Abstract
Background: Women have higher rates of obesity than men and develop more pronounced functional deficits as a result. Yet, little is known about how obesity reduction affects their functional status, including whether their responses differ when protein intake is enhanced.
Objective: The aim of this study was to confirm the feasibility of delivery of a higher-protein (balanced at each meal) calorie-restricted diet in obese women and determine its efficacy for influencing function and retention of lean mass.
Method: Obese community-dwelling women [n = 80; body mass index (in kg/m2), in means ± SDs: 37.8 ± 5.9; aged 45–78 y; 58.8% white] were enrolled in a weight-loss (−500 kcal/d) study and randomly assigned to either a Control–Weight-Loss (C-WL; 0.8 g protein/kg body weight) group or a High-Protein–Weight-Loss (HP-WL; 1.2 g protein/kg body weight; 30 g protein 3 times/d) group in a 1:2 allocation. Primary outcomes were function by 6-min walk test (6MWT) and lean mass by using the BodPod (Life Measurement, Inc.) at 0, 4, and 6 mo.
Results: Both groups reduced calorie intakes and body weights (P < 0.001), and the feasibility of the HP-WL intervention was confirmed. The 6MWT results improved (P < 0.01) at 4 mo in the HP-WL group and at 6 mo in both groups (P < 0.001). Both groups improved function by several other measures while slightly decreasing (P < 0.01) lean mass (−1.0 kg, C-WL; −0.6 kg, HP-WL). Weight loss was greater in white than in black women at both 4 mo (6.0 ± 3.6 compared with 3.7 ± 3.4 kg; P < 0.02) and 6 mo (7.2 ± 4.8 compared with 4.0 ± 4.7 kg; P < 0.04) and tended to be positively related to age (P < 0.06).
Conclusions: A clinically important functional benefit of obesity reduction was confirmed in both study groups, with no significant group effect. Our findings of racial differences in response to the intervention and a potential influence of participant age lend support for further studies sufficiently powered to explore the interaction of race and age with functional responses to obesity reduction in women. This trial was registered at clinicaltrials.gov as NCT02033655.
Keywords: dynapenic obesity, women, balanced protein, age, race, function, body composition
Introduction
The impact of obesity on physical function is keenly felt by women. Their obesity rates surpass those of men at both middle (44.6% compared with 37.2%) and older (39.4% compared with 37.5%) ages, and their susceptibility to obesity-related functional impairments is heightened by a higher proportion of fat to lean mass and lower muscle strength relative to men (1–3). Age magnifies the negative impact of obesity on function starting in midlife; there is a steady decline in muscle strength with age, 5% per year between 50 and 60 y of age, and 3% annually thereafter (4–6). Combined with excessive adiposity, this can lead to a precipitous decline in functional status (dynapenic obesity) (7, 8).
Although obesity reduction strongly benefits physical function (9, 10), the potential for >25% of weight lost during dieting to be lean mass raises concerns about the long-term impact on muscle (11). Current reports suggest that enhancing protein intake could help circumvent this problem (12–14). A “balanced” compared with a “skewed” daily protein intake has been suggested to prompt optimal muscle protein anabolism. Thus, increasing protein intake on a “per meal” or “balanced” basis has been endorsed as a way to optimize muscle protein synthesis (15–17). Few studies, to our knowledge, have explored protein supplementation during obesity reduction and almost all have used exercise along with diet; none have balanced protein across the day (14, 18–21). Mojtahedi et al. (18) provided 25 g whey protein 2 times/d in an exercise and weight-loss intervention for overweight and obese postmenopausal women. The protein increased lean mass relative to body mass, but did not lead to improvements in function. In contrast, our recent findings in a 6-mo randomized controlled trial of dietary obesity reduction in men and women aged ≥60 y [Measuring Eating, Activity, and Strength: Understanding the Response–Using Protein (MEASUR-UP)8 trial] showed that, relative to a weight-loss control, a higher-protein diet with 30 g protein consumed 3 times/d robustly benefited function relative to the control diet, with no group difference in the preservation of lean mass (22).
The Protein Optimization in Women Enables Results–Using Protein (POWR-UP) trial tests a balanced higher-protein intake in obese middle-aged and older women during a 6-mo weight-loss intervention. The objective was to confirm the feasibility and fidelity of delivery of the higher-protein diet and to determine its efficacy for influencing function and retention of lean mass during weight reduction in this population. We hypothesized that the protein-enhanced diet would be superior to the control diet for impact on function.
Methods
Trial design and participants
This 6-mo randomized controlled trial compared a protein-adequate diet with a higher-protein diet with regard to effects on the primary outcomes of function and lean mass during a weight-loss intervention for obese women. The study was approved by the Duke University Health System Institutional Review Board, and written informed consent was obtained from all participants. Interventions and assessments were conducted at Duke University Medical Center facilities. This trial was registered at clinicaltrials.gov as NCT02033655.
We used a computerized centralized randomization scheme blocking by race and marital or partner status. Because many, but not all, of the participants were functionally frail at baseline, the treatment groups were also blocked by functional status [≤549 or ≥550 m on the 6-min walk test (6MWT)]. Eligible participants were randomly assigned in a 1:2 allocation to 1) a Control–Weight-Loss (C-WL) group or 2) a High-Protein–Weight-Loss (HP-WL) group.
The 1:2 allocation to the C-WL and HP-WL groups was chosen because results from a number of published studies of traditional weight-reduction diets (without protein supplementation) are already available. Thus, we chose to oversample for the protein treatment to enhance our ability to make within-group comparisons in the protein group in subsequent analyses. This oversampling of the protein group did not cause a substantial loss of power for the between-group comparisons; the relative power for a 2:1 split is 0.94 (23). The 6-mo duration was selected to allow time for safe, gradual weight loss. An interim (4-mo) time point was also included to determine the trajectory of change in the primary outcomes and to assess whether a shorter period of treatment might work almost as well as the commonly conducted 6-mo trial (previous findings showed that 3 mo was insufficient to achieve physiologically important weight loss).
Participants
Community-dwelling women aged ≥45 y with a BMI (in kg/m2) ≥30 were recruited from Durham, North Carolina, and the surrounding areas; recruitment included an emphasis on enrolling black women. POWR-UP enrolled an all-female population because women have higher obesity rates and more pronounced functional frailty than men. We oversampled black women because they have the highest obesity rates of any demographic subgroup and yet are understudied with regard to the most effective obesity interventions (3, 24, 25). Participants aged ≥45 y were included because the precise timing of changes in the protein response with age has not been established. For example, the age at which the anabolic resistance of aging begins to take a clinically detectable toll on function is unknown (26). Exclusion criteria included dementia, functional limitations caused by neurological conditions, and unstable or terminal medical conditions. According to our previously established protocol (27), individuals with a glomerular filtration rate (GFR) of ≥60 mL · min−1 · 1.73 m−2 were eligible for enrollment without monitoring. Those with a GFR of 45–59 mL · min−1 · 1.73 m−2 were enrolled but monitored with a repeat GFR determination every 2 mo; if GFR decreased by ≥10% or to <45 mL · min−1 · 1.73 m−2 the participant was disqualified from the study.
