Summary
Survival outcome of patients with peripheral T-cell lymphoma-not otherwise specified (PTCL-NOS) and angioimmunoblastic T-cell lymphoma (AITL) who experience disease progression/relapse remains very poor. A total of 321 patients, newly diagnosed with PTCL-NOS (n=180) or AITL (n=141) between 1999 and 2015, were analysed. Failure-free survival (FFS) and overall survival (OS) were calculated from the time of first disease progression (FFS1, OS1), from second disease progression (FFS2, OS2) and from third progression (FFS3, OS3). With a median follow-up duration of 52 months, 240 patients (135 PTCL-NOS, 105 AITL) experienced progression/relapse. In patients with PTCL-NOS, the median durations of FFS1, FFS2 and FFS3 were 3.1, 2.5 and 2.1 months, respectively. In patients with AITL, they were 5.5, 2.9 and 2.3 months, respectively. There was no improvement in FFS1 and OS1 by the time of recurrence during this period (1999–2004–2005–2009 and 2010–2015). The median FFS after pralatrexate and romidepsin was only 3.0 and 2.5 months, respectively. The 5-year OS rates after salvage autologous and allogeneic transplant were 32% and 52%, respectively; while the 5-year OS rates for patients who did not undergo transplant was 10%. Further research for novel therapeutic approaches with higher efficacy and better safety profile are needed.
Keywords: Peripheral T-cell lymphoma, relapse/refractory, romidepsin, pralatrexate
Introduction
Peripheral T-cell lymphoma (PTCL) is a heterogeneous group of lymphomas that represents 5–10% of all lymphomas in the United States (Chihara, et al 2014, Morton, et al 2006). The two most common histological subtypes are PTCL, not otherwise specified (PTCL-NOS) and angioimmunoblastic T-cell lymphoma (AITL), accounting for 40–50% of cases of all PTCL. Patients with PTCL-NOS and AITL are most commonly treated with CHOP (cyclophosphamide, doxorubicin, vincristine and prednisone)-like therapy, which is associated with long-term progression-free survival (PFS) of about 30% (Reimer, et al 2009, Simon, et al 2010, Vose, et al 2008). Young and fit patients may be offered more intensive approaches, including frontline stem cell transplant (SCT) for consolidation of remission, resulting in longer disease control (d’Amore, et al 2012, Reimer, et al 2009), but for those patients who do not respond to initial therapy or who experience relapse, the prognosis remains poor.
In a report from the British Colombia Cancer Agency (BCCA), the median PFS and overall survival (OS) after the first recurrence or disease progression were only 3.1 and 5.5 months, respectively, without SCT (Mak, et al 2013). There was only a small difference in the OS between patients who received chemotherapy and those who did not (6.5 vs 3.7 months, respectively). The Swedish Lymphoma Registry (SLR) also reported survival of patients with PTCL after first relapse/progression (Ellin, et al 2014). The median OS after relapse or progression in patients who initially responded to first line therapy was 6.0 months. In this study, patients in these studies were treated for recurrent disease with conventional cytotoxic chemotherapy.
Over the last six years, the US Food and Drug Administration (FDA) approved four drugs with novel mechanisms of action for the treatment of patients with recurrent PTCL: these included pralatrexate in 2009, brentuximab vedotin (BV) for anaplastic large cell lymphoma (ALCL) in 2011, romidepsin in 2011 and belinostat in 2014. While these drugs are additions to the therapeutic options, response rates were generally less than 30% with the exception of BV for patients with ALCL, and an actual impact on long-term outcome has not been well described. Herein we report the outcome of patients with recurrence of the two most common subtypes of PTCL, which are PTCL-NOS and AITL, treated at The University of Texas MD Anderson Cancer Center. We performed our analysis according to the histology and the number of prior treatment regimens, and sought to assess the impact of new therapeutic options, particularly pralatrexate and romidepsin.
Patients and Methods
We analysed the results of 321 consecutive patients with newly diagnosed with PTCL-NOS (N=180) or AITL (N=141) between 1999 and 2014. The diagnosis was based on current consensus criteria at the time as summarized by the World Health Organization (Harris, et al 1994, Jaffe, et al 2001, Swerdlow, et al 2008). Patients with ALCL were excluded from the study due to the huge impact of BV for relapsed/refractory disease. Calculation, scoring and separation of risk groups using the International Prognostic Index (IPI) and the Prognostic Index for PTCL-NOS (PIT) were performed as previously described (The International Non-Hodgkin’s Lymphoma Prognostic Factors Project 1993, Gallamini, et al 2004). Continuous variables were compared using the Mann-Whitney U-test and categorical variables were compared using the Fisher exact test between the patients with PTCL-NOS and AITL. Response to the treatment was reported according to 1999 response criteria (Cheson, et al 1999), and according to 2007 revised criteria when a positron emission tomography (PET) scan was performed (Cheson, et al 2007). Response for patients who underwent SCT was recorded prior to SCT.
Failure-free survival (FFS: progression/relapse of lymphoma or initiation of next therapy or death from treatment-related mortality/myelodysplastic syndrome) and overall survival (OS: death from any cause) from the first progression/relapse (FFS1 and OS1), from the second progression/relapse (FFS2 and OS2) and from the third progression/relapse (FFS3 and OS3) were calculated using the Kaplan-Meier method.
