Abstract
Background
Lung-protective ventilation (LPV) has been demonstrated to improve clinical outcomes in surgical patients. There are very limited data on the current use of LPV for patients undergoing one lung ventilation (1LV) despite evidence that 1LV may be a particularly important setting for its use. In this multicenter study, we report trends in ventilation practice for patients undergoing 1LV.
Methods
The Multicenter Perioperative Outcomes Group database was used to identify patients undergoing 1LV. We retrieved and calculated median initial and overall tidal volume (VT) for the cohort and for high risk subgroups (female sex, obesity (body mass index (BMI) > 30), and short stature), percentage of patients receiving positive end expiratory pressure (PEEP) ≥ 5 cm H2O, LPV during 1LV (VT ≤ 6 ml/kg predicted body weight (PBW) and PEEP ≥ 5 cm H2O), and ventilator driving pressure (ΔP; plateau airway pressure - PEEP).
Results
Data from 5,609 patients across four institutions were included in the analysis. Median VT were calculated for each case and since the data were normally distributed the mean is reported for the entire cohort and subgroups. Mean of median VT during 1LV for the cohort was 6.49 ± 1.82 ml/kg PBW. VT (ml/kg PBW) for high risk subgroups was significantly higher; 6.86 ± 1.97 for BMI≥30, 7.05 ± 1.92 for female patients, and 7.33 ± 2.01 for short stature patients. Mean of the median VT declined significantly over the study period (from 6.88 to 5.72; p < 0.001), and the proportion of patients receiving LPV increased significantly over the study period (from 9.1% to 54.6%; p < 0.001). These changes coincided with a significant decrease in ΔP during the study period, from 19.4 cm H2O during period 1 to 17.3 cm H2O in period 12 (p=0.003).
Conclusions
Despite a growing awareness of the importance of protective ventilation, a large proportion of patients undergoing 1LV continue to receive VT PEEP levels outside of recommended thresholds. Moreover, VT remains higher and LPV less common in high risk subgroups, potentially placing them at elevated risk for iatrogenic lung injury.
Introduction
Despite advances in perioperative care, patients undergoing surgery remain at risk for the development of pulmonary complications. Mechanical ventilation during surgery can lead to clinically significant iatrogenic ventilator- induced lung injury through a number of mechanisms1. High quality evidence supports the use of lung protective ventilation (LPV) strategies during elective high-risk surgery. LPV regimens that combine a physiologically appropriate tidal volume (VT) with moderate levels of positive end-expiratory pressure (PEEP) have been demonstrated to improve biochemical2, physiological3, and clinical outcomes4. Nonetheless, a significant proportion of patients, particularly previously identified high risk subgroups (female patients, those with height < 165 cm and obese patients), continue to receive VT and PEEP outside of recommended practice parameters.5 Although prominent studies2,6,7, reviews1,8,9, and expert commentary8,9 support combining the use of lower physiologically appropriate VT with PEEP during 1LV, it is not clear that widespread adoption of these evidence-based practices has occurred.
Thoracic surgery patients may be at even greater risk for the injurious processes generated by positive pressure ventilation, and particularly by one lung ventilation (1LV), which is itself a risk factor for lung injury10. Indeed, the risk of major respiratory complications after thoracic surgery requiring 1LV remains much higher than that of other surgery classes11–13. There are, however, very limited data on the current use of LPV for patients undergoing 1LV14,15.
In this multicenter study we examined variations and trends in the management of 1LV with emphasis on the use of LPV strategies. We hypothesized that 1) a significant percentage of patients continue to be ventilated with potentially injurious larger tidal volumes (VT > 6 ml/kg PBW) and/or minimal PEEP (< 5 cm H2O) during 1LV, 2) that the use of protective 1LV (VT ≤ 6 ml/kg PBW; PEEP ≥ 5 cm H2O), varies as a function of patient characteristics – particularly high body mass index (BMI), short stature (<160 cm), and female sex, 3) that adoption of protective 1LV increased over the study period, and 4) that variability existed between institutions in the practice of protective 1LV.
Methods
Approval for this project was provided by the University of Michigan Institutional Review Board (IRB# HUM24166 and HUM33894, Ann Arbor, Michigan). The requirement for written consent was waived by the IRB. Data was extracted from anesthesia information system records that were transformed into a standardized format at each participating institution and submitted electronically to the Multicenter Perioperative Outcomes Group (MPOG) centralized database at the University of Michigan. Participation in the MPOG Project requires each participating institution to obtain institutional IRB approval for creation and transmission of a limited data set to the centralized coordinating center. Research was completed in a manner consistent with the STROBE Statement. A completed STROBE Checklist is supplied in the supplementary material.
Data quality is ensured by a number of sequential quality control processes. First, each MPOG participating institution is independently responsible for mapping anesthesia information system (AIMS) data into MPOG concept identifiers. When complete, a test upload of the data is sent to MPOG. Each participating site performs more than 70 automated MPOG data diagnostics, which were developed to ensure the accuracy of the key data elements being submitted to MPOG. Only after these tasks are completed and necessary corrections made, are the data uploaded into the MPOG research database. Third, for several months after the first upload, all sites are required to validate 20 random cases per month; thereafter 5 cases per month are validated. Finally, MPOG programmers regularly perform data diagnostics to ensure the accuracy of transmitted data elements. Further details of the function of MPOG are described elsewhere16,17.
All data analysis was performed at the University of Michigan. The study protocol, including primary and secondary outcomes, patient inclusion/exclusion criteria, and a proposed statistical analysis were presented, approved, and registered with the MPOG perioperative clinical research committee prior to data extraction.
The initial proposal was presented in May 2014, with revisions submitted in July 2014. The following minor modifications (made before data were collected and analyzed) should be noted:
The definition of lung protective ventilation was liberalized to include VT ≤ 6 ml/kg PBW (originally proposed as ≤ 5 ml/kg) to reflect the upper acceptable limit of this parameter as supported by expert recommendations during the study period8,10.
The time period used for analysis of “initial” 1LV data was 5-15 minutes post-initiation of 1LV (previously 0-10 minutes) to reflect the fact that ventilator setting changes frequently occur in the initial 5 minutes of 1LV.