Interventions
Registered dietitians who were experienced in obesity treatment implemented supervised weight-loss interventions in both study groups. All of the participants were prescribed a hypocaloric (−500 kcal) diet and met twice with an interventionist to receive individualized kilocalorie prescription and meal plans. After the 2 individual sessions, participants attended weekly group meetings (specific to study group but equivalent in structure and duration) for diet and health-related counseling, peer support, and weekly weigh-ins. All of the participants were supplied with a low-dose multivitamin supplement (GNC Teen Multivitamin), along with 400 mg Ca and 600 IU vitamin D (Bayer Citracal Calcium Supplement +D3) to ensure adequate nutrient intake and to standardize supplement use (participants were instructed to discontinue all other nutritional supplements).
C-WL and HP-WL diets
All of the participants were prescribed an energy intake ∼500 kcal below their calculated requirement, as derived from calculations of estimated total energy expenditure on the basis of weight, height, sex, age, and activity level with the use of published equations (28). Participants in the C-WL group were prescribed the RDA for protein of 0.8 g/kg body weight, with a distribution of calories of ∼15% protein, 30% fat, and 55% carbohydrates. HP-WL participants were prescribed a protein intake of 1.2 g/kg body weight, with a target of 30 g protein/meal and a distribution of ∼30% protein, 30% fat, and 40% carbohydrates. Because high-quality protein is superior for promoting anabolism (29, 30), the HP-WL meal plan emphasized protein from animal sources, primarily lean meats and poultry, low-fat dairy, fish, and eggs. To promote achievement of the protein intake target, participants were supplied with preportioned frozen or chilled lean meats (lean ground pork, pork tenderloins, pork chops, and low-sodium ham) sufficient to provide ≥420 g protein/wk (≥30 g for 2 meals/d) for the duration of the trial. To avoid monotony and allow flexibility, participants consumed other complete proteins (e.g., other lean meats, poultry and fish, low-fat dairy foods, and eggs) at the third meal of each day according to their prescribed meal plan.
Outcome measurements
Primary outcomes assessed at 0, 4, and 6 mo were function (6MWT) and lean mass. The 6MWT is commonly used for a wide range of ages, including older adults (31), and is the test method of choice for walking speed in clinical research (32). Lean and fat mass were measured by using the BodPod air-displacement plethysmography method (Life Measurement, Inc.) per our established protocol (22). The BodPod is considered to be as reliable as DXA for a repeat-measure comparison of changes in fat-free mass within the same individual over time (33), although, as with any whole-body measure, it lacks the sensitivity to directly quantify skeletal muscle mass. Waist circumference was assessed at the minimal waist by using a Gulick II tape measure. Secondary function measures included the Short Physical Performance Battery (SPPB), the 8-foot up-and-go, and 30-s chair stands. Isometric hand-grip strength was assessed for both hands by using the Jamar Hand Dynamometer, with the higher score being recorded.
Energy and protein intakes were assessed by using 3-d food records collected at 0, 4, and 6 mo. Records were checked for completeness, and participants were contacted for any missing information. The intake of food and beverages was analyzed by using Food Processor Nutrition Analysis software (version 10.10, 2012; ESHA Research) to determine daily intakes of calories and macronutrients, as well as protein intake per meal.
Adherence and safety
The study was conducted under “intent to treat” criteria. Weekly body weights provided a regular indication of kilocalorie restriction compliance, and attendance at weigh-ins and group meetings was also recorded. Interventionists reviewed participants' daily food journals each week and adjusted their menus to ensure that the target kilocalorie intake and, for the HP-WL group, 30 g protein/meal for breakfast, lunch, and dinner were regularly achieved, as previously described (27). A fasting blood sample was collected to evaluate renal function by GFR at the end of the trial (or every 2 mo as dictated by the renal function protocol). The estimated GFR was determined by using the Chronic Kidney Disease Epidemiology Collaboration equation (LabCorp, Inc.) (34). Adverse events were thoroughly documented throughout the trial.
Data and statistical analysis
All of the outcome data were double entered, with differences adjudicated and treatment codes revealed only after the study statistician locked the database at the end of the trial. The primary objective of the trial was to provide information on the efficacy of an enhanced-protein diet relative to a traditional weight-loss (control) diet on change in function and lean mass. Measurements were taken at baseline and at 2 follow-up times (4 and 6 mo). Descriptive analyses were conducted to summarize the distribution of the covariates and dependent variables. For the primary and secondary outcomes, we tested an overall change in both intervention groups and differences in that change between groups over time. Controlling for baseline values, a mixed-model repeated-measures approach was used to assess change from baseline at 4 and 6 mo. The mixed-model repeated-measures approach extends the standard repeated-measures ANOVA to allow for missing values, error structures other than compound symmetry, and measurements taken at nonequal intervals. The main effect of the outcomes was tested by the time effect, whereas the group difference was assessed by statistical significance of the group and the group × time interaction. Significance was declared at an α level of 0.05 (2-tailed).
Results
Baseline demographic and functional characteristics
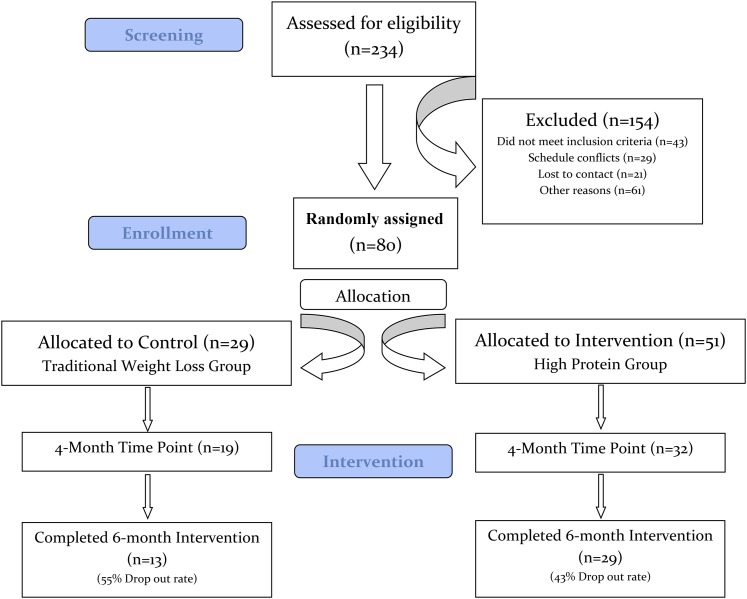
Of 234 eligible individuals identified for screening, 154 were excluded as shown in Figure 1. Baseline characteristics for the 80 participants who qualified and were randomly assigned to C-WL (n = 29) and HP-WL (n = 51) groups are shown in Table 1. Mean ± SD age was 60 ± 8.2 y, and 37.5% of enrolled women were black. Participants had class II (mean BMI = 37.8) obesity and their baseline protein intake was 0.83 ± 0.2 g/kg body weight.
