Abstract
Objective
Working memory (WM) deficits are consistently reported in schizophrenia and are related to poor functional outcomes. Functional magnetic resonance imaging (fMRI) studies of adult-onset schizophrenia (AOS) show reduced functional activations and connectivity in the WM network, but no prior fMRI study has examined WM in childhood-onset schizophrenia (COS). The aim of this study was to examine the neural correlates of WM in COS.
Method
Adult patients with COS (n=32, 21.3±1.1years), nonpsychotic siblings of COS (n=30, 19.4±0.8), and healthy controls (n=39, 20.0±0.7) completed 1- and 2-back working memory tasks during 3T fMRI scanning. Both functional activation and connectivity analyses were conducted. A separate group of 23 younger patients with COS (17.9±7.4) could not perform the tasks after twice completing a standard training and were not studied here.
Results
Patients with COS who were included scored significantly lower than controls on all tasks (p<.001). Patients with COS showed significantly lower activations in the dorsolateral prefrontal cortices, posterior parietal cortices, cerebellum and caudate, as well as reduced fronto-parietal and corticostriatal functional connectivity compared to controls (p<.05, corrected). Siblings had functional activations and connectivity intermediate between patients and controls in a similar set of regions (p<.05, corrected). In patients, functional connectivity strength in the left fronto-parietal network correlated positively with accuracy scores during the 1-back task (p=.0023, corrected).
Conclusion
Reduced functional activation and connectivity in the WM network in COS supports pathophysiologic continuity with AOS. The low participation rate and accuracy of the patients highlights the disease severity of COS. Hypo-activations and hypo-connectivity were shared by siblings of patients with COS, suggesting COS as a potential endophenotype.
Keywords: childhood-onset schizophrenia, fMRI, working memory
INTRODUCTION
Childhood-onset schizophrenia (COS, defined as onset prior to age 13) represents a rarer and more severe form relative to adult-onset schizophrenia (AOS, onset above age 18) and early-onset schizophrenia (EOS, onset between age 13 and 18).1–3 COS also has a more homogeneous phenotype (e.g., patients with COS are less likely than patients with AOS to be affected by substance abuse issues), and in general has greater genetic vulnerability than AOS.3,4 Therefore, studying COS may offer insights into the onset and course of schizophrenia.1,2,5
Most of our knowledge regarding COS was derived from a longitudinal COS study2 during which we continuously monitored a cohort of patients with COS from childhood to adolescence to adulthood. To date, COS studies have not examined the neural correlates of working memory (WM), a cognitive system of short-term storage and manipulation of information,6 which can be examined by tasks like n-back.7 While some EOS studies8,9 examined patients with the onset age between 12 and 18 years, which has one year overlap with the one (< 13) defined in COS, most of these patients had the onset age beyond 13. WM deficits have been consistently found in AOS and are linked to their functional impairments.10 It appears that WM impairments are even more severe in patients with COS.11 Furthermore, WM continues to develop in late childhood and early adolescence12,13 so the childhood onset of schizophrenia may impact working memory development in patients with COS potentially making their working memory deficits distinct from those of patients with later onset. Together, this highlights the need for a careful study of patients with COS across the lifespan.
AOS functional neuroimaging studies have identified the prefrontal and parietal cortex, specifically the dorsolateral prefrontal cortex (DLPFC), but also the ventrolateral prefrontal cortex (VLPFC) and anterior cingulate cortex (ACC) as key regions of WM dysfunction.7,14 Imaging studies have also found aberrant activation in patients with AOS in the cerebellum,15 anterior and superior temporal lobe,16 thalamus,15,16 and caudate17 in conjunction with prefrontal cortex aberrations during WM tasks. In general, these studies have shown reduced prefrontal cortex activation, or hypofrontality, during WM tasks in patients with AOS compared to healthy controls.16,18 However, some studies found increased DLPFC activation in patients with AOS.19 These discrepancies may be due to the relative load of the WM tasks (the “inverted U” hypothesis; see Manoach14).
There are similar patterns reported in the few WM studies of EOS reporting abnormal activations in regions of the prefrontal cortex (e.g. VLPFC, DLPFC, and ACC), as well as in the limbic and temporal lobes.8,9,20,21
Studies have examined the network associated with WM functions in AOS using functional connectivity analyses. Early investigations found disturbed fronto-temporal and fronto-parietal interactions using PET blood flow data22 and EEG correlation-coefficient estimations.23 More recently, fMRI studies have found reduced prefronto-hippocampal, fronto-parietal, parieto-occipital, and cortico-striatal connectivity and increased connectivity in other networks (e.g. in the medio-dorsal thalamus and frontal eye field with visual processing areas), possibly as compensatory strategies, revealing complex, aberrant connectivity patterns in these networks.24
One EOS study found reduced DLPFC connectivity within the WM network, specifically reduced coupling or less synchronization of the DLPFC with the ACC, inferior parietal lobule and middle occipital gyrus in patients compared to controls.8
Studying WM in first-degree relatives of schizophrenia patients may elucidate a familial genetic risk of schizophrenia. Similar, though often less severe, neural abnormalities during WM tasks have been observed in healthy siblings of patients with schizophrenia in studies of brain activation25 and functional connectivity.26 Our group has found cognitive deficits in COS siblings compared to healthy controls on the Trail Making Test, an executive function test involving WM,27 but to date no functional imaging studies have examined WM in COS siblings.
In this study, the first WM fMRI study in COS, we examine the brain activations and functional connectivity in adult patients with COS, their nonpsychotic siblings, and healthy volunteers during an fMRI n-back task with three loads (0-back, 1-back, 2-back). As earlier illness onset is associated with more severe cognitive deficits28 and COS shares neurobiological features with later-onset schizophrenia,4,5 we expected similar but possibly more severe behavioral and brain abnormalities to those of AOS. Specifically, we hypothesized patients with COS would show reduced activation and functional connectivity of the fronto-parietal network. Additionally, based on adult literature,25,26 we predicted siblings of patients with COS would also show reduced functional activation and connectivity, but to a lesser degree than their affected siblings, suggesting a genetic influence on WM deficits in schizophrenia.
