Abstract
Background
The US Preventive Services Task Force recommends routine screening for colorectal cancer (CRC) for adults 50-75 years. We generated small-area estimates for being current with CRC screening to examine socio-geographic differences among states and counties. To our knowledge, nationwide county-level estimates for CRC screening are rarely presented.
Methods
We used county data from the 2014 Behavioral Risk Factor Surveillance System (BRFSS) (n=251,360 adults), linked it to the American Community Survey poverty data, and fitted multilevel logistic regression mixed models. We post-stratified the data with the US census population data to run Monte-Carlo simulations. We generated county-level screening prevalence estimates nationally and by race/ethnicity, mapped the estimates and aggregated them into state- and national estimates. We evaluated internal consistency of our model state-specific estimates with BRFSS direct state estimates using Spearman correlation coefficients.
Results
Correlation coefficients were ≥0.95, indicating high internal consistency. We observed substantial variations in current CRC screening estimates among the states and counties within states. State mean estimates ranged from 58.2% in Wyoming to 75.03% in Massachusetts. County mean estimates ranged from 40.11% in Alaska to 79.76% in Florida. Larger county variations were observed in various race/ethnicity groups.
Conclusions
State estimates mask county variations. However, both state and county estimates indicate that the country is far behind the “80% by 2018” target.
Impact
County-modeled estimates help identify variation in CRC screening prevalence in the US and guide education and enhanced screening efforts in areas of need including areas without BRFSS direct-estimates.
Keywords: multilevel small area estimation, county-level estimates, colorectal cancer screening, colonoscopy, fecal occult blood test, internal consistency
Introduction
Routine testing for colorectal cancer (CRC), starting at age 50 years, saves lives through early detection and removal of precancerous polyps and early stage cancers. The Centers for Disease Control and Prevention (CDC) reported that in 2014, there were 139,992 new cases of and 51,651 deaths from CRC (1). A large proportion of premature deaths from CRC in the United States resulted from racial/ethnic, educational attainment, and geographical inequalities (2). The National Institutes of Health projected in 2010 that medical costs for CRC are expected to increase with the aging of the US population (3). Nevertheless, in 2012 only 65.1% of adults ages 50-75 were current with CRC screening (4). To reduce the burden of colorectal cancer, the National CRC Roundtable, with the support of the American Cancer Society (ACS) and the Centers for Disease Control and Prevention (CDC), proposed in 2014 a goal of reaching 80% of persons 50 years and older being screened for CRC by 2018 (“80% by 2018 initiative”) (5). This initiative is now supported by over 1000 organizations, including public, private and voluntary organizations (6).
National health surveys provide reliable estimates of CRC screening prevalence for the entire United States or for the states (7-9). State-level screening prevalence is often estimated using data from the Behavioral Risk Factor Surveillance System (BRFSS), administered by the CDC (10). The 2012 BRFSS survey revealed large variations among the states in adults 50 years and older who were current with screening, with a low of 55.7% in Arkansas and a high of 76.3% in Massachusetts (4). However, a recent study found substantial variation within the state of Missouri, where location (urban versus rural) had a large effect on CRC screening uptake (11). In addition to geographical location, local communities may show substantial variation in CRC screening from diversity in social and economic status and demographic and healthcare characteristics, which can be masked when data is aggregated at the state-level.
Our goal was to model BRFSS data to provide prevalence estimates of being current with CRC screening at the county level nationally and by race/ethnicity, which to our knowledge, are rarely presented. These estimates can potentially help with decisions about local prevention and control plans, and resource allocation. To achieve this goal, we used a model-based small area estimation (12) that generates county estimates. This method was internally and externally validated (13).
Materials and Methods
The BRFSS is an annual, state-based, random-digit-dial survey of non-institutionalized adults aged 18 years or older administered by CDC in collaboration with health departments in the 50 states, District of Columbia, and 3 territories. Our analysis included only data from the 50 states and the District of Columbia. Trained interviewers in each state collect demographic and health-related information to generate reliable direct estimates of health risk behaviors, preventive health practices, and access to care through landline or cell phone interviews. The 2014 survey combined landline and cell phone response rates ranged from 25.1% in California to 60.1% in South Dakota, with a median rate of 47%. Detailed information about the response rate can be found on the BRFSS website (10). We post-stratified the sampled population of the 2014 BRFSS data with the US Census 2010 population counts at the county level (14) to improve the information about the sampled population. The US Census provides detailed information about each county’s 2014 population, by age (5-year age groups from ages 50 to 74), sex, race/ethnicity (8 non-Hispanic (NH) and Hispanic or Latino origin groups: NH white, NH black, NH American Indian or Alaska Native, NH Asian, NH Native Hawaiian or other Pacific Islander, NH other single race, NH 2 or more races; or Hispanic). This information about adults aged 75 years was obtained from the National Center for Health Statistics (15). County-level poverty rates (≤150% of the federal poverty rate), which are associated with CRC (16,17), were extracted from the American Community Survey (ACS) 2010-2014 (18). Additional information included respondent county (n=3,142 counties) and state (n=51; 50 states and the District of Columbia) identifiers.
CRC screening test use
Because the latest BRFSS data available for analysis in our study was from 2014, we followed the 2008 USPSTF CRC screening guideline recommendations in effect at that time to routinely screen average risk adults ages 50-75 years, with one of three options: 1) fecal occult blood testing (FOBT) within one year, or 2) sigmoidoscopy within 5 years with FOBT within 3 years, or 3) colonoscopy within 10 years (19).
Respondents aged 50 years and older were asked 5 questions to assess CRC test use including blood stool test, sigmoidoscopy and colonoscopy. Each of the tests was described to help the respondent identify the test. The BRFSS questions did not include details about the indication for the test (i.e. for screening or for diagnosis/treatment purposes). As a result, the BRFSS questions are best considered a general measure of test use rather than a specific measure of screening.
The BRFSS described blood stool test (fecal occult blood test, or FOBT) as a test that may use a special kit at home to determine whether the stool contains blood. Sigmoidoscopy and colonoscopy were described as exams in which a tube is inserted in the rectum to view the colon for signs of cancer or other health problems. Respondents were asked whether they had had any of the 3 tests. Those who answered “yes” were further asked how long it had been since their most recent test.
Following the USPSTF recommendations, we defined CRC screening status for any CRC screening test for adults ages 50-75 years as follows:
Current: Respondents tested for at least one of the 3 test types according to recommendations.
Not current: Respondents who had at least one of the tests, but no test was within the recommended time interval, or those who had never had any of the three tests.
The outcome of being current with any CRC test type is used as a measure for comparing adherence with the 80% by 2018 goal. Additional analyses for colonoscopy and FOBT are to assess what is being practiced in reality and how the reported use of each test type is distributed at the county level.
Data analysis
We used the 2014 BRFSS individual-level county data (sample size=251,360 adults) and linked it with the ACS to construct and fit 3 multilevel logistic regression mixed models that estimated the expected probability of an individual being “current” with any CRC test use; with colonoscopy; and with FOBT, in each county in the US. Each model included individual-level fixed effects (age, sex, race/ethnicity), county-level poverty, and county- and state-level random effects. The results from each of the 3 models included parameters for each of the fixed and random variables.