In addition, FFS1 and OS1 were calculated according to three different time periods of progression/relapse (1999–2004–2005–2009 and 2010–2015). The OS after SCT was calculated from the date of transplant to death from any cause. Cox proportional hazard regression models were used to evaluate characteristics affecting FFS and OS. Cox proportional hazard model was used to identify the prognostic significance of risk factors for FFS1 and OS1, and dummy variables were used for missing data (Polissar and Diehr 1982). The degree of association was described by hazard ratio (HR) with a 95% confidence interval (95%CI). Multivariate analysis was performed using all available variables. All analyses were performed using STATA version 13.1 (StataCorp LP, College Station, TX), and the significant p-value was defined as <0.05. The study was performed in accordance with the declaration of Helsinki and approved by the institutional review board of MD Anderson Cancer Center.
Results
Patient characteristics and first-line treatment
First-line chemotherapy regimens included CHOP (cyclophosphamide, doxorubicin, vincristine and prednisone, n=202), CHOEP (CHOP plus etoposide, n=15), CEOP (cyclophosphamide, etoposide, vincristine and prednisone, n=14), hyper-CVAD/MA or hyper-CVIDD/MA hyper-fractionated cyclophosphamide, vincristine, dexamethasone and doxorubicin or liposomal doxorubicin alternating with methotrexate and cytarabine: Chihara, et al 2015) (n=50), alternating triple therapy (Cabanillas, et al 1998) (n=15), EPOCH (etoposide, prednisolone, vincristine, cyclophosphamide; doxorubicin; Wilson, et al 1993) (n=8) and others (n=19). The overall response rate (ORR) to first line therapy was 75% (95%CI: 69–79%) with a complete response (CR) rate of 60% (95%CI: 54–66%). Forty-two patients received consolidative SCT following first-line therapy (auto-SCT n=39 and allo-SCT n=3), either in CR (n=38) or in partial response (n=4).
With a median follow-up of 55.5 months, 240 patients (135 PTCL-NOS, 105 AITL) experienced progression/relapse after first-line therapy. Characteristics of patients at the time of first progression/relapse are summarized in Table I. The median age of patients at progression/relapse was 60 years (range, 23–83). At the time of first progression/relapse, 11%, 36%, 32% and 21% patients were in PIT risk groups 1, 2, 3 and 4, respectively.
Table 1.
Patient characteristics at the time of first progression/relapse
| PTCL-NOS | AITL | ||
|---|---|---|---|
| N | 135 | 105 | |
| Median age, years | (range) | 55 (23–79) | 64 (28–83) |
| Gender | Male | 93 (69) | 56 (53) |
| Performance status | 2–4 | 28 (29) | 17 (22) |
| Stage | 3–4 | 75 (78) | 72 (92) |
| Lactate dehydrogenase | Above normal | 46 (48) | 34 (44) |
| Bone marrow involvement | Present | 40 (42) | 34 (44) |
| Albumin | Median (range) | 3.8 (1.3–4.8) | 3.9 (1.8–4.9) |
| Beta-2 microglobulin | Median (range) | 3.6 (1.6–12.4) | 3.7 (1.7–9.3) |
| First line chemotherapy | CHOP | 79 (56) | 80 (76) |
| Etoposide-containing regimen | 17 (16) | 10 (9) | |
| HCVAD, HCVIDD | 26 (19) | 9 (9) | |
| Others | 13 (9) | 6 (6) | |
| Prior stem cell transplant | Autologous/Allogeneic | 10/1 | 8/0 |
| Duration of response to first line therapy | < 6 months | 69 (51) | 24 (23) |
| 6 – 12 months | 42 (31) | 39 (37) | |
| ≥ 12 months | 24 (18) | 42 (40) | |
| International Prognostic Index | Low | 32 (33) | 12 (16) |
| Low-Intermediate | 23 (24) | 34 (45) | |
| Intermediate-High | 26 (27) | 20 (26) | |
| High | 15 (16) | 10 (13) | |
| PIT | Group 1 | 13 (14) | 6 (8) |
| Group 2 | 34 (35) | 28 (36) | |
| Group 3 | 28 (29) | 28 (36) | |
| Group 4 | 21 (22) | 15 (20) | |
| Response to first line therapy | CR | 51 (38) | 62 (59) |
| PR | 25 (19) | 16 (15) | |
| SD/PD | 53 (39) | 25 (24) | |
AITL, angioimmunoblastic T-cell lymphoma; CHOP, cyclophosphamide, doxorubicin, vincristine, prednisone; CR, complete response, HCVAD, hyper-fractionated cyclophosphamide, vincristine, dexamethasone, doxorubicin; HCVIDD, hyper-fractionated cyclophosphamide, vincristine, dexamethasone, liposomal doxorubicin; PD, progressive disease; PIT, prognostic index for PTCL-NOS; PR, partial response; PTCL-NOS, peripheral T-cell lymphoma-not otherwise specified; SD, stable disease
Failure-free survival and overall survival post first progression/relapse
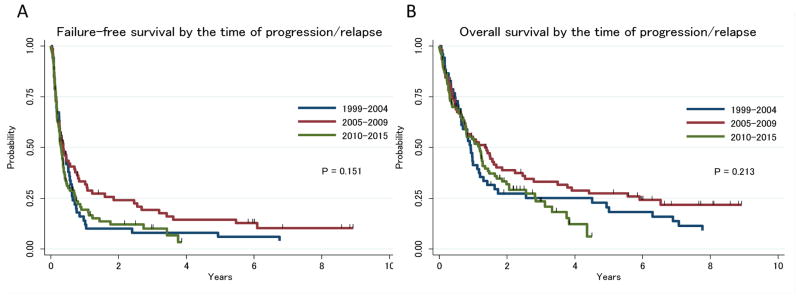
Second line treatment regimens included ICE (ifosphamide, carboplatin, etoposide, n=46, nine of whom also received romidepsin with their combination), ESHAP (etoposide, methylprednisolone, cytarabine, cisplatin, n=26, including one with BV), gemcitabine (n=25, including 18 with oxaliplatin and two with pralatrexate), single agent pralatrexate (n=7), single agent romidepsin (n=8), other investigational drugs (n=40), other chemotherapy (n=42) and unknown (treated outside MD Anderson Cancer Center, n=18). Twenty-eight patients did not receive lymphoma-specific treatment because of their poor overall condition. The ORR for second line therapy was 44% (95%CI: 36–51%) with a CR rate of 28% (95%CI: 22–36%). Twenty-five and 15 patients eventually underwent autologous and allogeneic SCT, respectively, after having had an adequate response (per treating physician) to the second line chemotherapy. There were no significant differences in response rate or FFS1 by second line regimen (Supplemental Figure 1). Time of second line treatment (1999–2005–2005–2010 and 2010–2015) did not significantly affect FFS1 and OS1 (Figure 1A, 1B). When multivatiate analysis was performed including baseline PIT risk or second PIT risk, response duration to first line therapy (as a continuous variable or <1 year vs >= 1 year), FFS1 and OS1 still remained unaffected by the time of treatment (data not shown).
