 1.35 (1.19–1.54) |
| Model 2 | 1 | 1.21 (0.92–1.70) | 1.63 (1.21–1.70) | 0.004 | 1.33 (1.14–1.55) |
| Model 3 | 1 | 1.28 (0.97–1.70) | 1.60 (1.22–2.11) | 0.003 | 1.23 (1.01–1.48) |
| Fas | |||||
| Model 1 | 1 | 1.06 (0.79–1.42) | 1.35 (1.02–1.79) | (0.063) | 1.30 (1.06–1.59) |
| Model 2 | 1 | 1.04 (0.77–1.39) | 1.29 (0.96–1.69) | n.s. | 1.21 (0.99–1.49) |
| Model 3 | 1 | 1.02 (0.76–1.38) | 1.23 (0.92–1.64) | n.s. | 1.10 (0.89–1.37) |
| Ischemic stroke (n = 180) | |||||
| TNFR-1 | 1s | 2nd | 3rd | P for trend | |
| Model 1 | 1 | 1.30 (0.87–1.95) | 1.69 (1.15–2.48) | 0.03 | 1.53 (1.14–2,03) |
| Model 2 | 1 | 1.25 (0.83–1.86) | 1.52 (1.03–2.24) | n.s. | 1.31 (0.99–1.75) |
| Model 3 | 1 | 1.14 (0.79–1.63) | 1.31 (0.92–1.86) | n.s. | 1.06 (0.78–1.43) |
| TRAILR-2 | |||||
| Model 1 | 1 | 1.56 (1.03–2.37) | 2.04 (1.37–3.05) | 0.002 | 1.37 (1.17–1.59) |
| Model 2 | 1 | 1.39 (0.91–0.12) | 1.76 (1.17–2.65) | 0.02 | 1.30 (1.07–1.57) |
| Model 3 | 1 | 1.29 (0.88–1.88) | 1.60 (1.11–2.32) | 0.04 | 1.17 (0.93–1.49) |
| Fas | |||||
| Model 1 | 1 | 1.57 (0.58–4.26) | 1.35 (0.52–3.51) | n.s. | 1.15 (0.90–1.46) |
| Model 2 | 1 | 1.04 (0.71–1.53) | 1.03 (0.71–1.50) | n.s. | 1.05 (0.81–1.34) |
| Model 3 | 1 | 0.94 (0.66–1.31) | 0.94 (0.67–1.32) | n.s. | 0.95 (0.73–1.24) |
Soluble death receptor tertile and per standard deviation (SD) hazard ratios (HR) and 95% confidence intervals for incident cardiovascular mortality, myocardial infarction and ischemic stroke were calculated using Cox regression models. Model 1; age and sex, model 2; age, sex, current smoking, LDL and systolic blood pressure, model 3; age, sex, current smoking, LDL, systolic blood pressure, BMI, glucose, triglycerides, HDL and hsCRP.
Controlling for age and sex in logistic regressions models there were no significant associations between SNPs demonstrating independent association with soluble death receptors and the incidence of myocardial infarction or stroke in this cohort.
4. Discussion
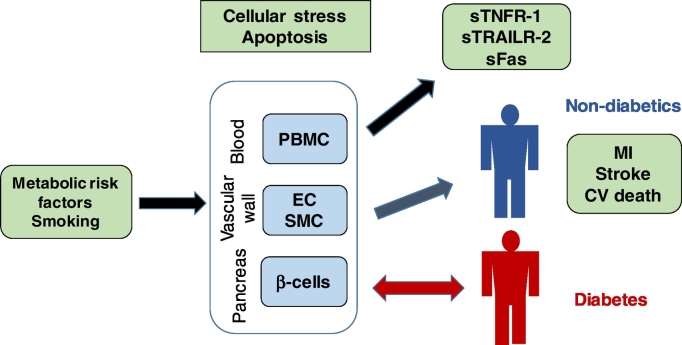
In the present study, we demonstrate that activation of apoptosis through the FasL/Fas pathway is associated with a release of soluble TNFR-1, TRAILR-2 and Fas from cells. We also provide evidence for a link between receptor-activated apoptotic cell death and risk for development of diabetes and CVD. Our findings are compatible with the notion that smoking and metabolic risk factors that causes cellular stress are associated with increased cell death by death receptor-activated apoptosis in exposed tissues which in turn is reflected by elevated plasma levels of soluble TNFR-1, TRAILR-2 and Fas. Accordingly, it is likely that the plasma level of these receptors act as a marker of the tissue injury caused by the risk factors. When pancreatic β-cells are affected the risk for development of diabetes increases while injury to vascular cells promotes atherosclerosis and increases the risk for cardiovascular events (Fig. 7).