Because some BRFSS counties had no direct estimates and no random effects, we defined a county-level random effect μic for any county-level i with a missing random effect, by spatially smoothing its adjacent counties’ random effects μjc (j ≠i) and averaging them. We then linked the newly created random effects back to the county random effects list. We post stratified the BRFSS data with US census county population counts and used them with the updated random effect list and the estimated parameters from each model in newly constructed Monte Carlo simulation programs. Each simulation included 1,000 randomly drawn samples for each of the parameters and their standard errors, to predict the individual-level expected probability of being current with the respective CRC screening type. Our choice of a flexible spatial smoothing method was driven by caution about over-smoothing health behavior outcome variations in adjacent counties and by BRFSS data, where direct estimate calculations for some counties were limited (20,21). We did not present county estimates for the option of sigmoidoscopy every 5 years with FOBT every 3 years because of small percentages.
Additional analysis included county-level models to generate estimates by race/ethnicity for being current with any CRC test type.
Our multilevel logistic regression models followed the generalized linear mixed models general formula (12):
yijkcs is the self-reported screening status (1=current, 0=not current or never been screened) for an individual in age group i, i=1 to 6, sex group j, j=1 to 2, and race/ethnicity group k, k=1 to 8, from county c in state s, and their respective regression coefficients. xc is a vector of county-level covariates and η is a vector of their respective regression coefficients. The prediction model included a product of the county-level poverty status x’c and its respective regression coefficient η. μc, and ѵs are the county- and state-level random effects, which were assumed to be independent and normally distributed.
We used the results from the generated respective samples of 1000 SAEs to summarize the prevalence estimates for each of the 3 screening types by county, by state and for the entire United States and generated predicted mean values, their standard errors, and 95% confidence intervals. The prevalence estimates calculations are described below:
For example, let P1cs = the county-level estimated prevalence of being current with any test use in county c within state s, Pijkcs = the individual probability of being in age group i, sex group j, and race/ethnicity group k, in county c and state s, Popijkcs = the respective population count, and Popcs = the total population count in county c within state s, then
Similarly, let P2cs = the county-level prevalence of not being current with CRC screening or never been screened for CRC in county c within state s, then
The above prevalence calculations utilize the county population counts from the census as weights.
We calculated summary statistics for the model-based county distributions (total and by race/ethnicity) with the univariate procedure. We also calculated direct estimates (means and 95% CIs) by race/ethnicity with the BRFSS data. For race/ethnicity analysis, we combined NH Native Hawaiian or other Pacific Islander with NH other single race and NH 2 or more races. Summary statistics for state estimates for both the 2014 BRFSS direct estimates and our model-based SAE estimates were calculated with the MEANS procedure.
We validated internal consistency between the BRFSS and the model-based state estimates and estimates of counties with 500 or more respondents with the Spearman correlation coefficients and mean absolute differences between the estimates. In addition, we used ArcGIS (Esri, Redlands, CA) to separately map the model-based county estimates for each of the estimated percentages of being current with any CRC screening test, with colonoscopy, and with FOBT. Summary statistics by race/ethnicity were only compared with reliable BRFSS direct-estimates such as means and medians. We used the SAS GLIMMIX procedure (SAS Institute, Inc., Cary, NC) to fit the BRFSS multilevel logistic models with unweighted data, based on a validation study showing that an unweighted model generated more accurate small area estimates (13). The multilevel simulation models were fitted with SAS Version 9.3. BRFSS states summary estimates calculations for internal consistency were performed with SAS-callable SUDAAN (Research Triangle Institute, Research Triangle Park, NC).
Results
Our 2014 BRFSS analytic file included information from a sample of 251,360 adults 50 to 75 years old. Our post-stratification included current US Census 2010 population data from all 3,142 US counties. The national 2014 model-based SAE prevalence estimate for being current with CRC screening was 67.28%, 95% CI (confidence interval), (66.83%―67.71%) while the direct 2014 BRFSS estimate was 66.24%, 95% CI, (65.83%―66.65%) (Table 1). Mean national estimates for the race/ethnicity groups ranged from 69.16% for NH white to 56.81% for Hispanic, with county estimates being lower by 1% to 5% except for NH AIAN, where the national and county means were similar. The overall ranges of county estimates were between 34.45% for NH white to 47.76% among NH Asians indicating a large variability. BRFSS mean estimates were very similar to the model-based means with 66.24% for the national estimate, 68.33% for NH white, 67.79% for NH black, and somewhat lower (50.82%) for Hispanic.
Table 1.
Model-based SAE summary statistics (%) for being current with CRC screening, overall, by county and by race/ethnicity and comparison with the mean (%) and 95% CI for BRFSS select race/ethnicity groups.
| Test | No of counties | Mean | 95% CI | Min | Lower quartile | Median | Upper quartile | Max | Inter-quartile range | Overall Range | BRFSS | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mean | 95% CI | |||||||||||
| Any CRC test type | ||||||||||||
| Overall | ||||||||||||
| U.S. | 67.28 | 66.83―67.71 | 66.24 | 65.83―66.65 | ||||||||
| Counties | 3142 | 65.23 | 40.11 | 62.04 | 65.53 | 68.88 | 79.76 | 6.84 | 39.65 | |||
| NH white | ||||||||||||
| U.S. | 69.16 | 68.77―69.55 | 68.33 | 67.92―68.73 | ||||||||
| counties | 3142 | 66.29 | 45.96 | 63.17 | 66.41 | 69.72 | 80.41 | 6.54 | 34.45 | |||
| NH black | ||||||||||||
| U.S. | 67.69 | 67.17―68.17 | 67.79 | 66.42―69.12 | ||||||||
| Counties | 3065 | 64.07 | 33.09 | 60.64 | 64.87 | 68.32 | 80.79 | 7.68 | 47.70 | |||
| NH AIANa | ||||||||||||
| U.S. | 57.81 | 57.19―58.41 | ||||||||||
| Counties | 3110 | 58.24 | 30.01 | 54.28 | 58.55 | 62.60 | 75.36 | 8.32 | 45.35 | |||
| NH Asian | ||||||||||||
| U.S. | 61.99 | 60.88―63.00 | ||||||||||
| Counties | 3075 | 56.90 | 28.67 | 52.43 | 56.89 | 61.03 | 75.43 | 8.60 | 47.76 | |||
| NH otherb | ||||||||||||
| U.S. | 63.12 | 62.44―63.76 | ||||||||||
| Counties | 3127 | 60.27 | 34.29 | 57.02 | 60.71 | 64.08 | 75.22 | 7.06 | 40.96 | |||
| Hispanic | ||||||||||||
| U.S. | 56.81 | 55.82―57.71 | 50.82 | 48.79―52.85 | ||||||||
| Counties | 3134 | 53.34 | 25.05 | 49.76 | 53.53 | 57.22 | 71.00 | 7.45 | 45.95 | |||
| Colonoscopy | ||||||||||||
| Overall | ||||||||||||
| U.S. | 63.69 | 63.20―64.16 | 62.48 | 62.06―62.90 | ||||||||
| Counties | 3142 | 62.01 | 35.36 | 58.65 | 62.23 | 65.76 | 76.73 | 7.11 | 41.36 | |||
| FOBT | ||||||||||||
| Overall | ||||||||||||
| U.S. | 9.67 | 9.43―9.90 | 9.86 | 9.59―10.14 | ||||||||
| Counties | 3142 | 8.47 | 3.28 | 7.29 | 8.19 | 9.34 | 20.54 | 2.05 | 17.26 | |||
Notes: CRC, colorectal cancer; SAE, small area estimates
Any CRC test type =Home fecal occult blood test (FOBT) within the past year; sigmoidoscopy within 5 years with FOBT within 3 years; or colonoscopy within 10 years
We used a modified Monte Carlo simulation method for estimating the model-based predicted standard errors.
