SUMMARY
The mechanisms underpinning integration of instructions that program naïve CD8+ T cells for effector and/or memory differentiation are not well understood. Herein, we demonstrate that IL-12, the critical determinant that instructs antigen-stimulated naïve CD8+ (OT-I) T cells for T-bet dependent heritable type I effector maturation, enhances and sustains Ag/B7.1 induced mTOR activity via PI3K and STAT4 pathways. Blocking mTOR activity by rapamycin reverses IL-12 induced heritable type I effector functions due to loss of persistent T-bet expression. Remarkably, rapamycin treatment of IL-12 conditioned OT-I cells promotes persistent Eomesodermin expression and produces memory-precursors that demonstrate enhanced sustenance and antigen-recall responses upon adoptive transfer. Surprisingly, the memory-precursors show greater tumor efficacy than IL-12 conditioned effector OT-I cells. These results identify mTOR as the “central” regulator of transcriptional programs that determine effector and/or memory cell-fates in CD8+ T cells. Targeting mTOR activity offers new opportunities to regulate CD8+ T cell mediated immunity.
Key words/phrases: CD8+ T cell, instructional programming, mTOR, T-bet, Eomesodermin, IL-12, IFN-γ, heritable effector maturation, antigen recall, memory and tumor immunity
INTRODUCTION
The nature and intensity of instructions received by a naïve CD8+ T cell orchestrates gene programs that guide their differentiation into various functional subsets (Iezzi et al., 1998; Joshi et al., 2007). The presence of cytokines during antigen stimulation is instrumental in regulating the transcriptional program of CD8+ T cells for effector and memory functions (Curtsinger et al., 2003; Xiao et al., 2009). However, the molecular mechanisms by which integration of cytokine generated signals determine antigen and co-stimulation induced T cell responses are not well defined. Based on the type of cytokine present during antigen stimulation, naive CD8+ T cells could give rise to either type I (IFN-γ), type II (IL-4), type 17 (IL-17) or regulatory (TGF-β, IL-10) functional outcomes, but the instructional requirements and the transcriptional regulators for CD8+ T cells are poorly defined and the understanding is based on information generated by the use of CD4+ T cells (Bettelli et al., 2007; Zhou et al., 2009). Besides the ability of effector CD8+ T cells to protect against variety of challenges, its ability to produce memory functions is crucial for host immunity. Emerging insights into CD8+ T cell memory generation demonstrates the variety of pathways that can give rise to memory cells (Kaech and Wherry, 2007; Lefrancois and Marzo, 2006). Moreover, the cell type and the pathway used for the emergent memory cells may impart functional diversity to the memory CD8+ T cells, thus emphasizing the role played by instructions early during antigen stimulation for not only effector differentiation but also for memory functions.
Several transcription factors have been shown to coordinate and regulate the balance between long-lived memory and terminally differentiated effector CD8+ T cells (Intlekofer et al., 2005; Joshi et al., 2007; Welsh, 2009). The transcription factor T-bet (Tbx21) is the master regulator of type I effector differentiation, whose expression is considerably enhanced and sustained in the presence of IL-12 (Joshi et al., 2007; Szabo et al., 2000). Recent evidence suggests that inflammation induced T-bet can control effector and memory fate decisions in CD8+ T cells, because increased T-bet expression promotes short-lived effector cells with a KLRG1hi and IL-7Rlo phenotype, whereas low T-bet expression promotes long-lived memory cells (Joshi et al., 2007). Eomesodermin (Eomes), another T-box containing transcription factor, whose expression increases from the effector to memory phases of an immune response, is proposed to promote memory formation (Intlekofer et al., 2005). Moreover, IL-12 induces T-bet, but inhibits Eomes expression to favor effector versus memory generation (Takemoto et al., 2006), suggesting the importance of understanding cell intrinsic factors that regulate T-bet and Eomes expression which may enable achieving desirable CD8+ T cell functional outcomes.
The energy sensitive kinase mammalian target of rapamycin (mTOR), has the ability to sense cellular metabolic status (ATP:AMP), extracellular nutrient availability (glucose, amino-acids) and growth factor/cytokines levels, which endows it with the unique capacity to control key cellular processes like ribosomal genesis, autophagy, cell growth and proliferation and determine cell fate (Dennis et al., 2001; Wullschleger et al., 2006). The immunosuppressive drug rapamycin (specific inhibitor of mTOR) is widely used to restrict allograft rejection reactions (Saunders et al., 2001), and most mechanistic studies to date have been focused on understanding the action of rapamycin on CD4+ T cell responses. These studies have demonstrated that mTOR inhibition by rapamycin induces CD4+ T cell anergy and/or differentiation into (FoxP3+) regulatory T cells (Kang et al., 2008; Zheng et al., 2007). Moreover, a recent report has implicated the requirement of mTOR kinase signaling in regulating effector versus regulatory cell lineage commitment in CD4+ T cells (Delgoffe et al., 2009). Interestingly, recent reports have demonstrated the role of mTOR in regulating CD8+ T cell trafficking (Sinclair et al., 2008) and memory differentiation (Araki et al., 2009; Pearce et al., 2009), but the molecular mechanisms by which mTOR regulates CD8+ T cell differentiation or trafficking remains uncharacterized.
In this study, we have identified the role of mTOR in instructional programming of naïve CD8+ T cells for effector and/or memory fate by regulating expression of T-bet and Eomes. Inhibition of mTOR activity blocks persistent Tbet expression and promotes memory-precursor generation that shows greater tumor efficacy than type I effector CD8+ T cells.
RESULTS
Instructions that program naïve CD8+ T cell for heritable type I effector differentiation augment mTOR activity
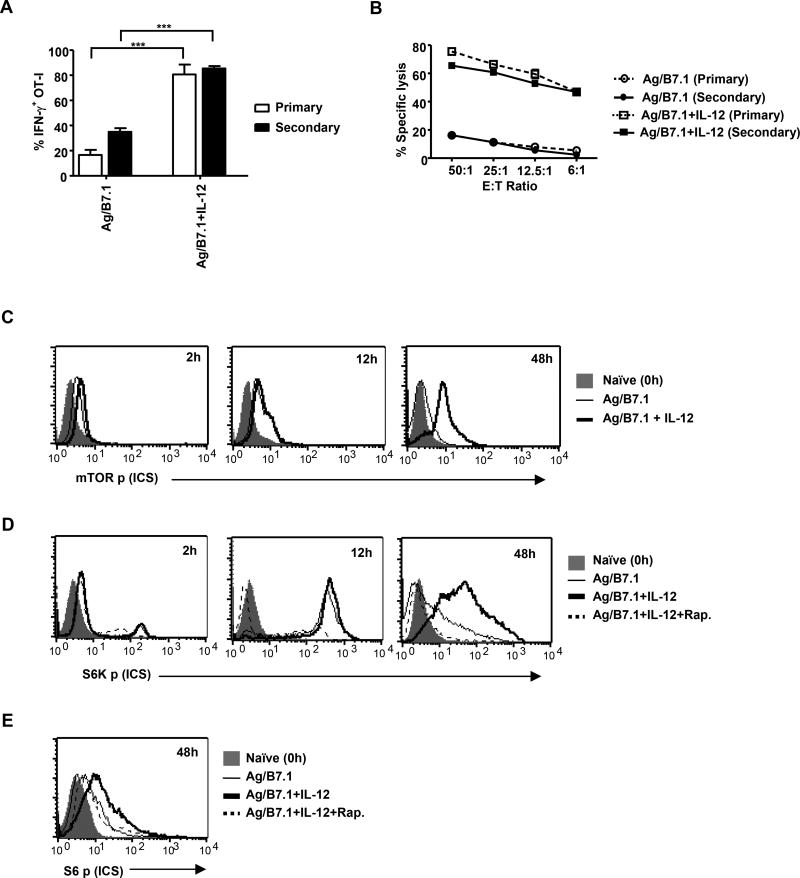
To characterize mechanisms underpinning instructional (signal 1, 2 and 3 – Ag, B7.1 and IL-12, respectively) programming of naïve CD8+ T cells for heritable type I effector functions, we initiated our studies to confirm the deterministic role of IL-12 in imparting heritable type I effector maturation in OT-I cells stimulated with adherent BOK cells (MEC.B7.SigOVA: expressing H-2Kb, OVAp (SIINFEKL) and B7.1). As anticipated, addition of IL-12 resulted in robust IFN-γ production and CTL activity in OT-I cells at 72h (Figure1A and 1B; primary). Furthermore, when the primary effector OT-I cells (72h) were rested with IL-7 for additional 72h (low; 12% IFN-γ detected at 144h) and re-stimulated with Ag/B7.1 alone (see methods), only the IL-12 conditioned (primary) OT-I cells re-induced IFN-γ and CTL activity (Figure1A and 1B; secondary). Thus, demonstrating the deterministic role of IL-12 in CD8+ T cell effector maturation with heritable type I functions.