Figure 1.
A, Failure-free survival by the time of progression/relapse; B, Overall survival by the time of progression/relapse
Third line regimens included ICE (n=6), ESHAP (n=15), gemcitabine (n=15, including 6 with oxaliplatin and 1 with dexamethasone and cisplatin), pralatrexate (n=5), romidepsin (n=13, including 2 with alisertib), other investigational drugs (n=29) and other chemotherapy regimens (n=29). Forty-one patients did not receive treatment. The ORR for third line therapy was 27% (95%CI: 19–36%) with a CR rate of 13% (95%CI: 7–20%). Fifteen patients received auto-SCT (n=8) or allo-SCT (n=7) after responding to the third line therapy. There were no significant differences in FFS2 according to choice of treatment regimen (data not shown).
Fourth line regimens included ICE (n=4), ESHAP (n=4), gemcitabine (n=10, including 6 with oxaliplatin and 2 with ifosfamide and vinorelbine), pralatrexate (n=5), romidepsin (n=8), other investigational drugs (n=6), other chemotherapy (n=28), and unknown (n=2). Forty-two patients did not receive treatment. The ORR for fourth line therapy was 24% (95%CI: 14–36%) with a CR rate of 14% (95%CI: 7–25%). Five patients received auto-SCT (n=2) or allo-SCT (n=3) after responding to fourth line therapy. There were no significant differences in FFS3 according to choice of treatment regimen (data not shown).
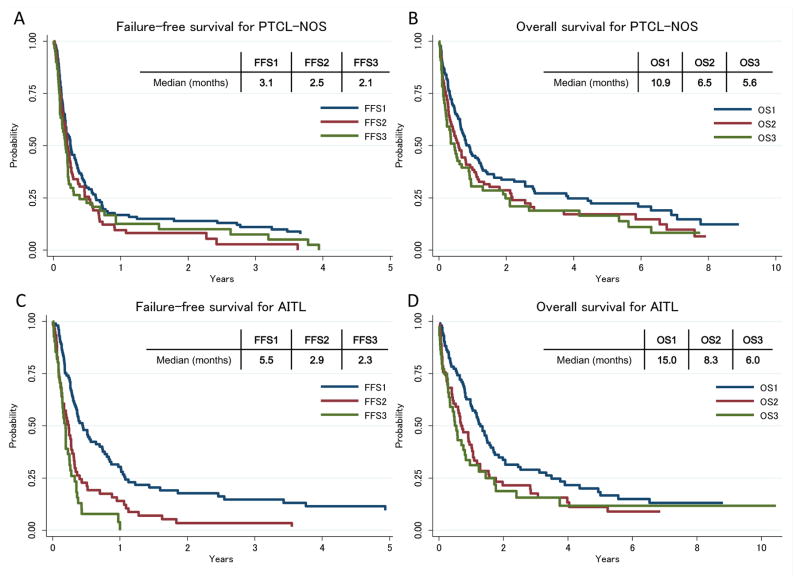
In patients with PTCL-NOS, the median FFS1 (n=135), FFS2 (n=92) and FFS3 (n=63) were 3.1, 2.5 and 2.1 months, respectively (Figure 2A): the corresponding median OS1, OS2 and OS3 were 10.9, 6.5 and 5.6 months (Figure 2B). In patients with AITL, the median FFS1 (n=105), FFS2 (n=62) and FFS3 (n=45) were 5.5, 2.9 and 2.3 months, respectively (Figure 2C), and median OS1, OS2 and OS3 were 15.0, 8.3 and 6.0 months (Figure 2D).
Figure 2. A, Failure-free survival after first, second and third progression/relapse for PTCL-NOS; B, Overall survival after first, second and third progression/relapse for PTCL-NOS; C, Failure-free survival after first, second and third progression/relapse for AITL; D, Overall survival after first, second and third progression/relapse for AITL.
AITL, angioimmunoblastic T-cell lymphoma; FFS, failure-free survival; OS, overall survival; PTCL-NOS, peripheral T-cell lymphoma-not otherwise specified.