AIAN = American Indian/Alaska Native.
NH other = Pacific Islander (PI), other one race, and 2 or more races.
An additional analysis comparing mean estimates of counties with ≥500 respondents shows that the mean abs difference between the model-based estimates and the BRFSS direct estimates for being current with any CRC test type is 2.3, SE=0.127 and interquartile range=2.43.
Our national model-based SAE prevalence estimate for colonoscopy within the past 10 years was 63.69%, and was 9.67% for FOBT within the past year. BRFSS estimates were 62.48%, and 9.86%, respectively (Table 1).
The Spearman correlation coefficients (CC) between our state-level model-based SAE estimates and BRFSS direct estimates was 0.95 for being current with any CRC test type, 0.96 for colonoscopy, and 0.97 for FOBT (Table 2). Spearman CC for county estimates with ≥500 respondents, was 0.86 for being current with any CRC test type (Pearson CC was 0.9).
Table 2.
State (n=51) summary statistics (%) for being current with CRC screening by screening type and race/ethnicity ― model-based SAE versus 2014 BRFSS.
| Test | ρa | Mean | Min | Lower quartile | Median | Upper quartile | Max | Interquartile range |
|---|---|---|---|---|---|---|---|---|
| Any CRC test type | ||||||||
| All | ||||||||
| Model-based | 0.95 | 67.11 | 58.92 | 63.00 | 67.47 | 70.38 | 75.03 | 7.38 |
| BRFSS | 66.24 | 56.52 | 61.81 | 66.35 | 69.95 | 76.37 | 8.15 | |
| NH white | ||||||||
| Model-based | 68.56 | 59.87 | 60.64 | 64.87 | 71.76 | 76.13 | 6.24 | |
| BRFSS | 67.82 | 56.99 | 64.63 | 67.58 | 71.87 | 78.03 | 7.24 | |
| NH black | ||||||||
| Model-based | 67.08 | 58.51 | 64.54 | 66.81 | 70.23 | 74.58 | 5.68 | |
| BRFSSb | 64.71 | 34.37 | 60.29 | 65.61 | 71.25 | 86.83 | 10.96 | |
| NH AIANc | ||||||||
| Model-based | 59.50 | 47.23 | 55.23 | 59.80 | 63.75 | 69.04 | 8.51 | |
| BRFSS | 57.70 | 30.77 | 49.84 | 57.67 | 64.38 | 79.25 | 14.54 | |
| NH Asian | ||||||||
| Model-based | 59.70 | 50.19 | 56.76 | 59.77 | 63.41 | 67.87 | 6.66 | |
| BRFSSd | 54.88 | 14.59 | 45.90 | 56.39 | 64.30 | 89.76 | 18.40 | |
| NH othere | ||||||||
| Model-based | 62.05 | 53.41 | 58.65 | 61.59 | 65.30 | 70.20 | 6.61 | |
| BRFSS | 61.29 | 32.13 | 54.76 | 62.78 | 68.62 | 80.52 | 13.86 | |
| Hispanic | ||||||||
| Model-based | 55.76 | 46.96 | 52.18 | 55.75 | 59.48 | 65.02 | 7.30 | |
| BRFSS | 52.47 | 26.63 | 43.66 | 52.26 | 62.51 | 82.35 | 18.85 | |
| Colonoscopy | ||||||||
| Model-based | 0.96 | 64.00 | 56.16 | 60.15 | 64.26 | 67.73 | 72.02 | 7.58 |
| BRFSS | 62.94 | 53.81 | 58.57 | 62.50 | 67.11 | 73.06 | 8.54 | |
| FOBT | ||||||||
| Model-based | 0.97 | 8.49 | 3.73 | 7.06 | 8.21 | 9.97 | 16.83 | 2.91 |
| BRFSS | 8.53 | 2.86 | 6.62 | 7.93 | 10.13 | 20.54 | 3.52 |
Notes: CRC, colorectal cancer; NH, non-Hispanic
Any CRC test type=Home fecal occult blood test (FOBT) within the past year; sigmoidoscopy within 5 years with FOBT within 3 years; or colonoscopy within 10 years; SAE=small area estimates; BRFSS=Behavioral Risk Factor Surveillance System.
ρ = The Spearman correlation coefficients. The correlation coefficient test was performed for the overall national estimates only. Because the BRFSS is a state-based survey and did not include data for all counties, the race groups were not assessed with correlation coefficients. The similarity between the SAE and BRFSS distributions was assessed by comparing the means and the medians.
The mean abs difference between the model-based state-estimates and the BRFSS direct state-estimates for each CRC screening type is as follows: 1) Any CRC test type: Mean=1.36%, SE=0.14; 2) Colonoscopy: Mean=1.5%, SE=0.161; and 3) FOBT: Mean=0.608%, SE=0.088.
We excluded one state with a minimum estimate=0.0.
AIAN = American Indian/Alaska Native.
We excluded one state with a maximum estimate=100.0.
NH other = Pacific Islander (PI), other one race, and 2 or more races.
Except for NH white, state model-based summary statistics for the remaining race/ethnicity groups show a much larger spread for the BRFSS estimates with interquartile estimates ranging from 10.96% to 18.85%. In contrast, the model-based estimates range from 5.68% to 8.51%.
Table 3 presents the model-based estimated prevalence for being current with any CRC test type by state in ranked order and for FOBT, and county estimates (%) summarized by state. Figure 1 presents county estimated prevalence (%) for being current with CRC screening by any CRC test, colonoscopy and FOBT.
Table 3.