Figure 1. Instructions that program naïve CD8+ T cell for heritable type I effector maturation enhances and sustains mTOR activity.
(A–B) OT-I cells stimulated with BOK (+/−) IL-12 were evaluated for (A); IFN-γ by ICS and (B); cytolytic activity (Primary–72h post-stimulation, Secondary–24h post-secondary stimulation); ***p<0.0002. (C–E) OT-I cells stimulated with antigen (Ag) (SIINFEKL, 10nM) plus B7.1 (100ug/ml) (Ag/B7.1) (+/−) IL-12 (2ng/ml) were evaluated by ICS at the indicated time points for (C); phosphorylated mTOR (D); phosphorylated S6K and (E); phosphorylated ribosomal S6. For mTOR inhibition, rapamycin (20ng/ml) was added 30 minutes prior to addition of antigen, cytokine. Data are representative of at least three independent experiments with identical outcomes. (Data are presented as mean +/− SEM)
Although, the energy sensitive kinase mTOR has been implicated as an integrator of various extracellular signals and internal energy levels for determination of cell fate (Hay and Sonenberg, 2004), the role for mTOR in integrating instructions that program naïve CD8+ T cells for type I effector differentiation is not reported. First, we tested the ability of Ag/B7.1 in the presence or absence of (+/−) IL-12 to activate mTOR in OT-I cells at various time-points after stimulation. The stimulation of naive OT-I cells by Ag/B7.1 induced mTOR phosphorylation (activation) by 2h, which was maximal at 12h and barely detectable by 48h (Figure 1C). Remarkably, IL-12 addition enhanced Ag/B7.1 induced mTOR phosphorylation at 2h, which was maintained at 48h post-stimulation (Figure 1C). Thus, demonstrating that although, Ag/B7.1 induces mTOR phosphorylation, the addition of IL-12; determinant for heritable type I effector maturation, enhances and sustains mTOR phosphorylation in OT-I cells. To verify that the instructionally induced mTOR phosphorylation also led to its kinase activity, we kinetically monitored the levels of p70 S6Kp (Thr 389), a direct target of mTOR kinase activity. Although, both Ag/B7.1 and Ag/B7.1 plus IL-12 induced similar levels of S6K phosphorylation at 12h (maximal), the presence of IL-12 was able to sustain the S6Kp up to 48h (Figure 1D), in correlation to mTOR phosphorylation (Figure 1C). Similarly, phosphorylation of S6 (Ser235/236), a downstream substrate of S6K was also enhanced and sustained in IL-12 conditioned OT-I cells (Figure 1E). The Ag/B7.1 +/− IL-12 induced S6K and S6 phosphorylation in OT-I cells was blocked by rapamycin (specific inhibitor of mTOR complex-1) (Figure 1D and 1E), thus confirming the ability of instructions to activate mTOR and its kinase activity in OT-I cells. In agreement, with the reported role for mTOR in regulating cell size (Schmelzle and Hall, 2000) and the expression of L-type amino acid transporter (CD98) (Cornish et al., 2006), the induction of blast transformation and CD98 expression in Ag/B7.1 stimulated OT-I cells was further augmented by IL-12 in a rapamycin sensitive manner (Figure S1A). These observations identify mTOR as a target of instructions that program CD8+ T cell responses and suggest a potential role for mTOR kinase in regulating IL-12 determined heritable type I differentiation of CD8+ T cells.
Instructions that program CD8+ T cells for effector responses enhance PI3K and STAT4 dependent mTOR activity
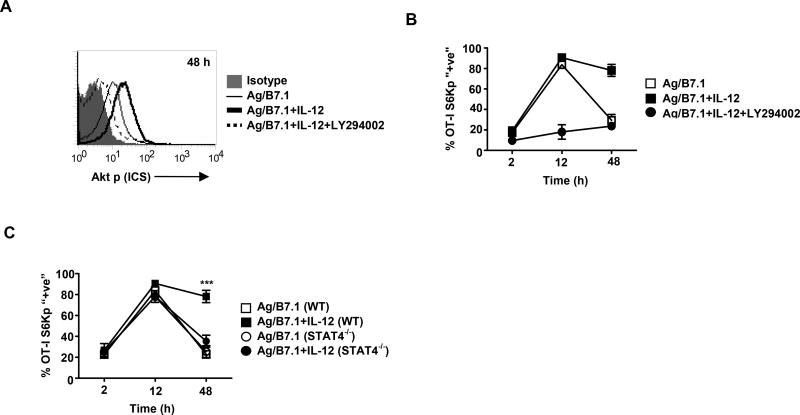
To determine the molecular pathways governing mTOR activity in CD8+ T cells, we explored whether the Ag, B7.1 and IL-12 induced PI3K-Akt pathway (Yoo et al., 2002) is required for mTOR signaling in CD8+ T cells. The OT-I cells stimulated with Ag/B7.1 +/− IL-12 were evaluated for Akt phosphorylation (Thr 308), as a functional measure of PI3K activity. Although, Ag/B7.1 in the presence or absence of IL-12 induced similar levels of Akt phosphorylation by 30 minutes (data not shown), the presence of IL-12 augmented Akt phosphorylation up to 48h, which was blocked by the PI3K inhibitor (LY294002) (Figure 2A), thereby confirming that IL-12 augments Ag/B7.1 induced PI3K activity in OT-I cells. Moreover, IL-12 augmented mTOR activity (S6K phosphorylation observed at 2, 12 and 48h) was blocked by PI3K inhibition (Figure 2B), demonstrating that Ag/B7.1 and IL-12 activated PI3K activity in antigen stimulated OT-I cells is required for induction of mTOR kinase activity.
Figure 2. IL-12 enhances antigen induced mTOR activity via PI3K and STAT4.
(A–B) OT-I cells stimulated with Ag/B7.1 (+/−) IL-12 and LY294002 (10µM) were evaluated by ICS for (A); phosphorylated Akt at 48h (B); phosphorylated S6K at the indicated time-points. (C) WT or STAT4−/− OT-I cells stimulated with Ag/B7.1 in the presence or absence of IL-12 were analyzed at the indicated time points for phosphorylated S6K; ***p<0.0001. Experiments shown are representative of three independent experiments with similar outcomes. (Data are presented as mean +/− SEM)
The ability of IL-12 to instruct CD8+ T cells for robust effector maturation requires STAT4 (Jacobson et al., 1995; Li et al., 2006). To determine whether IL-12 augmented mTOR activity in OT-I cells is STAT4 dependent, we tested the ability of WT or STAT4−/− OT-I cells to induce S6K phosphorylation upon stimulation with Ag/B7.1 +/− IL-12. In contrast to our observations with PI3K inhibition, the absence of STAT4 in OT-I cells did not affect IL-12 induced S6K phosphorylation at early time-points (2h & 12h), but failed to maintain the induced levels of S6K phosphorylation (48h) (Figure 2C). Thus indicating the differential roles for IL-12 induced PI3K and STAT4 in regulating mTOR activity in OT-I cells.