Four centers contributed data to this analysis (University of Michigan, University of Virginia, University of Vermont and University of Tennessee). The contributing centers and the study period (2009-2014) were chosen based on completeness of 1LV data (structured, timed documentation of 1LV) and consistent case volume throughout the study period with the anthropometric data required to complete the analysis. Institutions were selected prior to assessment of outcome data. Individual cases were included if the patient was greater than 18 years of age, the case included documentation of 1LV initiation and available data permitted the calculation of BMI. Thus, no cases with missing data elements were included in the study. No a priori power calculation was performed as this was a sample of available complete data in the registry. Analyzed ventilator data were as recorded in the Anesthesia Information System and transmitted to the coordinating center.
We defined two time periods for analysis: an “initial” period 5 to 15 minutes after the charted initiation of 1LV (a delayed start was included to allow for ventilator changes following initiation of 1LV) and an “overall” period which encompassed the entire duration of 1LV based on charted initiation of 1LV and resumption of two lung ventilation (2LV). In some institutions resumption of 2LV was not consistently charted; therefore we analyzed the dataset for differences between these two time periods. By definition, these time periods overlapped. We sought to determine whether studied variables differed between the two periods, and if not, planned to maximize available statistical power by using the “initial” period since more subjects would be available. Driving pressure (ΔP; PPLAT-PEEP) was calculated from ventilator data derived from two of the four institutions (University of Virginia and University of Vermont) which reported PPLAT data.
Tidal volumes were normalized based on calculated predicted body weight (PBW): males: 50 kg + 2.3 kg (height [inches] − 60) and females: 45.5 kg + 2.3 kg (height [inches] – 60) as used by ARDSNet investigators18. VT, PEEP and ΔP were calculated as median or mean values for each case during the time of 1LV. The co-primary outcomes were median tidal volume (ml/kg PBW) and use of PEEP ≥ 5 cm H2O. Secondary outcomes included ΔP and the use of LPV, defined as tidal volume ≤ 6 ml/kg combined with PEEP ≥ 5 cm H2O. Based on the results of prior work5 the following subgroups were included in the analysis: short stature - defined as height < 165 cm5; overweight and obese status using the World Health Organization criteria - BMI 25-29.9 and BMI ≥ 30, respectively; and female gender. If a patient underwent multiple procedures at different dates meeting inclusion criteria during the study period, each case was included.
Statistical Analysis
Statistical analysis was performed using SPSS version 21 and STATA MP version 14. All data were analyzed for normality. Normally distributed data are presented as means ± standard deviation. Non-normally distributed data are presented as medians [25th to 75th percentile]. The initial VT for each individual patient (case) was not normally distributed and therefore the median value was calculated for each patient (case). The distribution of initial VT for the study population was normally distributed and therefore the sample mean of the individual patient’s median initial VT is reported along with standard deviations. To assess the presence of statistically and clinically relevant differences between initial and overall tidal volumes (mL/kg PBW), a paired samples t-test was performed. To assess whether the proportion of cases with PEEP ≥ 5 cm H2O versus PEEP < 5 H2O and the proportion of cases with LPV versus those without LPV differed for initial VT and overall VT time periods, a Pearson Chi-square test was used. We assessed the association between mean driving pressure and the factors obesity (yes vs no), gender, and stature (short versus non-short) using the students t-test. To assess statistically significant differences in VT across the institutions for non-normally distributed data, independent samples Kruskal-Wallis Test was used with Bonferroni correction. A p-value of 0.0125 was considered statistically significant for these tests. We used a linear regression model to determine if there were significant interactions between the institution and each of the three subgroups; obesity (yes vs no), gender, and stature (short versus non-short). The dependent variable was the VT. ANOVA was used to assess statistically significant differences between institutions for VT and the use of protective 1LV.
A mixed effects logistic regression model was developed to assess independent predictors of LPV (dependent variable) controlling for patient factors (age, gender, BMI, height, ASA classification status) and individual year (where 2009 was the reference year) as fixed effects and the individual MPOG institution as a random effect. A p-value of <0.05 was considered statistically significant for fixed effects covariates. The intraclass correlation (ICC) is reported for the variation in LPV due to the variability between the institutions.
All cases from the participating institutions meeting the inclusion criteria during the study period (January 2, 2009 to December 30, 2014) were included in the analysis. There were 328 cases during the first 6 month study period and 445 subjects during the last 6 month period. There is a 91.2% power to detect a difference of 8% in lung protective ventilation between the two periods assuming a percentage of 9.1% (30/328) during the first 6 month study period using a two-sided Z-test with unpooled variance and a significance level of 0.05.
Results
Data from 5,609 patients across the four institutions were included in the analysis. The two data periods (overall versus initial) were compared. The median period of 1LV was 117 minutes (Interquartile range (IQR) 59 - 186). Mean of the median initial VT was not different from the mean of the median overall VT (6.49 ± 1.82 ml/kg PBW versus 6.47 ± 1.57ml/kg PBW, p = 0.856) (Table 1). Median PEEP in the initial and overall periods were 5 cm H2O (data not shown), suggesting that only minor modifications of PEEP occurred beyond the initial observation period. Use of PEEP ≥ 5 cm H2O in the initial intraoperative period was 50.2% versus 46% in the overall period (p < 0.001); no difference was found in the rate of LPV between intraoperative periods: 23% (initial) versus 20% (overall) (p = 0.174).
Table 1. Median Initial VT (ml/kg PBW) per Institution by Subgroup Analysis.
Patient counts for each institution and the entire cohort and median initial VT (ml/kg PBW or ABW) for the cohort and median initial VT (ml/kg PBW) for high risk subgroups – BMI > 30 kg/m2, height < 165 cm, and female sex.