FIGURE 1.
CONSORT flowchart. CONSORT, Consolidated Standards of Reporting Trials.
TABLE 1.
Baseline profile of participants by treatment group1
| C-WL group (n = 29) | HP-WL group (n = 51) | Both groups combined (n = 80) | |
|---|---|---|---|
| Age, y | 61.8 ± 7.6 | 58.9 ± 8.4 | 60.0 ± 8.2 |
| Range | 46.0–73.0 | 45.0–78.0 | 45.0–78.0 |
| Body weight, kg | 103.0 ± 15.6 | 98.6 ± 18.6 | 100.2 ± 17.6 |
| BMI, kg/m2 | 38.3 ± 5.8 | 37.5 ± 6.1 | 37.8 ± 5.9 |
| Race, n (%) | |||
| White | 18 (62) | 29 (60) | 47 (59) |
| African American | 11 (38) | 19 (37) | 30 (38) |
| Other | 0 (0) | 1 (2) | 1 (1) |
| Marital status, n (%) | |||
| Married | 17 (59) | 29 (57) | 46 (58) |
| Single | 6 (21) | 8 (16) | 14 (17) |
| Widow | 2 (7) | 3 (6) | 5 (6) |
| Education, n (%) | |||
| Completed high school | 1 (3) | 3 (6) | 4 (5) |
| Some college | 8 (28) | 15 (29) | 23 (29) |
| Completed college | 16 (55) | 29 (57) | 45 (56) |
| Energy intake, kcal | 2071.4 ± 832.9 | 1756.7 ± 476.9 | 1875.8 ± 648.4 |
| Protein intake, g/kg body weight | 0.8 ± 0.3 | 0.8 ± 0.2 | 0.8 ± 0.2 |
| Fasting blood glucose, mg/dL | 106.9 ± 31.2 | 111.7 ± 40.9 | 100.9 ± 37.5 |
| GFR,2 mL · min−1 · 1.73 m−2 | 83.9 ± 15.3 | 85.6 ± 18.0 | 85.0 ± 17.0 |
Values are means ± SDs unless otherwise indicated. C-WL, Control–Weight-Loss; GFR, glomerular filtration rate; HP-WL, High-Protein–Weight-Loss.
GFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation (34).
Efficacy of the interventions
Both groups reduced calorie intakes at 4 and 6 mo, with no group difference (Table 2). Three-day diet records confirmed achievement of an average intake of 30 g protein at each meal in the HP-WL group. At 6 mo, mean ± SD HP-WL protein intakes were 31.0 ± 11.6 g for breakfast, 34.5 ± 8.0 g for lunch, and 40.0 ± 8.5 g for dinner, with a total intake of 1.3 g protein · kg body weight−1 · d−1 at 4 and 6 mo. For the C-WL group, in contrast, protein intakes per meal and expressed as g · kg body weight−1 · d−1 were unchanged at 4 and 6 mo. Age was inversely associated with calories consumed (r = −0.25, P < 0.05) and total protein intake per day (r = −0.23, P < 0.05); however, protein intake per meal did not differ by age.
TABLE 2.
Baseline values and change scores at 4 and 6 mo for calorie and protein intakes by treatment group1
| C-WL group | HP-WL group | ||||
|---|---|---|---|---|---|
| Mean ± SD | P 2 | Mean ± SD | P 2 | P (C-WL vs. HP-WL) | |
| Calorie intake, kcal | |||||
| Baseline | 2071.4 ± 832.9 | 1744.4 ± 474.9 | |||
| Change at 4 mo | −704.2 ± 669.8 | <0.001 | −339.4 ± 441.6 | <0.001 | 0.37 |
| Change at 6 mo | −574.0 ± 588.5 | <0.001 | −341.7 ± 415.6 | <0.001 | 0.91 |
| Protein intake, g/d | |||||
| Baseline | 86.1 ± 25.9 | 80.5 ± 17.5 | |||
| Change at 4 mo | −9.4 ± 26.0 | 0.32 | 32.9 ± 22.8 | <0.001 | <0.001 |
| Change at 6 mo | −5.2 ± 21.9 | 0.65 | 32.8 ± 22.3 | <0.001 | <0.001 |
| At breakfast, g/meal | |||||
| Baseline | 16.5 ± 8.2 | 15.9 ± 8.2 | |||
| Change at 4 mo | −2.2 ± 9.6 | 0.14 | 17.6 ± 9.8 | <0.001 | <0.001 |
| Change at 6 mo | 2.0 ± 8.2 | 0.96 | 16.1 ± 11.0 | <0.001 | <0.001 |
| At lunch, g/meal | |||||
| Baseline | 26.7 ± 10.9 | 27.7 ± 12.4 | |||
| Change at 4 mo | −1.2 ± 11.5 | 0.43 | 9.5 ± 15.0 | <0.001 | <0.001 |
| Change at 6 mo | −1.0 ± 9.7 | 0.73 | 6.6 ± 15.0 | <0.001 | <0.01 |
| At dinner, g/meal | |||||
| Baseline | 36.1 ± 14.2 | 33.3 ± 11.3 | |||
| Change at 4 mo | −3.6 ± 18.2 | 0.29 | 2.9 ± 13.9 | 0.29 | 0.14 |
| Change at 6 mo | −5.7 ± 8.2 | 0.05 | 6.5 ± 14.5 | <0.01 | <0.001 |
| Protein intake, g/kg body weight | |||||
| Baseline | 0.8 ± 0.3 | 0.8 ± 0.2 | |||
| Change at 4 mo | −0.04 ± 0.3 | 0.75 | 0.4 ± 0.3 | <0.001 | <0.001 |
| Change at 6 mo | 0.01 ± 0.2 | 0.99 | 0.4 ± 0.3 | <0.001 | <0.001 |
Between- and within-group differences were detected by using mixed-model repeated-measures ANOVA with baseline values as covariates. C-WL, Control–Weight-Loss; HP-WL, High-Protein–Weight-Loss.
P values for within-group change from baseline to 4 and 6 mo.