METHOD
Participants
Thirty-two adult patients with childhood-onset schizophrenia (COS), 30 nonpsychotic siblings (Sib) of patients with COS, and 39 typically developing controls (C) participated in the study. Age and sex were matched between groups (Table 1). The study team obtained informed assent and consent from participants and/or their guardian in accordance with a National Institutes of Health (NIH) Institutional Review Board-approved protocol. Patients with COS examined in this study were nationally recruited as part of a longitudinal study of COS at the National Institute of Mental Health.2 Criteria for selection and inclusion have been described previously.2,29 Briefly, children and adolescents 6 to 18 years old who met DSM-III-R/DSM-IV criteria for schizophrenia with onset before 13 years of age were nationally recruited. Diagnoses were confirmed by two child psychiatrists after extensive inpatient observation in the NIH intramural hospital, including a 1- to 3-week medication washout period, using DSM-III-R/DSM-IV criteria for schizophrenia. Onset age was determined as the time of initial report of symptoms that were documented in medical records by a licensed psychiatrist. Exclusion criteria were medical or neurological illness, substance use, or full-scale IQ below 70 prior to the onset of psychosis.30 Detailed interviews were conducted by a psychiatrist at each follow-up about every two years for confirmation of diagnosis and assessment of substance use. All patients with COS still had the diagnosis of schizophrenia at the time of the experiment and none had ever had substance use issues. Medication status and illness duration are shown in Table 1. Child psychiatrists’ ratings on the Scale for the Assessment of Positive Symptoms (SAPS)31 and Scale for the Assessment of Negative Symptoms (SANS)32 were used to measure patients’ symptom severity.
Table 1.
Participant Demographics
| COS | Sibling (SIB) | Control (C) | P | |
|---|---|---|---|---|
| N | 32 | 30 | 39 | |
| Age (mean ± SE) | 21.3 ± 1.1 | 19.4 ± 0.8 | 20.0 ± 0.7 | .3 |
| Sex (F:M) | 16:16 | 13:17 | 18:21 | .87 |
| Parental SES33 | 56.9 ± 28.3 | 49.9 ± 21.9 | 41.1 ± 17.5 | |
| DVARS (mean ± SE BOLD%) | 0.87 ± 0.07 | 0.64 ± 0.03 | 0.77 ± 0.13 | .27 |
| Illness duration (years, mean [SE]) | 11.3 ± 1.1 | |||
| Medication (chlorpromazine equivalents, mg, mean [SE]) | 943 ± 113 |
Note. DVARS represents a measurement of degree of motion and other artefactual changes in image intensity. Illness duration was the time between initial reporting of symptoms and functional magnetic resonance imaging (fMRI) date. All patients were taking antipsychotics at the time of scanning, typically clozapine. Medication doses are converted chlorpromazine equivalents.45 Fisher’s exact tests were used for sex comparison, F tests for others. COS = childhood-onset schizophrenia; SE = standard error of the mean; SES = socioeconomic status. All p > .16
In this study, we screened a total of 55 patients with COS who had been selected from the original longitudinal study sample of 133 patients using the simple random sampling design. Only 32 of the 55 whom we screened were capable of performing the WM task and therefore participated in the final experiment. The rest of 23 (42%) were excluded because they were unable to understand or perform the WM task after two rounds of a standardized training protocol that included instruction and practice rounds or were unable to lie still in the scanner for the duration of the task (see Table S1, available online).
Age-, parental socioeconomic status- (SES)33, and sex-matched control participants were recruited locally (Table 1) and had no lifetime medical or psychiatric disorders or first-degree relatives with psychiatric illness as determined by standardized interviews.
Behavioral Comparison Analysis
Chi-square and Fisher’s exact tests were used to compare sex and race between groups. Analysis of variance (ANOVA) models and Welch two-sample t tests were used to compare other variables. Details are listed in legends of Tables 1, 2, and S1 (available online).
Table 2.
N-Back Task Performance
| N-back | Accuracy % mean ± SD (n) | Cohen's d, P | |||
|---|---|---|---|---|---|
|
| |||||
| COS | SIB | C | COS vs. C | SIB vs.C | |
| 1-back | 64.2 ± 30.0 (30) | 87.0 ± 23.8 (30) | 89.2 ± 21.5 (39) | 1.0, <0.001 | 0.1, 0.945 |
| 2-back | 56.8 ± 23.0 (12) | 74.7 ± 24.4 (29) | 84.9 ± 19.6 (39) | 1.3, <0.001 | 0.5, 0.161 |
Note. Only patients who completed scanning were included in analyses. Groups were compared using Welch two-sample t tests, and effects were measured by Cohen’s d. BOLD = blood oxygen level-dependent; C = controls; COS = childhood-onset schizophrenia; SIB = siblings.
Image Acquisition
T2*-weighted blood oxygen level-dependent (BOLD) images were acquired on a General Electric Signa HDxt 3.0 Tesla scanner (GE Healthcare, Waukesha, WI) with an 8-channel High Resolution Brain Coil. Anatomical images were acquired using a magnetization-prepared rapid gradient-echo sequence (MPRAGE). A single-shot gradient-echo echo planar imaging (EPI) sequence was used for functional imaging (TR=2.7s, TE=28ms, 3.75 x 3.75mm). 40–46 interleaved axial slices with thickness of 3 mm were used to cover the whole brain.