Sustained mTOR activity is essential for heritable type I effector differentiation of CD8+ T cells
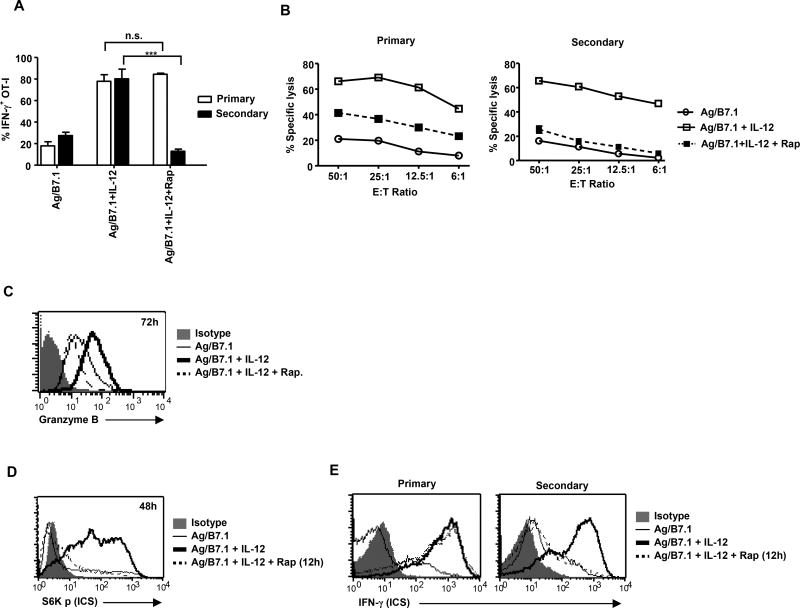
The fact that the presence of IL-12 during antigen stimulation augments mTOR activity and is deterministic for heritable type I effector maturation, we hypothesized that sustained mTOR kinase activity is required for IL-12 programmed heritable type I effector functions in OT-I cells. To test this notion, we stimulated naïve OT-I cells with BOK +/− IL-12 and rapamycin and effector functions were analyzed from the primary and secondary activated OT-I pool. Addition of rapamycin to IL-12 conditioned OT-I cells did not affect IFN-γ production from primary activated OT-I cells, but reduced their CTL activity associated with decreased Granzyme B expression (Figure 3A, 3B and 3C; primary). In contrast, when the rapamycin treated IL-12 conditioned OT-I cells were evaluated for heritable type I effector functions, we noted a complete reversal of IL-12 conditioned effector functions from the secondary activated pool (IFN-γ production and CTL activity) (Figure 3A and 3B; secondary), This blockade of IL-12 conditioned type I effector functions was not due to rapamycin induced inhibition of cell proliferation and/or protein synthesis, as re-activation of these cells in the presence of IL-12 resulted in considerable IFN-γ production (data not shown). These results indicate that IL-12 induced commitment of naïve CD8+ T cells for heritable type I effector functions requires mTOR activity. In addition, we observed a block in IL-12 induced IFN-γ production and CTL activity upon rapamycin treatment at 144h post-primary stimulation (Figure S2A and B). These results further confirm that rapamycin treatment blocks type I effector functions and the loss of effector functions observed in the secondary activated pool (168h) (Figure 3A and 3B; secondary) is solely due to the inability of these cells to re-induce IFN-γ production and not due to a refractory state of the rapamycin treated cells.
Figure 3. Sustained mTOR activity is essential for heritable type I effector differentiation of CD8+ T cells.
(A–C) OT-I cells stimulated with Ag/B7.1 (+/−) IL-12 and rapamycin were evaluated at the primary and secondary phase for (A); IFN-γ by ICS; ***p<0.0002, n.s.–not significant (B); cytolytic activity (C); granzyme B expression at 72h by ICS. (D–E) OT-I cells were stimulated with Ag/B7.1 (+/−) IL-12 and rapamycin was added 12 hr post-stimulation to evaluate cells for (D); S6K phosphorylation at 48h (E); IFN-γ production at the primary and secondary phase. Experiments shown are representative of at least three (A, B) and two (CE) independent experiments with similar outcomes. (Data are presented as mean +/− SEM)
To determine whether sustained mTOR activity achieved by IL-12 treatment was required for heritable type I effector maturation, we blocked persistence of IL-12 induced mTOR activity by adding rapamycin at 12h (mTOR activation peaks at 12h; Fig. 1C, D) (Figure 3D) after Ag/B7.1 stimulation and evaluated their ability to produce IFN-γ production from the primary and secondary activated OT-I pool. The addition of rapamycin at 12h blocked IL-12 induced heritable effector functions, just as observed with the treatment at 0h (Figure 3E; primary activated versus secondary activated responses). Thus, demonstrating that mTOR activity induced during the first 12h is not sufficient to program CD8+ T cells for heritable type I effector maturation and emphasizes the requirement of IL-12 induced persistence of mTOR activity (12h or later) to program heritable type I effector functions in CD8+ T cells.
The IL-12 augmented mTOR activity regulates T-bet expression for effector maturation of CD8+ T cells
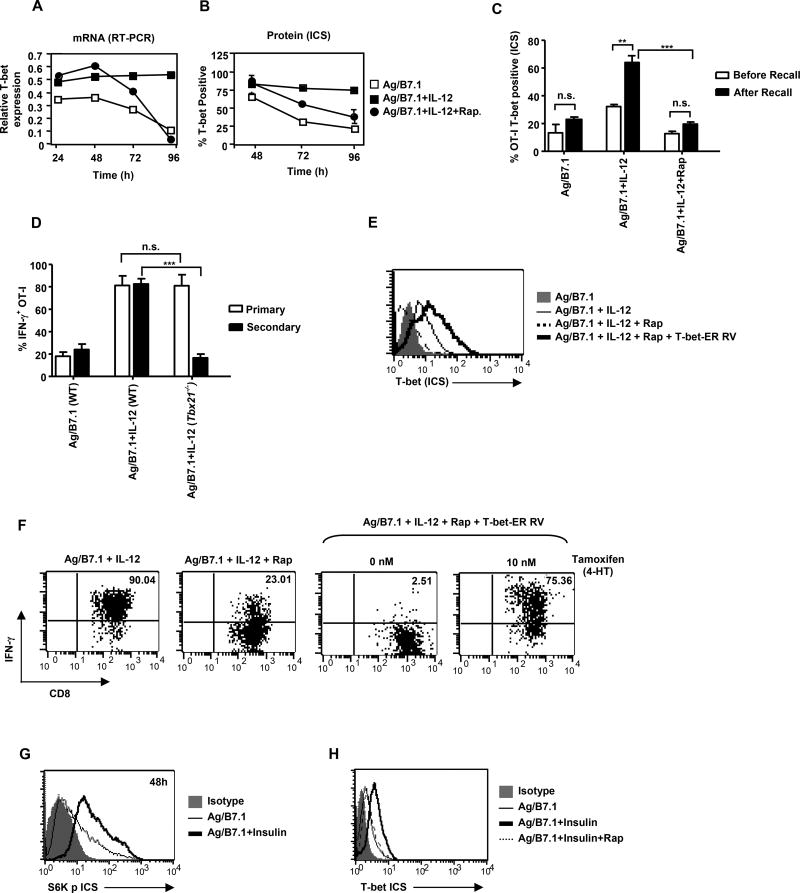
Since, the sustained expression of the master regulator T-bet is necessary and sufficient for imprinting type I effector cell fate (Matsuda et al., 2007) and mTOR inhibition reversed IL-12 imprinted heritable type I effector maturation in OT-I cells (Figure 3); we next sought to determine whether rapamycin treatment affects T-bet expression in OT-I cells by performing kinetic analysis of T-bet mRNA expression (Figure 4A). The addition of IL-12 enhanced and sustained Ag/B7.1 induced T-bet expression at all time-points tested (24–96h). Remarkably, mTOR inhibition did not affect Ag/B7.1 plus IL-12 induced early T-bet expression (24–48h), but blocked IL-12 induced sustained T-bet mRNA expression (barely detectable by 96h), and correspondingly the OT-I cells lost T-bet protein expression (Figure 4B). Moreover, inhibition of mTOR activity at 12h was also able to achieve loss in T-bet expression (Figure S3A), similar to the observed loss in heritable type I effector maturation (Figure 3E). Thus, demonstrating that IL-12 augmented (enhanced/sustained) mTOR activity is required for sustained T-bet expression in CD8+ T cells.
Figure 4. IL-12 enhanced mTOR phosphorylation is essential for T-bet determined type I effector maturation of CD8+ T cells.
(A–C) OT-I cells stimulated with Ag/B7.1 (+/−) IL-12 and rapamycin were evaluated for (A); mRNA for T-bet at the indicated time points by RT-PCR (B); Tbet protein expression at the indicated time-points by ICS (C); T-bet protein expression by ICS before and after antigen recall; **p<0.0035, ***p<0.0005. (D) WT and Tbx21−/− OT-I cells were stimulated with Ag/B7.1 (+/−) IL-12 and evaluated for IFN-γ production at the primary and secondary phase; ***p<0.0001. (E–F) OT-I cells stimulated with Ag/B7.1 (+/−) IL-12 and rapamycin were transduced with T-bet-ER retroviral vector (+/−) 4-HT (10nM) and evaluated by ICS for (E); T-bet protein expression by ICS (F); IFN-γ at secondary phase (168h). (G–H) OT-I cells stimulated with Ag/B7.1 (+/−) insulin (1 U/ml) and rapamycin were evaluated by ICS for (G); S6K phosphorylation at 48h (H); T-bet expression at 72h. Experiments shown are representative of at least three (A, B, D, E & F) and two (C, G and H) independent experiments with similar outcomes. (Data are presented as mean +/− SEM).
