Abstract
Systemic lupus erythematosus (SLE) is a chronic inflammatory autoimmune disorder with prevalent hypertension that significantly contributes to the mortality in this patient population. Pre-clinical and clinical evidence suggests that anti-CD3 antibody therapy may attenuate the development of autoimmune diseases like SLE. However, it is unclear whether this treatment impacts the development of the prevalent hypertension associated with SLE. The present study was designed to determine whether anti-CD3 antibody treatment attenuates the progression of hypertension in female SLE mice with already established renal disease (albuminuria ≥ 100 mg/dL). Female SLE (NZBWF1) and control (NZW) mice were administered either an antibody to CD3ε, a component of the T cell receptor complex expressed on all T cells, or IgG antibody (isotype control) for up to 4 weeks (intranasal; 25 μg/week). Spleen weight was lower in SLE mice treated with anti-CD3 antibody than in IgG-treated SLE mice, suggesting that immune system hyperactivity is decreased. Circulating anti-dsDNA autoantibodies were increased in SLE mice compared to controls and were blunted in the anti-CD3-treated SLE mice. The development of hypertension was attenuated in anti-CD3 treated mice with SLE independently of changes in renal injury (assessed by urinary albumin). These data suggest anti-CD3 therapy during autoimmune disease may have added clinical benefit to attenuate cardiovascular risk factors like hypertension.
Keywords: hypertension, autoimmune, T cell, antibody
Graphical abstract
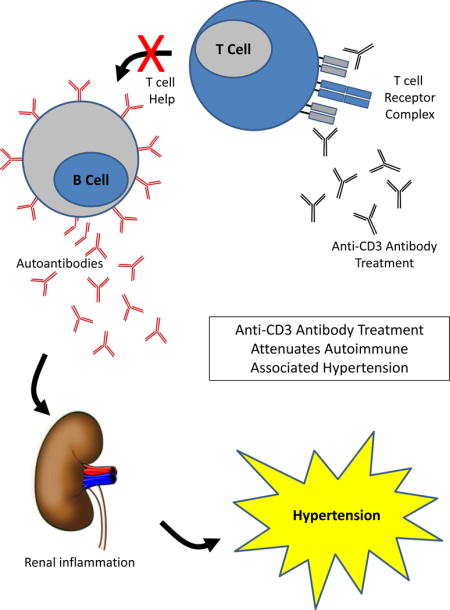
INTRODUCTION
Systemic lupus erythematosus (SLE) is an autoimmune disease that predominantly affects women of reproductive age. While loss of immune tolerance leading to the production of autoantibodies that promote tissue injury and inflammation is a hallmark of SLE, the major cause of mortality in these women is cardiovascular disease,1–5 and the prevalence of hypertension, a major cardiovascular risk factor, is markedly increased in this patient population6–11. An important clinical goal for patients with autoimmune disease is to induce tolerance. To achieve this goal, a monoclonal antibody to CD3ε, a subunit of the T-cell co-receptor complex which is expressed on the surface of all T cells, has been used in both pre-clinical and clinical studies to induce peripheral tolerance by expanding tolerogenic regulatory T cells (TREG) and by promoting the removal of apoptotic bodies12. Treatment with anti-CD3 antibodies reportedly attenuates disease in non-obese diabetic mice13, models of experimental autoimmune encephalitis14, 15, rheumatoid arthritis16, and SLE17. While the efficacy of these antibodies to attenuate autoimmunity has been widely established, it is unclear whether anti-CD3 therapy can attenuate the prevalent hypertension associated with autoimmune diseases like SLE. This is an important question to address given the now widely recognized role for adaptive immunity in the pathogenesis of hypertension18. The present study was designed to test the hypothesis that anti-CD3 therapy in female SLE mice with already established renal disease can attenuate the progression of hypertension.
METHODS
Animal Model
Female NZBWF1 and control (NZW/LacJ) mice were obtained from Jackson Laboratories (Bar Harbor, ME). The female NZBWF1 mouse is a well-established model of SLE with prevalent hypertension and renal injury19–25. Mice were randomly divided into 4 groups: control mice administered an isotype control (Control/IgG) or the monoclonal antibody to mouse CD3 (Control/Anti-CD3), and SLE mice administered isotype control (SLE/IgG) or the antibody to CD3 (SLE/Anti-CD3). Only NZBWF1 mice with already established renal disease (urinary albumin ≥ 100 mg/dL by dipstick) at 30 weeks of age were used in the SLE groups. Therefore the experiment is designed to determine whether the disease progression can be attenuated or reversed. All studies were approved by the University of Mississippi Medical Center Institutional Animal Care and Use Committee (IACUC) and were in accordance with National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals.
Antibody Administration
Mice were administered a monoclonal hamster anti-mouse CD3ε antibody (clone 145-2C11, BioXcell, #BE0001-1) or a polyclonal hamster IgG (vehicle) as the isotype control (5 μg, intranasal; West Lebanon, NH) for 5 consecutive days per week for 3–4 weeks. This route of administration, dose, and duration of anti-CD3 antibody was previously shown by others to attenuate disease severity in the (NZB × SWR)F1 (SNF1) model of SLE17. Spleen weight was assessed at the end of the study as an indirect surrogate marker to determine whether the antibody treatment was impacting systemic immune function.
Autoantibody Production
The presence of plasma anti-dsDNA autoantibodies (dsDNA), a clinical marker of SLE, was measured by ELISA (Alpha Diagnostics International, San Antonio, TX) and is presented as antibody activity index, as previously described by our laboratory25.
Blood Pressure Measurement
At 34 weeks of age, blood pressure was recorded in conscious, unrestrained mice by carotid artery catheter as previously described by our laboratory23, 24, 26–29.