Model-based SAE state estimated mean (%) and county statistics summarized by state for being current with any CRC test type and with FOBT screening
| Any CRC test type | FOBT | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| County summary statistic | County summary statistic | |||||||||||||||
|
|
|
|||||||||||||||
| State | State Mean | Min | Q1 | Mean | Median | Q3 | Max | Range | State Mean | Min | Q1 | Mean | Median | Q3 | Max | Range |
|
|
|
|||||||||||||||
| Massachusetts | 75.03 | 72.35 | 73.85 | 74.92 | 74.92 | 75.90 | 77.79 | 5.44 | 10.02 | 9.04 | 9.35 | 9.32 | 9.68 | 10.35 | 12.43 | 3.38 |
| Maine | 74.34 | 69.61 | 72.38 | 73.68 | 73.88 | 75.13 | 76.95 | 7.34 | 7.03 | 5.84 | 7.01 | 7.37 | 7.37 | 7.85 | 8.85 | 3.01 |
| Rhode Island | 74.18 | 71.47 | 76.47 | 76.29 | 77.18 | 77.40 | 78.92 | 7.44 | 8.22 | 7.95 | 8.05 | 8.32 | 8.14 | 8.60 | 8.85 | 0.90 |
| Connecticut | 73.11 | 71.94 | 72.78 | 73.83 | 74.00 | 74.80 | 75.55 | 3.60 | 8.54 | 7.63 | 7.92 | 8.35 | 8.21 | 8.80 | 9.31 | 1.68 |
| Delaware | 72.81 | 71.42 | 71.42 | 73.30 | 73.27 | 75.22 | 75.22 | 3.80 | 6.40 | 5.70 | 7.70 | 6.69 | 6.94 | 7.41 | 7.41 | 1.71 |
| New Hampshire | 72.40 | 67.14 | 71.08 | 71.72 | 71.59 | 73.58 | 73.97 | 6.83 | 6.49 | 5.99 | 6.27 | 6.72 | 6.64 | 6.87 | 8.26 | 2.28 |
| Maryland | 72.25 | 66.19 | 70.07 | 71.41 | 71.60 | 73.24 | 75.22 | 9.03 | 10.62 | 7.67 | 9.44 | 10.16 | 10.07 | 11.04 | 12.42 | 4.76 |
| Washington DC | 72.17 | 72.17 | 10.67 | 10.67 | ||||||||||||
| Michigan | 71.83 | 67.56 | 71.19 | 72.39 | 72.37 | 73.68 | 76.78 | 9.22 | 8.63 | 7.29 | 8.39 | 8.64 | 8.57 | 8.90 | 10.94 | 3.65 |
| Minnesota | 71.38 | 63.23 | 70.13 | 71.10 | 71.56 | 72.19 | 74.63 | 11.39 | 5.80 | 5.23 | 5.79 | 5.96 | 5.92 | 6.06 | 7.51 | 2.28 |
| North Carolina | 70.60 | 62.47 | 68.61 | 70.19 | 70.33 | 71.46 | 74.83 | 12.36 | 10.55 | 8.96 | 10.29 | 10.76 | 10.68 | 11.23 | 12.48 | 3.52 |
| Vermont | 70.47 | 64.82 | 68.60 | 69.96 | 69.81 | 71.57 | 73.41 | 8.60 | 6.79 | 5.52 | 6.48 | 7.18 | 7.06 | 7.80 | 9.29 | 3.77 |
| Wisconsin | 70.38 | 59.11 | 69.54 | 70.41 | 70.64 | 71.58 | 75.64 | 16.53 | 6.40 | 5.81 | 6.39 | 6.63 | 6.56 | 6.79 | 9.89 | 4.09 |
| Washington | 70.19 | 58.46 | 67.86 | 69.19 | 69.40 | 71.27 | 75.07 | 16.61 | 10.26 | 8.88 | 9.78 | 10.28 | 10.16 | 10.74 | 12.35 | 3.48 |
| Florida | 69.63 | 59.83 | 67.80 | 69.62 | 70.02 | 71.33 | 79.76 | 19.94 | 13.17 | 10.69 | 12.36 | 12.84 | 12.69 | 13.23 | 15.60 | 4.91 |
| Virginia | 69.57 | 58.78 | 66.69 | 68.68 | 69.18 | 70.80 | 75.61 | 16.83 | 7.39 | 6.36 | 7.24 | 7.51 | 7.46 | 7.75 | 8.79 | 2.43 |
| Utah | 69.27 | 53.35 | 65.78 | 66.79 | 66.94 | 68.96 | 72.84 | 19.50 | 3.73 | 3.28 | 3.94 | 4.12 | 4.11 | 4.29 | 5.33 | 2.04 |
| Oregon | 68.92 | 61.78 | 66.53 | 68.07 | 68.12 | 69.70 | 72.04 | 10.26 | 10.01 | 8.72 | 9.43 | 9.70 | 9.65 | 9.96 | 11.13 | 2.42 |
| South Carolina | 68.85 | 61.44 | 65.40 | 67.19 | 67.21 | 69.39 | 74.37 | 12.93 | 7.80 | 6.84 | 7.57 | 7.97 | 8.06 | 8.36 | 9.14 | 2.3 |
| Kentucky | 68.40 | 57.26 | 64.64 | 66.74 | 66.95 | 69.04 | 73.78 | 16.52 | 9.56 | 8.59 | 9.33 | 9.64 | 9.56 | 0.86 | 12.23 | 3.65 |
| Georgia | 68.06 | 58.25 | 64.84 | 66.92 | 66.86 | 68.65 | 74.31 | 16.07 | 9.97 | 8.83 | 9.64 | 10.00 | 10.00 | 10.34 | 11.40 | 2.57 |
| Iowa | 67.71 | 62.26 | 66.56 | 67.47 | 67.38 | 68.53 | 71.08 | 8.82 | 7.27 | 6.45 | 7.10 | 7.29 | 7.25 | 7.46 | 8.51 | 2.06 |
| California | 67.68 | 58.10 | 67.39 | 69.33 | 69.95 | 72.17 | 75.55 | 17.45 | 16.83 | 14.46 | 15.81 | 16.49 | 16.25 | 16.76 | 20.54 | 6.08 |
| New York | 67.61 | 60.91 | 68.22 | 69.22 | 69.45 | 70.55 | 73.83 | 12.93 | 8.30 | 7.44 | 8.06 | 8.20 | 8.19 | 8.31 | 9.04 | 1.60 |
| South Dakota | 67.58 | 43.80 | 64.93 | 65.84 | 68.21 | 69.32 | 72.41 | 28.61 | 8.21 | 6.47 | 7.90 | 8.55 | 8.14 | 8.56 | 12.72 | 6.25 |