Fig. 7.
Associations between metabolic stress, release of soluble death receptors and development of diabetes and cardiovascular disease. Smoking and metabolic risk factors such as hyperglycemia, hypertriglyceridemia and low HDL cause cellular stress leading to increased cell death by death receptor-activated apoptosis in exposed tissues. The activation of death receptor-activated apoptosis is associated with release of soluble (s)TNFR-1, sTRAILR-2 and sFas into the circulation suggesting that the plasma level of these receptors act as a marker of the tissue injury caused by the risk factors. When pancreatic β-cells are affected the risk for development of diabetes increases while injury to vascular cells promotes atherosclerosis and increases the risk for cardiovascular events.
The mechanisms responsible for loss of β-cells in diabetes remains a matter of controversy (Cnop et al., 2005, Halban et al., 2014), but several lines of evidence have implicated activation of β-cell apoptosis in response to the metabolic and oxidative stress caused by elevated levels of glucose and fatty acids as a key factor (Allen et al., 2005, Anuradha et al., 2014, Poitout et al., 2010). The present study provides clinical support for this notion by demonstrating that (Hetts, 1998) exposure of INS-1 β-cells to FasL activates the release of TNFR-1, TRAILR-2 and Fas from the cells, (Van Vre et al., 2012) associations between metabolic changes characteristic for an impaired glucose metabolism and elevated levels of these markers of receptor-activated apoptosis, (Butler et al., 2003) subjects with elevated levels of these markers have an increased risk for development of diabetes and (Allen et al., 2005) markers of receptor-activated apoptosis are significantly elevated in subjects with manifest diabetes. Both hypertriglyceridemia and HDL have previously been implicated in β-cell apoptosis in diabetes. In hypertriglyceridemia it is primarily high levels of saturated fatty acids that activate apoptosis by increasing endoplasmic reticulum stress (Sramek et al., 2016, Biden et al., 2014). HDL is known to protect β-cells against apoptosis induced by a variety of factors including saturated fatty acids, hyperglycemia, oxidized LDL as well as TNF-α and other pro-inflammatory cytokines (von Eckardstein and Widmann, 2014). The mechanism through which HDL protects β-cells against apoptosis remains to be fully understood, but HDL has been shown to down-regulate the expression of Fas on murine islets suggesting that it may inhibit activation through the extrinsic pathway (Rutti et al., 2009). Collectively, these observations support the notion that metabolic stress caused by hypertriglyceridemia and low HDL is associated with an increased rate of death receptor-activated apoptosis and that this involves pancreatic β-cells increasing the risk for development of diabetes. Analysis of soluble death receptors may be useful in selecting patients for new targeted new therapies in preventing the development of diabetes.