Renal Injury and Inflammation
Albuminuria was measured by ELISA (Alpha Diagnostics International) in 24-hour urine samples at the conclusion of the study as previously published by our laboratory23, 24,26–29. Renal expression of tumor necrosis factor alpha (TNF-α) was assessed by immunoblot as previously reported in our laboratory29, 30 and renal expression of the interleukin 17 receptor (IL-17RA) was assessed using a polyclonal goat anti mouse antibody (AF448, R&D Systems).
Statistical Analysis
Data are presented as mean ± standard error of means (SEM). Statistical analyses were performed using Sigmaplot 11.2 software (Systat, Richmond, CA). A two-way ANOVA was used to assess group and treatment effects followed by a one-way ANOVA with an appropriate post-hoc analysis to detect determine differences between groups. Values were considered statistically different at p values < 0.05.
RESULTS
Anti-CD3 treated animals have lower spleen weight
Spleen weight was assessed as a surrogate marker for the efficacy of the anti-CD3 treatment to reduce immune system hyperactivity. There was a trend for increased spleen weight in SLE mice relative to control animals; however, this did not reach statistical significance (Figure 1; 0.25 ± 0.06 vs. 0.16 ± 0.02 grams, p = 0.075) as it has in our previous studies25. However, spleen weight was significantly lower in SLE mice treated with anti-CD3 therapy (0.15 ± 0.02; p = 0.022) compared to SLE mice treated with IgG. Spleen weight was not altered in anti-CD3 treated control mice (0.14 ± 0.02).
Figure 1. Anti-CD3 therapy reduces spleen weight in SLE mice.
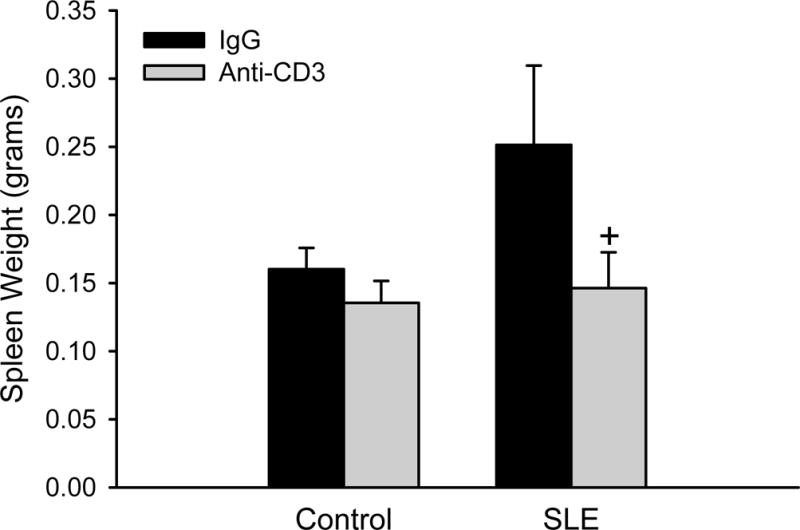
Spleen weight (g) in control and SLE mice administered anti-IgG or anti-CD3 antibodies (n = 6–11). +p <0.05 vs. SLE/IgG
Anti-CD3 treatment attenuates autoantibody production
Circulating levels of anti-dsDNA autoantibodies were greater in SLE compared to controls (Figure 2; 19.8 ± 7.3 vs. 0.7 ± 0.1 antibody units; p = 0.050, by two way ANOVA group effect) as previously reported25. Circulating anti-dsDNA autoantibodies were qualitatively lower in anti-CD3 treated SLE mice compared to vehicle treated SLE mice (8.4 ± 3.3), but the study was not sufficiently powered for this to reach statistical significance.
Figure 2. Anti-CD3 therapy attenuates autoantibody production in SLE mice.
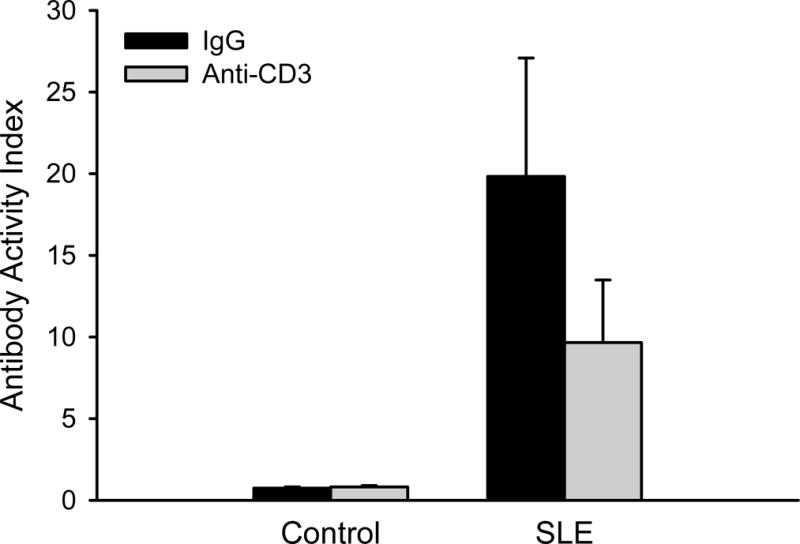
Circulating anti-dsDNA autoantibodies (presented as antibody activity index) measured in control and SLE mice administered anti-IgG or anti-CD3 (n = 3–12).
Anti-CD3 treatment attenuates the progression of hypertension in SLE mice
Mean arterial pressure was higher in SLE mice with established disease than controls (Figure 3; 156 ± 3 vs. 112 ± 4 mmHg; p < 0.001) as we previously reported23, 24, 26–29. Blood pressure was lower in anti-CD3 treated SLE mice (137 ± 4; p < 0.001) compared to vehicle treated SLE mice, but anti-CD3 did not change blood pressure in control mice (117 ± 2).