The median FFS1 and OS1 for patients who did not proceed to SCT were 4.0 months (95%CI: 3.1–4.7 months) and 9.2 months (95%CI: 6.9–11.8 months), respectively. The median FFS2 and OS2 for those who did not proceed to SCT at any time during the treatment was 2.5 months (95%CI: 2.0–3.1 months) and 5.5 months (95%CI: 3.5–7.4 months), respectively.
Pralatrexate and romidepsin, risk factors after relapse
A total of 56 patients received pralatrexate (total n=27, as second line therapy n=10, third line therapy n=6, fourth and after n=11) and/or romidepsin (total n=42, as second line therapy n=17, third line therapy n=12, fourth and after n=13) at some point during salvage therapy. Thirteen patients received both drugs. The ORR for pralatrexate and romidepsin as single agents was 41% (95%CI: 21–64%) and 42% (95%CI: 25–61%), respectively. The CR rates for pralatrexate and romidepsin as single agents were 23% (95%CI: 8–45%) and 24% (95%CI: 11–42%), respectively. All CRs were confirmed by PET scan. Because of the small numbers, there were no significant difference in response between PTCL-NOS and AITL; however only one AITL patient achieved CR with pralatrexate compared to four PTCL-NOS patients. The number of prior regimens had no significant impact on CR rate (Supplemental Table 1). However, the CR rate from pralatrexate or romidepsin was 0% when used for fifth line therapy or more. The median FFS after pralatrexate and romidepsin as single agents was 3.0 and 2.5 months, respectively. The median durations of response in responders after pralatrexate and romidepsin as single agents were 10.5 and 9.2 months, respectively.
Univariate analysis for FFS1 and OS1 are summarized in Table II. PIT high-risk group at time of relapse was associated with shorter OS1 in both PTCL-NOS and AITL (both P<0.001). Neither pralatrexate nor romidepsin was associated with longer FFS1 compared to other therapies when used as second line therapy. Multivariate analysis incorporating second PIT risk factors at relapse, the duration of the response to first line therapy and eventual application of auto-SCT or allo-SCT revealed that use of pralatrexate at some point during the treatment was associated with significantly longer OS1 in patients with PTCL-NOS (HR: 0.33, 95%CI: 0.16–0.65, p=0.001) and AITL (HR: 0.39, 95%CI: 0.16–0.97, p=0.044) (Table III). Use of romidepsin at any point during the treatment was not associated with longer OS1 in the patients with PTCL-NOS (HR: 1.09, 95%CI: 0.61–1.97) and AITL (HR: 0.99, 95%CI: 0.53–1.85) in multivariate analysis.
Table II.
Univariate analysis for the risk factors after first progression/relapse
| Prognostic factors | Failure-free survival after first progression/relapse (FFS1) | Overall survival after first progression/relapse (OS1) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| PTCL-NOS | AITL | PTCL-NOS | AITL | ||||||||||
| HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value | ||
| Elevated LDH at relapse | 1.36 | 0.88–2.10 | 0.172 | 1.50 | 0.92–2.47 | 0.107 | 1.92 | 1.19–3.09 | 0.007 | 1.37 | 0.81–2.30 | 0.236 | |
| Performance status ≥2 at relapse | 2.40 | 1.50–3.83 | <0.001 | 1.77 | 0.99–3.18 | 0.056 | 3.77 | 2.29–6.20 | <0.001 | 3.59 | 1.94–6.64 | <0.001 | |
| Bone marrow involvement at relapse | 1.19 | 0.77–1.85 | 0.428 | 2.16 | 1.31–3.54 | 0.002 | 1.34 | 0.83–2.15 | 0.233 | 2.06 | 1.23–3.47 | 0.006 | |
| Second PIT | Group 1 | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | ||||
| Group 2 | 1.24 | 0.60–2.56 | 0.561 | 1.14 | 0.39–3.34 | 0.815 | 2.09 | 0.85–5.13 | 0.109 | 0.68 | 0.23–2.03 | 0.488 | |
| Group 3 | 2.05 | 0.97–4.31 | 0.059 | 2.93 | 1.01–8.51 | 0.049 | 3.82 | 1.53–9.54 | 0.004 | 2.55 | 0.87–7.45 | 0.087 | |
| Group 4 | 1.93 | 0.90–4.16 | 0.093 | 3.03 | 0.99–9.31 | 0.052 | 4.79 | 1.89–12.1 | 0.001 | 2.85 | 0.93–8.71 | 0.067 | |
| Response to 1st line therapy | Complete response | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | ||||
| Partial response | 1.06 | 0.63–1.79 | 0.837 | 0.73 | 0.38–1.38 | 0.332 | 1.24 | 0.71–2.16 | 0.456 | 0.74 | 0.38–1.44 | 0.372 | |
| Progressive/stable disease | 1.34 | 0.88–2.05 | 0.168 | 1.51 | 0.91–2.49 | 0.107 | 1.67 | 1.05–2.67 | 0.029 | 1.32 | 0.78–2.23 | 0.294 | |
| Response duration to 1st therapy | < 6months | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | ||||
| 6 – 12months | 0.55 | 0.36–0.84 | 0.006 | 0.92 | 0.53–1.62 | 0.781 | 0.48 | 0.30–0.77 | 0.002 | 1.46 | 0.81–2.64 | 0.212 | |
| ≥ 12months | 0.49 | 0.28–0.85 | 0.011 | 0.61 | 0.34–1.07 | 0.085 | 0.47 | 0.27–0.84 | 0.010 | 0.91 | 0.50–1.67 | 0.767 | |
| Pralatrexate at 1st salvage | 0.91 | 0.37–2.25 | 0.845 | 0.94 | 0.29–2.99 | 0.914 | 0.32 | 0.08–1.30 | 0.111 | 0.64 | 0.20–2.04 | 0.447 | |
| Pralatrexate at any time | 0.47 | 0.25–0.89 | 0.020 | 0.55 | 0.24–1.26 | 0.158 | |||||||
| Romidepsin at 1st salvage | 0.75 | 0.31–1.86 | 0.540 | 1.21 | 0.58–2.53 | 0..608 | 0.61 | 0.19–1.94 | 0.405 | 1.72 | 0.81–3.63 | 0.155 | |
| Romidepsin at any time | 0.72 | 0.42–1.23 | 0.226 | 0.95 | 0.53–1.70 | 0.870 | |||||||
95% CI, 95% confidence interval; AITL, angioimmunoblastic T-cell lymphoma; HR, Hazard ratio; PIT, prognostic index for PTCL-NOS; PTCL-NOS, peripheral T-cell lymphoma-not otherwise specified.