Since the associations between hypertriglyceridemia, low HDL and high levels of soluble death receptors observed in the present study are unlikely to solely be a consequence of increased β-cell apoptosis they suggest a general effect of these metabolic factors on death receptor-activated apoptotic cell death. Such an effect could potentially play an important role in diabetic organ complications including those affecting the vascular system (Qiu et al., 1998). Unexpectedly, the associations between elevated levels of markers of death receptor-activated apoptotic cell death was stronger in subjects without than in those with diabetes. Without adjusting for other risk factors baseline plasma levels of TNFR-1, TRAILR-2 and Fas were significantly higher in those that suffered from MI or ischemic stroke during follow-up. When adjusting for age, sex, systolic blood pressure and smoking in Cox regressions models those in the highest tertile of soluble TNFR-1 in plasma had a 74% higher risk of MI and those in the highest tertile of soluble TRAILR-2 had a 65% higher risk of cardiovascular death and a 63% higher risk of MI as compared with those in the corresponding lowest tertile. Further adjustment for impaired glucose metabolism (BMI, glucose, triglycerides, HDL and insulin) weakened these associations, but they still remained significant. The stroke hazard ratios for those in the highest tertiles of all three types of soluble death receptors were comparable to those for MI, but the statistical significance of the findings were weaker most likely because of a lower power to detect such associations. Although the present observations do not prove any causal relationships they are well in line with the notion that metabolic factors associated with an impaired glucose metabolism contributes to an increased frequency of death receptor-activated apoptotic cell death in the cardiovascular system resulting in an increased risk for development of myocardial infarction and stroke. Cells undergoing apoptosis are frequently observed in atherosclerotic plaques (Kolodgie et al., 1999, Crisby et al., 1997, Kolodgie et al., 2000), but the functional importance of this remains to be fully understood (Van Vre et al., 2012). The role of apoptosis in advanced plaques is complex and can potentially be both beneficial and detrimental depending on the cells affected. Studies performed in experimental mouse models have shown that inhibition of macrophage apoptosis can lead to both reduced necrotic core formation (Thorp et al., 2009) as well as increased macrophage accumulation and lesion progression (Bolick et al., 2009). Apoptosis of endothelial cells may give rise to endothelial erosions (Tricot et al., 2000), while apoptosis of smooth muscle cells can contribute to plaque destabilization (Clarke et al., 2006).
To further address the role of death receptor-activated apoptosis in CVD we performed a GWAS to search for genetic variations affecting plasma levels of soluble death receptors and to determine if such genetic variants were linked to CVD risk. We identified several SNPs of genome wide significant association with the plasma level of soluble death receptors. These associations remained essentially unchanged when controlling for metabolic factors demonstrating that also genetic factors are of importance for the level of soluble death receptors into the circulation. Notably, we found a strong association between a SNP in the gene region for Fas with a minor allele frequency of 0.31 and soluble Fas as well as an association between an SNP in the gene region for TRAILR-2 with a minor allele frequency of 0.09 and soluble TRAILR-2. However, none of these SNPs were associated with an increased risk for CVD in the present study. Data available from the CARDIoGRAMplusC4D Consortium (www.cardiogramplusc4d.org) showed a weak association between SNP in the gene region for TRAILR-2 (rs13265018) and coronary artery disease (β = 0.037, SE = 0.016 and p = 0.018), but no association for the SNP in the gene region for Fas. A functional role of the SNP in the gene region for TRAILR-2 is supported by observations of a poor prognosis of lung cancer patients carrying this gene variant (Schabath et al., 2013). However, generally genetic polymorphisms affecting the plasma levels of soluble death receptors appear to have a limited effect on cardiovascular risk. Accordingly, it is unlikely that soluble death receptors in themselves play a functional role in CVD. More probably, they serve as markers of death receptor-activated apoptosis and that it is this process that increases cardiovascular risk. This concept is in line with the “response to injury” hypothesis according to which atherosclerosis is developed in response to injury caused by accumulation of lipoprotein-derived lipids in the artery wall (Ross, 1999). The plasma level of soluble death receptors could also be affected by genetic variations in the genes encoding the proteases responsible for their shedding from the cell surface. These proteases remain to be fully identified but a protease called A Disintegrin And Metallopeptidase (ADAM 17) has been shown to mediate shedding of TNFR-1 (Rowlands et al., 2011). It is an interesting possibility that the associations between a SNP in the ADAMTS 14 gene region and the plasma levels of TNFR-1, TRAILR-2 and Fas observed in the present study could reflect a role of this protease in death receptor shedding.