Figure 3. Anti-CD3 therapy attenuates the progression of hypertension in SLE mice.
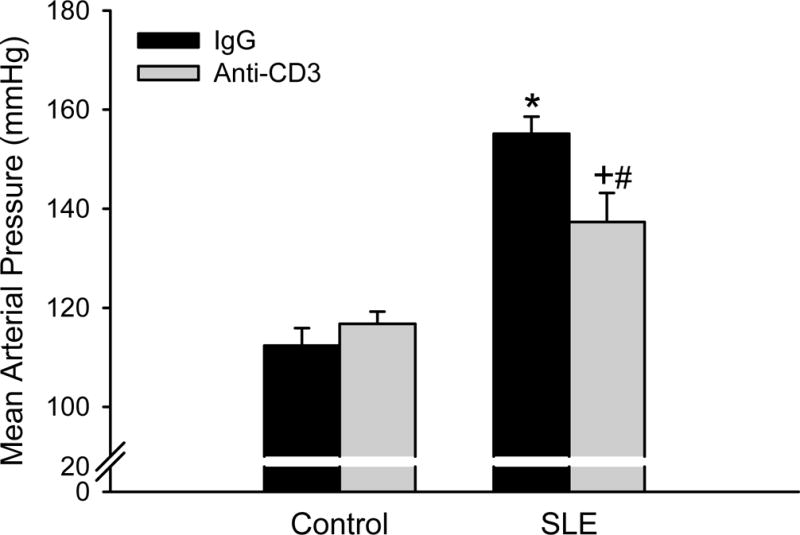
Mean arterial pressure measured in control and SLE mice administered anti-IgG or anti-CD3 (n = 6–7). *p<0.05 vs. Control/Anti-IgG; #p<0.05 vs. Control/Anti-CD3; +p<0.05 vs. SLE/Anti-IgG
Anti-CD3 treatment does not impact renal injury and inflammation
Albuminuria was increased in SLE mice with established disease compared to control animals (Figure 4; 304679 ± 110518 vs. 52 ± 6 μg/mg creatinine; SLE vs control; p = 0.008, two way ANOVA group effect). However, urinary albumin was not altered in anti-CD3 treated SLE animals (272227± 59129 μg/mg creatinine) or control animals (74±5 μg/mg creatinine). Renal TNF-α expression (normalized to β-actin) was increased in female SLE mice compared to controls as we previously showed (12 ± 1 vs. 7 ± 3; p = 0.016, two way ANOVA group effect)23, 25; however, expression was not reduced in the treated animals (Figure 5A). Renal expression of the IL-17RA was increased in female SLE mice compared to controls, and its expression was lower in SLE mice treated with anti-CD3 antibodies (Figure 5B).
Figure 4. Anti-CD3 therapy does not alter renal injury in SLE mice.
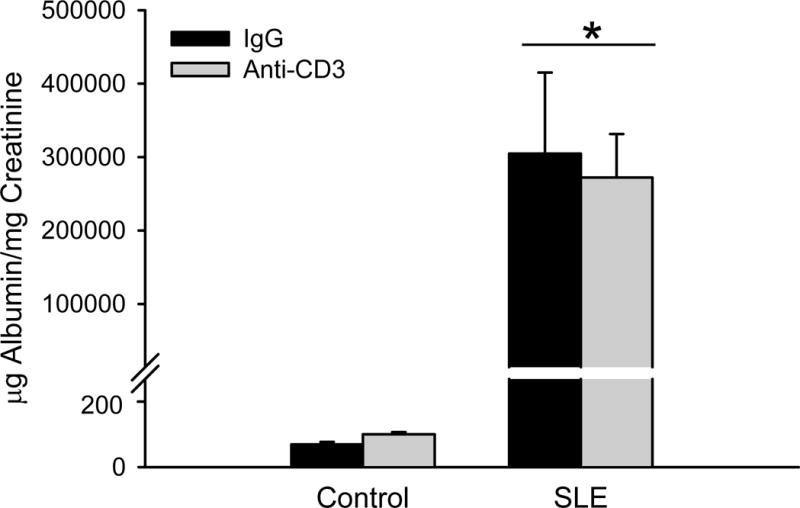
Urinary albumin excretion rate at 34 weeks measured by ELISA in control and SLE mice administered anti-IgG or anti-CD3 (n = 6–12). *p<0.05 vs. control.
Figure 5.