Table III.
Multivariate analysis for the risk factors after first progression/relapse
| Prognostic factors | Overall survival after first progression/relapse (OS1)
|
||||||
|---|---|---|---|---|---|---|---|
| PTCL-NOS | AITL | ||||||
| HR | 95% CI | P-value | HR | 95% CI | P-value | ||
| Second PIT | Group 1 | 1.00 | Reference | 1.00 | Reference | ||
| Group 2 | 1.74 | 0.70–4.36 | 0.236 | 0.58 | 0.19–1.83 | 0.357 | |
| Group 3 | 3.42 | 1.35–8.66 | 0.009 | 2.53 | 0.84–7.66 | 0.101 | |
| Group 4 | 4.58 | 1.71–12.3 | 0.002 | 2.92 | 0.92–9.26 | 0.069 | |
| Response duration to 1st therapy | < 6months | 1.00 | Reference | 1.00 | Reference | ||
| 6 – 12months | 0.43 | 0.26–0.72 | 0.002 | 1.29 | 0.68–2.44 | 0.432 | |
| ≥ 12months | 0.50 | 0.27–0.93 | 0.028 | 0.67 | 0.35–1.27 | 0.217 | |
| Pralatrexate at any time | 0.33 | 0.16–0.65 | 0.001 | 0.39 | 0.16–0.97 | 0.044 | |
| Romidepsin at any time | 1.09 | 0.61–1.97 | 0.226 | 0.99 | 0.53–1.85 | 0.979 | |
| Salvage transplant at any time | 0.23 | 0.14–0.40 | <0.001 | 0.29 | 0.16–0.53 | <0.001 | |
95 %CI, 95% confidence interval; AITL, angioimmunoblastic T-cell lymphoma; HR, Hazard ratio; PIT, prognostic index for PTCL-NOS; PTCL-NOS, peripheral T-cell lymphoma-not otherwise specified.
Results for patients undergoing SCT
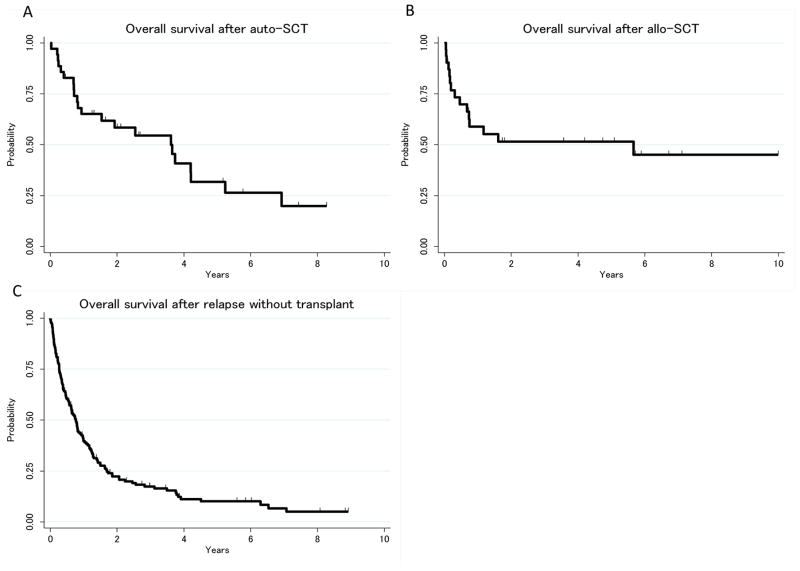
Overall, 36 patients (18 PTCL-NOS, 18 AITL) underwent auto-SCT and 31 patients (20 PTCL-NOS, 11 AITL) underwent allo-SCT after progression/relapse. Among the 31 patients who underwent allo-SCT, five patients underwent auto-SCT as part or initial therapy and three for salvage. The median number of prior regimens prior to SCT was 2 (range, 2–6) and 61% of the patients underwent SCT after the second line regimen. The OS1 for patients who received SCT after second line regimen (first progression/relapse) and OS2 for patients who received SCT after third line regimen (second progression/relapse) were 33.9 months (95%CI: 14.0–67.7 months) and 48.4 months (95%CI: 8.2 months-not reached), respectively. We did not observe a plateau of the survival curve in patients who received auto-SCT (Figure 3A), although the causes of death after 4 years post auto-SCT were not lymphoma (myelodysplastic syndrome, infection and pancreatic cancer). In contrast, only one death (lymphoma) occurred more than two years after allo-SCT (Figure 3B). The 5-year OS rates after auto-SCT and allo-SCT were 32% (95%CI: 15–50%) and 52% (95%CI: 32–68%), respectively, whereas the 5-year OS1 rates (after first progression/relapse) of patients who did not receive either SCT at any point during treatment was 10% (95%CI: 5–16%, Figure 3C).