It remains to be elucidated why the associations between high levels of soluble death receptors and risk for development of MI and ischemic stroke were observed only in subjects without but not in those with diabetes. Diabetes at baseline was associated with higher levels of soluble death receptors and an increased cardiovascular risk. Notably, the levels of soluble death receptors were higher in subjects with diabetes that remained free of cardiovascular events than in those non-diabetic subjects that did develop a cardiovascular event. One possible explanation to this could be lack of statistical power in the diabetes group as well as the circumstance that a more intense use of cardiovascular preventive medication in subjects with diabetes complicates biomarker prediction.
There are some other limitations of the present study that need to be considered. As discussed above the associations between metabolic risk factors, soluble death receptors and increased risk of diabetes and cardiovascular events do not prove causal relationships. Another important limitation is that we do not have information regarding changes in the plasma levels of these apoptosis markers during follow-up. Thus, we cannot exclude that changes in plasma death receptor levels subsequent to the baseline investigation has influenced the results. It is also not possible to conclude to what extent increased plasma levels of TNF1, TRAILR-2 and Fas reflect increased apoptosis specifically in the cardiovascular system. Moreover, it should be kept in mind that activation of death receptors also has been described to result in stimulation of inflammation and cell proliferation (Cullen and Martin, 2015), suggesting that the plasma levels of these receptors also may reflect other biological processes of importance for atherosclerosis development than apoptosis. Some support for this possibility comes from the present finding of associations between soluble death receptor levels and hsCRP. Finally, we did not study the association between plasma levels of death receptor ligands and risk for CVD. The findings of previous studies of these associations have been inconsistent. While Shimizu et al. (Shimizu et al., 2002) found increased levels of FasL in patients with acute coronary syndromes Michowitz et al. (Michowitz et al., 2005) reported decreased levels of TRAIL and no change in FasL in subjects with unstable angina. However, the importance of the plasma level of death receptor ligands is difficult to assess because these ligands are also active in their membrane-bound form (Wiley et al., 1995).
In conclusion, these findings show that subjects with high levels of circulating markers of apoptotic cells death have increased risk of cardiovascular mortality, MI and stroke. They also demonstrate that several cardiovascular risk factors are associated with increased circulating markers of apoptotic cells death. Collectively, these observations provide clinical support for the “response to injury” hypothesis of atherosclerosis and point to the possibility that the plasma level of soluble death receptors can be used as surrogate markers of arterial injury and atherosclerotic disease activity in cardiovascular interventions. Finally, our findings imply that soluble death receptors also may serve as biomarkers of the damage caused by metabolic stress to β-cells and risk for development of type 2 diabetes.
Sources of Funding
This study was supported by grants from the Swedish Medical Research Council (2015-02811), the Swedish Heart-Lung foundation (2014-0357), Swedish Foundation for Strategic Research (RBa08-0075) and the European Union's Seventh Framework Program (FP7/2007–2013) under grant agreement VIA n°603131.
Conflicts of Interest
None.
Author Contributions
IYM and JN designed the study, performed statistical analysis and drafted the manuscript. IYM also performed cell culture studies. HB, MW, AE, IG, EB, MOM, OM and GE participated in collection of data, statistical analyses and made critical revision of the manuscript. PA performed statistical analysis of genetic data and made critical revision of the manuscript.