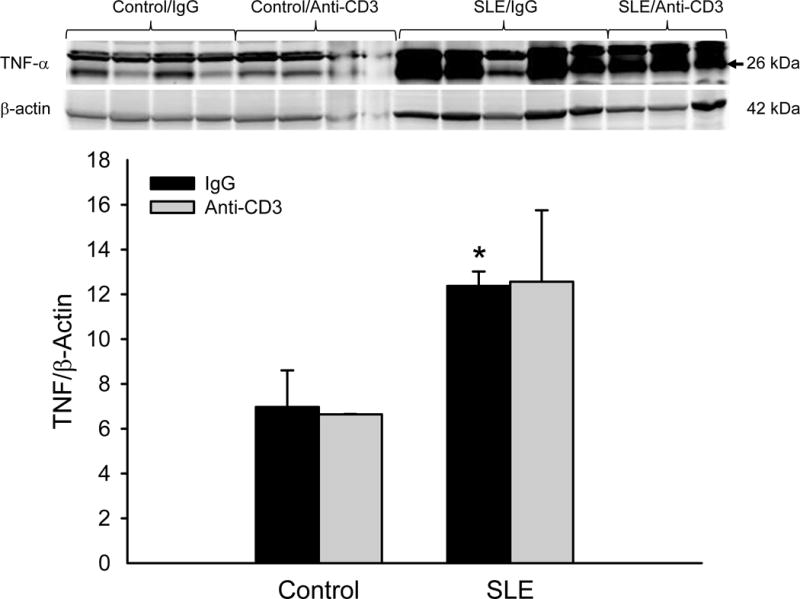
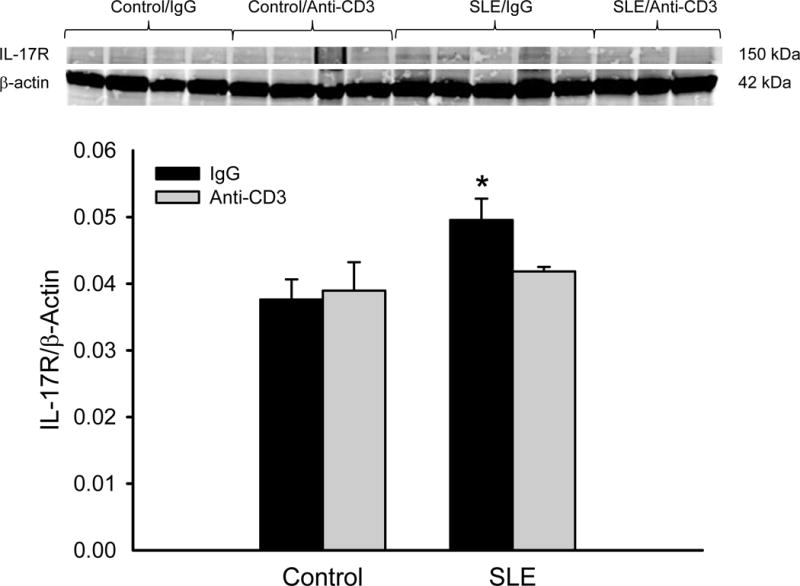
(A) Anti-CD3 therapy does not alter renal TNF-α protein expression in SLE mice. Renal TNF-α was not altered in anti-CD3 therapy, as assessed by immunoblot. *p<0.05 vs. control IgG. (B) Renal IL-17RA expression is attenuated in female SLE mice treated with anti-CD3 therapy. *p<0.05 vs. control IgG and SLE anti-CD3.
DISCUSSION
The prevalence of hypertension is much greater in women with SLE when compared to age matched healthy controls. Hypertension is a major cardiovascular risk factor and contributor to mortality in patients with SLE, and therefore should be considered as an important clinical endpoint that can improve care for this patient population. Previous studies have shown that anti-CD3 antibodies can attenuate the development of SLE and other autoimmune disorders; however, whether this treatment can reduce cardiovascular risk by attenuating the associated hypertension has not been tested. The major new finding of this study is that female SLE mice with established renal disease that are treated with an anti-CD3 antibody have lower blood pressure than vehicle treated animals. In addition, the attenuation of hypertension occurred independently of changes in renal injury and inflammation. Therefore, this study provides new insight into potential clinical benefits for patients with autoimmune disease that might arise from therapeutic approaches that target the immune system.
It is now widely recognized that abnormal immune system activation is associated with the pathogenesis of hypertension, both in humans and in experimental models. For example, numerous studies link inflammatory cytokines and blood pressure in humans31–35 and a growing body of literature suggests an association between autoantibodies, consistent with systemic autoimmunity, and hypertension36–40. In experimental models of spontaneous hypertension, non-specific immunosuppressive therapies such as cyclophosphamide have been reported not only to prevent the development of hypertension, but also to reduce blood pressure in animals with already established hypertension41. Similarly, mycophenolate mofetil (MMF), an immunosuppressive therapy commonly used in patients with SLE that depletes proliferating T and B lymphocytes, effectively attenuates the development of hypertension in several experimental models and in humans42–48. More recently, our laboratory demonstrated that the development of hypertension associated with SLE could be prevented by treatment with a mouse monoclonal antibody to CD20 (equivalent of rituximab approved for use in humans) intended to specifically deplete B lymphocytes25. However, consistent with human literature showing variability in the efficacy of rituximab to treat SLE49, 50, and in mouse models where efficacy of the anti-cd20 treatment of SLE was reduced with increasing age51, prophylactic treatment was required to effectively impact SLE disease, and this early intervention impacted both B and T lymphocytes. The present study makes an important advance towards understanding the impact of immunotherapies on hypertension because of the interventional experimental design focused on a therapy (anti-CD3) that consistently attenuates autoimmune disease in experimental animal models.
In addition to assessing the impact of anti-CD3 therapy on blood pressure during SLE, we examined markers of renal disease and inflammation. Immune complex mediated glomerulonephritis is common in patients with SLE and urinary albumin is an important clinical marker of glomerular disease. Female NZBWF1 mice represent a widely established experimental model that progressively develops renal disease easily detectable by measuring albumin in the urine. Data from the present study show that once SLE disease is established, anti-CD3 treatment may not afford renal protection given that urinary albumin was unchanged in the treated group. This data, however, does help to address the important experimental question of whether the albuminuria is simply driven by the hypertension. The fact that pressure is lower in SLE mice treated with anti-CD3, but urinary albumin is unchanged, suggests that these two characteristics are uncoupled in this model and that the renal disease is more likely to be driven by immune complex deposition rather than increased pressure. This is consistent with human studies suggesting that lupus nephritis and hypertension are not necessarily linked52, 53.