| Pennsylvania | 67.47 | 64.50 | 66.42 | 67.51 | 67.48 | 68.59 | 71.63 | 7.14 | 7.45 | 6.59 | 7.22 | 7.49 | 7.53 | 7.74 | 8.34 | 1.75 |
| Alabama | 67.09 | 58.23 | 63.82 | 65.29 | 65.33 | 66.80 | 70.80 | 12.57 | 8.28 | 7.58 | 8.01 | 8.51 | 8.53 | 8.88 | 9.98 | 2.40 |
| Tennessee | 67.01 | 60.20 | 64.43 | 65.81 | 65.59 | 67.01 | 71,76 | 11.56 | 9.22 | 8.28 | 8.94 | 9.15 | 9.10 | 9.32 | 10.89 | 2.61 |
| Hawaii | 66.78 | 61.23 | 64.40 | 64.60 | 64.42 | 64.96 | 68.00 | 6.75 | 15.33 | 11.67 | 14.80 | 15.87 | 15.05 | 18.69 | 19.12 | 7.44 |
| Colorado | 66.62 | 53.28 | 61.55 | 63.57 | 63.89 | 66.52 | 70.38 | 17.10 | 8.32 | 6.92 | 7.88 | 8.13 | 8.09 | 8.38 | 10.43 | 3.51 |
| Kansas | 66.43 | 54.33 | 62.68 | 64.25 | 64.70 | 66.15 | 71.81 | 17.48 | 8.10 | 6.53 | 8.21 | 8.39 | 8.38 | 8.61 | 9.40 | 2.88 |
| West Virginia | 66.20 | 57.80 | 63.98 | 65.20 | 65.41 | 66.46 | 70.18 | 12.38 | 9.55 | 8.62 | 9.29 | 9.62 | 9.57 | 9.96 | 10.66 | 2.04 |
| Louisiana | 65.73 | 54.02 | 63.03 | 64.35 | 64.30 | 65.99 | 69.11 | 15.09 | 9.45 | 8.26 | 9.06 | 9.42 | 9.47 | 9.78 | 11.00 | 2.74 |
| Arizona | 65.57 | 49.44 | 56.89 | 60.43 | 60.34 | 66.32 | 68.55 | 19.11 | 10.72 | 8.35 | 8.92 | 10.35 | 9.78 | 11.68 | 14.16 | 5.81 |
| Ohio | 65.17 | 58.75 | 63.06 | 64.17 | 64.32 | 65.38 | 70.09 | 11.34 | 7.75 | 6.88 | 7.31 | 7.56 | 7.51 | 7.72 | 9.08 | 2.20 |
| New Jersey | 65.15 | 56.36 | 63.11 | 65.18 | 66.54 | 67.22 | 68.97 | 12.61 | 7.71 | 6.67 | 7.31 | 7.69 | 7.73 | 8.11 | 8.82 | 2.15 |
| Missouri | 63.96 | 55.83 | 59.58 | 61.61 | 61.47 | 63.32 | 68.62 | 12.79 | 7.11 | 6.32 | 7.23 | 7.36 | 7.36 | 7.53 | 8.18 | 1.85 |
| Mississippi | 63.37 | 52.84 | 60.46 | 61.92 | 62.32 | 63.86 | 69.09 | 16.25 | 10.63 | 9.45 | 10.35 | 10.82 | 10.74 | 11.34 | 12.15 | 2.70 |
| North Dakota | 63.00 | 43.73 | 59.95 | 60.98 | 62.07 | 63.13 | 68.19 | 24.46 | 7.06 | 5.69 | 7.05 | 7.38 | 7.23 | 7.54 | 10.42 | 4.73 |
| Texas | 62.93 | 45.14 | 61.27 | 62.87 | 63.90 | 65.79 | 71.80 | 26.66 | 8.56 | 7.16 | 8.42 | 8.63 | 8.64 | 8.81 | 10.51 | 3.34 |
| Montana | 62.86 | 48.85 | 59.48 | 61.10 | 61.81 | 63.43 | 66.38 | 17.53 | 6.53 | 5.75 | 6.48 | 6.77 | 6.66 | 6.84 | 9.57 | 3.82 |
| Indiana | 62.84 | 52.65 | 61.16 | 62.95 | 62.67 | 64.54 | 69.68 | 17.03 | 7.90 | 7.01 | 7.62 | 7.80 | 7.76 | 7.93 | 9.46 | 2.45 |
| Nevada | 62.62 | 54.64 | 60.30 | 61.07 | 61.66 | 62.54 | 65.82 | 11.18 | 11.01 | 7.64 | 8.45 | 9.09 | 8.79 | 9.30 | 12.20 | 4.56 |
| Nebraska | 62.42 | 49.57 | 57.90 | 59.45 | 59.20 | 61.18 | 65.38 | 15.80 | 7.17 | 5.64 | 6.90 | 7.23 | 7.20 | 7.44 | 9.68 | 4.03 |
| Illinois | 62.12 | 51.78 | 60.97 | 62.11 | 62.29 | 63.26 | 66.71 | 14.93 | 6.36 | 5.60 | 6.24 | 6.34 | 6.33 | 6.44 | 7.07 | 1.47 |
| Arkansas | 61.74 | 50.96 | 58.57 | 60.04 | 60.11 | 61.73 | 69.16 | 18.20 | 7.18 | 6.67 | 7.07 | 7.37 | 7.37 | 7.58 | 8.27 | 1.60 |
| Idaho | 61.43 | 46.46 | 57.47 | 59.13 | 59.32 | 61.23 | 66.01 | 19.55 | 6.31 | 5.55 | 6.23 | 6.45 | 6.42 | 6.58 | 7.73 | 2.18 |
| Alaska | 60.75 | 40.11 | 52.14 | 56.60 | 59.12 | 60.98 | 66.29 | 26.17 | 5.50 | 4.83 | 5.46 | 6.28 | 5.83 | 7.06 | 8.56 | 3.73 |
| New Mexico | 60.71 | 45.96 | 54.85 | 57.63 | 57.18 | 61.20 | 69.80 | 23.85 | 7.53 | 6.24 | 7.22 | 7.47 | 7.40 | 7.65 | 10.59 | 4.35 |
| Oklahoma | 59.27 | 50.13 | 55.50 | 57.45 | 57.62 | 59.09 | 63.61 | 13.49 | 8.22 | 6.94 | 7.58 | 8.01 | 7.92 | 8.37 | 10.09 | 3.15 |
| Wyoming | 58.92 | 52.03 | 56.38 | 58.33 | 58.02 | 60.64 | 63.84 | 11.81 | 5.35 | 4.35 | 5.29 | 5.54 | 5.59 | 5.76 | 6.73 | 2.38 |
Notes: CRC, colorectal cancer; Q1=Lower quartile; Q3=upper quartile
Any CRC test type=Fecal occult blood test (FOBT) within the past year; sigmoidoscopy within 5 years with FOBT within 3 years; or colonoscopy within 10 years.
Percentages are in decreasing order of state prevalence of being current with any CRC test option. Percentages for FOBT match by state.
Ranges with percentages >=15% are highlighted.
Figure 1.