References
- Alberti K.G., Zimmet P.Z. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet. Med. 1998;15(7):539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Allen D.A., Yaqoob M.M., Harwood S.M. Mechanisms of high glucose-induced apoptosis and its relationship to diabetic complications. J. Nutr. Biochem. 2005;16(12):705–713. doi: 10.1016/j.jnutbio.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Anuradha R., Saraswati M., Kumar K.G., Rani S.H. Apoptosis of beta cells in diabetes mellitus. DNA Cell Biol. 2014;33(11):743–748. doi: 10.1089/dna.2014.2352. [DOI] [PubMed] [Google Scholar]
- Ashkenazi A., Dixit V.M. Death receptors: signaling and modulation. Science. 1998;281(5381):1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- Berglund G., Elmstahl S., Janzon L., Larsson S.A. The Malmo diet and cancer study. Design and feasibility. J. Intern. Med. 1993;233(1):45–51. doi: 10.1111/j.1365-2796.1993.tb00647.x. [DOI] [PubMed] [Google Scholar]
- Biden T.J., Boslem E., Chu K.Y., Sue N. Lipotoxic endoplasmic reticulum stress, beta cell failure, and type 2 diabetes mellitus. Trends Endocrinol. Metab. 2014;25(8):389–398. doi: 10.1016/j.tem.2014.02.003. [DOI] [PubMed] [Google Scholar]
- Blanco-Colio L.M., Martin-Ventura J.L., de Teresa E., Farsang C., Gaw A., Gensini G. Increased soluble Fas plasma levels in subjects at high cardiovascular risk: atorvastatin on inflammatory markers (AIM) study, a substudy of ACTFAST. Arterioscler. Thromb. Vasc. Biol. 2007;27(1):168–174. doi: 10.1161/01.ATV.0000250616.26308.d7. [DOI] [PubMed] [Google Scholar]
- Bolick D.T., Skaflen M.D., Johnson L.E., Kwon S.C., Howatt D., Daugherty A. G2A deficiency in mice promotes macrophage activation and atherosclerosis. Circ. Res. 2009;104(3):318–327. doi: 10.1161/CIRCRESAHA.108.181131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler A.E., Janson J., Bonner-Weir S., Ritzel R., Rizza R.A., Butler P.C. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52(1):102–110. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- Clarke M.C., Figg N., Maguire J.J., Davenport A.P., Goddard M., Littlewood T.D. Apoptosis of vascular smooth muscle cells induces features of plaque vulnerability in atherosclerosis. Nat. Med. 2006;12(9):1075–1080. doi: 10.1038/nm1459. [DOI] [PubMed] [Google Scholar]
- Cnop M., Welsh N., Jonas J.C., Jorns A., Lenzen S., Eizirik D.L. Mechanisms of pancreatic beta-cell death in type 1 and type 2 diabetes: many differences, few similarities. Diabetes. 2005;54(Suppl. 2):S97–107. doi: 10.2337/diabetes.54.suppl_2.s97. [DOI] [PubMed] [Google Scholar]
- Crisby M., Kallin B., Thyberg J., Zhivotovsky B., Orrenius S., Kostulas V. Cell death in atherosclerotic plaques involves both oncosis and apoptosis. Atherosclerosis. 1997;130:17–27. doi: 10.1016/s0021-9150(96)06037-6. [DOI] [PubMed] [Google Scholar]
- Cullen S.P., Martin S.J. Fas and TRAIL 'death receptors' as initiators of inflammation: implications for cancer. Semin. Cell Dev. Biol. 2015;39:26–34. doi: 10.1016/j.semcdb.2015.01.012. [DOI] [PubMed] [Google Scholar]
- Donath M.Y., Halban P.A. Decreased beta-cell mass in diabetes: significance, mechanisms and therapeutic implications. Diabetologia. 2004;47(3):581–589. doi: 10.1007/s00125-004-1336-4. [DOI] [PubMed] [Google Scholar]
- von Eckardstein A., Widmann C. High-density lipoprotein, beta cells, and diabetes. Cardiovasc. Res. 2014;103(3):384–394. doi: 10.1093/cvr/cvu143. [DOI] [PubMed] [Google Scholar]