The mechanism by which anti-CD3 therapy attenuated the hypertension during SLE remains uncertain. Previous work from our laboratory showed that treatment with etanercept to block the biological activity of TNF-α partially attenuated the hypertension29. The partial attenuation of the hypertension by etanercept mirrors the attenuation of hypertension reported here in the current study. However, renal TNF-α expression was not lower in the anti-CD3 treated animals, perhaps reflecting that that the current study design was interventional whereas our former work started treatment before evidence of albuminuria was present. IL-17, which is predominantly produced by TH17 cells and serves as a strong inducer of inflammation, has been implicated as an important cytokine in the pathogenesis of autoimmune diseases including SLE, and in the development of hypertension54, 55. The data showing that expression of the IL-17A receptor is lower in the kidneys from anti-CD3 treated mice suggests that TH17 cells, IL017, and downstream inflammation may be involved in the hypertension associated with this model. Another potential mechanism that could contribute to the attenuation of hypertension by anti-CD3 therapy is the expansion of the CD4+CD25+FoxP3+ inducible TREG (iTREG) that mature from CD4+FoxP3− conventional TH cells in the periphery, leading to reduced inflammation and the induction of peripheral tolerance. This would be consistent with the mechanism by which anti-CD3 antibodies attenuated SLE disease in previous studies17 and with our data suggesting that autoantibodies are reduced in anti-CD3 treated animals; however, direct measurement of this cell population is required for confirmation. TREG dysfunction is recognized as an important contributor to the pathogenesis of SLE56–61 and adoptive transfer of TREG reduces blood pressure in non-autoimmune mediated models of hypertension62–64. It will be important for future studies to explore the possibility that directly expanding these cells can attenuate the pathogenesis of hypertension during autoimmune disease.
In conclusion, data from this study add to the current understanding of the potential clinical benefits that anti-CD3 treatment may afford to patients with autoimmune disease. As part of this benefit, this study highlights the potential interventional utility of this therapy to control a hypertension, a major cardiovascular risk factor and contributor to mortality, during SLE.
Acknowledgments
The authors would like to thank Katie W. Corkern and C. Warren Masterson for their technical assistance with this study.
SOURCES OF FUNDING
This work was supported by the Department of Veterans Affairs (BX002604 to MJR), American Heart Association Scientist Development Grant (14SDG18320033 to KWM), the National Institutes of Health (P01HL051971, P20GM104357 to UMMC Physiology), and the University of Mississippi Medical Center-Intramural Research Support Program. EBT was supported by the National Institutes of Health (5T32HL105324).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
None
References
- 1.Abu-Shakra M, Urowitz MB, Gladman DD, Gough J. Mortality studies in systemic lupus erythematosus. Results from a single center. I. Causes of death. The Journal of rheumatology. 1995;22:1259–1264. [PubMed] [Google Scholar]
- 2.Bernatsky S, Clarke A, Gladman DD, Urowitz M, Fortin PR, Barr SG, Senecal JL, Zummer M, Edworthy S, Sibley J, Pope J, Ensworth S, Ramsey-Goldman R, Hanly JG. Mortality related to cerebrovascular disease in systemic lupus erythematosus. Lupus. 2006;15:835–839. doi: 10.1177/0961203306073133. [DOI] [PubMed] [Google Scholar]
- 3.Manzi S, Meilahn EN, Rairie JE, Conte CG, Medsger TA, Jr, Jansen-McWilliams L, D’Agostino RB, Kuller LH. Age-specific incidence rates of myocardial infarction and angina in women with systemic lupus erythematosus: Comparison with the framingham study. Am J Epidemiol. 1997;145:408–415. doi: 10.1093/oxfordjournals.aje.a009122. [DOI] [PubMed] [Google Scholar]
- 4.Manzi S, Wasko MC. Getting to the heart of the matter in systemic lupus and rheumatoid arthritis. Bull Rheum Dis. 2001;50:1–4. [PubMed] [Google Scholar]
- 5.Mody GM, Parag KB, Nathoo BC, Pudifin DJ, Duursma J, Seedat YK. High mortality with systemic lupus erythematosus in hospitalized african blacks. British journal of rheumatology. 1994;33:1151–1153. doi: 10.1093/rheumatology/33.12.1151. [DOI] [PubMed] [Google Scholar]
- 6.Al-Herz A, Ensworth S, Shojania K, Esdaile JM. Cardiovascular risk factor screening in systemic lupus erythematosus. J Rheumatol. 2003;30:493–496. [PubMed] [Google Scholar]
- 7.Budman DR, Steinberg AD. Hypertension and renal disease in systemic lupus erythematosus. Arch Intern Med. 1976;136:1003–1007. [PubMed] [Google Scholar]
- 8.Mandell BF. Cardiovascular involvement in systemic lupus erythematosus. Semin Arthritis Rheum. 1987;17:126–141. doi: 10.1016/0049-0172(87)90035-7. [DOI] [PubMed] [Google Scholar]
- 9.Petri M. Detection of coronary artery disease and the role of traditional risk factors in the hopkins lupus cohort. Lupus. 2000;9:170–175. doi: 10.1191/096120300678828226. [DOI] [PubMed] [Google Scholar]