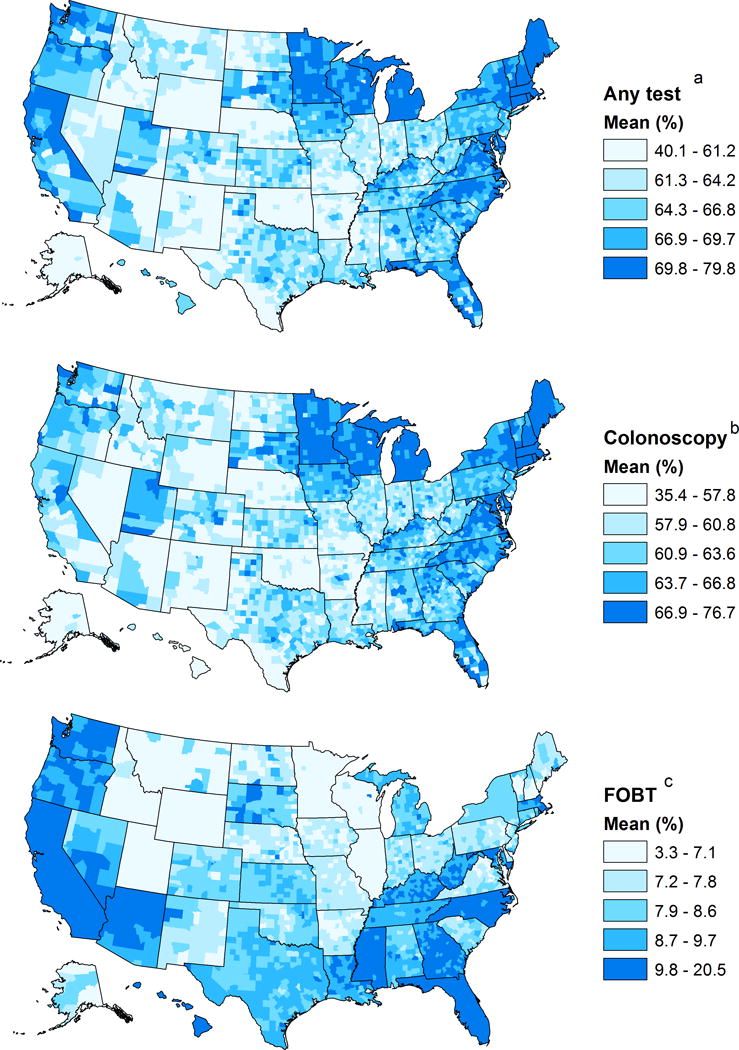
Model-based county estimated prevalence (%) maps for being current with 3 CRC test types. The maps show estimated prevalence for (A) any CRC test type, (B) Colonoscopy within 10 years, and (C) FOBT within the past year. Any CRC test type (A) includes FOBT within the past year; sigmoidoscopy within 5 years with FOBT within 3 years; or colonoscopy within 10 years. The County prevalence shown on the right of each map describes the prevalence by quintiles, each associated with a different color scale. Notes: CRC, colorectal cancer; FOBT, Fecal occult blood test
Being current with any CRC test type
Model-based states estimated prevalence ranged from 58.92% in Wyoming to 75.03% in Massachusetts with a mean of 67.11% and a median of 67.47% (Table 2, Table 3). States with the highest prevalence (>70%) were in the Northeast (Massachusetts, Maine, Rhode Island, Connecticut, New Hampshire, and Vermont), Midwest (Michigan, Minnesota, Wisconsin), South (Delaware, Maryland, Washington DC, North Carolina), and West (Washington) (Table 3). States with the lowest prevalence (<62%) included Wyoming, Oklahoma, New Mexico, Alaska, Idaho and Arkansas (Table 3). The overall range (minimum to maximum) of the county estimates within the states was lowest in Connecticut (3.60%), indicating low variability among these counties, and highest in South Dakota (28.61%). with Texas, Alaska, North Dakota, and New Mexico, each having a range >20%, indicating a high variability.
SAE county prevalence estimates ranged from 40.11% in Alaska to 79.76% in Florida with a median of 65.53% (Tables 1 and 3, Fig. 1a). The lowest ranking counties (≤49.50%) were in Alaska, South Dakota, North Dakota, Texas, Idaho, Montana, and Arizona. Counties with estimated prevalence of 75% or more (n=31) were in the Northeast (Rhode Island, Massachusetts, Maine, and Connecticut), Midwest (Michigan, Wisconsin), South (Florida, Virginia, Maryland and Delaware), and the West (California, Washington) (Table 3, Fig. 1a). Additional details about the 20 counties with the highest and lowest percentages and the predicted standard errors of county estimates for being current with any CRC test type are presented in the supplementary data in Table S1 and Figure S1.
Being current with colonoscopy
Model-based state estimated prevalence ranged from 56.16% in Oklahoma to 72.02% in Maine with a mean of 64.00% and a median of 64.26% (Table 2). States with the highest prevalence (≥70%) were in the Northeast (Maine, Rhode Island, Massachusetts, Connecticut, and New Hampshire) and Delaware. States with the lowest ranking percentages (≤60%) included Oklahoma, Wyoming, New Mexico, Hawaii, Nevada, Alaska, Arkansas, Idaho, Texas, Nebraska, Mississippi, and California.
County prevalence estimates ranged from 35.36% in Alaska to 76.73% in Rhode Island with a mean of 62.01% and a median of 62.23% (Table 1, Fig. 1b). The 20 lowest ranking counties (≤44.61) were in Alaska, South Dakota, North Dakota, New Mexico, Texas, Idaho, Arizona, Montana, and Nebraska, Counties with the highest prevalence (≥73%, n=20) were in the Northeast (Rhode Island, Maine, Massachusetts, and Connecticut), Midwest (Michigan, Wisconsin, and Minnesota), and South (Florida, and Virginia).
Being current with FOBT
Model-based state estimated prevalence ranged from 3.73% in Utah to 16.83% in California with a mean of 8.49% and a median of 8.21% (Table 2). States with the highest prevalence (≥11%) included California, Hawaii, Florida and Nevada. The lowest ranking states (<6%) included Utah, Wyoming, Alaska, and Minnesota (Table 3).
County prevalence estimates ranged from 3.28% in Utah to 20.54% in California (Table 3, Fig. 1c), with a mean of 8.47% and a median of 8.19% (Table 1). Most counties with the lowest prevalence (≤5.19%, n=34) were in Utah, Wyoming, and Alaska. Counties with the highest prevalence (≥16%, n=30) were in California and Hawaii.
Discussion
We present results from a small area estimation model, which generated 3142 county estimates nationally for being current with any CRC test type, with colonoscopy, and with FOBT. In addition, we presented model-based county estimates for being current with any CRC test type by race/ethnicity groups and by mean estimates of counties with ≥500 respondents. Our nationwide, race/ethnicity-specific modeled estimates were consistent with BRFSS direct estimates. These estimates can potentially provide useful information at the local and state levels and can inform decisions about resource allocation to geographically targeted prevention and control plans to increase colorectal cancer screening. Our study shows, that in 2014, most states and counties in the U.S. were still far from the “80% by 2018 initiative” target.
We found substantial geographic variation in the estimated prevalence of current CRC screening across states and among counties within states. Consistent with a previous study (4), the highest ranking state estimates of being current with any CRC test type were in the Northeast. The lowest ranking state estimates were in various regions of the West, South, and Alaska, some of which were also observed previously for their low estimated percentages (4). Estimated percentages for FOBT were much lower, and varied among states.