| Institution | Patient Count | Initial median VT (ml/kg PBW)a |
Initial Median VT (ml/kg ABW)a |
BMI > 30 Initial median VT (ml/kg PBW)b* |
BMI ≤ 30 Initial median VT (ml/kg PBW)a |
Height < 165cm Initial median VT (ml/kg PBW)c* |
Height ≥ 165cm Initial median VT (ml/kg PBW)a |
Female Initial median VT (ml/kg PBW)a* |
Male Initial median VT (ml/kg PBW)a |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 3,177 | 6.28 ± 1.69 | 5.14 ± 1.51 | 6.55 ± 1.81 | 6.22 ± 1.59 | 7.11 ± 1.87 | 6.04 ± 1.46 | 6.80 ± 1.77 | 5.92 ± 1.45 |
| 2 | 416 | 6.88 ± 1.97 | 5.55 ± 1.75 | 6.89 ± 1.89 | 6.89 ± 2.01 | 7.78 ± 2.45 | 6.77 ± 1.75 | 7.57 ± 1.94 | 6.59 ± 1.94 |
| 3 | 1,443 | 6.50 ± 1.94 | 5.34 ± 1.58 | 7.00 ± 2.11 | 6.42 ± 1.77 | 7.40 ± 2.10 | 6.14 ± 1.64 | 7.16 ± 2.09 | 6.11± 1.62 |
| 4 | 573 | 6.32 ± 2.17 | 5.32 ± 1.89 | 6.93 ± 2.45 | 6.31 ± 1.99 | 7.28 ± 2.38 | 6.06 ± 1.89 | 6.96 ± 2.34 | 5.99 ± 1.86 |
| All Institutions | 5,609 | 6.49 ± 1.82 | 5.22 ± 1.58 | 6.86 ± 1.97 (n=1,816) | 6.31 ± 1.70 | 7.33 ± 2.01 (n = 1,829) | 6.09 ± 1.57 | 7.05 ± 1.92 (n = 2,597) | 6.00 ± 1.56 |
There were no significant interactions between institution and BMI, height, or female sex. These subgroups were selected a priori based on their previous identification as risk factors for receiving high VT.
VT = tidal volume; ml = milliliter; kg = kilogram; PBW = predicted body weight; ABW = actual body weight; BMI = body mass index
Statistically significant inter-institution variability in overall initial VT (ml/kg PBW), p<0.001
Statistically significant inter-institution variability in overall initial VT (ml/kg PBW), p=0.004
Statistically significant inter-institution variability in overall initial VT (ml/kg PBW), p=0.008
The proportion of cases with PEEP ≥ 5 cm H2O increased from 53% during the first 6-month period in 2009 to 91% in the final period in 2014 (p < 0.001; Figure 1). Median VT (mL/kg PBW) declined during the study period from 6.88 ± 1.98 in 2009 to 5.72 ± 1.45 in 2014 (p < 0.001; Figure 2).
Figure 1.
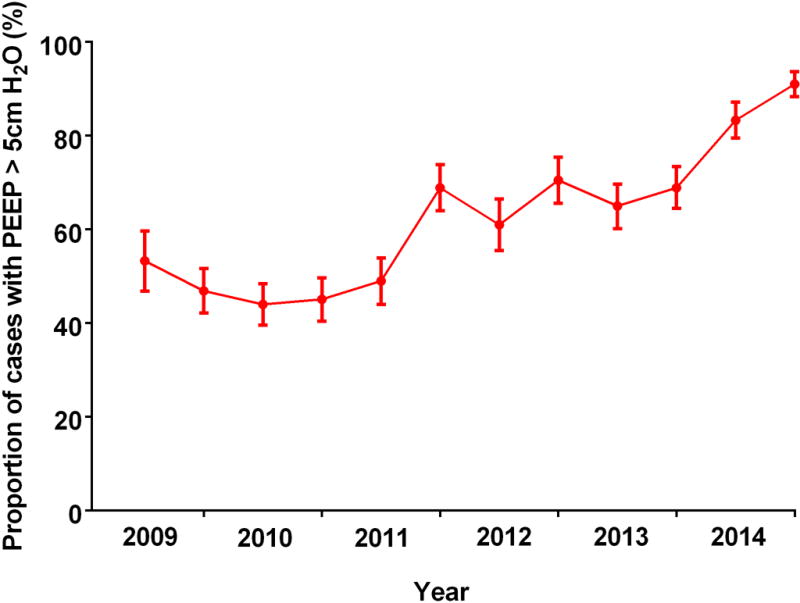
The proportion of patients receiving PEEP ≥ 5 cm H2O during a 10 minute epoch beginning 5 minutes after the start of 1LV is plotted at six month intervals. PEEP = positive end expiratory pressure; 1LV = one lung ventilation.
Figure 2.
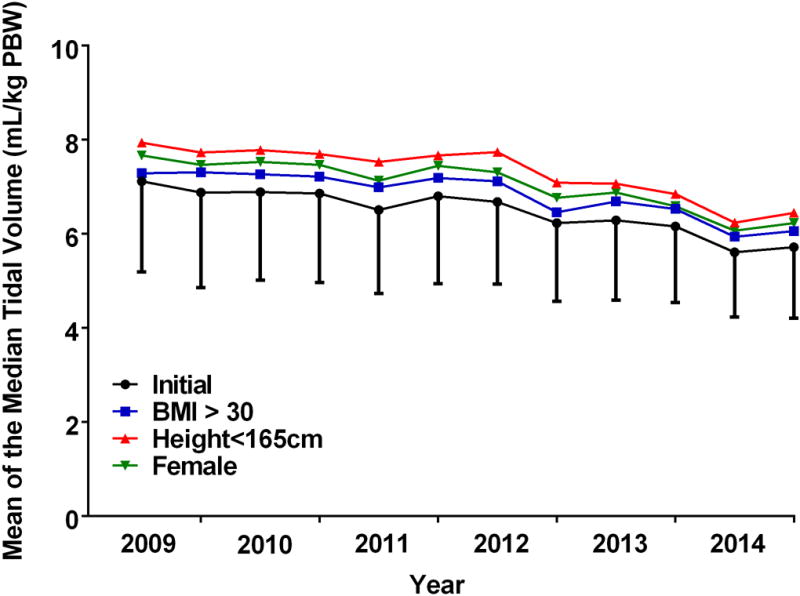
Tidal volumes during 1LV for the study cohort and subpopulations. Median tidal volumes (ml/kg PBW) during the entire period of 1LV (overall period) and during a 10 minute epoch of 1LV beginning 5 minutes after the start of 1LV (initial period) are plotted for the study cohort at six month intervals. Additionally, median tidal volumes for the initial period are plotted at six month intervals for the study subpopulations – patients with BMI >30, height < 165 cm, and females. 1LV = one lung ventilation; PBW = predicted body weight; PEEP = positive end expiratory pressure; BMI = body mass index.
The use of LPV rose during the study period from 9.1% in 2009 to 55% in 2014 (Figure 3). While PEEP ≥ 5 cm H2O was used in the majority of cases from 2011 onwards, median VT remained above 6ml/kg (PBW) until early 2014 (mean of the median VT ± SD for 2013 and 2014: 6.22 ± 1.66 and 5.67 ± 1.45, respectively).
Figure 3.