N-Back Experiment
The n-back experiment was in a blocked design at three loads: 0-, 1-, and 2-back (see Figure S1, available online). All participants received a standardized training of n-back tasks before the real experiment. Failure to demonstrate understanding or ability to perform the task after two rounds of training disqualified the patient from participation. There was one run for each load, and each run included 6 blocks. The 0-back condition was used as a sensorimotor control condition, and blocks of 0-back trials were inserted between working memory task blocks. Each block included 6–14 trials, resulting in 65 trials in total. The duration of each trial was 1.9s, and each stimulus was presented for 1.5s. Each run ended with a fixation period. Thus, the total duration of each run was 264.6 seconds, equal to 98 volumes of EPI images. The order of runs was randomized across participants. The task was controlled by a program in E-Prime (Psychology Software Tools, Inc., Sharpsburg, PA) and accuracy and reaction time were recorded.
Image Preprocessing
In-plane registration, slice-time correction, and volumetric rigid-body registration were sequentially applied to the functional images. The structural MRI scan was co-registered to the functional images and then segmented and normalized into Montreal Neurological Institute (MNI) space in SPM12.34 To further remove susceptibility artifacts generated by motion and physiological noise (blood pulsation, respiration, etc.), we applied the dual-mask spatial independent component analysis (sICA) to the motion and slice time-corrected functional data.35 The denoised functional data were then normalized into the MNI space at a voxel size of 3x3x3 mm and smoothed to a target full-width-half-max of 8mm. Using an ANOVA model, we compared groups on DVARS,36 a whole-brain measure of the temporal derivative (D) of image intensity, by computing the root-mean-square variance across voxels (VARS) to ensure that the amount of motion and other artifact-induced changes in image intensity was low and comparable between groups after preprocessing.
Functional Activation Analysis
At the participant level, the general linear model (GLM) was implemented using SPM12.34 0-back blocks were subtracted from all 1- and 2-back task conditions. For the group analysis, voxel-wise random effects 3(groups) x 2(loads) ANOVA models in SPM1234 were used to draw statistical inferences at the population level. Z-scores were used to represent the level of statistical significance. All statistical Z maps and tables were thresholded at a voxel-wise alpha level of p<.01 and then corrected for whole-brain comparisons using cluster size 61 (AFNI37 3dClustSim, October 2015, after the bug fix in May 2015, estimating the spatial autocorrelation function from the data) to ensure family-wise error (FWE) <0.05.
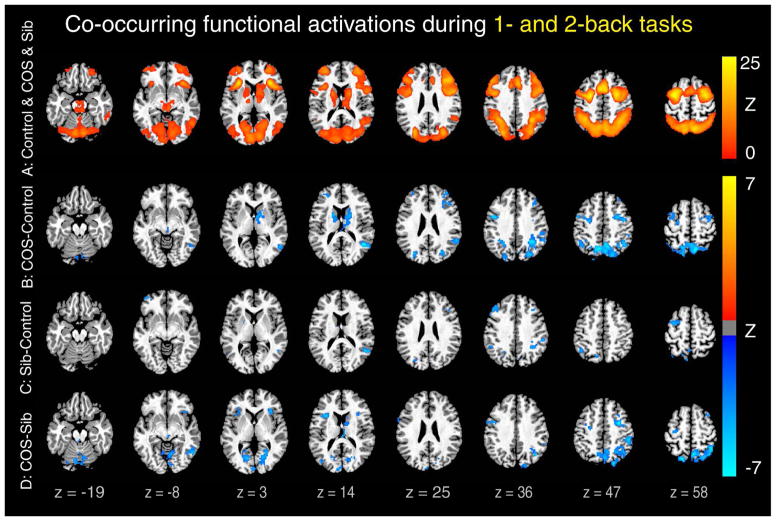
Group contrasts were masked by the union (Figure 1A) of activation maps in all patient, sibling, and control groups to refine group differences within regions that were activated during WM processing (Figure 1). For completeness, we also reported the unmasked activation maps of individual groups (see Figure S2, available online) and group contrasts (see Figure S3, available online) separately. In addition, we used accuracy scores as a covariate to examine the impact of poor WM performance on functional activity in patients (see Figure S4, available online) and examined the load effect on group contrasts (see Figure S5, available online). Finally, to unify the results at these two loads, we identified regions that showed co-occurring group differences at both 1- and 2-back loads using minimum Z conjunction, that is, calculating the minimum Z value of voxels that showed significant group differences at both 1- and 2-back loads (Figure 1).
Figure 1.
Decreased functional activation in patients and siblings compared to controls during 1- and 2-back tasks. Note: Colored Z-scores indicate co-occurring significant brain activations during both 1- and 2-back tasks versus 0-back task. A typical working memory (WM)-related activation pattern was found in patients with childhood-onset schizophrenia (COS), their siblings, and healthy controls as shown in the union activation map of these three groups (A). Within these activated regions, both patients (B) and siblings (C) showed reduced activations compared with controls. In addition, patients showed lower activations than their siblings (D). All clusters are significant (p < .05, corrected), and co-occurring maps were generated by minimum Z conjunction of 1- and 2-back contrasts. Montreal Neurological Institute coordinates of peak regions in (B), (C), and (D) are listed in Table S2, available online.
Functional Connectivity Analysis
A seed-based functional connectivity analysis was done to the residual time-series of the GLM analysis after removing variance explained by the task regressors. A finite impulse response band-pass filter (0.08–0.1 Hz) was applied to these residual time-series to remove high-frequency physiological noise or low-frequency fluctuations caused by scanner signal drifts and stimulus on-off manipulations. To account for the delay of hemodynamic response, the data for each condition was shifted by three volumes and concatenated.
Two sets of seeds were used: the first set (see Figure S6, available online) was defined by regions that showed significant differences in functional activations between groups, that is, the union of patients vs. controls (Figure 1B) and siblings vs. controls (Figure 1C); the second (see Figure S7, available online) was defined by regions that were activated but did not show group differences, that is, the union activation mask of all three groups (Figure 1A) minus the first set (see Figure S6, available online). These two sets of seeds were consistently applied to functional connectivity analyses in all three groups and at both loads.
