Abstract
A variety of nicotinic drug treatments have been found to decrease nicotine self-administration. However, interactions of drugs affecting different nicotinic receptor subtypes have not been much investigated. This study investigated the interactions between dextromethorphan, which blocks nicotinic α3β2 receptors as well as a variety of other receptors with sazetidine-A which is a potent and selective α4β2 nicotinic receptor partial agonist with desensitizing properties. This interaction was compared with dextromethorphan combination treatment with mecamylamine, which is a nonspecific nicotinic channel blocker. Co-administration of dextromethorphan (either 0.5 or 5 mg/kg) and lower dose of sazetidine-A (0.3 mg/kg) caused a significant reduction in nicotine SA. With regard to food-motivated responding, 3 mg/kg of sazetidine-A given alone caused a significant decrease in food intake. However, the lower 0.3 mg/kg sazetidine-A dose did not significantly affect food-motivated responding even when given in combination with the higher 5 mg/kg dextromethorphan dose which itself caused a significant decrease in food motivated responding. Interestingly, this higher dextromethorphan dose significantly attenuated the decrease in food motivated responding caused by 3 mg/kg of sazetidine-A. Locomotor activity was increased by the lower 0.3 mg/kg sazetidine-A dose and decreased by the 5 mg/kg dextromethorphan dose. Mecamylamine at the doses (0.1 and 1 mg/kg) did not affect nicotine SA, but at 1 mg/kg significantly decreased food-motivated responding. None of the mecamylamine doses augmented the effect of dextromethorphan in reducing nicotine self-administration. These studies showed that the combination of dextromethorphan and sazetidine- A had mutually potentiating effects, which could provide a better efficacy for promoting smoking cessation, however the strength of the interactions was fairly modest.
Keywords: Sazetidine-A, Dextromethorphan, Mecamylamine, Treatment, Addiction
Introduction
Several drug treatments have been developed to help people overcome tobacco addiction, including nicotine replacement of various sorts, varenicline and bupropion. Each of these treatments has been shown to improve tobacco smoking cessation to some extent, but the efficacy of each is relatively small and relapse rate is high. It is possible that combinations of effective treatments could provide greater efficacy. We and others have found that dextromethorphan, which among other actions blocks nicotinic α3β2 receptors, significantly reduced nicotine self-administration in rats (Glick et al. 2001, Briggs et al. 2016). Sazetidine-A is a selective ligand for nicotinic α4β2 receptors (Xiao et al. 2006) with agonist effects at one subtype and desensitizes at another subtype of α4β2 receptors. We have repeatedly shown that sazetidine-A significantly reduces nicotine self-administration in rats (Levin et al. 2010, Rezvani, Slade et al. 2010, Johnson et al. 2012). Mecamylamine, a non-specific nicotinic channel blocker, has also been shown to reduce nicotine self-administration in rats (Corrigall and Coen 1989, Shoaib et al. 1997, DeNoble and Mele 2006) and to have mixed effects on tobacco smoking in people (Pomerleau et al. 1987, Rose et al. 1989).
All three drugs tested in this set of studies, dextromethorphan, sazetidine-A and mecamylamine, have effects on nicotinic receptors with different specificities. Dextromethorphan has noncompetitive antagonist effects at α3β4, α4β2 and α7 nicotinic receptors (Hernandez et al. 2000, Damaj et al. 2005). Dextromethorphan also interacts with the glutamatergic receptors and similar to ketamine, it acts as an NMDA glutamate receptor antagonist (Netzer et al. 1993). Sazetidine-A is a desensitizing agent at nicotinic α4β2 receptors and an agonist at the high affinity concatemer of nicotinic α4β2 receptors and mecamylamine is a non-specific channel blocker at all nicotinic receptors.
The α4β2* nicotinic receptors play key roles in the modulation of dopamine innervation from the ventral tegmental area (VTA) to the nucleus accumbens (Zhao-Shea et al. 2011), a pathway involved in motivation and the action of addictive drugs such as nicotine. The hypothesized pathway for sazetidine-A effects on nicotine self-administration is that it is a selective partial agonist at α4β2* receptors and desensitizes them in the VTA rendering them resistant to subsequent stimulation by nicotine. This would then decrease nicotine-induced dopamine release in the nucleus accumbens, leading to less motivation to take nicotine (Levin et al. 2010, Rezvani et al. 2010)
In the current studies, we evaluated the combination of relatively low doses of dextromethorphan with sazetidine-A and mecamylamine. It was hypothesized that the combination of these drugs at sub-threshold doses would be more effective than individual drugs and will significantly reduce nicotine self-administration without significantly affecting food motivation responding.
Methods
Subjects
Adult female Sprague-Dawley rats (Charles River Labs, Raleigh, NC, USA) were used for the current studies. At the start of the study the rats were 60-70 days old and were naïve to nicotine. They were housed in a vivarium facility next to the testing rooms on a 12:12 hr reverse day night cycle with lights off at 7:00 AM so that they were in their active phase during behavioral testing. All rats had ad lib access to water and were fed rodent chow once daily 30 min after their testing to keep them at approximately 85% of their ad lib body weight. All procedures used in this study were approved by the Duke University Animal Care and Use Committee. Each of the two studies used separate sets of rats. The rats were singly housed after catheter implant surgery to prevent cage mates from gnawing on the rats’ catheters.