- 10.Sabio JM, Vargas-Hitos JA, Navarrete-Navarrete N, Mediavilla JD, Jimenez-Jaimez J, Diaz-Chamorro A, Jimenez-Alonso J. Prevalence of and factors associated with hypertension in young and old women with systemic lupus erythematosus. J Rheumatol. 2011;38:1026–1032. doi: 10.3899/jrheum.101132. [DOI] [PubMed] [Google Scholar]
- 11.Shaharir SS, Mustafar R, Mohd R, Mohd Said MS, Gafor HA. Persistent hypertension in lupus nephritis and the associated risk factors. Clinical rheumatology. 2015;34:93–97. doi: 10.1007/s10067-014-2802-0. [DOI] [PubMed] [Google Scholar]
- 12.Kuhn C, Weiner HL. Therapeutic anti-cd3 monoclonal antibodies: From bench to bedside. Immunotherapy. 2016;8:889–906. doi: 10.2217/imt-2016-0049. [DOI] [PubMed] [Google Scholar]
- 13.Chatenoud L, Thervet E, Primo J, Bach JF. Anti-cd3 antibody induces long-term remission of overt autoimmunity in nonobese diabetic mice. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:123–127. doi: 10.1073/pnas.91.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kohm AP, Williams JS, Bickford AL, McMahon JS, Chatenoud L, Bach JF, Bluestone JA, Miller SD. Treatment with nonmitogenic anti-cd3 monoclonal antibody induces cd4+ t cell unresponsiveness and functional reversal of established experimental autoimmune encephalomyelitis. Journal of immunology. 2005;174:4525–4534. doi: 10.4049/jimmunol.174.8.4525. [DOI] [PubMed] [Google Scholar]
- 15.Tran GT, Carter N, He XY, Spicer TS, Plain KM, Nicolls M, Hall BM, Hodgkinson SJ. Reversal of experimental allergic encephalomyelitis with non-mitogenic, non-depleting anti-cd3 mab therapy with a preferential effect on t(h)1 cells that is augmented by il-4. International immunology. 2001;13:1109–1120. doi: 10.1093/intimm/13.9.1109. [DOI] [PubMed] [Google Scholar]
- 16.Hughes C, Wolos JA, Giannini EH, Hirsch R. Induction of t helper cell hyporesponsiveness in an experimental model of autoimmunity by using nonmitogenic anti-cd3 monoclonal antibody. Journal of immunology. 1994;153:3319–3325. [PubMed] [Google Scholar]
- 17.Wu HY, Quintana FJ, Weiner HL. Nasal anti-cd3 antibody ameliorates lupus by inducing an il-10-secreting cd4+ J Immunol. 2008;181:6038–6050. doi: 10.4049/jimmunol.181.9.6038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ryan MJ. An update on immune system activation in the pathogenesis of hypertension. Hypertension. 2013;62:226–230. doi: 10.1161/HYPERTENSIONAHA.113.00603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Talal N. Natural history of murine lupus. Modulation by sex hormones. Arthritis Rheum. 1978;21:S58–S63. [PubMed] [Google Scholar]
- 20.Rudofsky UH, Dilwith RL, Roths JB, Lawrence DA, Kelley VE, Magro AM. Differences in the occurrence of hypertension among (nzb × nzw)f1, mrl-lpr, and bxsb mice with lupus nephritis. Am J Pathol. 1984;116:107–114. [PMC free article] [PubMed] [Google Scholar]
- 21.Okudaira H, Terada E, Okudaira K. Animal models utilized in the research of autoimmune disease control: Experimental therapy of glomerulonephritis in nzb/w f1 mice. Prog Clin Biol Res. 1987;229:157–174. [PubMed] [Google Scholar]
- 22.Burnett R, Ravel G, Descotes J. Clinical and histopathological progression of lesions in lupus-prone (nzb × nzw) f1 mice. Exp Toxicol Pathol. 2004;56:37–44. doi: 10.1016/j.etp.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 23.Venegas-Pont M, Sartori-Valinotti JC, Maric C, Racusen LC, Glover PH, McLemore GR, Jr, Jones AV, Reckelhoff JF, Ryan MJ. Rosiglitazone decreases blood pressure and renal injury in a female mouse model of systemic lupus erythematosus. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1282–R1289. doi: 10.1152/ajpregu.90992.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mathis KW, Venegas-Pont M, Masterson CW, Stewart NJ, Wasson KL, Ryan MJ. Oxidative stress promotes hypertension and albuminuria during the autoimmune disease systemic lupus erythematosus. Hypertension. 2012;59:673–679. doi: 10.1161/HYPERTENSIONAHA.111.190009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mathis KW, Wallace K, Flynn ER, Maric-Bilkan C, LaMarca B, Ryan MJ. Preventing autoimmunity protects against the development of hypertension and renal injury. Hypertension. 2014;64:792–800. doi: 10.1161/HYPERTENSIONAHA.114.04006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mathis KW, Venegas-Pont M, Flynn ER, Williams JM, Maric-Bilkan C, Dwyer TM, Ryan MJ. Hypertension in an experimental model of systemic lupus erythematosus occurs independently of the renal nerves. Am J Physiol Regul Integr Comp Physiol. 2013;305:R711–R719. doi: 10.1152/ajpregu.00602.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mathis KW, Venegas-Pont MR, Masterson CW, Wasson KL, Ryan MJ. Blood pressure in a hypertensive mouse model of sle is not salt-sensitive. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1281–R1285. doi: 10.1152/ajpregu.00386.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ryan MJ, McLemore GR, Jr, Hendrix ST. Insulin resistance and obesity in a mouse model of systemic lupus erythematosus. Hypertension. 2006;48:988–993. doi: 10.1161/01.HYP.0000243612.02929.df. [DOI] [PubMed] [Google Scholar]