Differences in estimated percentages between the highest and lowest ranking counties were approximately 40% for any CRC test type or for colonoscopy. The highest-ranking counties were most often in the Northeast with 70% or more of their counties being current with CRC screening. The lowest ranking counties were in the rural areas of Alaska, North Dakota, South Dakota, New Mexico, and Montana, where a large proportion of their populations were American Indians (22), had income below the poverty rate or had low education attainment. Other low ranking counties were in Texas, some of which had a large Hispanic or Latino population (23), had low income or low education. The above-mentioned states had the largest county variations in current CRC screening, which might indicate disparities in access to care between rural and metropolitan areas. Moreover, our model-based county estimates by race/ethnicity suggest even larger disparities among Non-Hispanic blacks, Asians, American Indians or Alaska Natives, and Hispanics, for whom culturally appropriate interventions are needed. We were not able to compare estimates for these subpopulation groups with BRFSS estimates because BRFSS is a state-level survey and is not designed to assess county reliability for these populations solely. Comparing county-level data with BRFSS can be potentially biased because of oversampling or having counties without BRFSS direct-estimates. Nevertheless, the mean and median estimates were consistent.
Despite CRC screening recommendations published in 2008 and 2016 by the USPSTF emphasizing that no one strategy is advantageous over the other (19,24), a survey of primary care providers found that most recommended colonoscopy to their patients (25), consistent with our findings. The 2016 recommendations extended the choices in screening tests to align more closely with a patient’s preferences, with the goal to maximize the total number of people screened (24). The Community Preventive Services Task Force encourages providers to use reminder systems, small media, such as brochures, videos or newsletters, to increase awareness about the different tests available, and engage their patients in decision making about a strategy of their choice and availability of services to increase completion rates and follow-up over time (26).
Using small area estimates analysis can potentially highlight barriers to CRC screening due to geographic accessibility such as travel time and distance to health care facilities, and identify specific geographic locations with these barriers. Several investigators have highlighted the importance of distance to CRC screening facilities as a barrier to health care (27,28). Lack of access to health care in rural areas has been well documented and these areas may have higher rates of poverty along with fewer physicians (29). These studies suggest that increasing access to transportation services for populations such as American Indians, or other rural populations living in areas with little or no access to health care facilities, may be an approach to help increase CRC screening.
Disparities in CRC screening can result from language or cultural barriers. A national study comparing Latinos and non-Latino whites revealed that Latinos were less likely to have received CRC screening than were non-Latino whites. Moreover, Latinos responding in Spanish had lower odds of receiving CRC screening than Latinos responding in English (30,31). A prospective study from six US states and 2 metropolitan areas found associations between self-reported education and CRC incidence at the census tract level (17). This study also observed higher prevalence of adverse health behaviors, such as poor diet, smoking, physical inactivity and unhealthy weight in this population. It is possible that other barriers to screening, such as widespread beliefs and lack of knowledge among county population may contribute to low screening. A literature review of cancer screening interventions in non-clinical community settings was used to derive lessons-learned about effective interventions including cost-sharing elimination for CRC screening, person-to person outreach, one-on-one or small groups’ education, and mass media interventions (32).
To increase the availability of data about chronic disease in small geographical areas, the CDC Foundation, in collaboration with the Robert Wood Johnson Foundation, launched in 2015 the 500 Cities Project. The project identifies and analyzes city and census tract-level data using small-area estimation methods for 27 chronic disease measures, five unhealthy behaviors, and nine prevention practices including CRC testing. The information characterizes the health conditions of these areas’ population and helps public health practitioners develop effective and targeted public health interventions for vulnerable populations.
The potential to increase screening rates exists if health-care providers consistently offer multiple screening options and help patients identify the tests they are willing to complete (24). Kaiser has reported being able to achieve screening rates close to or above 80% with an organized approach to screening, starting with FOBT (33,34). Additionally, states with no high quality county estimates can use our multilevel small area estimation method to do their own county-level analysis by only including county random effects but no state random effect. All counties in the state can be represented after post-stratification with the census population data, including counties in BRFSS with no direct estimates. County estimates can help with decisions about resource allocation for interventions on the local level.
Limitations and strengths
Our study has some limitations. First, the results of our study are based on self-reported information, which might be subject to bias. Second, our predicted percentages of being current with CRC screening might be more appropriate for program planning than for program evaluation. Our model-based estimates might have over-estimated sparsely-populated areas and under-estimated densely-populated areas. Third, we were not able to assess the model’s external validity because no other comparable national survey, such as the National Health Interview Survey, had information on CRC screening in 2014.
Using the large BRFSS data set in multilevel small area estimation models with post-stratification that included geographic and demographic characteristics, and county-level poverty data, is a strength of our study. Our method allows integration with other data sources at the county level, such as census county data and the American Community Survey, and provides estimates for all the counties. Lastly, our analysis generated consistent estimates when aggregated to the levels of reliable direct BRFSS estimates.
Conclusions
Our analysis highlights the value of having nation-wide large surveys, such as BRFSS with linkage to Census county data, to provide information that can be used at the local and state levels and identify patterns of adherence to screening recommendations. We found that state-level information about CRC screening masks substantial within-state variability. Our study shows that most states and counties in the U.S are still far from the “80% by 2018” goal. Our estimates may provide opportunities for municipal, state and federal public agencies to better identify areas in need of coordinated and targeted health promotion efforts, including areas with limited direct BRFSS estimates.
Supplementary Material
Acknowledgments
We thank Trevor Thompson for his help with the coding of being current with colorectal cancer screening.
Financial Disclosure; No financial support for this study
Note: The work was done in a government institution. No funds or grants were involved.