Behavioral Training
The rats were trained to lever press for food reinforcement using a standard chamber with two levers (Med Associates, St. Albans, VT, USA). The chambers were equipped with two levers (one active, one inactive), a house light, two cue lights located directly above each lever, and a white noise generator. Following lever pressing training, animals experienced three consecutive 45-min sessions of lever pressing for food under a fixed ratio (FR) 1 schedule of reinforcement. After reaching criterion of 50 pellets/session for three consecutive days, rats underwent surgery for implantation of a catheter into their jugular vein for nicotine self-administration.
Surgery
After training to lever press for food and before the initiation of nicotine self-administration sessions, rats were anesthetized by IP injection of ketamine (60 mg/kg) and dexdormitor (15 mg/kg). After establishment of total anesthesia, a catheter (Strategic Application Inc., Libertyville, IL, USA) was implanted into their jugular vein. The jugular catheter was attached to a harness that could be tethered to the infusion pump during the experimental sessions. Nicotine self-administration sessions were started the day after the surgery after full recovery (Levin et al. 2010, Rezvani et al. 2010, Rezvani et al. 2013).
Nicotine Self-Administration
Following full recovery from the surgery, rats were trained to self-administer nicotine (0.03 mg/kg/infusion, IV) via operant lever response with a visual secondary reinforcer. Two levers were available to be pressed but only pressing the lever on the active side resulted in the immediate delivery of one 50-µl infusion of nicotine in less than 1 second into the jugular vein. Each infusion was immediately followed by a one-minute period of timeout and presses on levers were recorded but not reinforced (Rezvani et al. 2010, Rezvani et al. 2013).
Locomotor Activity
Since changes in motor activity may interfere with nicotine self-administration, the effects of the drug treatment on motor activity was measured using a Figure-8 Apparatus (Levin et al. 2010). Animals were allowed to roam in the Figure-8 maze during a one-hour session and photo beam breaks were recorded in 12 five-minute blocks to measure their activity. The mazes had continuous enclosed alleys 10x10 cm in the shape of a Figure-8. Eight infrared photobeams, which crossed the alleys, indexed locomotion of the rat. One photobeam was located on each of the two blind alleys and three were located on each of two loops of the Figure-8 maze. The number of photo beam breaks was recorded and tallied during the one-hour session. The linear and quadratic trends across twelve five-min blocks in each session were calculated to determine locomotor activity over the course of one-hour session.
Food-Motivated Responding
Subsequent to the nicotine SA sessions, the rats were tested to assess drug effects on responding for food reinforcement. The drug treatments as well as the saline control were administered in a repeated measures counterbalanced order. The behavioral paradigm used FR1, with activation of a feedback tone for 0.5 s after reinforcement. Cue lights were on throughout the session with no house light illumination and no time out after reinforcement. The rewards were 45-mg food pellets. As with nicotine SA, the sessions for food SA were 45-min. long.
Preparation of Drugs
Using pyrogen-free glassware, all drugs were dissolved in saline and were injected sc in a volume of 1 mg//kg 10 min before nicotine SA session. The pH of the nicotine solution for SA was adjusted to 7.0 using NaOH solution and then the solution was filtered through a 0.22 µm Nalgene filter (Nalgene Nunc International, Rochester, NY, USA) to ensure sterilization. Between sessions nicotine solutions were refrigerated for no longer than two weeks before replacement. The dose of nicotine used for each rat was calculated as a function of the nicotine base weight.
Drug Treatment
Acute drug treatments were administered sc in a volume of 1 ml/kg. The same volume of saline was used as the control vehicle. Each study evaluated the interaction of two drug treatments using a 3 × 3 design with control, lower and higher doses of each drug. The dose combinations were given in a counterbalanced order to avoid confounding dose effects with dose order. There were three tests, for drug effects of nicotine self-administration, locomotor activity and food self-administration with nine dose combinations each. So, there were 27 total injections. The doses of sazetidine-A were 0, 0.3 and 3 mg/kg and the doses for dextromethorphan were 0, 0.5 and 5 mg/kg (N=13). In a separate set of rats, the doses of mecamylamine were 0.1 and 1 mg/kg. Each dose was given in a counterbalanced order with at least a day between consecutive doses (N=14). Then, the complete set of the nine doses and combinations was given again for a second phase. These dose ranges of dextromethorphan, sazstidine-A and mecamylamine has been shown in previous studies to span the behaviorally active doses for effects in rats on drug self-administration and cognitive function (Briggs et al. 2016, Levin et al. 2010, Rezvani et al. 2010, Rezvani et al 2012, Glick et al. 2002, Levin et al. 1987, Levin et al 1989, Levin, 2000 #12367, Glick et al. 2001, Pulvirenti et al. 1997).
Statistical Analysis
Analysis of variance was used to assess the data. A three factor within subjects design was used with sazetidine-A, dextromethorphan, mecamylamine and test phase used as factors to assess impacts on nicotine self-administration. For locomotor activity factors were the two drug treatments and 5-min block within the 1-hour session. With food self-administration the two drug treatments were the within subjects factors. Planned comparisons of the treated to control conditions were made as a follow-up to significant main effects. Interactions of p < 0.10 were followed up by tests of the simple main effects as recommended by Snedecor and Cochrane (Snedecor and Cochran 1967) with planned comparisons of the treated to control conditions. A threshold of p < 0.05 (two-tailed) was the threshold for significance. To guard against slippage of alpha with comparison of the dose groups back to control, Bonferroni corrections were used in comparisons of the dose effect functions of dextromethorphan, sazetidine-QA an mecamylamine back to controls.