Using AFNI,37 the Fisher’s z-transformed Pearson’s correlation maps were calculated between the average time series of all voxels within each seed region and each of the other voxels in the brain. The same ANOVA models used for the group-level functional activation analyses were used again to compare functional connectivity between groups at each load. To control for the number of seeds examined, the significance threshold used for functional activation (FWE < 0.05) was raised to FWE < 0.05/the number of seeds examined. First, all statistical Z maps were thresholded at a voxel-wise alpha level of p<.01 and then corrected for whole-brain comparisons using cluster size 87 corresponding to FWE < 0.05/12=0.0042 for the first set of 12 seeds, and 90 for the second set of 16 seeds corresponding to FWE <0.05/16=0.0031. Group differences in functional connectivity were masked by the union of functional activation maps of all three groups and the union of functional connectivity maps of all three to remove task-unrelated functional connections.
Behavioral Correlation Analysis
We evaluated whether functional activations and connectivity where group differences were identified above were linearly modulated by task performance, measured by accuracy, in patients and siblings. Using linear regression models, we examined the relationship between the averaged accuracy scores and amplitudes of activation (or Fisher’s Z transformed connectivity) across participants in each group. Using Bonferroni correction, the significance threshold was set to 0.05/the number of regions (or connections) examined. We also tested for correlations of illness duration and medication doses with the average amplitudes of functional activation and connectivity across patients.
RESULTS
The patient, sibling, and control groups did not significantly differ in age or sex (Table 1). Using an ICA-based de-noising algorithm, motion- and other noise-induced artifacts were low and did not significantly differ between groups.
Behavioral Comparison
Patients with COS who participated in the study had significantly lower accuracy scores in both identity and location conditions at both 1- and 2-back loads compared to healthy controls (Table 2). Additionally, over half of the patients with COS who could complete the 1-back tasks were not able to complete the 2-back tasks. Siblings did not show significantly lower accuracy scores than controls on any task; however, their accuracy was generally lower than controls, and effect sizes increased when working load went from 1- to 2-back.
Notably, other than the 32 patients with COS who participated in this study, 23 (42%) of the patients with COS screened were excluded from this study due to inability to perform even the 1-back task or remain still in the scanner. They scored significantly higher on positive and negative symptoms scales and were marginally younger than 32 patients with COS who participated in this study (see Table S1, available online), suggesting that this cohort, especially at an earlier age, could have even more severe WM deficits. This also explains why it was only feasible and necessary to study this subset of 32 patients with COS in adulthood (21.3 years). In the following imaging analyses, the 23 patients with COS unable to learn the task were not included.
Group Comparisons of Functional Activations
For both 1- and 2-back tasks (Figures S2A and D, available online), controls showed robust activations in the expected WM-related regions7: the bilateral DLPFC and VLPFC, medial and lateral posterior parietal, anterior cingulate (ACC), lateral and medial premotor cortices (pre-Supplementary Motor Area); cerebellum; and subcortical areas including thalamic and caudate nuclei. Control participants also demonstrated substantial deactivations in the default mode network, including the medial prefrontal cortex (mPFC), posterior dorsal cingulate, precuneus, and lateral temporal areas, as well as the hippocampus. A similar set of brain regions were significantly activated and deactivated in patients with COS and siblings (Figure S2: B, C, E, and F, available online). Group differences were shown in Figure S3 (available online), and there were a few regions (e.g., auditory cortices) that showed significantly higher activations in patients than in controls (Figure S3A and D, available online). Since these regions were strongly deactivated in both patients and controls (see Figure S2, available online), it is hard to understand the meaning of group differences in these regions. Therefore, to avoid bias, we applied a mask of the union of functional activations of all three groups (Figure 1A) to group differences (shown in Figure 1B–D) to restrict them to the regions that have been consistently reported to be involved in WM tasks. Compared to controls, both patients with COS and their siblings showed significantly lower activation in most of those brain regions including the DLPFC, posterior parietal cortices, cerebellum, and caudate (the last two only in patients) (Figure 1B, C, and Table S2, available online) for both working loads. Relative to siblings, patients with COS also showed hypoactivation in a similar set of regions including the posterior parietal cortices plus the motor and sensory related regions (Figure 1D and Table S2, available online), suggesting that the level of functional activations in siblings is in the middle between patients and controls.
As task performance in patients with COS was strikingly low, we did two parallel group comparison analyses to examine its effect on the results, one with accuracy score as a covariate of interest, and the other only in blocks with high accuracy scores. Using both methods, this hypoactivation pattern remained unchanged in patients with COS (see Figure S4, available online), indicating that these group differences were unlikely to have been driven only by poor performance. This hypoactivation pattern is consistent with reports in the AOS literature.38
We further examined the load effect on these group differences (see Figure S5, available online) and found that all three group differences were enlarged in 2-back than in 1-back. We were not able to test the inverted-U effect with this bi-load design and, as expected, group differences simply became larger as the task difficulty increased.