Figure 3.
A, Overall survival after autologous stem cell transplant (auto-SCT); B, Overall survival after allogeneic stem cell transplant (allo-SCT); C, Overall survival after relapse without transplant
Discussion
The survival outcome of patients with PTCL-NOS and AITL after progression/relapse remains very poor, even with newer treatment options, and has not significantly changed since 1999. The median FFS and OS after progression/relapse for patients who did not proceed to SCT were 3.0 and 8.3 months, respectively, which are generally in keeping with the data from the British Columbia Cancer Agency and the Swedish Lymphoma Registry (Ellin, et al 2014, Mak, et al 2013). In addition, we have shown that disease control becomes more difficult as patients experience multiple recurrences.
In order to obtain a comprehensive understanding of survival outcome after progression/relapse in patients with PTCL-NOS and AITL, we included the patients who proceeded to SCT in the analyses. Because of the retrospective nature of our study, we could not assess the actual impact of SCT on survival in patients who obtained responses to salvage treatment. Yet, it still seems reasonable to consider auto-SCT or allo-SCT for patients who experience good responses to salvage therapies, as these approaches were associated with up to 50% of the 5-year FFS rate. It should be noted that the survival curve of patients after auto-SCT continues to decline after 5 years, whereas the survival curve of patients who underwent allo-SCT reached a plateau at about 50% (Figure 3B), probably due to graft-versus lymphoma effect. Our finding is similar to those of several other studies of allo-SCT for PTCL, showing three- to five-year survival rates that approach 40–50% (Dodero, et al 2012, Jacobsen, et al 2011, Kim, et al 2013, Le Gouill, et al 2008, Shustov, et al 2010). The challenge of SCT is that these treatments are only available for limited numbers of patients who are relatively young and fit, who achieve good responses to the therapy and who have an available stem cell source. In fact, only 17% and 15% of the patients received auto-SCT and allo-SCT after progression/relapse, respectively. Better salvage treatment to achieve higher response rates to increase the number of patients who can proceed to SCT, better transplant strategies for elderly patients and ultimately, better treatments to achieve longer remission without SCT are needed to improve the survival outcome of patients with relapsed/refractory PTCL-NOS and AITL.
Pralatrexate and romidepsin have been approved by the FDA for treatment of relapsed/refractory PTCL. In a phase II study of pralatrexate for 115 patients with relapsed/refractory PTCL, the drug achieved an ORR of 29% with a CR rate of 11% (O’Connor, et al 2011). The median PFS was 3.5 months, with the median duration of response of 10.1 months. In a phase II study for romidepsin for 130 patients with relapsed/refractory PTCL, the ORR was 25% with CR a rate of 15% (Coiffier, et al 2012). The median PFS was 4 months, with the median duration of response of 28 months (Coiffier, et al 2014). Our retrospective data are generally similar to those reported in these studies of romidepsin and pralatrexate. Interestingly, the use of pralatrexate at some point during the treatment was associated with a longer OS in multivariate analysis, for both patients with PTCL-NOS and AITL. This result should be interpreted with caution given the retrospective nature of this analysis and based on a limited number of patients.
Overall, despite the limitation of retrospective analysis, our analysis provides a snapshot on survival outcomes for a large number of patients with relapsed/refractory PTCL-NOS and AITL. Even with the recent approval of new drugs, there has been no significant improvement in survival for our patient cohort following progression/relapse since 1999. Further research is needed to better understand the biology of PTCL and to identify a patient population that is likely to benefit from targeted agents. Obviously, novel therapeutic approaches with higher efficacy and better safety profile are needed.
Supplementary Material
Acknowledgments
Grant: NCI P30 CA16672
Footnotes
Authorship:
Y.O. and D.C. designed the research. D.C., M.N., and R.E.D. collected data. D.C. and Y.O. analysed and interpreted the data. M.A.F., R.N.M., J.R.W., L.J.N., F.B.F., L.E.F., J.E.R., F.S., F.T., H.J.L., S.S.N., A.M.R., M.W., N.H.F., L.J.M., C.H., Y.L.N. provided data and contributed to patient care. D.C. and Y.O. wrote draft of paper and all authors contributed in writing and approved the final version of the manuscript.