Results
Sazetidine-A – Dextromethorphan Interactions
Nicotine Self-administration
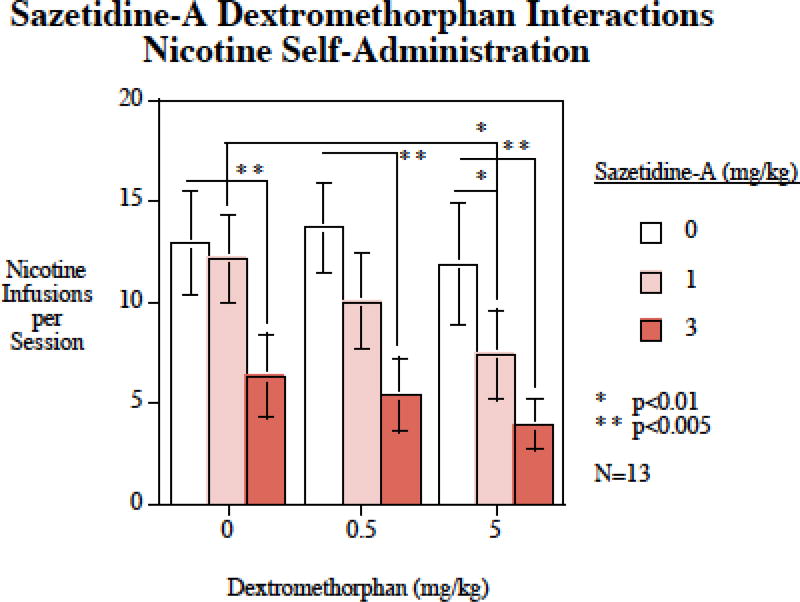
The main effects of dextromethorphan (F(2,24)=5.79, p < 0.01) sazetidine-A (F(2,12)=26.44, p < 0.0005) were significant. Sazetidine-A when given alone at a dose of 3 mg/kg caused a significant (p < 0.005) decrease in nicotine SA. The lower sazetidine-A dose of 0.3 mg/kg was not effective when given alone. Neither the 0.5 nor the 5 mg/kg dextromethorphan doses significantly affected nicotine self-administration when given alone. However, dextromethorphan significantly enhanced the effects of low-dose of sazetidine- A. The 0.3 mg/kg sazetidine-A dose significantly reduced nicotine self-administration when given together with the 5 mg/kg (p < 0.01) of dextromethorphan compared with these doses of dextromethorphan alone. The addition of 5 mg/kg of dextromethorphan also significantly (p < 0.01) enhanced the effectiveness of 0.3 mg/kg of sazetidine-A compared with this dose of sazetidine-A alone (Fig. 1). The interaction of dextromethorphan was tested twice as mentioned in the methods section in the drug treatment section. This has been clarified and detailed. The effects in the two phases did not significantly differ so the statistical analysis did not separately compare them. However, to check reproducibility, we did go back and make separate analyses of the drug interaction of Sazetidine-A (1 mg/kg) alone vs. sazetidine-A (1 mg/kg) + dextromethorphan (5 mg/kg). Phase 1: Saz=8.0±0.9; Saz+Dex=3.9±0.9; Phase 2: Saz=11.2±3.1; Saz+Dex=4.9±1.0, p < 0.01.
Figure 1.
Sazetidine-A – dextromethorphan interactions and nicotine self-administration, nicotine infusions per session (mean±sem), N=13.
Food Motivated Responding
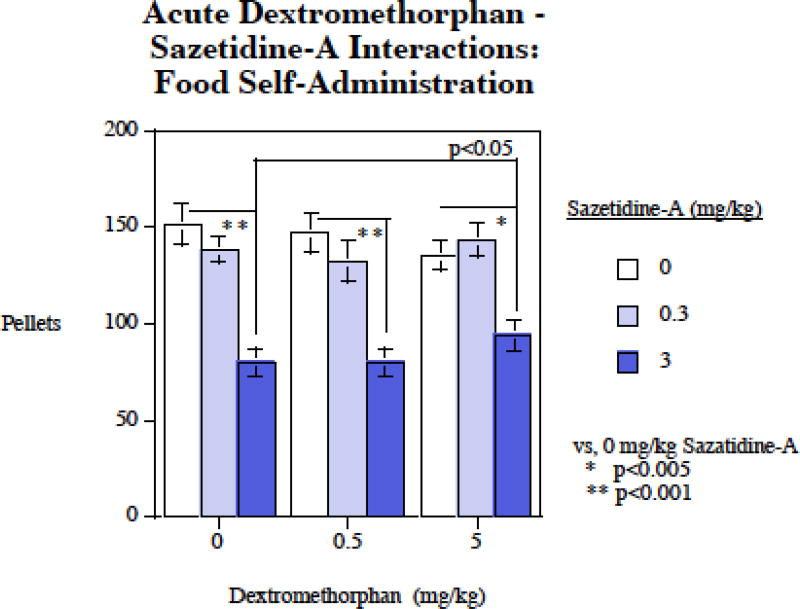
Figure 2 shows that a different pattern of interactions of dextromethorphan and sazetidine-A with regard to self-administration of food. There was a significant main effect of sazetidine-A (F2,30)=54.10, p < 0.0005). The 3 mg/kg sazetidine-A dose caused a significant (p < 0.005) decrease in food SA, whereas the 0.3 mg/kg dose was not effective in significantly reducing food-motivated responding. There was no significant main effect of dextromethorphan on food self-administration. There was a significant sazetidine-A×dextromethorphan interaction (F(4,60)=3.18, p < 0.025). Follow-up tests of the simple main effects shows that, unlike the mutually augmenting effects of sazetidine-A and dextromethorphan for decreasing nicotine self-administration, with food dextromethorphan significantly attenuated the sazetidine-A induced reduction in food intake. The comparison of the 3 mg/kg sazetidine-A effect on food intake to the effect of this treatment together with dextromethorphan showed that the addition of dextromethorphan modestly, though significantly (p < 0.05) attenuated the effect of sazetidine-A on food intake.