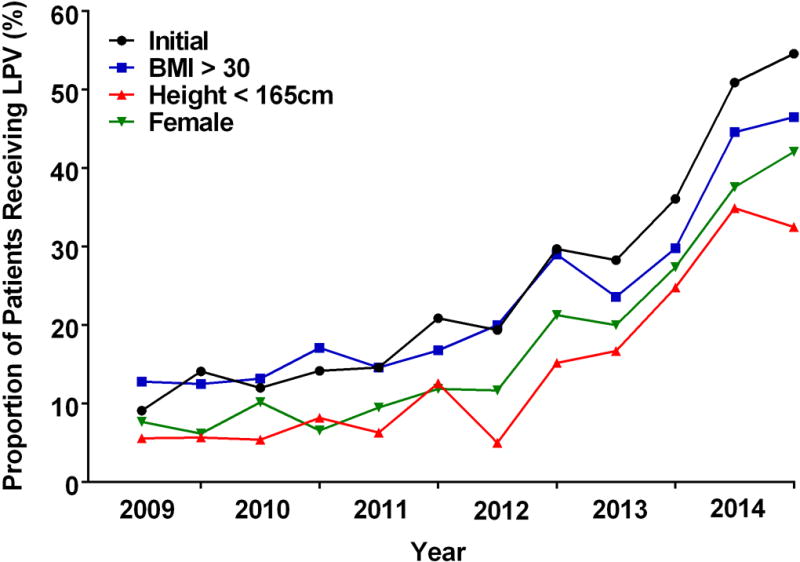
The proportion of patients receiving lung protective ventilation (VT ≤ 6 ml/kg PBW and PEEP ≥ 5 cm H2O) during 1LV for the study cohort and subpopulations during a 10 minute epoch beginning 5 minutes after the start of 1LV plotted at six month intervals. VT = tidal volume; PBW = predicted body weight; PEEP = positive end expiratory pressure; 1LV = one lung ventilation.
Each of the three subgroups previously identified as being at higher risk of receiving large VT5 during 2LV also received larger VT during 1LV relative to that of the overall cohort (Table 1, Figure 2). There were no statistically significant interactions between institution and each of the three subgroups with regard to the VT. Of 5,609 patients included in the study, 32% were obese (BMI > 30), 33% were of short stature (height < 165 cm), and 46% were female. Administered VT was larger for each of the high risk subgroups as compared with the cohort as a whole (6.49 ml/kg PBW) (Table 1). Administered VT was larger for female patients (compared to male patients; 7.05 ± 1.92 ml/kg PBW vs. 6.00 ± 1.56 ml/kg PBW; p < 0.001), those with short stature (< 165 cm compared to > 165 cm; 7.33 ± 2.01 ml/kg PBW vs. 6.09 ± 1.57 ml/kg PBW; p < 0.001) or obesity (compared to non obese patients; 6.86 ± 1.97 ml/kg PBW vs. 6.31 ± 1.70 ml/kg PBW; p < 0.001). The use of PEEP ≥ 5 cm H2O was more frequent in obese patients (68%) than in those who were non-obese (59%; p < 0.001), with short stature (59%) or female sex (61%) (p<0.001). A lower proportion of patients in the high risk subgroups received LPV when compared with the remainder of the cohort: female vs male (18% vs 32%; p < 0.001), short stature (< 165 cm vs. > 165 cm (14% vs 30%; p < 0.001), and obese vs non-obese (23% vs. 36%; p = 0.021).
Results of the mixed effects logistic regression model demonstrated the ICC for institution as a random effect was 0.46 (95% CI 0.09 – 0.88) which means that an estimated 46% of the variation in LPV is due to variability between hospitals and 54% of the variation is due to variability within hospitals.
Inter-institutional differences were seen in median tidal volumes, PEEP and the use of LPV. The absolute difference in the median initial VT for the duration of the study between institutions was 0.6 ml/kg (range: 6.28 – 6.88 ml/kg; p <0.001; Table 1). The use of PEEP ≥ 5 cm H2O varied significantly between institutions (32.3% - 76.8%, p-value <0.001; data not shown). The use of protective 1LV also varied markedly (range: 0% to 31%) between institutions throughout the study period (p <0.001; data not shown).
Data on driving pressure was available from two institutions within the study with a total of 1890 cases analyzed. Driving pressure fell during the study period from 19.4 ± 6.4 cm H2O during period 1 to 16.4 ± 5.7cm H2O in period 11 and 17.3 ± 5.9 cm H2O in period 12 (p=0.003, F statistic 2.625)(Figure 4). Patients with BMI ≥ 30 received driving pressures which were markedly higher than patients with a BMI < 30 (20.5 ± 5.8 cm H2O versus 16.2 ± 5.5 cm H2O, p <0.001). Patients with height < 165 cm (18.2 ± 6.0 cm H2O versus patients with height ≥ 165 cm 17.2 ± 5.8 cm H2O; p = 0.001) but not female patients (17.6 ± 5.9 cm H2O versus male patients 17.3 ± 6.0 cm H2O, p = 0.366) received higher driving pressures than the cohort as a whole (Figure 4).
Figure 4.
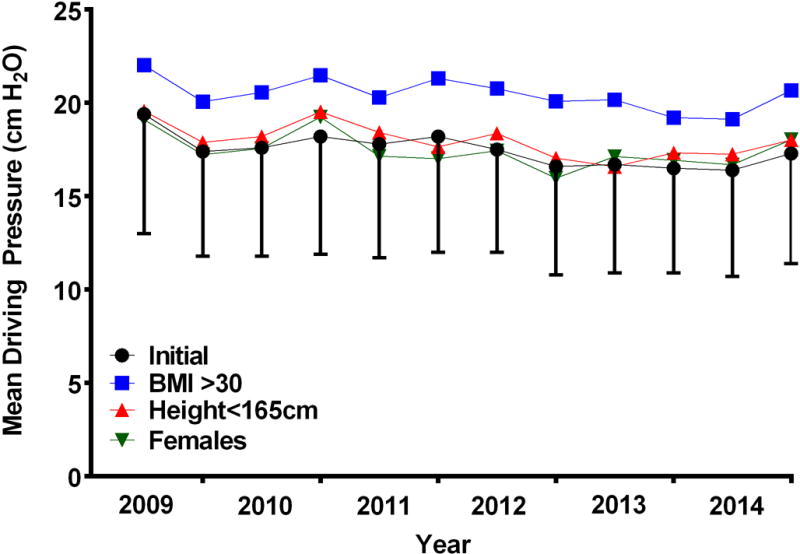
Mean driving pressure ((ΔP) calculated as PPLAT – PEEP; cm H2O) during a 10 minute epoch beginning 5 minutes after the initiation of 1LV for the study cohort and subpopulations plotted at six month intervals. PPLAT = plateau airway pressure; PEEP = positive end expiratory pressure; 1LV = one lung ventilation.