Group Comparisons of Functional Connectivity
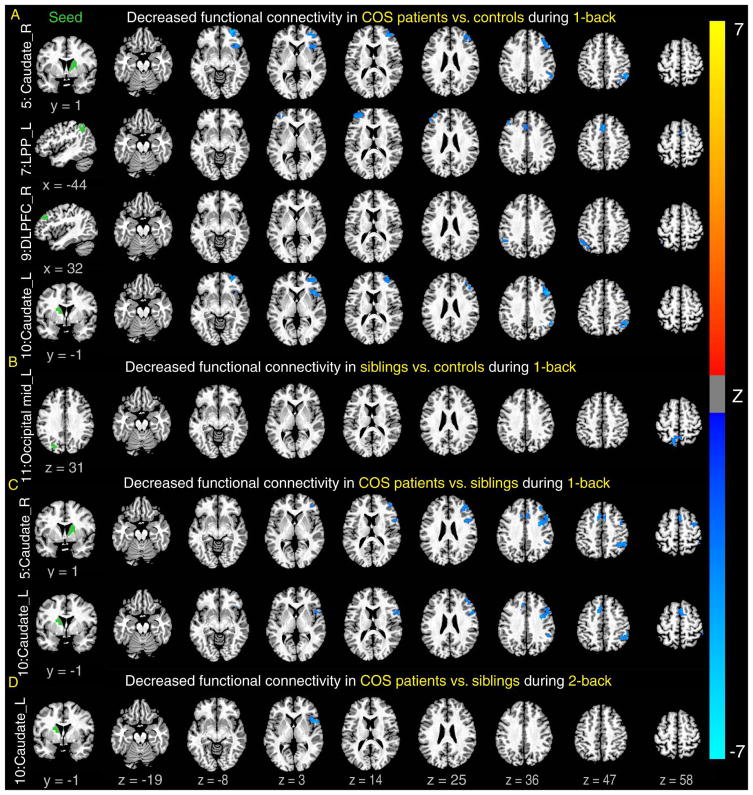
We used two sets of seeds to examine whether patients with COS and their siblings show impairments in functional connectivity in regions, identified above (Figure 1A), that showed significant group differences in brain activations. When using the first set of seeds (see Figure S6, available online) where either patients or siblings showed reduced activations compared to controls, compared to both controls (Figure 2A) and siblings (Figure 2C, D), patients with COS showed significantly lower functional connectivity, mainly between regions within the fronto-parietal network, e.g., the lateral posterior parietal cortex and DLPFC, and between this network and regions outside of this network primarily in the caudate. Although not as widely as in patients, siblings also showed significantly reduced functional connectivity compared to controls in one pair of regions, between the medial posterior parietal cortex and the visual processing related area (Figure 2B). This suggests that functional integrations of these regions in and out of the fronto-parietal network were attenuated in patients compared with controls and again, with siblings in between patients and controls.
Figure 2.
Decreased functional connectivity in patients compared to controls and siblings. Note: Patients showed significantly lower functional connectivity between seeds in the left column and many of brain regions on the right (that were activated, shown in Figure 1A) than controls during 1-back (A) and siblings during 1-back (C) and 2-back (D). In addition, siblings also showed lower functional connectivity than controls (B). Seeds were defined by regions showing lower activations in patients or siblings compared with controls (see details in Figure S6, available online). All clusters are significant (p < .05, corrected for both the number of voxels and seeds examined). LPP = lateral posterior parietal cortex.
When using the second set of seeds (see Figure S7, available online) where patients with COS or siblings showed functional activations without reductions compared with controls, compared to both controls and siblings, patients with COS still showed reduced functional connectivity in most pairs. But in a few (1st, 4th, and 6th rows in Figure S7A, available online), mainly associated with motor and sensory regions, we found significantly higher functional connectivity in patients with COS than in controls, implying that there may be an attempted compensatory mechanism at work in patients.
Correlation With Task Performance
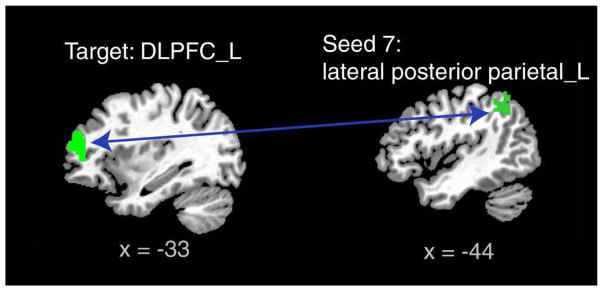
We examined whether task performance, meaning accuracy scores, of patients with COS and their siblings could be predicted by their activation amplitudes or functional connectivity strength where group differences were identified above. After Bonferroni correction for the number of pairs of functional connectivity examined, we found patients’ functional connectivity strength between the left DLPFC and lateral posterior parietal area were positively correlated with their performance during 1-back task (Figure 3). No relationship between sibling task performance and either activation or connectivity levels was found. No associations were seen between medication dose or illness duration and activation or connectivity.
Figure 3.
Correlation between task performance and functional connectivity in patients with children-onset schizophrenia. Note: Functional connectivity between the left lateral posterior parietal (LPP) and the left dorsal lateral prefrontal cortex (DLPFC, see the second row of Figure 2 for its group difference) correlated positively with accuracy scores of patients during 1-back task, Spearman correlation r = 0.53, p=.0023 < .05/12=.0042, Bonferroni correction of a total of twelve examined pairs of seed and target areas (in Figure 2A).
DISCUSSION
In this study, we examined neural activations and functional connectivity during WM tasks across patients with COS, their nonpsychotic siblings, and typically-developing controls. First, we found significantly lower WM accuracy scores in patients compared to controls. Second, consistent with many AOS studies, patients with COS showed hypoactivations in a wide set of WM-related regions centered in the frontal and parietal cortices and caudate compared to controls, with activation levels of siblings between those of COS and controls. Finally, similar to AOS studies, patients with COS had reduced fronto-parietal functional connectivity during WM tasks compared to controls, and the functional connectivity within this fronto-parietal network was correlated with WM performance in patients with COS.
The behavioral results of this study underscore the severity of working memory deficits in our COS sample. Patients with COS scored significantly lower in WM task accuracy than healthy controls, averaging less than 65% accuracy on the 1-back task. Notably, almost half the patients screened were unable to perform even the 1-back task. Furthermore, among those who could, only 44% accomplished 2-back tasks. While most AOS studies also find significantly lower accuracy in patients than controls, they generally include large samples of patients capable of performing both 1- and 2-back tasks.16,18,26,39 Similarly, EOS studies did not have to exclude patients even in 3-back20,21 and found lower accuracy rates in 2-back, but not significantly, between patients and controls.8,9 As the task used in the current study is similar to ones used in these studies in EOS and AOS, these results suggest patients with COS may be more severely impaired than patients with later onset. Siblings’ performance was intermediate between patients and controls, although not significantly lower than controls, which is consistent with previous AOS findings.40
Consistent with AOS and several EOS studies, we found patients with COS and their siblings had lower activations in the DLPFC and posterior parietal cortices compared to controls.8,16,18,21 Additionally, we found lower activation in the cerebellum and caudate in patients than in controls. Similarly, in comparable studies of AOS samples, Koch et al.17 found hypoactivation in the caudate, while Andreasen et al.15 found hypoactivation in the cerebellum and the prefrontal cortex. While the caudate and cerebellum are traditionally considered to be involved in motor control, increasing evidence suggests that they also contribute to cognitive processing, the cerebellum in encoding and maintenance during WM tasks, and the caudate in selection of relevant information during memory retrival.41 Siblings did not show hypoactivation of these regions and instead only showed hypoactivation in primary WM regions: the lateral PFC and posterior parietal cortex. In addition, compared with siblings, patients also showed hypoactivation in the posterior parietal cortex and other motor and visual-related regions. This finding is directly in line with previous WM studies of patients with AOS and their unaffected first-degree relatives that found similar but less severe aberrations in siblings than in patients.25,40 The intermediate neural abnormalities found in siblings suggest that WM likely has a genetic influence.