Figure 2.
Sazetidine-A – dextromethorphan interactions and food-motivated responding, food pellets earned per session (mean±sem), N=13
Locomotor Activity
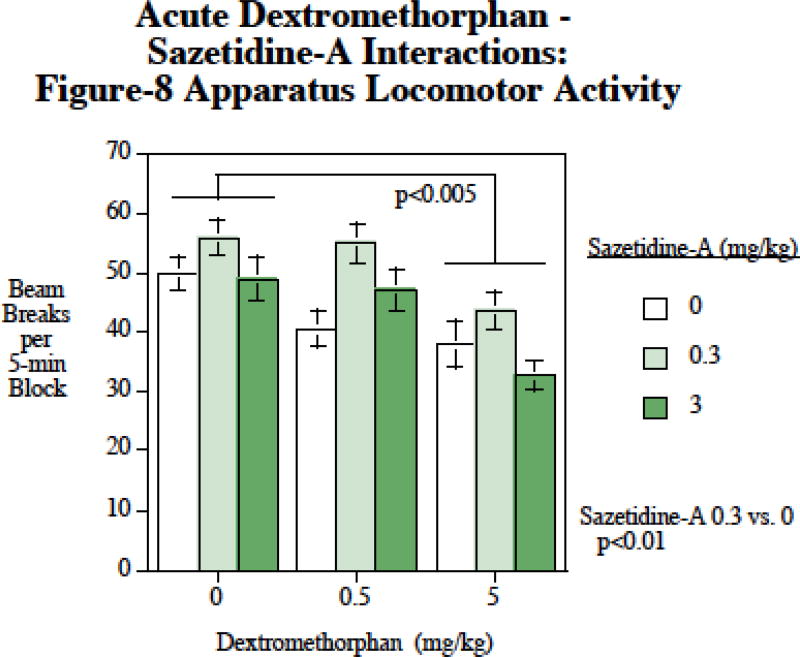
There were significant main effects of both sazetidine-A (F(2,24)=26.44, p < 0.0005) and dextromethorphan (F(2,24)=5.70, p < 0.01). Follow-up planned comparisons showed that sazetidine-A at 0.3 mg/kg caused a significant (p < 0.01) increase in locomotion and that the 5 mg/kg dextromethorphan dose caused a significant (p < 0.005) decrease in locomotion. There was no significant interaction of dextromethorphan × sazetidine-A with regard to locomotor activity.
Mecamylamine - Dextromethorphan Interactions
Nicotine Self-administration
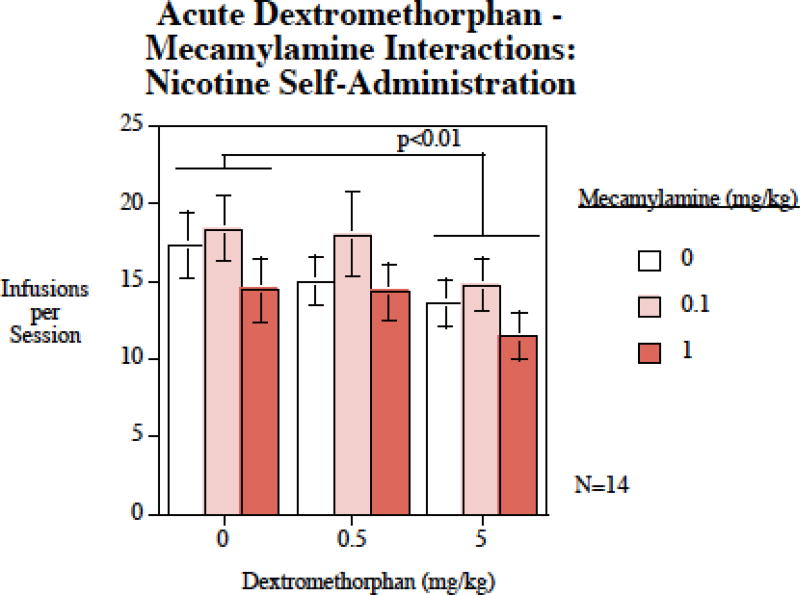
The main effect of dextromethorphan was significant (F(2,26)=6.24, p < 0.01). Planned comparisons of the doses of dextromethorphan to the control conditions showed that the 5 mg/kg dextromethorphan treatment significantly (p < 0.01) reduced nicotine self-administration (Fig. 4). The main effect of mecamylamine was also significant (F(1,26)=3.50, p < 0.05), but it is important to note that neither of the individual mecamylamine doses significantly differed from controls. Thus, despite a significant main effect of mecamylamine on nicotine self-administration, there was not evidence for either of the doses showing an impact relative to control. The interaction of dextromethorphan × mecamylamine was not significant.
Figure 4.
Mecamylamine – dextromethorphan interaction in nicotine self-administration, nicotine infusions per session (mean±sem), N=14.