Weight reduction and primary outcomes
At 6 mo, physiologically important weight losses were achieved (mean ± SD: C-WL, −6.3% ± 4.8%; HP-WL, −6.2% ± 5.3%; P < 0.001; Table 3) and function (6MWT) was improved in both groups. With regard to within-group improvements in function (6MWT), a significant change occurred at both 4 mo (P < 0.01) and 6 mo (P < 0.001) for the HP-WL group and only at 6 mo (P < 0.01) for the C-WL group. Controlling for baseline, 6MWT increased by 45.2 m (P < 0.001) at 4 mo and by 46.9 m (P < 0.001) at 6 mo, whereas 6MWT in the C-WL group increased by 46.8 m (P = 0.01) at 6 mo only. However, there was no significant difference detected in 6MWT results between treatment groups. Lean mass decreased in both groups (C-WL: −1.0 ± 1.1 kg; HP-WL: −0.6 ± 1.1 kg; P < 0.01; Table 3). The percentage of body lean mass also decreased in the C-WL group (at 6 mo) (P = 0.03) but was unchanged in the HP-WL group (P = 0.49). However, the comparison for changes in lean mass between treatment groups was nonsignificant.
TABLE 3.
Baseline values and change scores at 4 and 6 mo for primary and secondary outcome variables by treatment group1
| C-WL group | HP-WL group | ||||
|---|---|---|---|---|---|
| Mean ± SD | P 2 | Mean ± SD | P 2 | P (C-WL vs. HP-WL) | |
| Primary outcomes | |||||
| 6MWT, m | |||||
| Baseline | 478.7 ± 79.3 | 494.1 ± 86.1 | |||
| Change at 4 mo | 23.2 ± 54.4 | 0.09 | 45.2 ± 61.1 | <0.001 | 0.19 |
| Change at 6 mo | 46.8 ± 89.0 | 0.01 | 56.9 ± 68.0 | <0.001 | 0.58 |
| Lean body mass, kg | |||||
| Baseline | 51.1 ± 6.2 | 49.0 ± 7.3 | |||
| Change at 4 mo | −0.8 ± 1.0 | <0.01 | −0.8 ± 1.0 | <0.001 | 0.87 |
| Change at 6 mo | −1.0 ± 1.1 | <0.001 | −0.6 ± 1.1 | <0.01 | 0.20 |
| Secondary outcomes | |||||
| Body weight, kg | |||||
| Baseline | 103.0 ± 15.6 | 98.6 ± 18.6 | |||
| Change at 4 mo | −5.7 ± 3.2 | <0.001 | −5.2 ± 3.9 | <0.001 | 0.70 |
| Change at 6 mo | −6.4 ± 4.9 | <0.001 | −6.2 ± 5.9 | <0.001 | 0.92 |
| Body weight, % | |||||
| Change at 4 mo | −5.6 ± 3.3 | <0.001 | −5.2 ± 4.0 | <0.001 | 0.77 |
| Change at 6 mo | −6.3 ± 4.8 | <0.001 | −6.2 ± 5.3 | <0.001 | 0.92 |
| Lean body mass, % | |||||
| Change at 4 mo | −1.5 ± 1.9 | 0.07 | −1.0 ± 4.1 | 0.11 | 0.62 |
| Change at 6 mo | −1.9 ± 2.0 | 0.03 | −0.5 ± 4.5 | 0.49 | 0.17 |
| Fat mass, kg | |||||
| Baseline | 51.9 ± 11.9 | 49.4 ± 13.0 | |||
| Change at 4 mo | −4.9 ± 3.2 | <0.001 | −4.1 ± 3.8 | <0.001 | 0.45 |
| Change at 6 mo | −5.9 ± 3.7 | <0.001 | −5.2 ± 5.2 | <0.001 | 0.68 |
| Fat mass, % | |||||
| Change at 4 mo | −2.2 ± 1.9 | <0.001 | −2.0 ± 2.1 | <0.001 | 0.68 |
| Change at 6 mo | −2.7 ± 2.3 | <0.001 | −2.7 ± 2.8 | <0.001 | 0.81 |
| SPPB total, 0–12 | |||||
| Baseline | 10.1 ± 1.5 | 10.2 ± 1.5 | |||
| Change at 4 mo | 1.2 ± 1.0 | <0.001 | 0.9 ± 1.4 | <0.001 | 0.68 |
| Change at 6 mo | 1.2 ± 1.0 | <0.001 | 1.0 ± 1.4 | <0.001 | 0.94 |
| SPPB balance, 0–4 | |||||
| Baseline | 3.8 ± 0.6 | 3.9 ± 0.4 | |||
| Change at 4 mo | 0.2 ± 0.8 | 0.20 | −0.03 ± 0.4 | 0.37 | 0.65 |
| Change at 6 mo | 0.2 ± 0.8 | 0.38 | −0.03 ± 0.5 | 0.85 | 0.55 |
| SPPB gait speed, 0–4 | |||||
| Baseline | 3.8 ± 0.6 | 3.8 ± 0.4 | |||
| Change at 4 mo | 0.05 ± 0.4 | 0.12 | 0.1 ± 0.3 | <0.01 | 0.52 |
| Change at 6 mo | 0.2 ± 0.4 | 0.11 | 0.1 ± 0.4 | <0.01 | 0.82 |
| SPPB chair stands, 0–4 | |||||
| Baseline | 2.5 ± 1.0 | 2.6 ± 1.2 | |||
| Change at 4 mo | 1.0 ± 0.8 | <0.001 | 0.8 ± 1.1 | <0.001 | 0.80 |
| Change at 6 mo | 0.9 ± 1.0 | <0.001 | 1.0 ± 1.1 | <0.001 | 0.62 |
| Waist circumference, cm | |||||
| Baseline | 104.7 ± 10.0 | 102.5 ± 11.6 | |||
| Change at 4 mo | −4.6 ± 3.7 | <0.001 | −4.2 ± 4.2 | <0.001 | 0.66 |
| Change at 6 mo | −7.4 ± 3.8 | <0.001 | −6.0 ± 3.3 | <0.001 | 0.19 |
| 8-Foot up-and-go, s | |||||
| Baseline | 7.9 ± 2.3 | 7.8 ± 1.7 | |||
| Change at 4 mo | −0.9 ± 1.9 | 0.01 | −0.6 ± 1.3 | 0.01 | 0.71 |
| Change at 6 mo | −0.6 ± 1.5 | 0.01 | −1.2 ± 1.8 | <0.001 | 0.26 |
| 30-s Chair stands, n | |||||
| Baseline | 11.2 ± 3.1 | 11.2 ± 2.7 | |||
| Change at 4 mo | 1.1 ± 2.1 | <0.01 | 1.6 ± 1.8 | <0.001 | 0.59 |
| Change at 6 mo | 1.2 ± 3.1 | 0.06 | 1.9 ± 2.9 | <0.01 | 0.84 |
Between- and within-group differences were detected by using mixed-model repeated-measures ANOVA with baseline values as covariates. C-WL, Control–Weight-Loss; HP-WL, High-Protein–Weight-Loss; SPPB, Short Physical Performance Battery; 6MWT, 6 Minute Walk Test.
P values for within-group change from baseline to 4 and 6 mo.