Unlike our findings, some AOS and EOS studies report hyperactivation in this set of WM regions as opposed to hypoactivations.9,19,20 This discrepancy may be due to the WM load of the task, which can affect DLPFC activation. In general, task difficulty increases with the increase of the WM load level, e.g., 2-back is harder than 1-back. In controls, WM tasks cause DLPFC activation that follows an inverted-U trend, where activation increases until a task-difficulty threshold after which it begins to decline.14 Studies suggest this curve is left-shifted (toward lower loads)42 or flattened43 (i.e., more restricted changes in amplitude over different load levels) in patients with schizophrenia, which may account for discrepancies in hypo- verses hyper-activations in patients. Our study was unable to address load because it requires an experimental design with multiple levels of WM load, and we could not collect loads higher than 2-back due to the severity of impairment experienced by patients with COS, as only 58% could perform the tasks and only 44% of those could complete the 2-back task. Nevertheless, while some uncertainty remains regarding the direction of the aberrant activations, our findings are generally consistent with the majority of AOS studies.
Our functional connectivity results also resemble the EOS and AOS literature. When using seeds that showed funcational activation differences between groups, we found patients with COS showed significantly reduced functional connectivity in the fronto-parietal and cortico-striatal networks. This finding aligns with previous adult studies showing that fronto-parietal and cortico-striatal connectivity is lower in patients with AOS during WM tasks,62 and one similar EOS study showing decreased fronto-parietal connectivity, specifically between the DLPFC and the inferior parietal lobule.8 Compared to controls, siblings showed deficits, although less severe, in functional connectivity in several similar networks to patients. This is consistent with AOS studies that find functional connectivity differences in siblings that are intermediate between AOS and controls.26 When using seeds where functional activations were normal in patients and siblings, the hypofunctional connectivity pattern still dominated in patients. A few pairs of regions related to motor and sensory function showed hyperfunctional connectivity, which has been seen in AOS studies as well,24 and suggests the existence of an insufficient compensatory system. Thus, our findings demonstrate continuity (i.e., a similar pattern) of functional connectivity deficits with comparable adult studies both in terms of affected networks and the severity of sibling deficit.
Finally, we found that the strength of patients’ functional connectivity between the left DLPFC and lateral posterior parietal area was positively correlated with higher performance during the location 1-back task, in accordance with Henseler et al. (2010) regarding AOS.24 This observation suggests that decreased fronto-parietal functional connectivity may underlie WM dysfunction in patients with COS. Interestingly, the strength of fronto-parietal connectivity during a working memory task has been associated with genetic variations that are also linked to psychosis,44 which complements our results and suggests that disturbed fronto-parietal connectivity may be a potential marker of schizophrenia.
This study had several limitations. First, many of the patients with COS in our larger cohort were too impaired to return for follow-up, remain still in the scanner, or complete the task. Consequently, the subset reported here represents higher-functioning patients, which may have resulted in an underestimate of the severity of WM deficits in COS. Furthermore, many younger patients were unable to complete the task, so our average age at the time of study (21.3±1.1 years) was also high for a COS sample and limits the neurodevelopmental interpretations of our findings. The combination of these conditions led to a reduced number of patients able to be included in the analysis. Lastly, patients were generally taking antipsychotics at the time of scanning and had been for extended periods, which could affect our findings. However, we did not find an association between illness duration or medication doses and functional activity or connectivity, suggesting minimal impact of these factors. Meanwhile, we could not completely exclude the possibility that this lack of correlation may be caused by the saturation effect, as most of our patients had both long illness duration and high medication doses. This warrants careful examination in the future.
In conclusion, we found reduced functional activations and connectivity in patients with COS during an fMRI WM study, supporting the notion of continuity with AOS. Additionally, we found reduced functional activation and functional connectivity in siblings intermediate between COS and controls, suggesting a genetic component to WM. Finally, our participation rate and accuracy of patient performance demonstrates the severity of symptoms and deficits in COS and should serve as a reminder to clinicians to consider executive function deficits when treating COS.
Supplementary Material
Acknowledgments
The Intramural Research Program of the National Institute of Mental Health supported this research; Annual Report Number ZIAMH002581, ClinicalTrials.gov Identifier NCT00001198, Protocol ID 84-M-0050.
The authors thank all participants and their families for their support to this project and three anonymous referees for their insightful questions and comments.
Footnotes
Disclosure: Drs. Clasen, Lalonde, R. Berman, K. Berman, Rapoport, Liu, Mss. Loeb, Zhou, Craddock, Shora, Broadnax, and Mr. Gochman report no biomedical financial interests or potential conflicts of interest.
As all authors are US government employees and working for NIH intramural research program, the authors must comply with the NIH Public Access Policy. Please see the attached NIH Publishing Agreement & Manuscript Cover Sheet.
Clinical trial registration information—Evaluating Genetic Risk Factors for Childhood-Onset Schizophrenia; http://ClinicalTrials.gov/; NCT00001198
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Ms. Frances F. Loeb, Child Psychiatry Branch, at the National Institute of Mental Health, National Institutes of Health, Bethesda, MD.