Food Motivated Responding
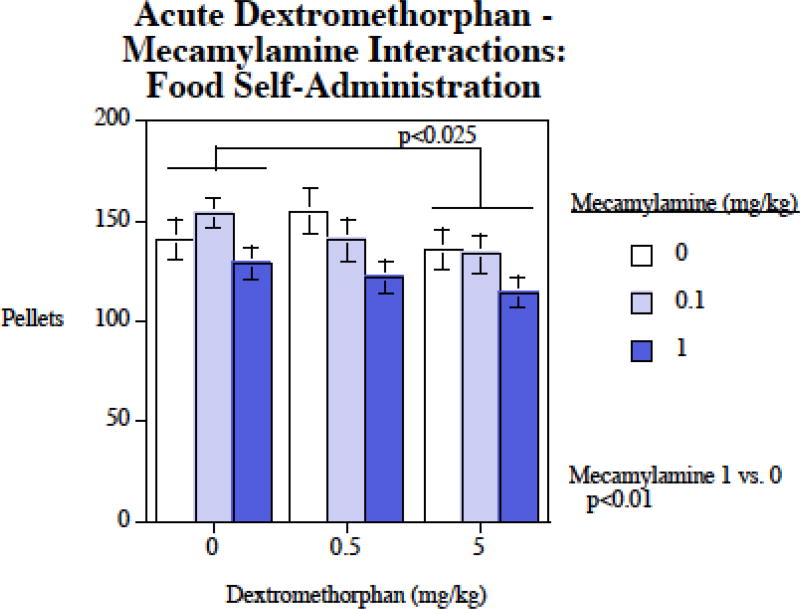
There were significant main effects of both dextromethorphan ((F2,26)=4.82, p < 0.025) and mecamylamine (F(2,26)=7.78, p < 0.005). Planned comparisons showed that compared with control, 1 mg/kg mecamylamine significantly (p < 0.01) reduced food self-administration. Also, 5 mg/kg dextromethorphan significantly (p < 0.025) reduced food self-administration (Fig. 5). The main effects of dextromethorphan and mecamylamine on food motivated responding were not seen to significantly interact with each other.
Figure 5.
Mecamylamine – dextromethorphan interactions and food-motivated responding, food pellets earned per session (mean±sem), N=14.
Locomotor Activity
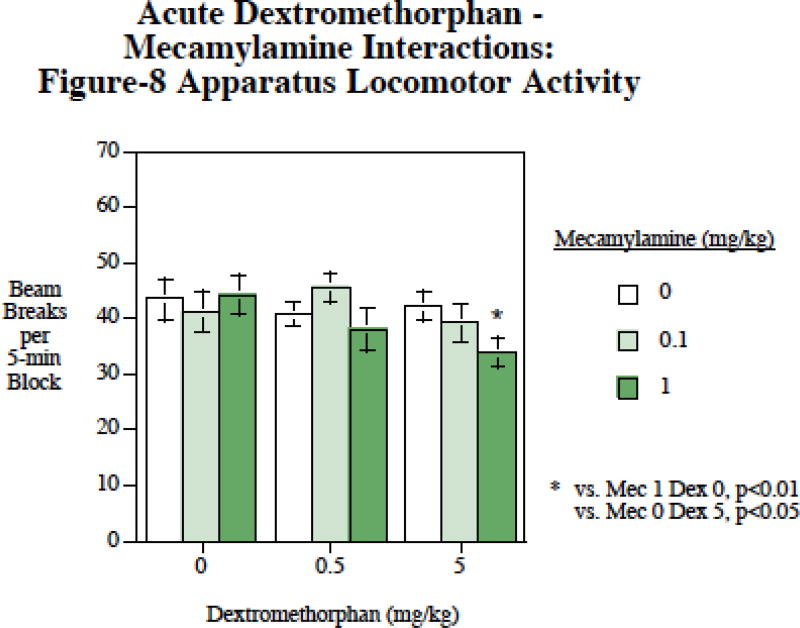
There was a significant main effect of mecamylamine (F2,30)=4.06, p < 0.05). Planned comparisons showed that it was the higher 5 mg/kg mecamylamine dose that significantly (p < 0.05) slowed activity compared with the condition in which no mecamylamine was administered, whereas the lower dose did not significantly affect locomotion. There was also a mecamylamine×dextromethorphan interaction (F(4,60)=2.09, p < 0.10) that was followed up by tests of the simple main effects. These comparisons (Fig. 6) showed that the 1 mg/kg mecamylamine + 5 mg/kg dextromethorphan dose caused significantly slower locomotion than with 1 mg/kg mecamylamine alone (p < 0.01) or 5 mg/kg dextromethorphan alone (p < 0.05).
Figure 6.
Mecamylamine – dextromethorphan interactions and locomotor activity, photobeam breaks per five-min time block (mean±sem), N=14.
Discussion
It has become apparent that among the major FDA-approved medications for smoking cessation including, nicotine replacement, varenicline and bupropion, single drug cannot help all smokers to quit. Hence, there is a critical need for the development of more efficient medications for this addiction. Previously it has been shown that dextromethorphan (Glick, Maisonneuve et al. 2001, Briggs, Wells et al. 2016), sazetidine-A (Levin, Rezvani et al. 2010, Rezvani, Slade et al. 2010, Johnson, Slade et al. 2012) and mecamylamine (Corrigall and Coen 1989, Shoaib, Schindler et al. 1997, DeNoble and Mele 2006) can significantly reduce nicotine self-administration in rats. We also have shown that the interaction of dextromethorphan with histaminergic and serotonergic receptors in reducing nicotine self-administration. The combination of dextromethorphan with pyrilamine, the H1 histaminergic antagonist, as well as the combination of dextromethorphan and lorcaserin, a serotonin 5H2c agonist, was more effective in reducing nicotine-self-administration than each individual drug in rats (Briggs, Wells et al. 2016). One possible speculation for augmenting effects of combination of these two drugs is that in addition to interaction on specific nicotinic receptors, dextromethorphan interacts with the glutamatergic receptors. Similar to ketamine, it acts as an NMDA glutamate receptor antagonist (Netzer, Pflimlin et al. 1993). It is of relevance to note that numerous studies have implicated the glutamatergic system in a variety of actions of nicotine, including its rewarding and addictive effects (Tizabi 2007, Li, Semenova et al. 2014).