Discussion
In this multi-institutional study of 1LV practice, we found that median VT declined and the use of PEEP ≥ 5 cm H2O increased during the study period. This decline in median VT and adoption of PEEP during 1LV accounted for a dramatic increase in the proportion of patients receiving LPV.
It is important to note that the current study does not attempt to demonstrate the clinical efficacy of LPV, but rather to describe clinical practice patterns of 1LV. The definition of LPV used herein reflects limits derived from clinical trial data6 and recommendations by experts8 and represents a relatively liberal approach to the definition. Not surprisingly, small variations in the upper limit of acceptable 1LV VT exist. Several clinical studies appear to support a lower VT limit (5 ml/kg)2,7,19 than the definition used in this study (VT ≤ 6 ml/kg PBW; PEEP ≥ 5 cm H2O). The use of a lower VT limit would certainly have resulted in an even lower reported proportion of patients receiving LPV.
These reported practice changes also explain the decrease in ΔP during 1LV observed in our study population. However, despite increasing adherence to recommended ventilation practices, large variations in clinical practice between institutions was observed with only approximately half of the cohort receiving LPV at the end of the study period. Moreover, high risk subgroups – patients with obesity, short stature, and females received larger median VT and a lower frequency of LPV relative to the overall cohort and obese patients were also exposed to higher ΔP.
1LV and Lung Injury
1LV contributes to the development of lung injury and complications after thoracic surgery1,10. Mechanisms by which such injury may occur include lung strain, atelectasis, cyclic recruitment/de-recruitment phenomena, oxidative stress, and capillary shear stress. Although the ideal ventilation management regimen for 1LV has not yet been identified, support for putative protective 1LV comes from trials in patients with pre-established lung injury18, surgical patients receiving two lung ventilation3,4, and small studies of patients undergoing thoracic surgery with 1LV2,6,7. Most prospective studies examining protective 1LV have demonstrated improvements in clinical outcomes or surrogate markers of lung injury2,6,7,19. A retrospective clinical study examining outcomes before and after institution of an LPV protocol also support a LPV strategy utilizing reduced VT, increased PEEP, limited airway pressures and recruitment maneuvers20.
It is important to note that low VT regimens per se may not be inherently protective. That is, in the absence of other strategies needed to prevent atelectasis - adequate PEEP and recruitment maneuvers - low VT may predispose to atelectasis. Possibly because of greater compressive forces exerted upon the dependent ventilated lung during 1LV and the use of higher inspiratory oxygen fractions, atelectasis and atelectrauma resulting from cyclic recruitment/derecruitment phenomena may be more common and problematic during 1LV. Atelectasis of the ventilated lung is a prominent feature of 1LV, has been demonstrated in an elegant animal model21, and is supported by studies demonstrating improved gas exchange22,23 and pulmonary mechanics21 after recruitment maneuvers. Once adequately recruited, the dependent ventilated lung during 1LV may require a critical level of PEEP to prevent cyclic recruitment and/or collapse. A recent study of surgical patients receiving either high or low VT regimens with an equivalent level of PEEP revealed no difference in postoperative pulmonary function24. Low VT with minimal PEEP (< 5 cm H2O) has been identified as 1) a risk factor for mortality in a large surgical cohort receiving 2LV25, 2) is associated with the risk of postoperative respiratory complications in thoracic surgical patients receiving 1LV26, and 3) leads to pulmonary inflammation and impaired oxygenation in patients undergoing hepatectomy27. Taken together, the bulk of available evidence supports the view that protective 1LV requires physiologically appropriate (low) VT and a level of PEEP sufficient to prevent both an injurious level of tidal strain as well as tidal recruitment/derecruitment phenomena.
Practice Patterns of 1LV Management
Recommendations reflecting theoretical, experimental and clinical evidence for protective 1LV have been published1,8 and may be contributing to a gradual change in clinical practice. In the current study we saw statistically and clinically significant decreases in median VT over the six year study period from 2009 to 2014, and significant increases in the proportion of patients receiving PEEP ≥ 5 cm H2O and ventilated in a manner that met our criteria for LPV.
Although very little is known about practice patterns of 1LV, it appears that changes in clinical practice have been slow. Surveys of self-reported 1LV practice in the UK and Italy suggest that approximately half14 or fewer15 respondents target VT of 6 ml/kg or less, but these results are inherently subjective and subject to reporting bias. In a single center study of 1LV practice patterns, we have previously found that practitioners continued to use high VT and low PEEP relative to recommendations26. When delivered VT was normalized to PBW, 43% of patients in the studied cohort were ventilated with VT greater than 6 ml/kg and only 47% received PEEP greater than 5 cm H2O. In the present multicenter cohort study, the overall median VT normalized to PBW was 6.49 ml/kg, but a significant decrease was seen over the six-year study period.
Numerous factors may contribute to the slow implementation of LPV in the management of surgical patients. First, there is a perception that the control groups used in recent prominent prospective trials3,4,28 did not reflect modern practice with regard to PEEP (zero or very low PEEP), potentially confounding interpretation. Secondly, the use of low VT per se (that is, without adequate PEEP) does not appear to be intrinsically protective24–27, potentially confounding a simple interpretation of the available data. Additionally, reader skepticism, practice inertia, and the need for accrual of a critical mass of clinically relevant information and expert opinion may all contribute to the slow adoption of LPV strategies.
Previous studies have demonstrated that, in surgical patients undergoing 2LV, female patients, obese patients, or short stature patients received larger median VT on the basis of PBW and consequently, that a smaller proportion of these subgroups received LPV5,29. A similar phenomenon was observed in the present study of thoracic surgery patients receiving 1LV. Previously defined high risk subgroups received significantly larger VT on the basis of PBW; 6.86 for BMI> 30, 7.05 for female patients, and 7.33 for patients of short stature (height < 165 cm). The delivery of greater VT in these patient subgroups may be unintentional and may occur for a number of reasons. First, many anesthesia machines do not calculate PBW. Secondly, the anesthesiologist may fail to correct for the discrepancy between actual and predicted body weight because of a lack of awareness regarding its significance. Third, modern anesthesia machines are generally programmed with preset default values for ventilator parameters such as VT and PEEP. In many cases, these default values may not reflect modern clinical practice standards but may continue to influence clinical practice. Clinically significant changes in ventilatory practice can be accomplished by simply modifying these default values (unpublished observations) or modernizing anesthesia equipment30.