Secondary outcomes, race, and age
At 6 mo, both the C-WL and HP-WL groups showed clinically meaningful improvements in SPPB scores (C-WL: 1.2 ± 1.0 units; HP-WL: 1.0 ± 1.4 units; P < 0.001). The SPPB subcomponent analysis showed no change in balance score but an improvement in the number of chair stands in both treatment groups; however, gait speed improved (P < 0.01) only in the HP-WL group (Table 3). The number of chair stands in 30 s increased (P < 0.01) at 4 mo in the HP-WL group and at 6 mo in both groups (P < 0.001), and performance of the 8-foot up-and-go test improved in both groups at both time points (P < 0.01). BodPod results indicated a reduction in fat mass in both groups. Likewise, waist circumference was decreased at both time points for both C-WL and HP-WL groups (P < 0.001). We did not find a significant group effect for any of these measures of function or for body fat or waist circumference.
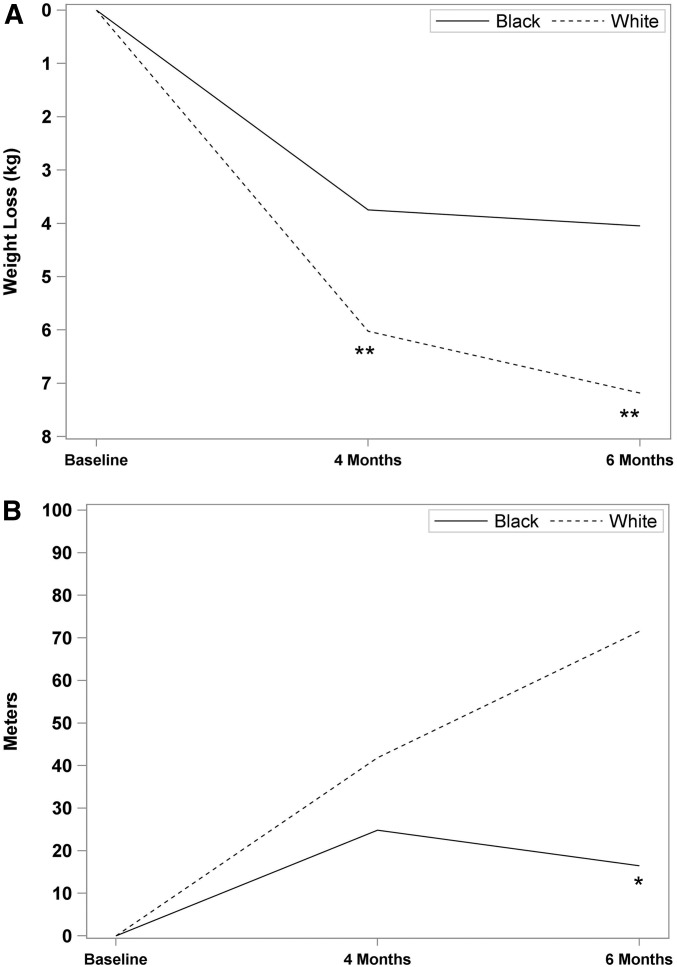
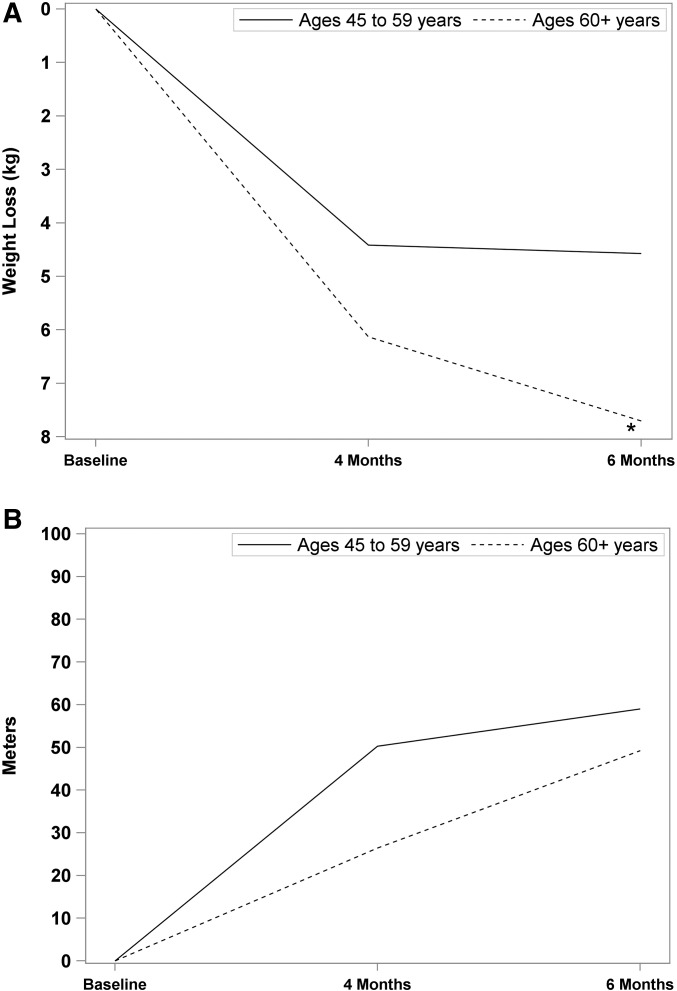
Our analyses of main study outcomes did show differences by race (Figure 2A). White women lost more weight than did black women at both 4 mo (6.0 ± 3.6 compared with 3.7 ± 3.4 kg; P < 0.02) and 6 mo (7.2 ± 4.8 compared with 4.0 ± 4.7 kg; P < 0.04). Black participants also tended (P = 0.07) to have smaller improvements in 6MWT than white participants (Figure 2B). As shown in Figure 3A, age tended (P = 0.06) to be positively associated with the amount of weight lost and there was a time × age interaction, such that middle-aged women lost more weight at 4 than at 6 mo, whereas older women lost more weight at 6 than at 4 mo [F(1, 48) = 4.71, P < 0.03]. Change in 6MWT did not differ by age (Figure 3B).
FIGURE 2.
Changes in body weight (A) and distance walked in 6 min (B) at 4 and 6 mo by race. (A) White women lost more weight than did black women at both 4 (P < 0.02) and 6 (P < 0.04) mo. (B) Black participants tended (P = 0.07) to show smaller improvements in 6-min walk distance at the 6-mo end point than did white participants. **P < 0.05; *P < 0.10.
FIGURE 3.
Changes in body weight (A) and distance walked in 6 min (B) at 4 and 6 mo by age. (A) Age tended to be positively (P = 0.06) associated with amount of weight lost. There was a time × age interaction, such that middle-aged women had lost more weight at 4 than at 6 mo, whereas older women had lost more weight at 6 than at 4 mo [F(1, 48) = 4.71, P < 0.03]. (B) There was no association of age with 6-min walk results. *P < 0.10.