- 29.Venegas-Pont M, Manigrasso MB, Grifoni SC, LaMarca BB, Maric C, Racusen LC, Glover PH, Jones AV, Drummond HA, Ryan MJ. Tumor necrosis factor-alpha antagonist etanercept decreases blood pressure and protects the kidney in a mouse model of systemic lupus erythematosus. Hypertension. 2010;56:643–649. doi: 10.1161/HYPERTENSIONAHA.110.157685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gilbert EL, Mathis KW, Ryan MJ. 17beta-estradiol protects against the progression of hypertension during adulthood in a mouse model of systemic lupus erythematosus. Hypertension. 2014;63:616–623. doi: 10.1161/HYPERTENSIONAHA.113.02385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bautista LE. Inflammation, endothelial dysfunction, and the risk of high blood pressure: Epidemiologic and biological evidence. J Hum Hypertens. 2003;17:223–230. doi: 10.1038/sj.jhh.1001537. [DOI] [PubMed] [Google Scholar]
- 32.Chae CU, Lee RT, Rifai N, Ridker PM. Blood pressure and inflammation in apparently healthy men. Hypertension. 2001;38:399–403. doi: 10.1161/01.hyp.38.3.399. [DOI] [PubMed] [Google Scholar]
- 33.Derhaschnig U, Shehata M, Herkner H, Bur A, Woisetschlager C, Laggner AN, Hirschl MM. Increased levels of transforming growth factor-beta1 in essential hypertension. Am J Hypertens. 2002;15:207–211. doi: 10.1016/s0895-7061(01)02327-5. [DOI] [PubMed] [Google Scholar]
- 34.Fernandez-Real JM, Vayreda M, Richart C, Gutierrez C, Broch M, Vendrell J, Ricart W. Circulating interleukin 6 levels, blood pressure, and insulin sensitivity in apparently healthy men and women. J Clin Endocrinol Metab. 2001;86:1154–1159. doi: 10.1210/jcem.86.3.7305. [DOI] [PubMed] [Google Scholar]
- 35.Manabe S, Okura T, Watanabe S, Higaki J. Association between carotid haemodynamics and inflammation in patients with essential hypertension. J Hum Hypertens. 2005;19:787–791. doi: 10.1038/sj.jhh.1001898. [DOI] [PubMed] [Google Scholar]
- 36.Khraibi AA. Association between disturbances in the immune system and hypertension. American journal of hypertension. 1991;4:635–641. doi: 10.1093/ajh/4.7.635. [DOI] [PubMed] [Google Scholar]
- 37.Luther HP, Homuth V, Wallukat G. Alpha 1-adrenergic receptor antibodies in patients with primary hypertension. Hypertension. 1997;29:678–682. doi: 10.1161/01.hyp.29.2.678. [DOI] [PubMed] [Google Scholar]
- 38.Patel R, Johnson J, Ansari A. Immunogenetic studies in essential hypertension among black patients. I. Correlative studies of serum autoantibody formation. International archives of allergy and applied immunology. 1982;67:145–148. doi: 10.1159/000233005. [DOI] [PubMed] [Google Scholar]
- 39.Walther T, Stepan H. Agonist autoantibodies against the angiotensin at1 receptor in renal and hypertensive disorders. Current hypertension reports. 2007;9:128–132. doi: 10.1007/s11906-007-0023-5. [DOI] [PubMed] [Google Scholar]
- 40.Zhou ZH, Wang J, Xiao H, Chen ZJ, Wang M, Cheng X, Liao YH. A novel autoantibody in patients with primary hypertension: Antibody against l-type ca2+ channel. Chinese medical journal. 2008;121:1513–1517. [PubMed] [Google Scholar]
- 41.Khraibi AA, Norman RA, Jr, Dzielak DJ. Chronic immunosuppression attenuates hypertension in okamoto spontaneously hypertensive rats. Am J Physiol. 1984;247:H722–H726. doi: 10.1152/ajpheart.1984.247.5.H722. [DOI] [PubMed] [Google Scholar]
- 42.Rodriguez-Iturbe B, Pons H, Quiroz Y, Gordon K, Rincon J, Chavez M, Parra G, Herrera-Acosta J, Gomez-Garre D, Largo R, Egido J, Johnson RJ. Mycophenolate mofetil prevents salt-sensitive hypertension resulting from angiotensin ii exposure. Kidney Int. 2001;59:2222–2232. doi: 10.1046/j.1523-1755.2001.00737.x. [DOI] [PubMed] [Google Scholar]
- 43.Rodriguez-Iturbe B, Quiroz Y, Nava M, Bonet L, Chavez M, Herrera-Acosta J, Johnson RJ, Pons HA. Reduction of renal immune cell infiltration results in blood pressure control in genetically hypertensive rats. Am J Physiol Renal Physiol. 2002;282:F191–F201. doi: 10.1152/ajprenal.0197.2001. [DOI] [PubMed] [Google Scholar]
- 44.Herrera J, Ferrebuz A, MacGregor EG, Rodriguez-Iturbe B. Mycophenolate mofetil treatment improves hypertension in patients with psoriasis and rheumatoid arthritis. J Am Soc Nephrol. 2006;17:S218–S225. doi: 10.1681/ASN.2006080918. [DOI] [PubMed] [Google Scholar]
- 45.Mattson DL, James L, Berdan EA, Meister CJ. Immune suppression attenuates hypertension and renal disease in the dahl salt-sensitive rat. Hypertension. 2006;48:149–156. doi: 10.1161/01.HYP.0000228320.23697.29. [DOI] [PubMed] [Google Scholar]
- 46.Bravo Y, Quiroz Y, Ferrebuz A, Vaziri ND, Rodriguez-Iturbe B. Mycophenolate mofetil administration reduces renal inflammation, oxidative stress, and arterial pressure in rats with lead-induced hypertension. Am J Physiol Renal Physiol. 2007;293:F616–F623. doi: 10.1152/ajprenal.00507.2006. [DOI] [PubMed] [Google Scholar]