In the current study, dextromethorphan and sazetidine-A were found to have mutually potentiating effects in reducing nicotine self-administration. Curiously, the 5 mg/kg dextromethorphan dose which is higher than the 3 mg/kg dose that we previously found to significantly reduce nicotine self-administration (Briggs et al. 2016) was not found in the current study to by itself to significantly impact nicotine self-administration. The enhances efficacy in the earlier study may have resulted from the fact that the 3 mg/kg dextromethorphan dose was given in a repeated measures study that included higher dextromethorphan given as well. The 5 mg/kg dextromethorphan dose was effective in significantly potentiating the effect of 1 mg/kg of sazetidine-A in reducing nicotine self-administration. The 1 mg/kg sazetidine-A dose was previously and in the current study subthreshold for significantly reducing nicotine self-administration (Levin, Rezvani et al. 2010, Rezvani, Slade et al. 2010). The effects in this study were relatively modest.
Sazetidine (3 mg/kg) effects on nicotine self-administration do not appear to be simply due to sedation inasmuch as locomotor activity was not found to be affected by this dose. It is true that food motivated responding was significantly reduced by the 3 mg/kg of sazetidine-A, but there did appear to be more specific interactions of sazetidine-A with dextromethorphan with regard to nicotine vs. food self-administration. Dextromethorphan co-treatment potentiated sazetidine-A induced reduction of nicotine self-administration, making the lower (0.3 mg/kg) sazetidine-A dose significantly reducing nicotine self-administration. In contrast, with food self-administration, sazetidine-A and dextromethorphan did not potentiate each other’s effects but rather reduced them.
However, the combination of dextromethorphan and mecamylamine did not produce a significant potentiating effect on nicotine self-administration possibly because the dose of mecamylamine was not large enough to cause an effect. In this study, the 5 mg/kg dextromethorphan dose did significantly reduce nicotine self-administration as has been previously reported (Briggs, Wells et al. 2016). This was seen both as a part of the main effect analysis and in exploratory analysis of just the overall control condition to 5 mg/kg of dextromethorphan alone (p < 0.025). Mecamylamine when given alone was not found to significantly reduce nicotine self-administration. The dose range of mecamylamine tested in this study may have too low to completely test the potential interactive effects of mecamylamine and dextromethorphan.
A caveat in the interpretation of the current studies is that the behavioral effects of dextromethorphan was exactly the same in the two studies. When given in a repeated measures study with sazetidine-A, dextromethorphan when given alone was found to decrease locomotor activity and food self-administration, but was not found to decrease nicotine self-administration. In comparison, when given in a repeated measures study with mecamylamine, dextromethorphan was found to decrease nicotine and food self-administration, but not locomotor activity. However, dextromethorphan was seen to significantly potentiate the decrease in nicotine self-administration caused by sazetidine-A and dextromethorphan was seen to significantly decrease in locomotor activity when given together with mecamylamine. It may be that the modest effects of dextromethorphan at the dose range investigated produce modest effects that are not always seen as main effects.
There were interactions of sazetidine-A and dextromethorphan with food motivated responding. The extent of the attenuation was modest though clearly significant (p < 0.025). Interestingly both sazetidine-A and dextromethorphan alone at the high doses decreased food self-administration. The fact that the sazetidine-A interaction with dextromethorphan was in the opposite direction of the additive effect of sazetidine-A + dextromethorphan effect on nicotine self-administration is the key in the interpretation of a specific effect on nicotine self-administration. The sazetidine-A interactions with dextromethorphan with food and nicotine self-administration differed in important ways. Whereas with nicotine self-administration there were mutually augment interaction between the two drugs, with food self-administration dextromethorphan significantly attenuated the decrease in food self-administration caused by the 3 mg/kg dose of sazetidine-A. This supports the specificity of the augmentation of sazetidine-A and dextromethorphan to nicotine self-administration rather than a more general reduction in all motivated behavior. In terms of locomotor activity, dextromethorphan at the 5 mg/kg dose caused significant reduction on locomotor activity. Sazetidine-A at the dose range tested did not decrease locomotor speed. In fact, the 0.3 mg/kg sazetidine-A dose caused a significant increase in locomotor behavior.
In the mecamylamine dextromethorphan interaction study, there was a significant main effect of dextromethorphan with the 5 mg/kg dose causing a significant reduction in nicotine self-administration. The same effect of dextromethorphan was seen with food self-administration. The 1 mg/kg mecamylamine dose also caused a significant reduction in food motivated responding, but not nicotine self-administration. With locomotor activity, it was only the combination of the high doses of both drugs that significantly reduced activity. The interaction study with mecamylamine is instructive. Mecamylamine is a nonspecific nicotinic receptor channel blocker. Mecamylamine had no observed advantage over sazetidine-A, which is specific to α4β2 receptors.