Another important finding of this study is the variability in 1LV practice between institutions. Despite similarities between the four institutions from which the data were derived (all are large tertiary, academic centers), median VT during the study period varied significantly. Much more variability was seen in the proportion of cases for which LPV was utilized. LPV utilization varied from approximately 30% in Institution 1 to 0% in Institution 2, reflecting differences in application of both VT and PEEP. Even after adjustment for patient fixed effects, including patient complexity using the ASA classification, the large ICC observed in the mixed effects model demonstrate the significant impact of institution on patient management. A significant variation in the use of high VT 2LV between institutions has been reported in a prior MPOG report and is likely related to differences in equipment, culture, academic interests, and familiarity with the pertinent literature.
The reduction in VT and increased utilization of moderate PEEP levels over the study period was also associated with a decrease in levels of ΔP seen in the two institutions from which these data were available. This finding is likely to be of clinical significance, given the relatively recent identification of ΔP as an important predictor of survival in ARDS31, the association between ΔP and pulmonary complications in surgical patients32,33, and the recently reported relationship between ΔP during 1LV and morbidity after thoracic surgery26. While it is not clear what level of ΔP is protective or “safe” during 1LV, data presented herein provide evidence that improved adherence to LPV recommendations are associated with reductions in ΔP. Further studies controlling for ΔP will be required to identify levels needed to limit iatrogenic lung injury during both 2LV and 1LV.
Limitations
Several study limitations should be noted. First, this study reports upon practice patterns and is not able to provide information on the putative relationship between observed ventilation trends and resultant changes in clinical outcomes that may have resulted from these changes. Such an endeavor would require an interface between the intraoperative electronic medical record (EMR) and a database containing information on postoperative outcomes. Efforts are being made to establish such an interface and pursue this line of investigation. However, it is worthwhile to restate the well established role of, and recommendations for, LPV in improving clinical outcomes in both general surgical as well as thoracic surgical patients undergoing 1LV. Second, this is a multicenter study utilizing data from MPOG member institutions. These contributing centers use different electronic medical record (EMR) systems and data from these medical records are subsequently combined into a single database. Although the methodologies for mapping objective concepts is rigorously validated, there is a potential for error. Third, the four contributing centers are relatively homogeneous in the sense that they are large, tertiary academic medical centers. As such, practice within these centers over the study period may not be representative of current practice at other institutions. Although the inclusion of the chosen centers was guided exclusively by the aforementioned inclusion criteria, a selection bias is possible. It should be noted that none of the centers contributing data for the current study had protocols guiding the management of 1LV during the study period. The large degree of variation in practice with regard to 1LV among these institutions raises the possibility that practice at other types of centers may be even more disparate in nature. Although, ASA physical status classification data were used to adjust for patient complexity, we were unable to adjust for surgical complexity in our mixed-effect logistic regression model. Fourth, while the analyzed dataset contained date of surgery data, no protected health information was available. Therefore, it is possible that some subjects may have had multiple operations. Including patients with multiple procedures without adjusting for the likely within-subject correlation would tend to give results with artificially small standard errors and artificially narrow confidence intervals. Finally, by study design, initial search algorithms excluded cases with missing anthropometric data. This approach, while an essential component of study design, could conceivably have introduced an additional selection bias.
Summary
The primary findings of this multi-institutional study of 1LV practice were that median VT declined and the use of PEEP ≥ 5 cm H2O increased during the study period. These changes in 1LV practice accounted for a dramatic increase in the proportion of patients receiving LPV and also appear to account for a decrease in observed ΔP during 1LV. However, despite increasing adherence with recommended ventilation practices, large variations in clinical practice between institutions were observed with only approximately half of the cohort receiving LPV at the end of the study period. Moreover, obese patients, patients of short stature, and female patients received larger median VT and a lower frequency of LPV relative to that of non-obese, non short-stature, and male patients, respectively, and thus may be at higher risk of iatrogenic lung injury during 1LV.
Key Points.
Question
In light of evidence linking potentially injurious ventilation practice (supraphysiologic tidal volume and low positive end expiratory pressure) to poorer outcomes after high risk surgery, we asked whether the clinical management of one lung ventilation for thoracic surgery over the study period in several major academic centers reflected changes in this evidence base.
Findings
Although median tidal volume and driving pressure during one lung ventilation declined during the study period, a large proportion of patients, particularly those in high risk subgroups – patients who were female, obese or of short stature – were ventilated with higher than recommended tidal volumes.
Meaning
Results of the present study confirm slowly improving adherence to lung protective ventilation recommendations for one lung ventilation but also demonstrate that specific patient subgroups are at risk for receiving higher than recommended tidal volumes, possibly because of the discrepancy between actual and predicted body weight, the failure of anesthesia ventilators to calculate predicted body weight, and tendency of practitioners to use default ventilator settings.
Acknowledgments
Sources of Financial Support: Work was supported in part by NIH/General Medical Sciences grant T32 GM103730. All additional funding attributed to the Departments of Anesthesiology, University of Virginia School of Medicine, University of Vermont College of Medicine and University of Michigan Medical School.