- Federici M., Hribal M., Perego L., Ranalli M., Caradonna Z., Perego C. High glucose causes apoptosis in cultured human pancreatic islets of Langerhans: a potential role for regulation of specific Bcl family genes toward an apoptotic cell death program. Diabetes. 2001;50(6):1290–1301. doi: 10.2337/diabetes.50.6.1290. [DOI] [PubMed] [Google Scholar]
- Halban P.A., Polonsky K.S., Bowden D.W., Hawkins M.A., Ling C., Mather K.J. Beta-cell failure in type 2 diabetes: postulated mechanisms and prospects for prevention and treatment. Diabetes Care. 2014;37(6):1751–1758. doi: 10.2337/dc14-0396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson G.K. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 2005;352(16):1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- Hedblad B., Nilsson P., Janzon L., Berglund G. Relation between insulin resistance and carotid intima-media thickness and stenosis in non-diabetic subjects. Results from a cross-sectional study in Malmo, Sweden. Diabet. Med. 2000;17(4):299–307. doi: 10.1046/j.1464-5491.2000.00280.x. [DOI] [PubMed] [Google Scholar]
- Hetts S.W. To die or not to die: an overview of apoptosis and its role in disease. JAMA. 1998;279(4):300–307. doi: 10.1001/jama.279.4.300. [DOI] [PubMed] [Google Scholar]
- Kolodgie F.D., Narula J., Guillo P., Virmani R. Apoptosis in human atherosclerotic plaques. Apoptosis. 1999;4(1):5–10. doi: 10.1023/a:1009645730270. [DOI] [PubMed] [Google Scholar]
- Kolodgie F.D., Narula J., Burke A.P., Haider N., Farb A., Hui-Liang Y. Localization of apoptotic macrophages at the site of plaque rupture in sudden coronary death. Am. J. Pathol. 2000;157(4):1259–1268. doi: 10.1016/S0002-9440(10)64641-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maedler K., Spinas G.A., Lehmann R., Sergeev P., Weber M., Fontana A. Glucose induces beta-cell apoptosis via upregulation of the Fas receptor in human islets. Diabetes. 2001;50(8):1683–1690. doi: 10.2337/diabetes.50.8.1683. [DOI] [PubMed] [Google Scholar]
- Mao H., Zhang L., Yang Y., Sun J., Deng B., Feng J. RhoBTB2 (DBC2) functions as tumor suppressor via inhibiting proliferation, preventing colony formation and inducing apoptosis in breast cancer cells. Gene. 2011;486(1–2):74–80. doi: 10.1016/j.gene.2011.07.018. [DOI] [PubMed] [Google Scholar]
- Mattey D.L., Glossop J.R., Nixon N.B., Dawes P.T. Circulating levels of tumor necrosis factor receptors are highly predictive of mortality in patients with rheumatoid arthritis. Arthritis Rheum. 2007;56(12):3940–3948. doi: 10.1002/art.23075. [DOI] [PubMed] [Google Scholar]
- Michowitz Y., Goldstein E., Roth A., Afek A., Abashidze A., Ben Gal Y. The involvement of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) in atherosclerosis. J. Am. Coll. Cardiol. 2005;45(7):1018–1024. doi: 10.1016/j.jacc.2004.12.065. [DOI] [PubMed] [Google Scholar]
- Muhammad I.F., Borne Y., Hedblad B., Nilsson P.M., Persson M., Engstrom G. Acute-phase proteins and incidence of diabetes: a population-based cohort study. Acta Diabetol. 2016;53(6):981–989. doi: 10.1007/s00592-016-0903-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poitout V., Amyot J., Semache M., Zarrouki B., Hagman D., Fontes G. Glucolipotoxicity of the pancreatic beta cell. Biochim. Biophys. Acta. 2010;1801(3):289–298. doi: 10.1016/j.bbalip.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Y., Sutton L., Riggs A.F. Identification of myoglobin in human smooth muscle. J. Biol. Chem. 1998;273(36):23426–23432. doi: 10.1074/jbc.273.36.23426. [DOI] [PubMed] [Google Scholar]