Adherence and safety
Active participants in both groups had good or very good attendance at the weekly group and weigh-in meetings (C-WL = 69.4%; HP-WL = 77.0%). However, drop-out rates were relatively high: 55% for controls and 43% for HP-WL participants. There was no difference in drop-out rate by group (P = 0.30); however, compared with dropouts, study completers were more likely to be white (P < 0.05) and to have a higher educational level (P < 0.05).
There were no adverse events related to the protocol and no serious adverse events occurred during the trial. Two HP-WL participants were disqualified from study participation when their GFR fell below the inclusion level; however, both cases occurred very early in the trial (within 1 mo of starting the higher protein intake) and were deemed unlikely to be protocol related. Otherwise, there were no clinically important changes in GFR during the study in either group (mean GFR was 85.0 ± 17.0 mL · min−1 · 1.73 m−2 at baseline and 84.9 ± 14.4 mL · min−1 · 1.73 m−2 at 6 mo).
Discussion
“Physical resilience” is defined as the ability to optimize or recover physical function when faced with a health stressor (35). For many women, obesity reduces physical resilience through a negatively reinforcing cycle of reduced mobility (3, 36–38). This study explored the feasibility and efficacy of a hypocaloric regimen with generous, balanced high-quality protein intake (“protein-centric” meals) to improve physical resilience in a high-risk population of women. Our diet approach was founded on reports by Symons et al. (39) and others (40) that showed that ∼30 g high-quality protein/meal optimizes the anabolic response in aging muscle. A recent cross-sectional analysis of NHANES 1999–2001 (1081 adults aged 50–85 y of age) found that consuming meals containing 30–45 g protein was associated with greater leg lean mass and strength (41). Thus, we hypothesized that a protein regimen of ∼30 g high-quality protein/meal would help counteract the tendency to lose muscle mass during weight loss and promote better function.
Our results support the feasibility of implementation of a meal-balanced higher-protein diet for obesity reduction. However, the hypothesis that the HP-WL group would achieve greater improvements in function and lean mass was not confirmed by a significant group effect. There were earlier and more positive within-group findings for the HP-WL than for the C-WL group, and this could hint that higher protein intake led to more robust improvements in function. However, it is important to note that, due to the 2:1 randomization, the number of completers in the HP-WL group (n = 29) was more than twice that in the C-WL group (n = 13), which could explain our ability to detect more changes in the HP-WL group. In the MEASUR-UP study, our first trial of 30 g protein/meal, there was a robust protein effect on function (22). However, several important differences between these 2 trials could explain the difference in findings. MEASUR-UP included both sexes, and the participants were older (mean ± SD age: 68.2 ± 5.6 y compared with 60.0 ± 8.2 y in POWR-UP) and achieved greater weight loss (∼8% compared with ∼6% in POWR-UP). The tendency for older participants to achieve more weight loss aligns with our present results as well as with findings from the Look AHEAD (42) and Diabetes Prevention Program (43) trials, which showed that older participants lost more weight than their younger counterparts. Although we lack sufficient numbers in this trial to answer the question, it could be that the amount of weight lost and/or age and sex modulates the impact of higher protein intake on function.
The absence of a group effect on retention of lean mass in POWR-UP is less surprising and could reflect the reported disconnect between changes in muscle mass and changes in muscle strength and function (44, 45). Moreover, although our measurement conditions were carefully standardized, the BodPod assesses total lean body mass and may be insufficiently sensitive to assess group differences in skeletal muscle mass.
It is intriguing that black women in the POWR-UP trial lost less body weight and showed smaller improvements in 6MWT than white participants. Although the study was not designed or powered to conduct a direct race comparison, our findings agree with reports in the literature of a blunted effectiveness of weight-loss interventions in black women (46–48). This is particularly unfortunate because black women face the highest obesity rates of any demographic group (3). Our findings on 6MWT also agree with reports of higher rates of obesity-related disability in black women than in whites (1, 49, 50). In view of the very limited research to date, further investigations aimed at understanding these racial differences are urgently needed, especially in older populations.
Strengths and limitations
The POWR-UP trial was a closely supervised intensive intervention conducted in a well-characterized population by an experienced clinical research team and included a battery of robust outcome measures. Detailed meal plans, supervision by registered dietitians, and weekly food logs ensured dietary compliance; and weekly body weight measurements objectively reflected adherence to the weight-loss regimen. The provision of high-quality protein for 2 of 3 daily meals for the HP-WL group helped ensure compliance with the protein intake goals, as we have previously shown (22), and as documented in 3-d diet records. The successful implementation of the protein regimen has important implications, because a meal-balanced, higher protein intake is being widely advocated for older adults for a host of diverse metabolic roles, including biosynthesis of nitrogen-containing compounds and mRNA translation (51).
The most obvious limitation of the trial is the relatively modest number of participants who completed the study. Drop-out rates were higher than anticipated, especially in the middle-aged women. We examined the reasons for dropout to inform future trials and found that 56% of dropouts had difficulty meeting study requirements due to family commitments or health issues; another 11% developed new health problems unrelated to the study. In particular, the tendency for more dropouts among minorities and those with less education indicates a need for future interventions to address adherence strategies for these groups. Another limitation concerns the limited ability to measure change in muscle mass because, as already noted, the BodPod measurement lacks sensitivity to quantify specific changes in skeletal muscle mass.
Summary
The findings of the POWR-UP trial showed clinically important functional benefits of weight reduction in middle-aged and older obese women but failed to conclusively confirm whether or not the higher-protein regimen provided more benefit to function than an RDA level of protein intake. Future studies in larger numbers of participants are warranted in both men and women, and especially in older age groups, who find it easier to lose weight and for whom extra protein at meals is being recommended (41, 52, 53). In addition, our findings of racial differences in response to the intervention lend strong support for further studies sufficiently powered to explore the interaction of race with functional responses to obesity reduction in middle-aged and older women.
Acknowledgments
The authors' responsibilities were as follows—CWB, KNPS, SRM, and CFP: created the study concept and design; CWB, KNPS, MCO, KM, AKJ, AO, HM, and CFP: acquired, analyzed, or interpreted the data; HM and CFP: performed the statistical analysis; CWB and KNPS: drafted the manuscript and supervised the study; CWB, KNPS, MEP, SRM, and CFP: critically revised the manuscript; CWB, KNPS, MCO, SRM, KM, AKJ, and AO: provided administrative, technical, or material support; and all authors: read and approved the final manuscript.