Appendix
The authors gratefully acknowledge the valuable contributions to protocol development and final manuscript review by the MPOG Perioperative Clinical Research Committee. Members of the Committee:
Joshua Berris, D.O. - Beaumont Health, Farmington Hills, MI, USA
Steven Lins, M.D. - Bronson Healthcare, Battle Creek, MI, USA
Peter Coles, M.D. - Bronson Healthcare, Kalamazoo, MI, USA
Kenneth C. Cummings, M.D. - Anesthesiology Institute, Cleveland Clinic, Cleveland, OH, USA
Kamal Maheshwari, M.D., M.P.H. - Anesthesiology Institute, Cleveland Clinic, Cleveland, OH, USA
Mitchell F. Berman, M.D. - Department of Anesthesiology, Columbia University Medical Center, New York, NY, USA
Christopher Wedeven, M.D. - Holland Hospital, Holland, MI, USA
John LaGorio, M.D. - Mercy Health, Muskegon, MI, USA
Peter M. Fleishut, M.D. - Department of Anesthesiology, New York-Presbyterian Hospital, Weill Cornell Medicine, New York, NY, USA
Terri A. Ellis II, M.D. - St. Joseph Mercy Oakland, Pontiac, MI, USA
Susan Molina, M.D. - St. Mary Mercy Hospital, Livonia, MI, USA
Curtis Carl, M.D. - Sparrow Health System, Lansing, MI, USA
Bassam Kadry, M.D. - Department of Anesthesiology, Perioperative and Pain Medicine, Stanford University School of Medicine, Stanford, CA, USA
Wilton A van Klei, M.D., Ph.D. - Department of Anesthesiology, University Medical Center Utrecht, Utrecht, The Netherlands
Wietze Pasma, D.V.M. - Department of Anesthesiology, University Medical Center Utrecht, Utrecht, The Netherlands
Leslie C. Jameson, M.D. - Department of Anesthesiology, University of Colorado, Aurora, CO, USA
Daniel L. Helsten, M.D. - Department of Anesthesiology, Washington University School of Medicine, St. Louis, MO, USA
Michael S. Avidan, MBBCh - Department of Anesthesiology, Washington University School of Medicine, St. Louis, MO, USA
Footnotes
Contributions:
Douglas A. Colquhoun, M.B. Ch.B., M.Sc., M.P.H.: This author helped in study design, data collection, analysis and interpretation of data and writing of the manuscript.
Bhiken I. Naik, M.B.B.Ch.: This author helped in study design, analysis and interpretation of data and writing of the manuscript.
Marcel E. Durieux, M.D., Ph.D: This author helped in study design, analysis and interpretation of data and writing of the manuscript.
Amy M. Shanks, Ph.D.: This author helped in study design, analysis and interpretation of data and writing of the manuscript.
Sachin Kheterpal, M.D., M.B.A.: This author helped in study design, analysis and interpretation of data and writing of the manuscript.
S. Patrick Bender, M.D., M.P.H: This author helped in study design, analysis and interpretation of data and writing of the manuscript.
Randal S. Blank, M.D., Ph.D.: This author had primary responsibility for, and helped in study design, analysis and interpretation of data, and writing of the manuscript.
Conflicts of Interest: The authors declare no conflicts of interest.
Contributor Information
Douglas A. Colquhoun, Clinical Lecturer in Anesthesiology, Department of Anesthesiology, University of Michigan Medical School, Ann Arbor, MI, USA
Bhiken I. Naik, Associate Professor of Anesthesiology, Departments of Anesthesiology and Neurosurgery, University of Virginia Health System, Charlottesville, VA, USA
Marcel E. Durieux, Professor of Anesthesiology, Department of Anesthesiology, University of Virginia Health System, Charlottesville, VA, USA
Amy M. Shanks, Associate Research Scientist, Department of Anesthesiology, University of Michigan Medical School, Ann Arbor, MI, USA
Sachin Kheterpal, Associate Professor of Anesthesiology, Department of Anesthesiology, University of Michigan Medical School, Ann Arbor, MI, USA
S. Patrick Bender, Associate Professor of Anesthesiology, Department of Anesthesiology, University of Vermont College of Medicine, Burlington, VT, USA
Randal S. Blank, Associate Professor of Anesthesiology, Department of Anesthesiology, University of Virginia Health System, Charlottesville, VA, USA
References
- 1.Lohser J, Slinger P. Lung Injury After One-Lung Ventilation: A Review of the Pathophysiologic Mechanisms Affecting the Ventilated and the Collapsed Lung. Anesth Analg. 2015;121:302–318. doi: 10.1213/ANE.0000000000000808. [DOI] [PubMed] [Google Scholar]
- 2.Michelet P, D’Journo XB, Roch A, et al. Protective ventilation influences systemic inflammation after esophagectomy: a randomized controlled study. Anesthesiology. 2006;105:911–919. doi: 10.1097/00000542-200611000-00011. [DOI] [PubMed] [Google Scholar]
- 3.Severgnini P, Selmo G, Lanza C, et al. Protective mechanical ventilation during general anesthesia for open abdominal surgery improves postoperative pulmonary function. Anesthesiology. 2013;118:1307–1321. doi: 10.1097/ALN.0b013e31829102de. [DOI] [PubMed] [Google Scholar]
- 4.Futier E, Constantin JM, Paugam-Burtz C, et al. A trial of intraoperative low-tidal-volume ventilation in abdominal surgery. N Engl J Med. 2013;369:428–437. doi: 10.1056/NEJMoa1301082. [DOI] [PubMed] [Google Scholar]
- 5.Bender SP, Paganelli WC, Gerety LP, et al. Intraoperative Lung-Protective Ventilation Trends and Practice Patterns: A Report from the Multicenter Perioperative Outcomes Group. Anesth Analg. 2015;121:1231–1239. doi: 10.1213/ANE.0000000000000940. [DOI] [PubMed] [Google Scholar]
- 6.Yang M, Ahn HJ, Kim K, et al. Does a protective ventilation strategy reduce the risk of pulmonary complications after lung cancer surgery?: a randomized controlled trial. Chest. 2011;139:530–537. doi: 10.1378/chest.09-2293. [DOI] [PubMed] [Google Scholar]
- 7.Shen Y, Zhong M, Wu W, et al. The impact of tidal volume on pulmonary complications following minimally invasive esophagectomy: a randomized and controlled study. J Thorac Cardiovasc Surg. 2013;146:1267–1273. doi: 10.1016/j.jtcvs.2013.06.043. discussion 1273-1264. [DOI] [PubMed] [Google Scholar]
- 8.Brassard CL, Lohser J, Donati F, Bussieres JS. Step-by-step clinical management of one-lung ventilation: continuing professional development. Can J Anaesth. 2014;61:1103–1121. doi: 10.1007/s12630-014-0246-2. [DOI] [PubMed] [Google Scholar]