To demonstrate that rapamycin mediated blockade of IFN-γ production during antigen-recall was due to their inability to re-induce T-bet expression, we rendered IL-12 conditioned OT-I cells (72h-type I effector cells) quiescent by IL-7 treatment for 72h (144h) and evaluated their T-bet expression before (144h) and after (168h) antigen-recall. As shown in Figure 4C, moderate levels of T-bet expression were detected in OT-I cells conditioned primarily with Ag/B7.1 plus IL-12 at 144h, which was blocked upon rapamycin treatment. Notably, upon antigen-recall, the IL-12 conditioned OT-I cells re-induced significantly high levels of T-bet protein, which was sensitive to rapamycin treatment (Figure 4C). These observations demonstrate that rapamycin treatment blocks persistent T-bet expression, which may result in the block of IL-12 mediated heritable type I effector maturation. This notion is supported by the fact that IL-12 conditioning of Tbx21−/− OT-I cells also fail to generate heritable type I effector functions (Figure 4D; secondary), although their ability to produce IFN-γ in the primary phase is not affected (Figure 4D; primary). These observations are in agreement with rapamycin treated IL-12 conditioned OT-I cells (Figure 3A) and lends further support to our argument that the loss of persistent T-bet expression upon mTOR inhibition blocks IL-12 conditioned heritable type I effector differentiation in CD8+ T cells.
To directly confirm that the loss of T-bet expression upon rapamycin treatment led to loss of heritable type I effector functions, we induced ectopic expression of T-bet in rapamycin treated IL-12 conditioned OT-I cells and evaluated their ability to re-induce IFN-γ production from the secondary activated OT-I pool. The retroviral vector, T-bet-ER (T-bet-ER RV) was employed wherein the expression of T-bet is regulated by tamoxifen (4-HT) (Matsuda et al., 2007). Indeed, addition of tamoxifen (Tm, 10nM) to T-bet-ER transduced OT-I cells led to a significant increase in T-bet expression (Figure 4E) and restored IFN-γ production in rapamycin treated IL-12 conditioned OT-I cells (Figure 4F). Thus, demonstrating that IL-12 induced persistent mTOR phosphorylation is essential for sustained T-bet expression and T-bet dependent type I effector commitment of CD8+ T cells.
The metabolic hormone insulin acts via insulin receptor substrate (IRS) to activate mTOR kinase (Harris and Lawrence, 2003), whereas 2-deoxyglucose (2-DG) a glycolytic inhibitor, leads to a blockade of mTOR activity (Dennis et al., 2001). Therefore, we employed insulin and 2-DG to metabolically regulate mTOR activity and test whether they could impact T-bet expression in OT-I cells. Indeed, insulin addition to Ag/B7.1 stimulated OT-I cells enhanced mTOR activity (S6Kp) and mTOR dependent increase in T-bet expression (Figure 4G and 4H), whereas 2-DG addition to Ag/B7.1/IL-12 stimulated OT-I cells led to loss of mTOR activity and T-bet expression (Figure S4A and 4B). These results identify mTOR as a critical integrator of instructions to regulate T-bet expression in CD8+ T cells.
Differential requirement of mTOR kinase in CD4+ and CD8+ T cells
Since, treatment of CD4+ T cells with rapamycin induces anergy and/or deviation to the Foxp3 expressing T regulatory cells (Delgoffe et al., 2009; Kang et al., 2008; Zheng et al., 2007), it can be envisaged that inhibition of Ag/B7.1 and IL-12 induced mTOR activity interferes with CD8+ T cell type I effector differentiation, due to a block in activation, proliferation and/or causes deviation to different effector subtypes. In agreement with the published observations in CD4+ T cells, our results demonstrate that rapamycin treatment significantly reduced activation (CD44 expression), proliferation (CFSE dilution) and cell recovery of CD4+ T cells (OT-II) (Figure S5A and 5B). However, rapamycin treatment did not affect CD8+ T cell (OT-I) early (CD69, 12h; and data not shown) and late activation (CD44), and only marginally affected proliferation (CFSE) and cell recovery (Figure S5C and 5D). Moreover, in contrast to the reported expression of FoxP3 in CD4+ T cells, the rapamycin treated OT-I cells failed to persistently express FoxP3, which is required for imparting T cells with regulatory function (Figure S6A). Furthermore, the loss of T-bet upon mTOR inhibition did not induce deviation into the type-2 or type-17 subset (Figure S6B & 6C). These observations were also confirmed at varying doses of rapamycin (20ng/ml–2ug/ml). At higher doses, rapamycin efficiently blocked mTOR activity in OT-I cells (S6Kp and S6p), but unlike CD4+ T cells it failed to block activation, proliferation or deviation into Treg subset (data not shown). These results indicate that rapamycin has different effects on CD4+ and CD8+ T cells and its ability to block IL-12 induced heritable type I CD8+ effector differentiation is not due to induction of anergy or deviation to other effector sub-types.
Rapamycin mediated loss of T-bet induces persistent Eomes expression and produces memory-precursor CD8+ T cells
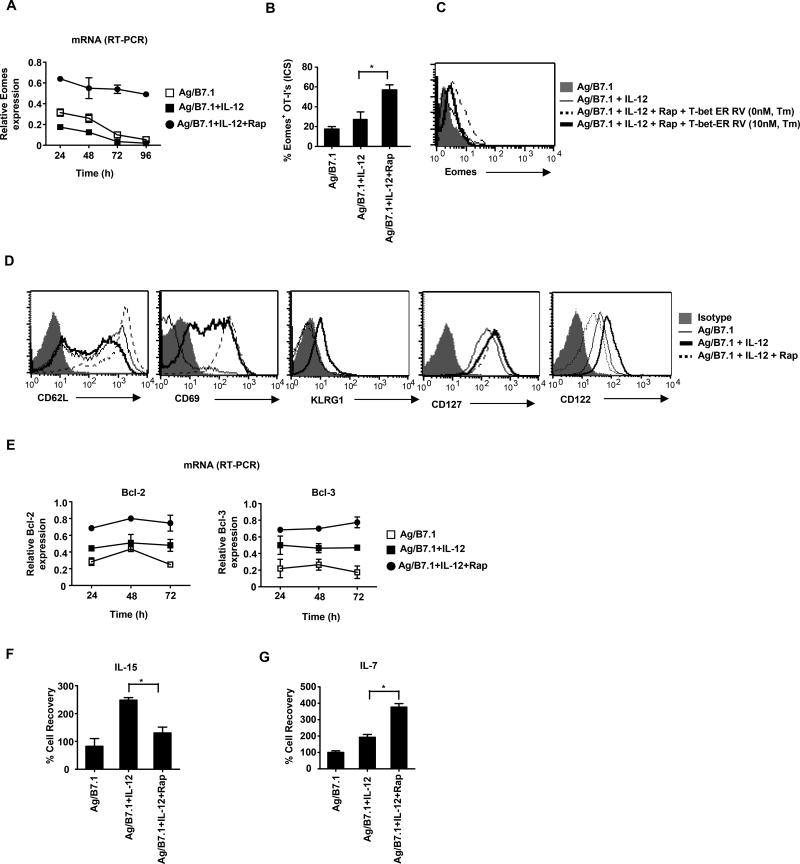
Since, rapamycin treatment blocked type I effector differentiation and failed to induce anergy or expression of other master transcriptional regulators, we next sought to characterize the fate of rapamycin treated IL-12 conditioned OT-I cells. The closely related transcription factors T-bet and Eomes are inversely regulated in effector and memory CD8+ T cells (Takemoto et al., 2006). To determine whether mTOR inhibition, which curtailed T-bet expression, led to induction of Eomes, we systematically analyzed Eomes mRNA expression in OTI cells. We observed modest levels of Eomes expression in naive OT-I cells (data not shown), which was enhanced when reacted with Ag/B7.1 and reduced upon IL-12 addition (Figure 5A). Surprisingly, addition of rapamycin to Ag/B7.1 plus IL-12 conditioned OT-I cells significantly enhanced Eomes mRNA levels, which was maintained at all time-points tested (24–96h) (Figure 5A). The increase in Eomes mRNA was confirmed at the protein level, as rapamycin treated IL-12 conditioned OT-I cells produced significant increases in Eomes protein (Figure 5B). It is noteworthy, that we consistently observe marginal increases (non-significant) in Eomes protein expression without mRNA induction in Ag/B7.1 plus IL-12 conditioned OT-I cells (Figure 5A, B).