Ms. Xueping Zhou, Child Psychiatry Branch, at the National Institute of Mental Health, National Institutes of Health, Bethesda, MD.
Ms. Kirsten E. S. Craddock, Child Psychiatry Branch, at the National Institute of Mental Health, National Institutes of Health, Bethesda, MD.
Ms. Lorie Shora, Child Psychiatry Branch, at the National Institute of Mental Health, National Institutes of Health, Bethesda, MD.
Ms. Diane D. Broadnax, Child Psychiatry Branch, at the National Institute of Mental Health, National Institutes of Health, Bethesda, MD.
Mr. Peter Gochman, Child Psychiatry Branch, at the National Institute of Mental Health, National Institutes of Health, Bethesda, MD.
Dr. Liv S. Clasen, Child Psychiatry Branch, at the National Institute of Mental Health, National Institutes of Health, Bethesda, MD.
Dr. Francois M. Lalonde, Child Psychiatry Branch, at the National Institute of Mental Health, National Institutes of Health, Bethesda, MD.
Dr. Rebecca A. Berman, Child Psychiatry Branch, at the National Institute of Mental Health, National Institutes of Health, Bethesda, MD.
Dr. Karen F. Berman, Section on Integrative Neuroimaging, Clinical & Translational Neuroscience Branch, at the National Institute of Mental Health, National Institutes of Health, Bethesda, MD.
Dr. Judith L. Rapoport, Child Psychiatry Branch, at the National Institute of Mental Health, National Institutes of Health, Bethesda, MD.
Dr. Siyuan Liu, Child Psychiatry Branch, at the National Institute of Mental Health, National Institutes of Health, Bethesda, MD.
References
- 1.Nicolson R, Rapoport JL. Childhood-onset schizophrenia: rare but worth studying. Biol Psychiatry. 1999;46:1418–1428. doi: 10.1016/s0006-3223(99)00231-0. [DOI] [PubMed] [Google Scholar]
- 2.Gordon CT, Frazier JA, McKenna K, et al. Childhood-onset schizophrenia: an NIMH study in progress. Schizophr Bull. 1994;20:697–712. doi: 10.1093/schbul/20.4.697. [DOI] [PubMed] [Google Scholar]
- 3.Ahn K, Gotay N, Andersen TM, et al. High rate of disease-related copy number variations in childhood onset schizophrenia. Mol Psychiatry. 2014;19(5):568–572. doi: 10.1038/mp.2013.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gogtay N, Rapoport JL. Childhood-onset schizophrenia: insights from neuroimaging studies. J Am Acad Child Adolesc Psychiatry. 2008;47:1120–1124. doi: 10.1097/CHI.0b013e31817eed7a. [DOI] [PubMed] [Google Scholar]
- 5.Jacobsen LK, Rapoport JL. Research update: childhood-onset schizophrenia: implications of clinical and neurobiological research. J Child Psychol Psychiatry. 1998;39:101–113. [PubMed] [Google Scholar]
- 6.Baddeley A. Working memory. Science. 1992;255:556–559. doi: 10.1126/science.1736359. [DOI] [PubMed] [Google Scholar]
- 7.Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp. 2005;25:46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kyriakopoulos M, Dima D, Roiser JP, Corrigall R, Barker GJ, Frangou S. Abnormal functional activation and connectivity in the working memory network in early-onset schizophrenia. J Am Acad Child Adolesc Psychiatry. 2012;51:911–920. e912. doi: 10.1016/j.jaac.2012.06.020. [DOI] [PubMed] [Google Scholar]
- 9.Thormodsen R, Jensen J, Holmen A, et al. Prefrontal hyperactivation during a working memory task in early-onset schizophrenia spectrum disorders: an fMRI study. Psychiatry Res. 2011;194:257–262. doi: 10.1016/j.pscychresns.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 10.Dickinson D, Bellack AS, Gold JM. Social/communication skills, cognition, and vocational functioning in schizophrenia. Schizophr Bull Sep. 2007;33:1213–1220. doi: 10.1093/schbul/sbl067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karatekin C, Asarnow RF. Working memory in childhood-onset schizophrenia and attention-deficit/hyperactivity disorder. Psychiatry Res. 1998;80:165–176. doi: 10.1016/s0165-1781(98)00061-4. [DOI] [PubMed] [Google Scholar]
- 12.Cowan N. Working Memory Maturation: Can We Get at the Essence of Cognitive Growth? Perspect Psychol Sci. 2016;11:239–264. doi: 10.1177/1745691615621279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cowan N. Working Memory Underpins Cognitive Development, Learning, and Education. Educational psychology review. 2014;26:197–223. doi: 10.1007/s10648-013-9246-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manoach DS. Prefrontal cortex dysfunction during working memory performance in schizophrenia: reconciling discrepant findings. Schizophr Res. 2003;60:285–298. doi: 10.1016/s0920-9964(02)00294-3. [DOI] [PubMed] [Google Scholar]
- 15.Andreasen NC, O'Leary DS, Cizadlo T, et al. Schizophrenia and cognitive dysmetria: a positron-emission tomography study of dysfunctional prefrontal-thalamic-cerebellar circuitry. Proc Natl Acad Sci U S A. 1996;93:9985–9990. doi: 10.1073/pnas.93.18.9985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schneider F, Habel U, Reske M, et al. Neural correlates of working memory dysfunction in first-episode schizophrenia patients: an fMRI multi-center study. Schizophr Res. 2007;89:198–210. doi: 10.1016/j.schres.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 17.Koch K, Wagner G, Nenadic I, et al. Fronto-striatal hypoactivation during correct information retrieval in patients with schizophrenia: an fMRI study. Neuroscience. 2008;153:54–62. doi: 10.1016/j.neuroscience.2008.01.063. [DOI] [PubMed] [Google Scholar]