- Roehrich M.E., Mooser V., Lenain V., Herz J., Nimpf J., Azhar S. Insulin-secreting beta-cell dysfunction induced by human lipoproteins. J. Biol. Chem. 2003;278(20):18368–18375. doi: 10.1074/jbc.M300102200. [DOI] [PubMed] [Google Scholar]
- Ross R. Atherosclerosis - an inflammatory disease. N. Engl. J. Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- Rowlands D.J., Islam M.N., Das S.R., Huertas A., Quadri S.K., Horiuchi K. Activation of TNFR1 ectodomain shedding by mitochondrial Ca2 + determines the severity of inflammation in mouse lung microvessels. J. Clin. Invest. 2011;121(5):1986–1999. doi: 10.1172/JCI43839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutti S., Ehses J.A., Sibler R.A., Prazak R., Rohrer L., Georgopoulos S. Low- and high-density lipoproteins modulate function, apoptosis, and proliferation of primary human and murine pancreatic beta-cells. Endocrinology. 2009;150(10):4521–4530. doi: 10.1210/en.2009-0252. [DOI] [PubMed] [Google Scholar]
- Schabath M.B., Giuliano A.R., Thompson Z.J., Amankwah E.K., Gray J.E., Fenstermacher D.A. TNFRSF10B polymorphisms and haplotypes associated with increased risk of death in non-small cell lung cancer. Carcinogenesis. 2013;34(11):2525–2530. doi: 10.1093/carcin/bgt244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimabukuro M., Zhou Y.T., Levi M., Unger R.H. Fatty acid-induced beta cell apoptosis: a link between obesity and diabetes. Proc. Natl. Acad. Sci. U. S. A. 1998;95(5):2498–2502. doi: 10.1073/pnas.95.5.2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu M., Fukuo K., Nagata S., Suhara T., Okuro M., Fujii K. Increased plasma levels of the soluble form of Fas ligand in patients with acute myocardial infarction and unstable angina pectoris. J. Am. Coll. Cardiol. 2002;39(4):585–590. doi: 10.1016/s0735-1097(01)01800-9. [DOI] [PubMed] [Google Scholar]
- Sramek J., Nemcova-Furstova V., Kovar J. Kinase signaling in apoptosis induced by saturated fatty acids in pancreatic beta-cells. Int. J. Mol. Sci. 2016;17(9) doi: 10.3390/ijms17091400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorp E., Li G., Seimon T.A., Kuriakose G., Ron D., Tabas I. Reduced apoptosis and plaque necrosis in advanced atherosclerotic lesions of Apoe −/− and Ldlr −/− mice lacking CHOP. Cell Metab. 2009;9(5):474–481. doi: 10.1016/j.cmet.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricot O., Mallat Z., Heymes C., Belmin J., Leseche G., Tedgui A. Relation between endothelial cell apoptosis and blood flow direction in human atherosclerotic plaques. Circulation. 2000;101(21):2450–2453. doi: 10.1161/01.cir.101.21.2450. [DOI] [PubMed] [Google Scholar]
- Van Vre E.A., Ait-Oufella H., Tedgui A., Mallat Z. Apoptotic cell death and efferocytosis in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2012;32(4):887–893. doi: 10.1161/ATVBAHA.111.224873. [DOI] [PubMed] [Google Scholar]
- Wiley S.R., Schooley K., Smolak P.J., Din W.S., Huang C.P., Nicholl J.K. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3(6):673–682. doi: 10.1016/1074-7613(95)90057-8. [DOI] [PubMed] [Google Scholar]
- Zia E., Hedblad B., Pessah-Rasmussen H., Berglund G., Janzon L., Engstrom G. Blood pressure in relation to the incidence of cerebral infarction and intracerebral hemorrhage. Hypertensive hemorrhage: debated nomenclature is still relevant. Stroke. 2007;38(10):2681–2685. doi: 10.1161/STROKEAHA.106.479725. [DOI] [PubMed] [Google Scholar]