Figure 5. Inhibition of mTOR promotes persistent Eomes expression and phenotypic markers of memory in CD8+ T cells.
(A–B) OT-I cells stimulated with Ag/B7.1 (+/−) IL-12 and rapamycin were evaluated for (A); mRNA for Eomes at the indicated time points by RT-PCR (B); Eomes protein expression at 72h by ICS; *p<0.03. (C) OT-I cells stimulated with Ag/B7.1 (+/−) IL-12 and rapamycin were transduced with T-bet-ER retroviral vector (+/−) 4-HT (10nM) and evaluated for Eomes protein expression at 96h. (D); OT-I cells stimulated with Ag/B7.1 (+/−) IL-12 and rapamycin were evaluated for CD62L, CD69, KLRG1, CD127 and CD122 expression at 72h (E); Bcl-2 and Bcl-3 mRNA expression at the indicated time points (F–G); OT-I cells stimulated with Ag/B7.1 (+/−) IL-12 and rapamycin for 72h were washed twice and rested for an additional 72h in the presence of (F); IL-7 (10ng/ml); *p<0.02 (G); IL-15 (10ng/ml); *p<0.02 and % cell recovery was calculated at 144h;. Experiments shown are representative of three independent experiments with similar outcomes. (Data are presented as mean +/− SEM).
To test whether rapamycin mediated up-regulation of Eomes in OT-I cells is a direct consequence of mTOR inhibition or a consequence of its ability to inhibit sustained T-bet expression, we ectopically induced T-bet expression in rapamycin conditioned OT-I cells and analyzed for Eomes expression in the presence or absence of tamoxifen. Indeed, induction of T-bet in rapamycin treated OT-I cells decreased Eomes expression (Figure 5C). Furthermore, we consistently observe increased Eomes expression in Tbx21−/− OT-I cells treated with Ag/B7.1 and IL-12 (data not shown). Taken together, these results demonstrate that mTOR inhibition selectively switches the transcriptional program from sustained T-bet to Eomes expression in IL-12 conditioned OT-I cells. Since, some studies suggest that type I IFN’s (IFN-α/β), like IL-12 can also promote type I effector CD8+ T cell responses (Agarwal et al., 2009), we determined whether IFN-α could also regulate mTOR activity and T-bet expression in OT-I cells. As shown in Figure S7A and S7B, IFN-α was unable to enhance mTOR activity and T-bet expression in Ag/B7.1 stimulated OT-I cells; however, we observed increases in Eomes expression and IFN-γ production. (Figure S7C and data not shown). These results confirm that IL-12 has the unique ability to imprint type I effector maturation by promoting persistent mTOR and T-bet expression and that IFN-α may lack this activity due to its inability to promote persistent mTOR activity and mTOR dependent T-bet expression.
Since, Eomes expression has been shown to favor memory precursor CD8+ T cell generation (Intlekofer et al., 2005), we next sought to determine whether rapamycin induced switch in T-bet to Eomes expression as well as block in type I maturation resulted in their transition to memory-precursors. We performed phenotypic analysis of OT-I cells using markers associated with memory precursor CD8+ T cells, i.e. CD62L (lymph node homing) (Wherry et al., 2003), CD69 (lymph node retention) (Shiow et al., 2006), CD127 (IL-7Rα; essential for memory T cell maintenance) (Schluns et al., 2000), CD122 (IL-15Rβ and essential for memory CD8+ T cell homeostatic renewal), (Schluns et al., 2002), KLRG1 (inversely co-related with memory CD8+ T cell generation) (Joshi et al., 2007) and Bcl-2 (anti-apoptotic and increased expression in memory T cells) (Grayson et al., 2000). The IL-12 conditioned OT-I cells treated with rapamycin expressed significantly higher levels of CD62L and also demonstrated persistent CD69 expression in comparison to non-rapamycin conditioned cells (Figure 5D). The increases in CD62L and CD69 expression could imply that rapamycin treated OT-I cells will have greater capacity for lymph node homing and retention. Moreover, rapamycin treated cells had a higher frequency of KLRG1low cells compared to the non-treated controls, along with increased and sustained expression of pro-survival genes (Bcl-2 and Bcl-3) at all time points observed (Figure 5D and 5E). Thus, demonstrating that indeed rapamycin treatment promotes phenotype indicative of memory precursor CD8+ T cells. Surprisingly, rapamycin treatment decreased CD122 expression and they showed a defect in their ability to respond to IL-15 stimulation in vitro (Figure 5D and 5F). However, this is in agreement with the fact that rapamycin treatment causes a loss in T-bet expression and CD122 is a direct gene target of T-bet in CD8+ T cells (Matsuda et al., 2007). Although, we did not observe any changes in CD127 expression upon rapamycin treatment; however, these cells were better sensitized for IL-7 responsiveness in vitro (Figure 5D and 5G). Overall, these data indicates that mTOR inhibition imparts a memory-like phenotype on IL-12 conditioned effector CD8+ T cells along with persistent expression of memory-fate transcription factor Eomes. We next investigated whether the reculture of 72h conditioned OT-I cells with IL-7 for an additional 72h or antigen recall (168h) affected their memory-like phenotype. As shown in Figure S8A and B, rapamycin treated OT-I cells maintained their CD62Lhi and KLRG1lo phenotype, but the CD69hi phenotype was lost. Notably, the CD122lo phenotype observed at 72h was restored and we observed no changes in CD127 expression. Thus confirming that resting the rapamycin treated OT-I cells with IL-7 essentially maintained their memory-precursor phenotype, baring their inability to maintain the CD69hi phenotype.
Inhibition of mTOR enhances memory CD8+ T cell generation
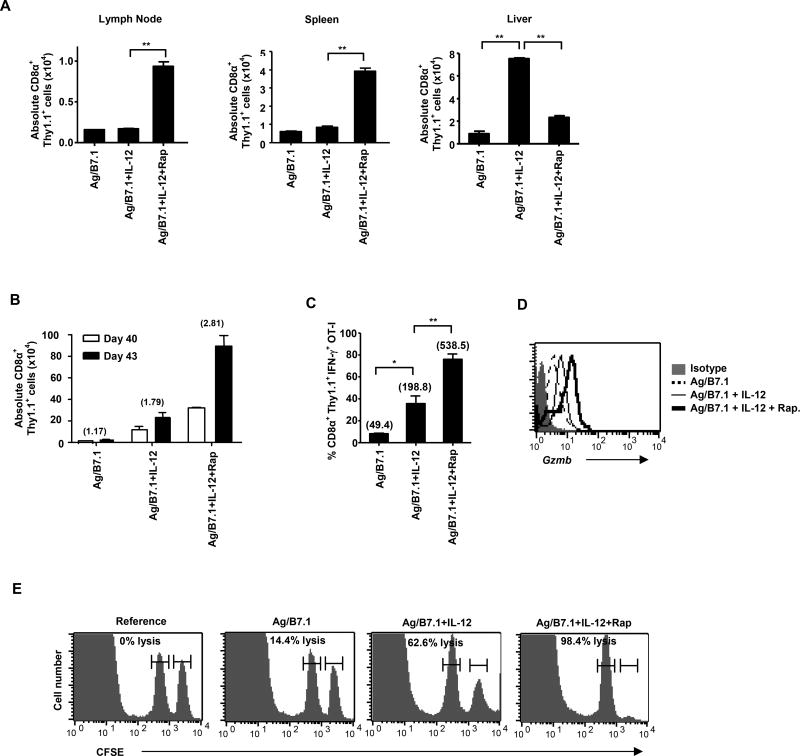
Based on the ability of rapamycin to block IL-12 mediated type I effector functions, switch persistent T-bet for Eomes expression and induce memory-like phenotype in OT-I cells, we predicted that rapamycin treated IL-12 conditioned OT-I cells would produce memory responses after adoptive transfer. To test this notion, we first investigated if rapamycin treated OT-I cells show changes in their ability localize within secondary lymphoid organs as suggested by their increased CD62L and CD69 expression. The adoptively transferred Ag/B7.1 +/− IL-12 and rapamycin conditioned OT-I cells (Thy1.1+) were detected in C57BL/6 (Thy1.2+) recipients after 24h. The rapamycin treated OT-I cells demonstrated increased localization in secondary lymphoid compartments (lymph node and spleen) and correspondingly lesser numbers were observed in tertiary sites such as liver (Figure 6A) and blood (Figure S9A). The non-rapamycin treated OT-I cells did not show this pattern of localization (Figure 6A). However, we did not observe any significant differences in the frequency of cells in the lung (Figure S9A). Thus, block in mTOR activity shifts the localization of antigen plus IL-12 conditioned CD8+ T cells to the secondary lymphoid compartment.