There are a variety of drugs that have been found in preclinical studies to significantly reduce nicotine self-administration. Some have been found to improve tobacco cessation. However, the magnitude of the effectiveness of any one drug is limited. It may be the case that combination drug treatments mediated via different mechanisms may have mutually potentiating effects. In the current studies, it was shown that this strategy may be effective within nicotinic systems with α4β2 and α3β4 receptors. However, dextromethorphan has actions at a variety of other receptors in addition to α3β4 nicotinic receptors, including actions at NMDA glutamate and sigma receptors (Musacchio, Klein et al. 1989, Netzer, Pflimlin et al. 1993, Jozwiak, Hernandez et al. 2003). The complex nature of dextromethorphan’s actions at a variety of receptors leaves open the question of which are key to its effects on nicotine self-administration alone or in combination with other drugs, although nicotinic α3β4 receptors are likely involved.
Overall our findings suggest that the combination of sazetidine-A and dextromethorphan had mutually potentiating effects on reducing nicotine self-administration. These interactive effects were on par with other combinations we have evaluated such as dextromethorphan interactions with lorcaserin or pyrilamine (Briggs, Wells et al. 2016), but weaker than varenicline interactions with bupropion (Hall, Slade et al. 2015). The effects were relatively modest. Further studies of chronic dextromethorphan treatment will help determine the extent and duration of the effects in reducing nicotine self-administration. Importantly these chronic studies could include the effect of dextromethorphan treatment on resumption of nicotine self-administration after enforced abstinence which models the experience that most people who attempt to quit tobacco smoking. This and other combination pharmacotherapies could provide a better approach for aiding smoking cessation.
Figure 3.
Sazetidine-A – dextromethorphan interactions and locomotor activity, photobeam breaks per five-min time block (mean±sem), N=13
Highlights.
Dextromethorphan can decrease nicotine self-administration (SA).
Sazetidine-A decreases nicotine SA
Dextromethorphan has mutually augmenting effects with sazetidine-A to decrease nicotine SA.
Combination therapy may be more effective smoking cessation treatments than monotherapy.
Acknowledgments
This research was supported by NIDA P50 (DA027840).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Briggs S, Wells C, Slade S, Jaskowski P, Morrison M, Hall BJ, Rezvani AH, Rose JE, Levin ED. Dextromethorphan interactions with serotonergic and histaminergic treatments to reduce nicotine self-administration. Pharmacology, Biochemistry and Behavior. 2016;142:1–7. doi: 10.1016/j.pbb.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM. Nicotine maintains robust self-administration in rats on a limited-access schedule. Psychopharmacology. 1989;99(4):473–478. doi: 10.1007/BF00589894. [DOI] [PubMed] [Google Scholar]
- Damaj MI, Flood P, Ho KK, May EL, Martin BR. Effect of dextrometorphan and dextrorphan on nicotine and neuronal nicotinic receptors: in vitro and in vivo selectivity. Journal of Pharmacology & Experimental Therapeutics. 2005;312(2):780–785. doi: 10.1124/jpet.104.075093. [DOI] [PubMed] [Google Scholar]
- DeNoble VJ, Mele PC. Intravenous nicotine self-administration in rats: effects of mecamylamine, hexamethonium and naloxone. Psychopharmacology. 2006;184(3-4):266–272. doi: 10.1007/s00213-005-0054-z. [DOI] [PubMed] [Google Scholar]
- Glick SD, Maisonneuve IM, Dickinson HA, Kitchen BA. Comparative effects of dextromethorphan and dextrorphan on morphine, methamphetamine, and nicotine self-administration in rats. European Journal of Pharmacology. 2001;422:87–90. doi: 10.1016/s0014-2999(01)01066-4. [DOI] [PubMed] [Google Scholar]
- Glick SD, Maisonneuve IM, Kitchen BA. Modulation of nicotine self-administration in rats by combination therapy with agents blocking alpha 3 beta 4 nicotinic receptors. European Journal of Pharmacology. 2002;448:185–191. doi: 10.1016/s0014-2999(02)01944-1. [DOI] [PubMed] [Google Scholar]
- Hall BJ, Slade S, Wells C, Rose JE, Levin ED. Bupropion-varenicline interactions and nicotine self-administration behavior in rats. Pharmacology, Biochemistry, and Behavior. 2015;130:84–89. doi: 10.1016/j.pbb.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez SC, Bertolino M, Xiao Y, Pringle KE, Caruso FS, Kellar KJ. Dextromethorphan and its metabolite dextrorphan block alpha3beta4 neuronal nicotinic receptors. The Journal of Pharmacology and Experimental Therapeutics. 2000;293:962–967. [PubMed] [Google Scholar]
- Johnson JE, Slade S, Wells C, Petro A, Sexton H, Rezvani AH, Brown ML, Paige MA, McDowell BE, Xiao Y, Kellar KJ, Levin ED. Chronic sazetidine-A infusion reduces nicotine self-administration in male and female rats. Psychopharmacology. 2012;222:269–276. doi: 10.1007/s00213-012-2642-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jozwiak K, Hernandez SC, Kellar KJ, Wainer IW. Enantioselective interactions of dextromethorphan and levomethorphan with the alpha 3 beta 4-nicotinic acetylcholine receptor: comparison of chromatographic and functional data. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;797(1-2):373–379. doi: 10.1016/s1570-0232(03)00608-1. [DOI] [PubMed] [Google Scholar]