- 18.Minzenberg MJ, Laird AR, Thelen S, Carter CS, Glahn DC. Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch Gen Psychiatry. 2009;66:811–822. doi: 10.1001/archgenpsychiatry.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Callicott JH, Bertolino A, Mattay VS, et al. Physiological dysfunction of the dorsolateral prefrontal cortex in schizophrenia revisited. Cereb Cortex. 2000;10:1078–1092. doi: 10.1093/cercor/10.11.1078. [DOI] [PubMed] [Google Scholar]
- 20.White T, Hongwanishkul D, Schmidt M. Increased anterior cingulate and temporal lobe activity during visuospatial working memory in children and adolescents with schizophrenia. Schizophr Res. 2011;125:118–128. doi: 10.1016/j.schres.2010.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bittner RA, Linden DEJ, Roebroeck A, et al. The When and Where of Working Memory Dysfunction in Early-Onset Schizophrenia—A Functional Magnetic Resonance Imaging Study. Cerebral Cortex. 2015;25:2494–2506. doi: 10.1093/cercor/bhu050. [DOI] [PubMed] [Google Scholar]
- 22.Meyer-Lindenberg A, Poline JB, Kohn PD, et al. Evidence for abnormal cortical functional connectivity during working memory in schizophrenia. Am J Psychiatry. 2001;158:1809–17. doi: 10.1176/appi.ajp.158.11.1809. [DOI] [PubMed] [Google Scholar]
- 23.Peled A, Geva AB, Kremen WS, Blankfeld HM, Esfandiarfard R, Nordahl TE. Functional connectivity and working memory in schizophrenia: an EEG study. Int J Neurosci. 2001;106:47–61. doi: 10.3109/00207450109149737. [DOI] [PubMed] [Google Scholar]
- 24.Henseler I, Falkai P, Gruber O. Disturbed functional connectivity within brain networks subserving domain-specific subcomponents of working memory in schizophrenia: relation to performance and clinical symptoms. J Psychiatr Res. 2010;44:364–372. doi: 10.1016/j.jpsychires.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 25.Meda SA, Bhattarai M, Morris NA, et al. An fMRI study of working memory in first-degree unaffected relatives of schizophrenia patients. Schizophr Res. 2008;104:85–95. doi: 10.1016/j.schres.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Repovs G, Barch DM. Working memory related brain network connectivity in individuals with schizophrenia and their siblings. Front Hum Neurosci. 2012;6:137. doi: 10.3389/fnhum.2012.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gochman PA, Greenstein D, Sporn A, et al. Childhood onset schizophrenia: familial neurocognitive measures. Schizophr Res. 2004;71:43–47. doi: 10.1016/j.schres.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 28.Rajji TK, Ismail Z, Mulsant BH. Age at onset and cognition in schizophrenia: meta-analysis. Brit J Psychiat. 2009;195:286–293. doi: 10.1192/bjp.bp.108.060723. [DOI] [PubMed] [Google Scholar]
- 29.Kumra S, Wiggs E, Bedwell J, et al. Neuropsychological deficits in pediatric patients with childhood-onset schizophrenia and psychotic disorder not otherwise specified. Schizophrenia Research. 2000;42:135–144. doi: 10.1016/s0920-9964(99)00118-8. [DOI] [PubMed] [Google Scholar]
- 30.McKenna K, Gordon CT, Lenane M, Kaysen D, Fahey K, Rapoport JL. Looking for childhood-onset schizophrenia: the first 71 cases screened. J Am Acad Child Adolesc Psychiatry. 1994;33:636–644. doi: 10.1097/00004583-199406000-00003. [DOI] [PubMed] [Google Scholar]
- 31.Andreasen NC. Scale for the assessment of positive symptoms. Iowa City: University of Iowa; 1984. [Google Scholar]
- 32.Andreasen NC. Scale for the assessment of negative symptoms. Iowa City: University of Iowa; 1983. [Google Scholar]
- 33.Hollingshead AB. Social Class and Mental Illness. New York: Wiley; 1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 35.Xu Y, Tong Y, Liu S, et al. Denoising the speaking brain: toward a robust technique for correcting artifact-contaminated fMRI data under severe motion. Neuroimage. 2014;103:33–47. doi: 10.1016/j.neuroimage.2014.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 38.Eisenberg DP, Berman KF. Executive function, neural circuitry, and genetic mechanisms in schizophrenia. Neuropsychopharmacology. 2010;35:258–277. doi: 10.1038/npp.2009.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Quide Y, Morris RW, Shepherd AM, Rowland JE, Green MJ. Task-related fronto-striatal functional connectivity during working memory performance in schizophrenia. Schizophr Res. 2013;150:468–475. doi: 10.1016/j.schres.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 40.Karlsgodt KH, Glahn DC, van Erp TG, et al. The relationship between performance and fMRI signal during working memory in patients with schizophrenia, unaffected co-twins, and control subjects. Schizophr Res. 2007;89(1–3):191–197. doi: 10.1016/j.schres.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 41.McNab F, Klingberg T. Prefrontal cortex and basal ganglia control access to working memory. Nat Neurosci. 2008;11:103–107. doi: 10.1038/nn2024. [DOI] [PubMed] [Google Scholar]
- 42.Jansma JM, Ramsey NF, van der Wee NJ, Kahn RS. Working memory capacity in schizophrenia: a parametric fMRI study. Schizophr Res. 2004;68:159–171. doi: 10.1016/S0920-9964(03)00127-0. [DOI] [PubMed] [Google Scholar]
- 43.Van Snellenberg JX, Girgis RR, Horga G, et al. Mechanisms of Working Memory Impairment in Schizophrenia. Biol Psychiatry. 2016;80:617–626. doi: 10.1016/j.biopsych.2016.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Buckholtz JW, Meyer-Lindenberg A, Honea RA, et al. Allelic variation in RGS4 impacts functional and structural connectivity in the human brain. J Neurosci. 2007;27:1584–93. doi: 10.1523/JNEUROSCI.5112-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64:663–667. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.