Conflict-of-interest Disclosure:
Yasuhiro Oki
Honoraria: Takeda Millenium
Research funding: Seattle Genetics, Takeda Millenium and Spectrum
Michelle Fanale
Honoraria: Celgene, Spectrum
Research funding: Celgene, Seattle Genetics
Consulting or Advisory Role: Celgene, Spectrum
Nathan Fowler
Research funding: Celgene
Consulting or Advisory Role: Celgene
Jorge Romaguera
Research funding: Celgene
Chitra Hosing
Research funding: Celgene
References
- Cabanillas F, Rodriguez-Diaz Pavon J, Hagemeister FB, McLaughlin P, Rodriguez MA, Romaguera JE, Dong K, Moon T. Alternating triple therapy for the treatment of intermediate grade and immunoblastic lymphoma. Ann Oncol. 1998;9:511–518. doi: 10.1023/a:1008214629544. [DOI] [PubMed] [Google Scholar]
- Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, Lister TA, Vose J, Grillo-Lopez A, Hagenbeek A, Cabanillas F, Klippensten D, Hiddemann W, Castellino R, Harris NL, Armitage JO, Carter W, Hoppe R, Canellos GP. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999;17:1244. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, Coiffier B, Fisher RI, Hagenbeek A, Zucca E, Rosen ST, Stroobants S, Lister TA, Hoppe RT, Dreyling M, Tobinai K, Vose JM, Connors JM, Federico M, Diehl V International Harmonization Project on L. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- Chihara D, Ito H, Matsuda T, Shibata A, Katsumi A, Nakamura S, Tomotaka S, Morton LM, Weisenburger DD, Matsuo K. Differences in incidence and trends of haematological malignancies in Japan and the United States. Br J Haematol. 2014;164:536–545. doi: 10.1111/bjh.12659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chihara D, Pro B, Loghavi S, Miranda RN, Medeiros LJ, Fanale MA, Hagemeister FB, Fayad LE, Romaguera JE, Samaniego F, Neelapu SS, Younes A, Fowler NH, Rodriguez MA, Wang M, Kwak LW, McLaughlin P, Dang NH, Oki Y. Phase II study of HCVIDD/MA in patients with newly diagnosed peripheral T-cell lymphoma. Br J Haematol. 2015;171:509–516. doi: 10.1111/bjh.13628. [DOI] [PubMed] [Google Scholar]
- Coiffier B, Pro B, Prince HM, Foss F, Sokol L, Greenwood M, Caballero D, Borchmann P, Morschhauser F, Wilhelm M, Pinter-Brown L, Padmanabhan S, Shustov A, Nichols J, Carroll S, Balser J, Balser B, Horwitz S. Results from a pivotal, open-label, phase II study of romidepsin in relapsed or refractory peripheral T-cell lymphoma after prior systemic therapy. J Clin Oncol. 2012;30:631–636. doi: 10.1200/JCO.2011.37.4223. [DOI] [PubMed] [Google Scholar]
- Coiffier B, Pro B, Prince HM, Foss F, Sokol L, Greenwood M, Caballero D, Morschhauser F, Wilhelm M, Pinter-Brown L, Padmanabhan Iyer S, Shustov A, Nielsen T, Nichols J, Wolfson J, Balser B, Horwitz S. Romidepsin for the treatment of relapsed/refractory peripheral T-cell lymphoma: pivotal study update demonstrates durable responses. J Hematol Oncol. 2014;7:11. doi: 10.1186/1756-8722-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d’Amore F, Relander T, Lauritzsen GF, Jantunen E, Hagberg H, Anderson H, Holte H, Osterborg A, Merup M, Brown P, Kuittinen O, Erlanson M, Ostenstad B, Fagerli UM, Gadeberg OV, Sundstrom C, Delabie J, Ralfkiaer E, Vornanen M, Toldbod HE. Up-front autologous stem-cell transplantation in peripheral T-cell lymphoma: NLG-T-01. J Clin Oncol. 2012;30:3093–3099. doi: 10.1200/JCO.2011.40.2719. [DOI] [PubMed] [Google Scholar]
- Dodero A, Spina F, Narni F, Patriarca F, Cavattoni I, Benedetti F, Ciceri F, Baronciani D, Scime R, Pogliani E, Rambaldi A, Bonifazi F, Dalto S, Bruno B, Corradini P. Allogeneic transplantation following a reduced-intensity conditioning regimen in relapsed/refractory peripheral T-cell lymphomas: long-term remissions and response to donor lymphocyte infusions support the role of a graft-versus-lymphoma effect. Leukemia. 2012;26:520–526. doi: 10.1038/leu.2011.240. [DOI] [PubMed] [Google Scholar]
- Ellin F, Landstrom J, Jerkeman M, Relander T. Real-world data on prognostic factors and treatment in peripheral T-cell lymphomas: a study from the Swedish Lymphoma Registry. Blood. 2014;124:1570–1577. doi: 10.1182/blood-2014-04-573089. [DOI] [PubMed] [Google Scholar]
- Gallamini A, Stelitano C, Calvi R, Bellei M, Mattei D, Vitolo U, Morabito F, Martelli M, Brusamolino E, Iannitto E, Zaja F, Cortelazzo S, Rigacci L, Devizzi L, Todeschini G, Santini G, Brugiatelli M, Federico M. Peripheral T-cell lymphoma unspecified (PTCL-U): a new prognostic model from a retrospective multicentric clinical study. Blood. 2004;103:2474–2479. doi: 10.1182/blood-2003-09-3080. [DOI] [PubMed] [Google Scholar]
- Harris NL, Jaffe ES, Stein H, Banks PM, Chan JK, Cleary ML, Delsol G, De Wolf-Peeters C, Falini B, Gatter KC, Grogan TM, Isaacson PG, Knowles DM, Mason DY, Muller-Hermelink HK, Warnke RA. A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood. 1994;84:1361–1392. [PubMed] [Google Scholar]