Figure 6. Inhibition of mTOR enhances memory CD8+ T cell generation.
OT-I cells (Thy1.1+) stimulated with Ag/B7.1 (+/−) IL-12 and rapamycin were harvested at 72h and adoptively transferred (2 × 106 cells) into BL/6 recipients. (A) The absolute number of adoptively transferred OT-I cells in the lymph node; **p<0.0052, spleen; **p<0.0037 and liver; **p<0.0012, **p<0.0011, at 24h post transfer. (B–E) The recipient mice were immunized with IFA-OVA on day 40 post transfer and secondary CD8+ T cell responses were measured 3 days later (B); The absolute numbers of adoptively transferred cells before (day 40) and after (day 43) immunization in the spleen. The numbers in parenthesis indicate fold expansion of CD8α+/Thy1.1+ from day 40 to day 43 (C); absolute numbers of IFN-γ secreting CD8α+/Thy1.1+ cells in the spleen on day 43; *p<0.01, **p<0.008. The numbers in parenthesis indicate the MFI of IFN-γ expression (D); Granzyme B expression on CD8α+/Thy1.1+ cells in the spleen on day 43 (E); the in vivo antigen specific cytolysis on day 43. A representative of two independent experiments is shown. (Data are presented as mean +/− SEM).
To confirm whether rapamycin treatment that produces memory precursor OT-I cells enables them for memory functions, we evaluated the persistence of the adoptively transferred cells (day 40) and tested their antigen recall response (day 43). The OT-I cells conditioned with Ag/B7.1 plus IL-12 demonstrate greater persistence than Ag/B7.1 stimulated OT-I cells (Figure 6B). Remarkably, rapamycin treatment significantly enhanced the ability of OT-I cells to persist as demonstrated by the increased numbers detected on day 40 (Figure 6B). The increased persistence of OT-I cells was largely due to their differential ability to survive rather than undergo greater homeostatic proliferation, as rapamycin treated OT-I cells show identical CFSE dilution as the non-treated controls (Figure S10A and S10B), but have higher expression of survival associated gene expressions (Figure 5E). Moreover, the rapamycin treated OT-I cells produced vigorous antigen recall responses as assessed by clonal expansion upon antigen re-challenge (Figure 6B) and effector responses; IFN-γ, gzmb expression and CTL activity (Figure 6C, 6D and 6E). More importantly, there is increased expression of IFN-γ and Granzyme B on a per-cell basis in the rapamycin treated group, which indicates that the increases in vivo cytolytic killing observed in this group is not only due to increased cell numbers, but also due to increased effector maturation upon antigen-recall. Therefore, rapamycin treatment not only enhances CD8+ T cell persistence, but also empowers them for greater effector capacities upon antigenic re-challenge. Careful phenotypic analysis of the adoptively transferred OT-I cells at early (day5) and late (day 40; memory) time-points show that rapamycin treated cells expressed higher levels of CD127, CD62L and CD69 expression on day 5, maintaining their memory precursor-phenotype (Figure S11A), but this phenotype was altered at day 40 post-transfer (Figure S11B). In addition, no changes in T-bet and CD122 expression were noted on day 40 (Figure S11C). Collectively, these observations demonstrate that rapamycin treatment promotes CD8+ T cell memory precursor generation that can localize within the secondary compartments and persist upon adoptive transfer. However, they alter their phenotype over-time and produce robust antigen–recall effector responses.
Rapamycin treated IL-12 conditioned OT-I cells demonstrate augmented tumor efficacy
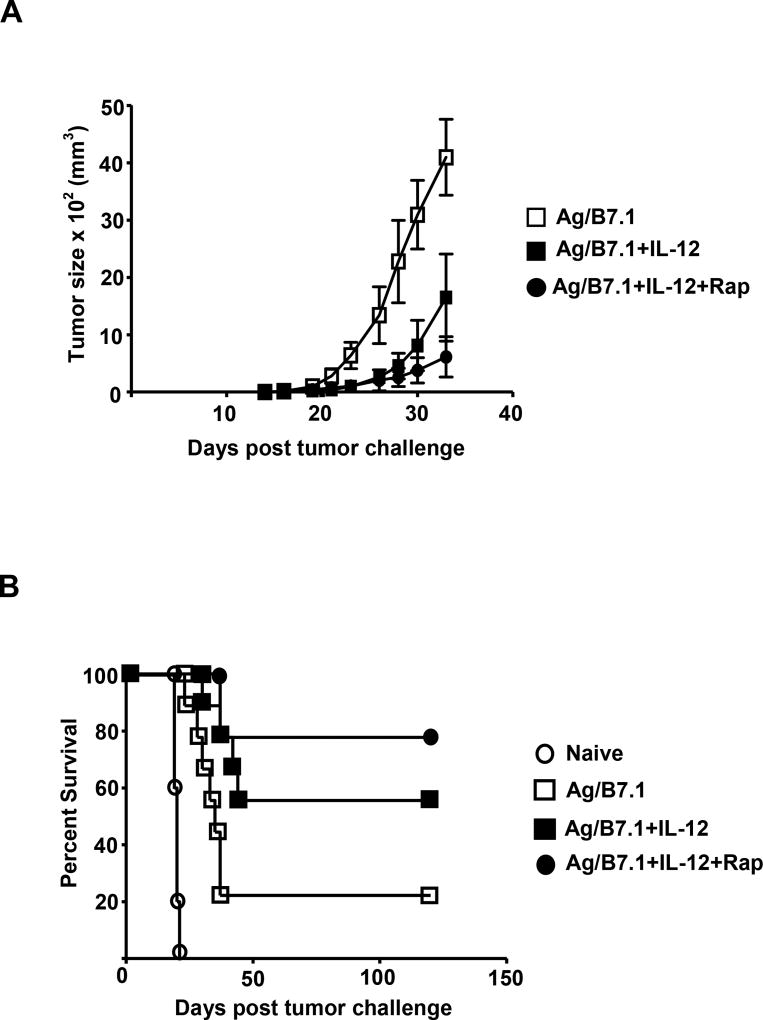
The use of ex vivo generated tumor-antigen specific effector CD8+ T cells in adoptive cell transfer (ACT) has produced tumor regressions in the clinical setting (Morgan et al., 2006). To test the tumor efficacy of rapamycin treated IL-12 conditioned OT-I cells, we adoptively transferred IL-12 conditioned OT-I cells (72h) that were either treated with or without rapamycin into intact C57BL/6 recipients bearing E.G7 tumor cells and their tumor size (s.c.) and survival was monitored over time. In comparison to naïve OT-I cell recipients, the mice receiving Ag/B7.1 stimulated OT-I cells, showed marginal benefits (100% to 80% fatality by day 30), which was further enhanced by the IL-12 conditioned OT-I cells (50% fatality by day 30). Remarkably, rapamycin treated IL-12 conditioned OT-I cells showed significantly enhanced tumor efficacy as more than 78% of the recipient animals survived tumor-free till day 120 (Figure 7B). Moreover, the rapamycin treated IL-12 conditioned OT-I cells also show significantly enhanced control of tumor size when compared to non-rapamycin treated counterparts (Figure 7A). These results demonstrate that inhibition of mTOR programs antigen and IL-12 conditioned CD8+ T cells for memory responses that show greater tumor efficacy than IL-12 conditioned effector CD8+ T cells.
Figure 7. mTOR inhibition promotes CD8+ T cell mediated anti-tumor immunity.
(A–B) Naive or 72 h conditioned OT-I cells were adoptively transferred into BL/6 recipients. Mice were inoculated with 2 × 106 E.G7 tumor cells, 24h post adoptive transfer of OT-I cells (A); tumor size (mm3) over time from tumor inoculation (B); Percent of tumor free survival over time from tumor inoculation. A representative of two independent experiments is shown.
DISCUSSION
Durable immunity requires CD8+ T cells to demonstrate both effector as well as memory functions. Instructions received by a naïve CD8+ T cell imprint differentiation into short lived effectors and/or long lived memory fates by regulating gene programs governed by master transcriptional factors (Kaech and Wherry, 2007). Many elegant studies have successfully elucidated the requirements for three distinct instructions; namely MHC-I/antigen, co-stimulatory molecules and cytokine, to achieve full activation and differentiation of naïve CD8+ T cells (Curtsinger et al., 2003), but the mechanism by which they are integrated to regulate transcriptional programs for CD8+ T cell functional differentiation is not entirely clear. Since, cellular energy status and the energy sensitive kinase mTOR, have been implicated in T cell activation/anergy, differentiation and survival (Araki et al., 2009; Cham and Gajewski, 2005; Delgoffe et al., 2009; Pearce et al., 2009), we predicted that the mTOR kinase activity is instrumental to the integration of instructions that program differentiation of CD8+ T cells. In this report, we demonstrate that Ag/B7.1 and IL-12 require the PI3K and STAT4 signaling pathways to augment mTOR activity, which determines effector and memory functional outcomes in CD8+ T cells by regulating T-bet and Eomes expression.