- 47.Boesen EI, Williams DL, Pollock JS, Pollock DM. Immunosuppression with mycophenolate mofetil attenuates the development of hypertension and albuminuria in deoxycorticosterone acetate-salt hypertensive rats. Clin Exp Pharmacol Physiol. 2010;37:1016–1022. doi: 10.1111/j.1440-1681.2010.05428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tipton AJ, Baban B, Sullivan JC. Female spontaneously hypertensive rats have greater renal anti-inflammatory t lymphocyte infiltration than males. Am J Physiol Regul Integr Comp Physiol. 2012 doi: 10.1152/ajpregu.00246.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Duxbury B, Combescure C, Chizzolini C. Rituximab in systemic lupus erythematosus: An updated systematic review and meta-analysis. Lupus. 2013;22:1489–1503. doi: 10.1177/0961203313509295. [DOI] [PubMed] [Google Scholar]
- 50.Terrier B, Amoura Z, Ravaud P, Hachulla E, Jouenne R, Combe B, Bonnet C, Cacoub P, Cantagrel A, de Bandt M, Fain O, Fautrel B, Gaudin P, Godeau B, Harle JR, Hot A, Kahn JE, Lambotte O, Larroche C, Leone J, Meyer O, Pallot-Prades B, Pertuiset E, Quartier P, Schaerverbeke T, Sibilia J, Somogyi A, Soubrier M, Vignon E, Bader-Meunier B, Mariette X, Gottenberg JE, Club Rhumatismes et al. Safety and efficacy of rituximab in systemic lupus erythematosus: Results from 136 patients from the french autoimmunity and rituximab registry. Arthritis and rheumatism. 2010;62:2458–2466. doi: 10.1002/art.27541. [DOI] [PubMed] [Google Scholar]
- 51.Bekar KW, Owen T, Dunn R, Ichikawa T, Wang W, Wang R, Barnard J, Brady S, Nevarez S, Goldman BI, Kehry M, Anolik JH. Prolonged effects of short-term anti-cd20 b cell depletion therapy in murine systemic lupus erythematosus. Arthritis Rheum. 2010;62:2443–2457. doi: 10.1002/art.27515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Petrin J, Rozman B, Dolenc P, Logar D, Bozic B, Vizjak A, Ferluga D, Jezersek P. The dissociation of arterial hypertension and lupus glomerulonephritis in systemic lupus erythematosus. Blood Press. 1993;2:108–112. doi: 10.3109/08037059309077537. [DOI] [PubMed] [Google Scholar]
- 53.Ward MM, Studenski S. Clinical prognostic factors in lupus nephritis. The importance of hypertension and smoking. Arch Intern Med. 1992;152:2082–2088. [PubMed] [Google Scholar]
- 54.Gaffen SL. Structure and signalling in the il-17 receptor family. Nature reviews Immunology. 2009;9:556–567. doi: 10.1038/nri2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McMaster WG, Kirabo A, Madhur MS, Harrison DG. Inflammation, immunity, and hypertensive end-organ damage. Circulation research. 2015;116:1022–1033. doi: 10.1161/CIRCRESAHA.116.303697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bagavant H, Tung KS. Failure of cd25+ t cells from lupus-prone mice to suppress lupus glomerulonephritis and sialoadenitis. Journal of immunology. 2005;175:944–950. doi: 10.4049/jimmunol.175.2.944. [DOI] [PubMed] [Google Scholar]
- 57.Crispin JC, Martinez A, Alcocer-Varela J. Quantification of regulatory t cells in patients with systemic lupus erythematosus. Journal of autoimmunity. 2003;21:273–276. doi: 10.1016/s0896-8411(03)00121-5. [DOI] [PubMed] [Google Scholar]
- 58.Liu MF, Wang CR, Fung LL, Wu CR. Decreased cd4+cd25+ t cells in peripheral blood of patients with systemic lupus erythematosus. Scandinavian journal of immunology. 2004;59:198–202. doi: 10.1111/j.0300-9475.2004.01370.x. [DOI] [PubMed] [Google Scholar]
- 59.Valencia X, Yarboro C, Illei G, Lipsky PE. Deficient cd4+cd25high t regulatory cell function in patients with active systemic lupus erythematosus. Journal of immunology. 2007;178:2579–2588. doi: 10.4049/jimmunol.178.4.2579. [DOI] [PubMed] [Google Scholar]
- 60.Wu HY, Staines NA. A deficiency of cd4+cd25+ t cells permits the development of spontaneous lupus-like disease in mice, and can be reversed by induction of mucosal tolerance to histone peptide autoantigen. Lupus. 2004;13:192–200. doi: 10.1191/0961203303lu1002oa. [DOI] [PubMed] [Google Scholar]
- 61.Yan B, Ye S, Chen G, Kuang M, Shen N, Chen S. Dysfunctional cd4+,cd25+ regulatory t cells in untreated active systemic lupus erythematosus secondary to interferon-alpha-producing antigen-presenting cells. Arthritis and rheumatism. 2008;58:801–812. doi: 10.1002/art.23268. [DOI] [PubMed] [Google Scholar]
- 62.Barhoumi T, Kasal DA, Li MW, Shbat L, Laurant P, Neves MF, Paradis P, Schiffrin EL. T regulatory lymphocytes prevent angiotensin ii-induced hypertension and vascular injury. Hypertension. 2011;57:469–476. doi: 10.1161/HYPERTENSIONAHA.110.162941. [DOI] [PubMed] [Google Scholar]
- 63.Matrougui K, Abd Elmageed Z, Kassan M, Choi S, Nair D, Gonzalez-Villalobos RA, Chentoufi AA, Kadowitz P, Belmadani S, Partyka M. Natural regulatory t cells control coronary arteriolar endothelial dysfunction in hypertensive mice. The American journal of pathology. 2011;178:434–441. doi: 10.1016/j.ajpath.2010.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mian MO, Barhoumi T, Briet M, Paradis P, Schiffrin EL. Deficiency of t-regulatory cells exaggerates angiotensin ii-induced microvascular injury by enhancing immune responses. Journal of hypertension. 2016;34:97–108. doi: 10.1097/HJH.0000000000000761. [DOI] [PubMed] [Google Scholar]