- Jacobsen ED, Kim HT, Ho VT, Cutler CS, Koreth J, Fisher DC, Armand P, Alyea EP, Freedman AS, Soiffer RJ, Antin JH. A large single-center experience with allogeneic stem-cell transplantation for peripheral T-cell non-Hodgkin lymphoma and advanced mycosis fungoides/Sezary syndrome. Ann Oncol. 2011;22:1608–1613. doi: 10.1093/annonc/mdq698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe E, Harris N, Stein H, Vardiman J. WHO Classification Tumors of Hematopoietic and Lymphoid Tissues. International Agency for Research on Cancer (IARC); Lyon, France: 2001. [Google Scholar]
- Kim SW, Yoon SS, Suzuki R, Matsuno Y, Yi HG, Yoshida T, Imamura M, Wake A, Miura K, Hino M, Ishikawa T, Kim JS, Maeda Y, Lee JJ, Kang HJ, Lee HS, Lee JH, Izutsu K, Fukuda T, Kim CW, Yoshino T, Ohshima K, Nakamura S, Nagafuji K, Suzumiya J, Harada M, Kim CS. Comparison of outcomes between autologous and allogeneic hematopoietic stem cell transplantation for peripheral T-cell lymphomas with central review of pathology. Leukemia. 2013;27:1394–1397. doi: 10.1038/leu.2012.321. [DOI] [PubMed] [Google Scholar]
- Le Gouill S, Milpied N, Buzyn A, De Latour RP, Vernant JP, Mohty M, Moles MP, Bouabdallah K, Bulabois CE, Dupuis J, Rio B, Gratecos N, Yakoub-Agha I, Attal M, Tournilhac O, Decaudin D, Bourhis JH, Blaise D, Volteau C, Michallet M Societe Francaise de Greffe de Moelle et de Therapie Cellulaire. Graft-versus-lymphoma effect for aggressive T-cell lymphomas in adults: a study by the Societe Francaise de Greffe de Moelle et de Therapie Cellulaire. J Clin Oncol. 2008;26:2264–2271. doi: 10.1200/JCO.2007.14.1366. [DOI] [PubMed] [Google Scholar]
- Mak V, Hamm J, Chhanabhai M, Shenkier T, Klasa R, Sehn LH, Villa D, Gascoyne RD, Connors JM, Savage KJ. Survival of patients with peripheral T-cell lymphoma after first relapse or progression: spectrum of disease and rare long-term survivors. J Clin Oncol. 2013;31:1970–1976. doi: 10.1200/JCO.2012.44.7524. [DOI] [PubMed] [Google Scholar]
- Morton LM, Wang SS, Devesa SS, Hartge P, Weisenburger DD, Linet MS. Lymphoma incidence patterns by WHO subtype in the United States, 1992–2001. Blood. 2006;107:265–276. doi: 10.1182/blood-2005-06-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor OA, Pro B, Pinter-Brown L, Bartlett N, Popplewell L, Coiffier B, Lechowicz MJ, Savage KJ, Shustov AR, Gisselbrecht C, Jacobsen E, Zinzani PL, Furman R, Goy A, Haioun C, Crump M, Zain JM, Hsi E, Boyd A, Horwitz S. Pralatrexate in patients with relapsed or refractory peripheral T-cell lymphoma: results from the pivotal PROPEL study. J Clin Oncol. 2011;29:1182–1189. doi: 10.1200/JCO.2010.29.9024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polissar L, Diehr P. Regression analysis in health services research: the use of dummy variables. Med Care. 1982;20:959–966. doi: 10.1097/00005650-198209000-00008. [DOI] [PubMed] [Google Scholar]
- Reimer P, Rudiger T, Geissinger E, Weissinger F, Nerl C, Schmitz N, Engert A, Einsele H, Muller-Hermelink HK, Wilhelm M. Autologous stem-cell transplantation as first-line therapy in peripheral T-cell lymphomas: results of a prospective multicenter study. J Clin Oncol. 2009;27:106–113. doi: 10.1200/JCO.2008.17.4870. [DOI] [PubMed] [Google Scholar]
- Shustov AR, Gooley TA, Sandmaier BM, Shizuru J, Sorror ML, Sahebi F, McSweeney P, Niederwieser D, Bruno B, Storb R, Maloney DG. Allogeneic haematopoietic cell transplantation after nonmyeloablative conditioning in patients with T-cell and natural killer-cell lymphomas. Br J Haematol. 2010;150:170–178. doi: 10.1111/j.1365-2141.2010.08210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon A, Peoch M, Casassus P, Deconinck E, Colombat P, Desablens B, Tournilhac O, Eghbali H, Foussard C, Jaubert J, Vilque JP, Rossi JF, Lucas V, Delwail V, Thyss A, Maloisel F, Milpied N, le Gouill S, Lamy T, Gressin R. Upfront VIP-reinforced-ABVD (VIP-rABVD) is not superior to CHOP/21 in newly diagnosed peripheral T cell lymphoma. Results of the randomized phase III trial GOELAMS-LTP95. Br J Haematol. 2010;151:159–166. doi: 10.1111/j.1365-2141.2010.08329.x. [DOI] [PubMed] [Google Scholar]
- Swerdlow S, Campo E, Harris N, Swerdlow S, Campo E, Harris N. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. International Agency for Research on Cancer; Lyon, France: 2008. [Google Scholar]
- The International Non-Hodgkin’s Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin’s lymphoma. The International Non-Hodgkin’s Lymphoma Prognostic Factors Project. N Engl J Med. 1993;329:987–994. doi: 10.1056/NEJM199309303291402. [DOI] [PubMed] [Google Scholar]
- Vose J, Armitage J, Weisenburger D. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26:4124–4130. doi: 10.1200/JCO.2008.16.4558. [DOI] [PubMed] [Google Scholar]
- Wilson WH, Bryant G, Bates S, Fojo A, Wittes RE, Steinberg SM, Kohler DR, Jaffe ES, Herdt J, Cheson BD. EPOCH chemotherapy: toxicity and efficacy in relapsed and refractory non-Hodgkin’s lymphoma. J Clin Oncol. 1993;11:1573–1582. doi: 10.1200/JCO.1993.11.8.1573. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.