Abbreviations
- C-WL
Control–Weight-Loss
- GFR
glomerular filtration rate
- HP-WL
High-Protein–Weight-Loss
- MEASUR-UP
Measuring Eating, Activity, and Strength: Understanding the Response–Using Protein
- SPPB
Short Physical Performance Battery
- 6MWT
6-min walk test
References
- 1. Visser M, Kritchevsky SB, Goodpaster BH, Newman AB, Nevitt M, Stamm E, Harris TB.. Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: the Health, Aging and Body Composition Study. J Am Geriatr Soc 2002;50:897–904. [DOI] [PubMed] [Google Scholar]
- 2. Lafortuna CL, Maffiuletti NA, Agosti F, Sartorio A.. Gender variations of body composition, muscle strength and power output in morbid obesity. Int J Obes (Lond) 2005;29:833–41. [DOI] [PubMed] [Google Scholar]
- 3. Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL.. Trends in obesity among adults in the United States, 2005 to 2014. JAMA 2016;315:2284–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stenholm S, Sainio P, Rantanen T, Alanen E, Koskinen S.. Effect of co-morbidity on the association of high body mass index with walking limitation among men and women aged 55 years and older. Aging Clin Exp Res 2007;19:277–83. [DOI] [PubMed] [Google Scholar]
- 5. Friedmann JM, Elasy T, Jensen GL.. The relationship between body mass index and self-reported functional limitation among older adults: a gender difference. J Am Geriatr Soc 2001;49:398–403. [DOI] [PubMed] [Google Scholar]
- 6. von Haehling S, Morley JE, Anker SD.. From muscle wasting to sarcopenia and myopenia: update 2012. J Cachexia Sarcopenia Muscle 2012;3:213–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Prado CM, Wells JC, Smith SR, Stephan BC, Siervo M.. Sarcopenic obesity: a critical appraisal of the current evidence. Clin Nutr 2012;31:583–601. [DOI] [PubMed] [Google Scholar]
- 8. Evans WJ. Skeletal muscle loss: cachexia, sarcopenia, and inactivity. Am J Clin Nutr 2010;91(Suppl):1123S–7S. [DOI] [PubMed] [Google Scholar]
- 9. Villareal DT, Chode S, Parimi N, Sinacore DR, Hilton T, Armamento-Villareal R, Napoli N, Qualls C, Shaw K.. Weight loss, exercise, or both and physical function in obese older adults. N Engl J Med 2011;364:1218–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Beavers KM, Miller ME, Rejeski WJ, Nicklas BJ, Krichevsky SB.. Fat mass loss predicts gain in physical function with intentional weight loss in older adults. J Gerontol A Biol Sci Med Sci 2013;68:80–6. Erratum in: J Gerontol A Biol Sci Med Sci 2014;69(10):1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Weinheimer EM, Sands LP, Campbell WW.. A systematic review of the separate and combined effects of energy restriction and exercise on fat-free mass in middle-aged and older adults: implications for sarcopenic obesity. Nutr Rev 2010;68:375–88. [DOI] [PubMed] [Google Scholar]
- 12. Li Z, Heber D.. Sarcopenic obesity in the elderly and strategies for weight management. Nutr Rev 2012;70:57–64. [DOI] [PubMed] [Google Scholar]
- 13. Pasiakos SM, Cao JJ, Margolis LM, Sauter ER, Whigham LD, McClung JP, Rood JC, Carbone JW, Comb GF Jr, Young AJ.. Effects of high-protein diets on fat-free mass and muscle protein synthesis following weight loss: a randomized controlled trial. FASEB J 2013;27:3837–47. [DOI] [PubMed] [Google Scholar]
- 14. Kim JE, O'Connor LE, Sands LP, Slebodnik MB, Campbell WW.. Effects of dietary protein intake on body composition changes after weight loss in older adults: a systematic review and meta-analysis. Nutr Rev 2016;74:210–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bauer J, Biolo G, Cederholm T, Cesari M, Cruz-Jentoft AJ, Morley JE, Phillips S, Sieber C, Stehle P, Teta D, et al. Evidence-based recommendations for optimal dietary protein intake in older people: a position paper from the PROT-AGE Study Group. J Am Med Dir Assoc 2013;14:542–59. [DOI] [PubMed] [Google Scholar]
- 16. Deutz NE, Bauer JM, Barazzoni R, Biolo G, Boirie Y, Bosy-Westphal A, Cederholm T, Cruz-Jentoft A, Krznaric Z, Nair KS, et al. Protein intake and exercise for optimal muscle function with aging: recommendations from the ESPEN Expert Group. Clin Nutr 2014;33:929–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Murphy CH, Oikawa SY, Phillips SM.. Dietary protein to maintain muscle mass in aging: a case for per-meal protein recommendations. J Frailty Aging 2016;5:49–58. [DOI] [PubMed] [Google Scholar]
- 18. Mojtahedi MC, Thorpe MP, Karampinos DC, Johnson CL, Layman DK, Georgiadis JG, Evans EM.. The effects of a higher protein intake during energy restriction on changes in body composition and physical function in older women. J Gerontol A Biol Sci Med Sci 2011;66:1218–25. [DOI] [PubMed] [Google Scholar]
- 19. Verreijen AM, Verlaan S, Engberink MF, Swinkels S, de Vogel-van den Bosch J, Weijs PJ.. A high whey protein-, leucine-, and vitamin D-enriched supplement preserves muscle mass during intentional weight loss in obese older adults: a double-blind randomized controlled trial. Am J Clin Nutr 2015;101:279–86. [DOI] [PubMed] [Google Scholar]
- 20. Coker RH, Miller S, Schutzler S, Deutz N, Wolfe RR.. Whey protein and essential amino acids promote the reduction of adipose tissue and increased muscle protein synthesis during caloric restriction-induced weight loss in elderly, obese individuals. Nutr J 2012;11:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Smith GI, Yoshino J, Kelly SC, Reeds DN, Okunade A, Patterson BW, Klein S, Mittendorfer B.. High-protein intake during weight loss therapy eliminates the weight-loss-induced improvement in insulin action in obese postmenopausal women. Cell Reports 2016;17:849–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Porter Starr KN, Pierper CF, Orenduff MC, McDonald SR, McClure LB, Zhou R, Payne ME, Bales CW.. Improved function with enhanced protein intake per meal: a pilot study of weight reduction in frail, obese older adults. J Gerontol A Biol Sci Med Sci 2016;71:1369–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fleiss JL. Design and analysis of clinical experiments. Hoboken: (NJ): John Wiley & Sons; 1986. [Google Scholar]