Footnotes
Conflict of interest: No potential conflicts of interest exist
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or the Economic Research Service, U.S. Department of Agriculture
References
- 1.U.S. Cancer Statistics Working Group. United States Cancer Statistics: 1999-2014 Incidence and Mortality Web-based Report. Atlanta (GA): U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; 2017. [Google Scholar]
- 2.Jemal A, Siegel R, Desantis C, Sauer AG. Inequalities in premature death from colorectal cancer by state. J Clin Oncol. 2015;33(8):829–35. doi: 10.1200/JCO.2014.58.7519. [DOI] [PubMed] [Google Scholar]
- 3.Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010-2020. JNCI J Natl Cancer Inst. 2011;103:117–28. doi: 10.1093/jnci/djq495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Vital signs: colorectal cancer screening test use―United States, 2012. MMWR Morb Mortal Wkly Rep. 2013;62(44):881–8. [PMC free article] [PubMed] [Google Scholar]
- 5.Simon S. Organizations commit to goal of 80% colon cancer testing rate by 2018. American Cancer Society; website. http//www.cancer.org/cancer/news/organizations-commit-to-goal-of-80-percent-coloncancer-testing-rate-by-2018. Published March17, 2014. [Google Scholar]
- 6.Organizations working together to advance colorectal cancer control efforts. National Colorectal Cancer Roundtable; Available at: http://nccrt.org/tools/80-percent-by-2018. [Google Scholar]
- 7.American Cancer Society. Colorectal cancer facts and figures 2014-2016. Atlanta: American Cancer Society; 2014. [Google Scholar]
- 8.Steele BC, Rim SH, Joseph DA, King JB, Seeff LC. Colorectal cancer incidence and screening-United states, 2008 and 2010. MMWR Morb Mortal Wkly Rep. 2013 Nov 22;62(03):53–60. [PubMed] [Google Scholar]
- 9.Shapiro JA, Klabunde CN, Thompson TD, Nadel MR, Seeff LC. Patterns of colorectal cancer test use, including CT colonography, in the 2010 national Health Interview Survey. Cancer Epidemiol Biomarkers Prev. 2012;21(6):895–904. doi: 10.1158/1055-9965.EPI-12-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Office of Surveillance, Epidemiology and Laboratory Services. Centers for Disease Control and Prevention. Behavioral Risk Factor Surveillance System: 2014 Summary Data Quality Report. Atlanta, GA: Centers for Disease Control and Prevention; 2014. [Google Scholar]
- 11.Colditz GA, McGowen LD, James AS, Bohike K, Goodman AS. Screening for colorectal cancer: using data to set prevention priorities. Cancer Causes and Control. 2014;25(1):93–8. doi: 10.1007/s10552-013-0311-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xingyou Zhang, Holt JB, Lu H, et al. Multilevel regression and post stratification for small-area estimation population health outcomes: A case study of chronic obstructive pulmonary disease prevalence using the Behavioral Risk Factor Surveillance System. Am J Epidemiol. 2014;179(8):1025–33. doi: 10.1093/aje/kwu018. [DOI] [PubMed] [Google Scholar]
- 13.Xingyou Zhang, Holt JB, Yun S, Lu H, Greenlund KJ, Croft JB. Validation of multilevel regression and poststratification methodology for small area estimation of health indicators from the Behavioral Risk Factor Surveillance System. Am J Epidemiol. 2015;182:127–37. doi: 10.1093/aje/kwv002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.United States Census Bureau. Population Division. Annual Estimates of the Resident Population by Sex, Race, and Hispanic Origin for the United States, States, and Counties: April 1, 2010 to July 1, 2014 [Google Scholar]
- 15.Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System, Bridged Race Categories. Vintage 2014 Bridged-Race Postcensal Population Estimates. Available at: http://www.cdc.gov/nchs/nvss/bridged_race/data_documentation.htm.
- 16.Boscoe FP, Henry KA, Sherman RL, Johnson CJ. The relationship between cancer incidence, stage and povery in the United States. Int J Cancer. 2016;139(3):607–12. doi: 10.1002/ijc.30087. [DOI] [PubMed] [Google Scholar]
- 17.Doubeni CA, Major JM, Laiyemo AO, Schootman M, Zauber AG, Hollenbeck AR, et al. Contribution of behavioral risk factors and obesity to socioeconomic differences in colorectal cancer incidence. JNCI J Natl Cancer Inst. 2012;104(18):1353–62. doi: 10.1093/jnci/djs346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.United States Census Bureau. American Community survey 2010-2014 state level estimation details.
- 19.Screening for colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149(9):627–37. doi: 10.7326/0003-4819-149-9-200811040-00243. [DOI] [PubMed] [Google Scholar]
- 20.Ryan, Louise Spatial Epidemiology: Some Pitfalls and Opportunities. Epidemiology. 2009;20(2):242–4. doi: 10.1097/EDE.0b013e318198a5fb. [DOI] [PubMed] [Google Scholar]
- 21.Condon P. Mixture of spatial and unstructured effects for spatially discontinuous health outcomes. Computational Statistics & data analysis. 2007;51:3197–212. [Google Scholar]
- 22.Norris T, Vines PL, Hoeffel EM. 2010 Census briefs. United States Census Bureau; 2012. The American Indian and Alaska Native Population: 2010. Available at: http://www.census.gov/prod/cen2010/briefs/c2010br-10.pdf. [Google Scholar]
- 23.Norris T, Vines PL, Hoeffel EM. 2010 Census briefs. United States Census Bureau; The Hispanic Population: 2010. 201. Available at: http://www.census.gov/prod/cen2010/briefs/c2010br-04.pdf. [Google Scholar]
- 24.Screening for colorectal cancer: U.S. Preventive Services Task Force recommendation statement. JAMA. 2016;315(23):2564–75. doi: 10.1001/jama.2016.5989. [DOI] [PubMed] [Google Scholar]
- 25.Zapka J, Klabunde CN, Taplin S, Yuan G, Ransohoff D, Kobrin S. Screening colonoscopy in the US: attitudes and practices of primary care physicians. J Gen Intern Med. 2012;27(9):1150–8. doi: 10.1007/s11606-012-2051-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.The Community Guide. Cancer screening: multicomponent interventions–colorectal cancer. 2016 Available at: http://www.thecommunityguide.org/cancer.
- 27.Wheeler SB, Kuo T, Goyal RK, Myer A, Lich K, Gillen EM, et al. Regional variation in colorectal cancer testing and geographic availability in a publicly insured population. Health & Place. 2014;29:114–23. doi: 10.1016/j.healthplace.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sherman RL, Henry KA, Tannenbaum SL, Feaster DJ, Kobetz E, Lee DJ. Applying Spatial Analysis Tools in Public Health: An Example Using SaTScan to Detect Geographic Targets for Colorectal Cancer Screening Interventions. Prev Chronic Dis. 2014;11:E41. doi: 10.5888/pcd11.130264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Caldwell JT, Ford CL, Wallace SP, Wang MC, Takahashi LM. Intersection of living in rural versus urban area and race/ethnicity in explaining access to health care in the United States. Am J Public Health. 2016;106(8):1463–9. doi: 10.2105/AJPH.2016.303212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Diaz JA, Roberts MB, Goldman RE, Weitzen S, Eaton CB. Effect of language on colorectal cancer screening among Latinos and non-Latinos. Cancer Epidemiol Biomarkers Prev. 2008;17:2169–73. doi: 10.1158/1055-9965.EPI-07-2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Diaz JA, Roberts MB, Clarke JG, Simmons EM, Goldman RE, Rakowski W. Colorectal Cancer Screening: Language is a Greater Barrier for Latino Men than Latino Women. J Immigrant Minority Health. 2013;15:472–475. doi: 10.1007/s10903-012-9667-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pasick RJ, Hiatt RA, Paskett ED. Lessons learned from community-based cancer screening intervention research. Cancer. 2004;(5 suppl):1146–64. doi: 10.1002/cncr.20508. [DOI] [PubMed] [Google Scholar]
- 33.Levin TR, Jamieson L, Burley DA, Reyes J, Oehrli M, Caldwell C. Organized colorectal cancer screening in integrated health care systems. Epidemiol Rev. 2011;33:101–10. doi: 10.1093/epirev/mxr007. [DOI] [PubMed] [Google Scholar]
- 34.Lee JK, Levin TR, Corley DA. The road ahead: what if gastroenterologists were accountable for preventing colorectal cancer? Clin Gastroenterol Hepatol. 2013;11:204–7. doi: 10.1016/j.cgh.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