- 9.Senturk M, Slinger P, Cohen E. Intraoperative mechanical ventilation strategies for one-lung ventilation. Best Pract Res Clin Anaesthesiol. 2015;29:357–369. doi: 10.1016/j.bpa.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 10.Lohser J. Evidence-based management of one-lung ventilation. Anesthesiol Clin. 2008;26:241–272. doi: 10.1016/j.anclin.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 11.Licker MJ, Widikker I, Robert J, et al. Operative mortality and respiratory complications after lung resection for cancer: impact of chronic obstructive pulmonary disease and time trends. Ann Thorac Surg. 2006;81:1830–1837. doi: 10.1016/j.athoracsur.2005.11.048. [DOI] [PubMed] [Google Scholar]
- 12.Zingg U, Smithers BM, Gotley DC, et al. Factors associated with postoperative pulmonary morbidity after esophagectomy for cancer. Ann Surg Oncol. 2011;18:1460–1468. doi: 10.1245/s10434-010-1474-5. [DOI] [PubMed] [Google Scholar]
- 13.Tandon S, Batchelor A, Bullock R, et al. Peri-operative risk factors for acute lung injury after elective oesophagectomy. Br J Anaesth. 2001;86:633–638. doi: 10.1093/bja/86.5.633. [DOI] [PubMed] [Google Scholar]
- 14.Della Rocca G, Langiano N, Baroselli A, Granzotti S, Pravisani C. Survey of thoracic anesthetic practice in Italy. J Cardiothorac Vasc Anesth. 2013;27:1321–1329. doi: 10.1053/j.jvca.2013.03.026. [DOI] [PubMed] [Google Scholar]
- 15.Shelley B, Macfie A, Kinsella J. Anesthesia for thoracic surgery: a survey of UK practice. J Cardiothorac Vasc Anesth. 2011;25:1014–1017. doi: 10.1053/j.jvca.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 16.Freundlich RE, Kheterpal S. Perioperative effectiveness research using large databases. Best Pract Res Clin Anaesthesiol. 2011;25:489–498. doi: 10.1016/j.bpa.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 17.Kheterpal S. Clinical research using an information system: the multicenter perioperative outcomes group. Anesthesiol Clin. 2011;29:377–388. doi: 10.1016/j.anclin.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 18.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 19.Schilling T, Kozian A, Huth C, et al. The pulmonary immune effects of mechanical ventilation in patients undergoing thoracic surgery. Anesth Analg. 2005;101:957–965. doi: 10.1213/01.ane.0000172112.02902.77. [DOI] [PubMed] [Google Scholar]
- 20.Licker M, Diaper J, Villiger Y, et al. Impact of intraoperative lung-protective interventions in patients undergoing lung cancer surgery. Crit Care. 2009;13:R41. doi: 10.1186/cc7762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kozian A, Schilling T, Schutze H, Senturk M, Hachenberg T, Hedenstierna G. Ventilatory protective strategies during thoracic surgery: effects of alveolar recruitment maneuver and low-tidal volume ventilation on lung density distribution. Anesthesiology. 2011;114:1025–1035. doi: 10.1097/ALN.0b013e3182164356. [DOI] [PubMed] [Google Scholar]
- 22.Tusman G, Bohm SH, Sipmann FS, Maisch S. Lung recruitment improves the efficiency of ventilation and gas exchange during one-lung ventilation anesthesia. Anesth Analg. 2004;98:1604–1609. doi: 10.1213/01.ANE.0000068484.67655.1A. [DOI] [PubMed] [Google Scholar]
- 23.Unzueta C, Tusman G, Suarez-Sipmann F, Bohm S, Moral V. Alveolar recruitment improves ventilation during thoracic surgery: a randomized controlled trial. Br J Anaesth. 2012;108:517–524. doi: 10.1093/bja/aer415. [DOI] [PubMed] [Google Scholar]
- 24.Treschan TA, Kaisers W, Schaefer MS, et al. Ventilation with low tidal volumes during upper abdominal surgery does not improve postoperative lung function. Br J Anaesth. 2012;109:263–271. doi: 10.1093/bja/aes140. [DOI] [PubMed] [Google Scholar]
- 25.Levin MA, McCormick PJ, Lin HM, Hosseinian L, Fischer GW. Low intraoperative tidal volume ventilation with minimal PEEP is associated with increased mortality. Br J Anaesth. 2014;113:97–108. doi: 10.1093/bja/aeu054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blank RS, Colquhoun DA, Durieux ME, et al. Management of One-lung Ventilation: Impact of Tidal Volume on Complications after Thoracic Surgery. Anesthesiology. 2016;124:1286–1295. doi: 10.1097/ALN.0000000000001100. [DOI] [PubMed] [Google Scholar]
- 27.Sato H, Nakamura K, Baba Y, Terada S, Goto T, Kurahashi K. Low tidal volume ventilation with low PEEP during surgery may induce lung inflammation. BMC Anesthesiol. 2016;30(16):47. doi: 10.1186/s12871-016-0209-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hemmes SN, Gama de Abreu M, Pelosi P, Schultz MJ. High versus low positive end-expiratory pressure during general anaesthesia for open abdominal surgery (PROVHILO trial): a multicentre randomised controlled trial. Lancet. 2014;384:495–503. doi: 10.1016/S0140-6736(14)60416-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fernandez-Bustamante A, Wood CL, Tran ZV, Moine P. Intraoperative ventilation: incidence and risk factors for receiving large tidal volumes during general anesthesia. BMC Anesthesiol. 2011;11:22. doi: 10.1186/1471-2253-11-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blum JM, Davila V, Stentz MJ, Dechert R, Jewell E, Engoren M. Replacement of anesthesia machines improves intraoperative ventilation parameters associated with the development of acute respiratory distress syndrome. BMC Anesthesiol. 2014;14:44. doi: 10.1186/1471-2253-14-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amato MB, Meade MO, Slutsky AS, et al. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med. 2015;372:747–755. doi: 10.1056/NEJMsa1410639. [DOI] [PubMed] [Google Scholar]
- 32.Neto AS, Hemmes SN, Barbas CS, et al. Association between driving pressure and development of postoperative pulmonary complications in patients undergoing mechanical ventilation for general anaesthesia: a meta-analysis of individual patient data. Lancet Respir Med. 2016;4:272–80. doi: 10.1016/S2213-2600(16)00057-6. [DOI] [PubMed] [Google Scholar]
- 33.Blum JM, Stentz MJ, Dechert R, et al. Preoperative and intraoperative predictors of postoperative acute respiratory distress syndrome in a general surgical population. Anesthesiology. 2013;118:19–29. doi: 10.1097/ALN.0b013e3182794975. [DOI] [PMC free article] [PubMed] [Google Scholar]