Recent reports have demonstrated a role for mTOR activity in memory CD8+ T cell differentiation (Araki et al., 2009; Pearce et al., 2009). However, the mechanisms by which mTOR integrates extracellular instructions and regulates gene programs to achieve distinct functional fates in CD8+ T cells is unknown. By stimulating naïve CD8+ T cells with defined quantity and quality of instructions, we have established that IL-12 provides the deterministic signal for CD8+ heritable effector maturation by enhancing and maintaining mTOR activity. Moreover, the stringent requirement for IL-12 induced STAT4 activity for CD8 type I effector functions is in large part due to its ability to maintain mTOR activity, which in turn produces persistent T-bet expression (Figure S12). Although, at this juncture, we do not fully understand how STAT4 maintains mTOR activity, it can be reasoned that STAT4 sustains transcription of mTOR whereby enhancing/sustaining its activity. Moreover, the ability of mTOR to sustain T-bet expression (mRNA and protein) is intriguing and deserves further studies. Nevertheless, by identifying the pathways by which instructions regulate mTOR activity for T-bet mediated functional maturation, we have generated new opportunities to modulate CD8+ T cell responses for desirable outcomes.
The selective ability of rapamycin to inhibit IFN-γ production in the secondary (144h & 168h) but not the primary (72h) phase of antigen stimulation was perplexing, but its interesting to note that rapamycin treatment did not affect the expression of homeobox transcription factor Hlx (data not shown), which has been shown to regulate IFN-γ production in the primary phase (Mullen et al., 2002). Therefore, the selective blockade of secondary IFN-γ production, but not primary upon rapamycin treatment is due to its ability to block persistent T-bet expression. This is consistent with the observation that primary IFN-γ production is not perturbed in Tbx21−/− OT-I T cells (this report) and with IFN-α being able to promote primary IFN-γ production in OT-I cells without promoting sustained T-bet expression, indicates the role played by factors other than T-bet in regulating primary IFN-γ production in CD8+ T cells. Indeed, our ability to restore antigen recall IFN-γ expression by ectopic T-bet expression, confirms the causal role of T-bet deficiency in rapamycin mediated loss of heritable effector functions. Although, the mechanisms by which mTOR inhibition curtails IL-12 sustained Tbet expression is not readily apparent, it is not due to rapamycin induced inhibition of protein translation as expression of several proteins like Eomes and CD62L are enhanced upon rapamycin treatment. It is possible that mTOR, which has been shown to tether several transcriptional factors (both activators and repressors) (Wullschleger et al., 2006) may regulate T-bet expression based on its activity.
The balance between transcriptional factors T-bet and Eomes has been shown to determine effector and memory cell fate in CD8+ T cells (Intlekofer et al., 2005). The fact that ectopic expression of T-bet led to loss of increased Eomes expression in rapamycin treated OT-I cells and Tbx21−/− OT-I cells show higher Eomes expression, indicates that rapamycin mediated up-regulation of Eomes is due to its ability to block persistent T-bet expression. Notably, we did not observe any changes in Eomes expression in Ag/B7.1 +/− IL-12 and rapamycin conditioned STAT4−/− OT-I cells, suggesting that regulation of Eomes by IL-12 and rapamycin in CD8+ T cells is STAT4 independent (data not shown). The ability to instructionally modulate the level of mTOR activity for relative expression of T-bet and Eomes offers new means to target transcriptional programs in CD8+ T cells.
The treatment of antigen plus IL-12 conditioned OT-I cells with rapamycin induced changes in phenotypic markers representing memory-precursors (72h, 144h and day 5). However, these changes were transient as by day 40 the phenotype was similar to non-treated controls. These observations and the recent report (Araki et al., 2009), suggests that continuous presence of rapamycin may be required to maintain the memory-like phenotype to day 40. Evidently, the in vitro rapamycin treated OT-I cells upon adoptive transfer undergo homeostatic proliferation in the absence of rapamycin, which may permit restoration of phenotype and functions. Exploring the notion that the extent of rapamycin exposure may affect effector versus memory functional maturation is provocative, as it is likely to identify new strategies to generate functionally distinct types of memory CD8+ T cells with heterogeneous efficacy against tumor and/or infectious challenges.
Strikingly, the in vitro rapamycin generated memory-precursor OT-I cells were more effective at promoting tumor immunity than IL-12 conditioned type I effector OT-I cells after adoptive transfer. The enhanced efficacy could be ascribed to several changes noted in their phenotype, localization, greater survival due to Bcl-2 related gene expression, and robust effector functions upon antigen re-challenge. Although, it would be interesting and challenging to delineate the relative contributions of all the changes produced by rapamycin treatment, the reported findings represent an important first step towards identifying the use of rapamycin to generate durable tumor immunity. The recent reports showing the ability of stem cell like or central memory CD8+ T cells to have greater tumor efficacy (Gattinoni et al., 2009), supports the paradigm that memory CD8+ T cells rather than effector CD8+ T cells enable greater tumor immunity.
In summary, we have identified mTOR as an important mediator of instructionally programmed CD8+ effector and memory cell fate by demonstrating its ability to regulate T-bet and Eomes expression. The information offers new targets to modulate CD8 T cell responses in infectious diseases, tumors, autoimmunity and/or GVHD.
EXPERIMENTAL PROCEDURES
Mice and reagents
The C57BL/6, CD4+ TCR transgenic Rag−/− (OT-II), CD8+ TCR transgenic Rag−/− (OT-I, WT), STAT4−/− OT-I Rag−/− and T-bet−/− OT-I Rag−/− mice were bred, housed and used according to IACUC guidelines at RPCI. The T-bet-ER RV was a kind gift from L. Gapin (Univ of Colorado). The rmIL-12 (2ng/ml) was a gift from Wyeth, Inc. (Cambridge, MA). IFN-α was a gift from T. Tomasi (RPCI). rmIL-7 was purchased from Peprotech (Rocky Hill, NJ). 2-DG, 4-HT and rapamycin were purchased from Sigma Aldrich (St. Louis, MO). LY290042 was purchased from Calbiochem. Insulin was purchased from Novo Nordisk Inc. (Princeton, NJ).
Stimulation of OT-I cells
Naïve OT-I cells were stimulated with latex microspheres expressing H-2Kb/SIINFEKL and B7.1 as described previously (Goldberg et al., 2003). Naïve OT-II cells were stimulated with anti-CD3/anti-CD28 coated latex beads. In some experiments, the cell line derived from embryonic fibroblasts namely; BOK (MEC.B7.SigOVA: expressing H-2Kb, OVAp (SIINFEKL) and B7.1), were used as antigen-presenting cells to stimulate naïve OT-I cells as previously described (Li et al., 2006). For evaluation of secondary antigen recall responses in vitro, see Supplemental experimental procedures.
Statistical analysis
For statistical analysis, the unpaired Student’s t test was applied. Tumor survival between various groups was compared using Kaplan Meier survival curves and log-rank statistics. Significance was set at p < 0.05.
Supplementary Material
Acknowledgments
We are grateful to entire Shrikant laboratory and Dr.’s Y. Liu, J. Kesterson and N. Yu for their assistance. The advice from Dr.’s J. Powell (JHU) and N. Bangia (RPCI) on these studies is appreciated. Our special thanks to Dr. Laurent Gapin, Univ. of Colorado for providing us with T-bet-ER retroviral vector. This work was supported by the grants from NIH-NCI (RO1 CA104645) and Alliance Foundation of Roswell Park Cancer Institute (P.A.S.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
COMPETING INTERESTS STATEMENTS
The authors declare that they have no competing financial interests.