- 24. Fitzgibbon ML, Tussing-Humphreys LM, Porter JS, Martin IK, Odoms-Young A, Sharp LK.. Weight loss and African-American women: a systematic review of the behavioural weight loss intervention literature. Obes Rev 2012;13:193–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kumanyika S, Fassbender J, Phipps E, Tan-Torres S, Localio R, Morales KH, Sarwer DB, Harralson T, Allison K, Wesby L, et al. Design, recruitment and start up of a primary care weight loss trial targeting African American and Hispanic adults. Contemp Clin Trials 2011;32:215–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sahni S, Mangano KM, Hannan MT, Kiel DP, McLean RR.. Higher protein intake is associated with higher lean mass and quadriceps muscle strength in adult men and women. J Nutr 2015;145:1569–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McDonald SR, Porter Starr KN, Mauceri L, Orenduff M, Granville E, Ocampo C, Payne ME, Pieper CF, Bales CW.. Meal-based enhancement of protein quality and quantity during weight loss in obese older adults with mobility limitations: rationale and design for the MEASUR-UP trial. Contemp Clin Trials 2015;40:112–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.FAO/WHO/United Nations University. Human energy requirements: report of a joint FAO/ WHO/UNU Expert Consultation. Food Nutr Bull 2005;26:166. [PubMed] [Google Scholar]
- 29. Phillips SM. The science of muscle hypertrophy: making dietary protein count. Proc Nutr Soc 2011;70:100–3. [DOI] [PubMed] [Google Scholar]
- 30. McLean RR, Mangano KM, Hannan MT, Kiel DP, Sahni S.. Dietary protein intake is protective against loss of grip strength among older adults in the Framingham offspring cohort. J Gerontol A Biol Sci Med Sci 2016;71:356–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rikli RE, Jones CJ.. The reliability and validity of a 6-minute walk test as a measure of physical endurance in older adults. J Aging Phys Act 1998;6:363–75. [Google Scholar]
- 32. Solway S, Brooks D, Lacasse Y, Thomas S.. A qualitative systematic overview of the measurement properties of functional walk tests used in the cardiorespiratory domain. Chest 2001;119:256–70. [DOI] [PubMed] [Google Scholar]
- 33. Frisard MI, Greenway FL, Delany JP.. Comparison of methods to assess body composition changes during a period of weight loss. Obes Res 2005;13:845–54. [DOI] [PubMed] [Google Scholar]
- 34. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Whitson HE, Duan-Porter W, Schmader KE, Morey MC, Cohen HJ, Colon-Emeric CS.. Physical resilience in older adults: systematic review and development of an emerging construct. J Gerontol A Biol Sci Med Sci 2016;71:489–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Topinková E. Aging, disability and frailty. Ann Nutr Metab 2008;52(Suppl 1):6–11. [DOI] [PubMed] [Google Scholar]
- 37. Rolland Y, Lauwers-Cances V, Cristini C, Abellan van Kan G, Janssen I, Morley JE, Vellas B.. Difficulties with physical function associated with obesity, sarcopenia, and sarcopenic-obesity in community-dwelling elderly women: the EPIDOS (EPIDemiologie de l'OSteoporose) study. Am J Clin Nutr 2009;89:1895–900. [DOI] [PubMed] [Google Scholar]
- 38. Vincent HK, Vincent KR, Lamb KM.. Obesity and mobility disability in the older adult. Obes Rev 2010;11:568–79. [DOI] [PubMed] [Google Scholar]
- 39. Symons TB, Sheffield-Moore M, Wolfe RR, Paddon-Jones D.. A moderate serving of high-quality protein maximally stimulates skeletal muscle protein synthesis in young and elderly subjects. J Am Diet Assoc 2009;109:1582–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pennings B, Groen B, de Lange A, Gijsen AP, Zorenc AH, Senden JM, van Loon LJ.. Amino acid absorption and subsequent muscle protein accretion following graded intakes of whey protein in elderly men. Am J Physiol Endocrinol Metab 2012;302:E992–9. [DOI] [PubMed] [Google Scholar]
- 41. Loenneke JP, Loprinzi PD, Murphy CH, Phillips SM.. Per meal dose and frequency of protein consumption is associated with lean mass and muscle performance. Clin Nutr 2016;35:1506–11. [DOI] [PubMed] [Google Scholar]
- 42. Look AHEAD Research Group Eight-year weight losses with an intensive lifestyle intervention: the Look AHEAD study. Obesity (Silver Spring) 2014;22:5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wing RR, Hamman RF, Bray GA, Delahanty L, Edelstein SL, Hill JO, Horton ES, Hoskin MA, Kriska A, Lachin J, et al. Achieving weight and activity goals among Diabetes Prevention Program lifestyle participants. Obes Res 2004;12:1426–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Newman AB, Kupelian V, Visser M, Simonsick EM, Goodpaster BH, Kritchevsky SB, Tylavsky FA, Rubin SM, Harris TB.. Strength, but not muscle mass, is associated with mortality in the Health, Aging and Body Composition study cohort. J Gerontol A Biol Sci Med Sci 2006;61:72–7. [DOI] [PubMed] [Google Scholar]
- 45. McGregor RA, Cameron-Smith D, Poppitt SD.. It is not just muscle mass: a review of muscle quality, composition and metabolism during ageing as determinants of muscle function and mobility in later life. Longev Healthspan 2014;3:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. DeLany JP, Jakicic JM, Lowery JB, Hames KC, Kelley DE, Goodpaster BH.. African American women exhibit similar adherence to intervention but lose less weight due to lower energy requirements. Int J Obes (Lond) 2014;38:1147–52. [DOI] [PubMed] [Google Scholar]
- 47. Wingo BC, Carson TL, Ard J.. Differences in weight loss and health outcomes among African Americans and whites in multicentre trials. Obes Rev 2014;15(Suppl 4):46–61. [DOI] [PubMed] [Google Scholar]
- 48. Fisher G, Hyatt TC, Hunter GR, Oster RA, Desmond RA, Gower BA.. Markers of inflammation and fat distribution following weight loss in African-American and white women. Obesity (Silver Spring) 2012;20:715–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mendes de Leon CF, Barnes LL, Bienias JL, Skarupski KA, Evans DA.. Racial disparities in disability: recent evidence from self-reported and performance-based disability measures in a population-based study of older adults. J Gerontol B Psychol Sci Soc Sci 2005;60:S263–71. [DOI] [PubMed] [Google Scholar]
- 50. Ostchega Y, Harris TB, Hirsch R, Parsons VL, Kington R.. The prevalence of functional limitations and disability in older persons in the US: data from the National Health and Nutrition Examination Survey III. J Am Geriatr Soc 2000;48:1132–5. [DOI] [PubMed] [Google Scholar]
- 51. Layman DK, Anthony TG, Rasmussen BB, Adams SH, Lynch CJ, Brinkworth GD, Davis TA.. Defining meal requirements for protein to optimize metabolic roles of amino acids. Am J Clin Nutr 2015;101(Suppl):1330S–8S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Paddon-Jones D, Rasmussen BB.. Dietary protein recommendations and the prevention of sarcopenia. Curr Opin Clin Nutr Metab Care 2009;12:86–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Beasley JM, Shikany JM, Thomson CA.. The role of dietary protein intake in the prevention of sarcopenia of aging. Nutr Clin Pract 2013;28:684–90. [DOI] [PMC free article] [PubMed] [Google Scholar]