- Levin ED, Castonguay M, Ellison GD. Effects of the nicotinic receptor blocker, mecamylamine, on radial-arm maze performance in rats. Behavioral and Neural Biology. 1987;48:206–212. doi: 10.1016/s0163-1047(87)90752-7. [DOI] [PubMed] [Google Scholar]
- Levin ED, McGurk SR, Rose JE, Butcher LL. Reversal of a mecamylamine-induced cognitive deficit with the D2 agonist, LY 171555. Pharmacology, Biochemistry and Behavior. 1989;33:919–922. doi: 10.1016/0091-3057(89)90494-2. [DOI] [PubMed] [Google Scholar]
- Levin ED, Mead T, Rezvani AH, Rose JE, Gallivan C, Gross R. The nicotinic antagonist mecamylamine preferentially inhibits cocaine vs. food self-administration in rats. Physiology and Behavior. 2000;71:565–570. doi: 10.1016/s0031-9384(00)00382-6. [DOI] [PubMed] [Google Scholar]
- Levin ED, Rezvani AH, Xiao Y, Slade S, Cauley M, Wells D, Hampton D, Petro A, Rose JE, Brown ML, Paige MA, McDowell BE, Kellar K. Sazetidine-A, a selective α4β2 nicotinic receptor desensitizing agent and partial agonist reduces nicotine self-administration in rats. Journal of Pharmacology and Experimental Therapeutics. 2010;332:933–939. doi: 10.1124/jpet.109.162073. [DOI] [PubMed] [Google Scholar]
- Li X, Semenova S, D'Souza MS, Stoker AK, Markou A. Involvement of glutaminergic and GABAergic systems in nicotine dependence: Implications for smoking cessation. Neuropharmacology. 2014;76(Pt B):554–565. doi: 10.1016/j.neuropharm.2013.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musacchio JM, Klein M, Canoll PD. Dextromethorphan and sigma ligands: common sites but diverse effects. Life Sciences. 1989;45(19):1721–1732. doi: 10.1016/0024-3205(89)90510-9. [DOI] [PubMed] [Google Scholar]
- Netzer R, Pflimlin P, Trube G. Dextromethorphan blocks N-methyl-D-aspartate-induced currents and voltage-operated inward currents in cultured cortical neurons. European Journal of Pharmacology. 1993;238:209–216. doi: 10.1016/0014-2999(93)90849-d. [DOI] [PubMed] [Google Scholar]
- Pomerleau CS, Pomerleau OF, Majchrzak MJ. Mecamylamine pretreatment increases subsequent nicotine self-administration as indicated by changes in plasma nicotine level. Psychopharmacology (Berl) 1987;91(3):391–393. doi: 10.1007/BF00518198. [DOI] [PubMed] [Google Scholar]
- Pulvirenti L, Balducci C, Koob GF. Dextromethorphan reduces intravenous cocaine self-administration in the rat. European Journal of Pharmacology. 1997;321:279–283. doi: 10.1016/s0014-2999(96)00970-3. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Cauley M, Xiao Y, Kellar KJ, Levin ED. Effects of chronic sazetidine-A, a selective β2* nicotinic receptor desensitizing agent on pharmacologically-induced impaired sustained attention in rats. Psychopharmacology. 2012;222:269–276. doi: 10.1007/s00213-012-2895-6. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Sexton HG, Johnson J, Wells C, Gordon K, Levin ED. Caffeine effects on alcohol consumption and nicotine self-administration in rats. Alcoholism: Clinical and Experimental Research. 2013;37:1609–1617. doi: 10.1111/acer.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezvani AH, Slade S, Wells C, Petro A, Li TK, Lumeng L, Xiao Y, Brown ML, Paige MA, McDowell BE, Kellar KJ, Rose JE, Levin ED. Sazetidine-A, a selective α4β2 nicotinic acetylcholine receptor desensitizing agent and partial agonist reduces both alcohol and nicotine self-administration in selectively-bred alcohol preferring (P) rats. Psychopharmacology. 2010;211:161–174. doi: 10.1007/s00213-010-1878-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JE, Sampson A, Levin ED, Henningfield JE. Mecamylamine increases nicotine preference and attenuates nicotine discrimination. Pharmacol Biochem Behav. 1989;32(4):933–938. doi: 10.1016/0091-3057(89)90061-0. [DOI] [PubMed] [Google Scholar]
- Shoaib M, Schindler CW, Goldberg SR. Nicotine self-administration in rats: Strain and nicotine pre-exposure effects on acquisition. Psychopharmacology. 1997;129(1):35–43. doi: 10.1007/s002130050159. [DOI] [PubMed] [Google Scholar]
- Snedecor GW, Cochran WG. Statistical Methods. Ames, Iowa: Iowa State University Press; 1967. [Google Scholar]
- Tizabi Y. Nicotine and nicotinic systm in hypoglutaminergic models of schizophrenia. Neurotoxicity Research. 2007;12(4):233–246. doi: 10.1007/BF03033907. [DOI] [PubMed] [Google Scholar]
- Xiao Y, Fan H, Musachio JL, Wei ZL, Chellappan SK, Kozikowski AP, Kellar KJ. Sazetidine-A, a novel ligand that desensitizes alpha4beta2 nicotinic acetylcholine receptors without activating them. Molecular Pharmacology. 2006;70:1454–1460. doi: 10.1124/mol.106.027318. [DOI] [PubMed] [Google Scholar]
- Zhao-Shea R, Liu L, Soll LG, Improgo MR, Meyers EE, McIntosh JM, Grady SR, Marks MJ, Gardner PD, Tapper AR. Nicotine-mediated activation of dopaminergic neurons in distinct regions of the ventral tegmental area. Neuropsychopharmacology. 2011;36:1021–1032. doi: 10.1038/npp.2010.240. [DOI] [PMC free article] [PubMed] [Google Scholar]