References
- Agarwal P, Raghavan A, Nandiwada SL, Curtsinger JM, Bohjanen PR, Mueller DL, Mescher MF. Gene regulation and chromatin remodeling by IL-12 and type I IFN in programming for CD8 T cell effector function and memory. J Immunol. 2009;183:1695–1704. doi: 10.4049/jimmunol.0900592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki K, Turner AP, Shaffer VO, Gangappa S, Keller SA, Bachmann MF, Larsen CP, Ahmed R. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009 doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8:345–350. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- Cham CM, Gajewski TF. Glucose availability regulates IFN-gamma production and p70S6 kinase activation in CD8+ effector T cells. J Immunol. 2005;174:4670–4677. doi: 10.4049/jimmunol.174.8.4670. [DOI] [PubMed] [Google Scholar]
- Cornish GH, Sinclair LV, Cantrell DA. Differential regulation of T-cell growth by IL-2 and IL-15. Blood. 2006;108:600–608. doi: 10.1182/blood-2005-12-4827. [DOI] [PubMed] [Google Scholar]
- Curtsinger JM, Lins DC, Mescher MF. Signal 3 determines tolerance versus full activation of naive CD8 T cells: dissociating proliferation and development of effector function. J Exp Med. 2003;197:1141–1151. doi: 10.1084/jem.20021910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgoffe GM, Kole TP, Zheng Y, Zarek PE, Matthews KL, Xiao B, Worley PF, Kozma SC, Powell JD. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity. 2009;30:832–844. doi: 10.1016/j.immuni.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis PB, Jaeschke A, Saitoh M, Fowler B, Kozma SC, Thomas G. Mammalian TOR: a homeostatic ATP sensor. Science. 2001;294:1102–1105. doi: 10.1126/science.1063518. [DOI] [PubMed] [Google Scholar]
- Gattinoni L, Zhong XS, Palmer DC, Ji Y, Hinrichs CS, Yu Z, Wrzesinski C, Boni A, Cassard L, Garvin LM, et al. Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nat Med. 2009;15:808–813. doi: 10.1038/nm.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg J, Shrikant P, Mescher MF. In vivo augmentation of tumor-specific CTL responses by class I/peptide antigen complexes on microspheres (large multivalent immunogen) J Immunol. 2003;170:228–235. doi: 10.4049/jimmunol.170.1.228. [DOI] [PubMed] [Google Scholar]
- Grayson JM, Zajac AJ, Altman JD, Ahmed R. Cutting edge: increased expression of Bcl-2 in antigen-specific memory CD8+ T cells. J Immunol. 2000;164:3950–3954. doi: 10.4049/jimmunol.164.8.3950. [DOI] [PubMed] [Google Scholar]
- Harris TE, Lawrence JC., Jr TOR signaling. Sci STKE. 2003;2003:re15. doi: 10.1126/stke.2122003re15. [DOI] [PubMed] [Google Scholar]
- Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- Iezzi G, Karjalainen K, Lanzavecchia A. The duration of antigenic stimulation determines the fate of naive and effector T cells. Immunity. 1998;8:89–95. doi: 10.1016/s1074-7613(00)80461-6. [DOI] [PubMed] [Google Scholar]
- Intlekofer AM, Takemoto N, Wherry EJ, Longworth SA, Northrup JT, Palanivel VR, Mullen AC, Gasink CR, Kaech SM, Miller JD, et al. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat Immunol. 2005;6:1236–1244. doi: 10.1038/ni1268. [DOI] [PubMed] [Google Scholar]
- Jacobson NG, Szabo SJ, Weber-Nordt RM, Zhong Z, Schreiber RD, Darnell JE, Jr, Murphy KM. Interleukin 12 signaling in T helper type 1 (Th1) cells involves tyrosine phosphorylation of signal transducer and activator of transcription (Stat)3 and Stat4. J Exp Med. 1995;181:1755–1762. doi: 10.1084/jem.181.5.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaech SM, Wherry EJ. Heterogeneity and cell-fate decisions in effector and memory CD8+ T cell differentiation during viral infection. Immunity. 2007;27:393–405. doi: 10.1016/j.immuni.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J, Huddleston SJ, Fraser JM, Khoruts A. De novo induction of antigen-specific CD4+CD25+Foxp3+ regulatory T cells in vivo following systemic antigen administration accompanied by blockade of mTOR. J Leukoc Biol. 2008;83:1230–1239. doi: 10.1189/jlb.1207851. [DOI] [PubMed] [Google Scholar]
- Lefrancois L, Marzo AL. The descent of memory T-cell subsets. Nat Rev Immunol. 2006;6:618–623. doi: 10.1038/nri1866. [DOI] [PubMed] [Google Scholar]
- Li Q, Eppolito C, Odunsi K, Shrikant PA. IL-12-programmed long-term CD8+ T cell responses require STAT4. J Immunol. 2006;177:7618–7625. doi: 10.4049/jimmunol.177.11.7618. [DOI] [PubMed] [Google Scholar]
- Matsuda JL, George TC, Hagman J, Gapin L. Temporal dissection of T-bet functions. J Immunol. 2007;178:3457–3465. doi: 10.4049/jimmunol.178.6.3457. [DOI] [PubMed] [Google Scholar]
- Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, Royal RE, Topalian SL, Kammula US, Restifo NP, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen AC, Hutchins AS, High FA, Lee HW, Sykes KJ, Chodosh LA, Reiner SL. Hlx is induced by and genetically interacts with T-bet to promote heritable T(H)1 gene induction. Nat Immunol. 2002;3:652–658. doi: 10.1038/ni807. [DOI] [PubMed] [Google Scholar]
- Pearce EL, Walsh MC, Cejas PJ, Harms GM, Shen H, Wang LS, Jones RG, Choi Y. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 2009;460:103–107. doi: 10.1038/nature08097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders RN, Metcalfe MS, Nicholson ML. Rapamycin in transplantation: a review of the evidence. Kidney Int. 2001;59:3–16. doi: 10.1046/j.1523-1755.2001.00460.x. [DOI] [PubMed] [Google Scholar]
- Schluns KS, Kieper WC, Jameson SC, Lefrancois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat Immunol. 2000;1:426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- Schluns KS, Williams K, Ma A, Zheng XX, Lefrancois L. Cutting edge: requirement for IL-15 in the generation of primary and memory antigen-specific CD8 T cells. J Immunol. 2002;168:4827–4831. doi: 10.4049/jimmunol.168.10.4827. [DOI] [PubMed] [Google Scholar]
- Schmelzle T, Hall MN. TOR, a central controller of cell growth. Cell. 2000;103:253–262. doi: 10.1016/s0092-8674(00)00117-3. [DOI] [PubMed] [Google Scholar]
- Shiow LR, Rosen DB, Brdickova N, Xu Y, An J, Lanier LL, Cyster JG, Matloubian M. CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature. 2006;440:540–544. doi: 10.1038/nature04606. [DOI] [PubMed] [Google Scholar]
- Sinclair LV, Finlay D, Feijoo C, Cornish GH, Gray A, Ager A, Okkenhaug K, Hagenbeek TJ, Spits H, Cantrell DA. Phosphatidylinositol-3-OH kinase and nutrient-sensing mTOR pathways control T lymphocyte trafficking. Nat Immunol. 2008;9:513–521. doi: 10.1038/ni.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–669. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- Takemoto N, Intlekofer AM, Northrup JT, Wherry EJ, Reiner SL. Cutting Edge: IL-12 inversely regulates T-bet and eomesodermin expression during pathogen-induced CD8+ T cell differentiation. J Immunol. 2006;177:7515–7519. doi: 10.4049/jimmunol.177.11.7515. [DOI] [PubMed] [Google Scholar]
- Welsh RM. Blimp hovers over T cell immunity. Immunity. 2009;31:178–180. doi: 10.1016/j.immuni.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wherry EJ, Teichgraber V, Becker TC, Masopust D, Kaech SM, Antia R, von Andrian UH, Ahmed R. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol. 2003;4:225–234. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Xiao Z, Casey KA, Jameson SC, Curtsinger JM, Mescher MF. Programming for CD8 T cell memory development requires IL-12 or type I IFN. J Immunol. 2009;182:2786–2794. doi: 10.4049/jimmunol.0803484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo JK, Cho JH, Lee SW, Sung YC. IL-12 provides proliferation and survival signals to murine CD4+ T cells through phosphatidylinositol 3-kinase/Akt signaling pathway. J Immunol. 2002;169:3637–3643. doi: 10.4049/jimmunol.169.7.3637. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Collins SL, Lutz MA, Allen AN, Kole TP, Zarek PE, Powell JD. A role for mammalian target of rapamycin in regulating T cell activation versus anergy. J Immunol. 2007;178:2163–2170. doi: 10.4049/jimmunol.178.4.2163. [DOI] [PubMed] [Google Scholar]
- Zhou L, Chong MM, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009;30:646–655. doi: 10.1016/j.immuni.2009.05.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.