Abstract
Context
Immune checkpoint inhibitors, including anti–programmed cell death-1 (PD-1) antibodies, have become promising treatments for a variety of advanced malignancies. However, these medicines can cause immune-related adverse events (irAEs), including endocrinopathies.
Objective
This study examined the incidence of endocrine irAEs induced by nivolumab.
Patients and Main Outcome Measured
Sixty-six patients treated with nivolumab at Nagoya University Hospital were prospectively evaluated for pituitary hormones, thyroid function, antithyroid antibodies (Abs), and glucose levels every 6 weeks after the initiation of nivolumab for 24 weeks.
Results
Four out of 66 patients developed destructive thyroiditis, and three patients developed hypothyroidism requiring levothyroxine replacement. The prevalence of positive anti-thyroglobulin Abs (TgAbs) and/or anti–thyroid peroxidase Abs (TPOAbs) at baseline was significantly higher in the group that developed destructive thyroiditis (3/4) compared with the group that did not develop thyroiditis (3/62; P = 0.002). There were no significant differences in other clinical variables between the groups. There were no endocrine irAEs other than destructive thyroiditis during the 24 weeks. The prevalence of TgAbs and/or TPOAbs at baseline was not associated with the development of other irAEs, including pneumonitis, colitis, or skin reactions.
Conclusions
Our real-world data showed that destructive thyroiditis was an endocrine irAE that was frequently induced by nivolumab and was significantly associated with positive TgAbs and/or TPOAbs before treatment. Our findings indicate that evaluating these Abs before treatment may help identify patients with a high risk of thyroidal irAEs and may have important clinical benefit.
Keywords: immune checkpoint inhibitor, immunotherapy, irAE, nivolumab, PD-1, thyroiditis
We prospectively examined endocrine irAEs induced by nivolumab and clarified that patients with antithyroid antibodies are prone to develop destructive thyroiditis.
Immune checkpoint inhibitors have recently risen to prominence as promising treatments for advanced malignancies. Among them, anti–programmed cell death-1 (PD-1) antibodies (Abs) enhance immune responses against several malignancies through inhibition of PD-1 signaling, which inhibits T-cell activation [1, 2]. Nivolumab, an anti–PD-1 monoclonal Ab, is an effective treatment of unresectable metastatic melanoma (MM) [3], non–small cell lung cancer (NSCLC) [4], renal cell carcinoma (RCC) [5], head and neck cancer [6], Hodgkin lymphoma (HL) [7], and gastric cancer [8]. On the other hand, immune checkpoint inhibitors, including nivolumab, have been reported to cause adverse events, termed “immune-related adverse events” (irAEs). irAEs are considered a consequence of enhanced autoimmunity. Lung, skin, intestinal tract, liver, and endocrine glands have been reported to be affected by irAEs induced by nivolumab [9].
Nivolumab has caused several endocrine irAEs, including thyroid dysfunction [10], pituitary dysfunction [3, 11], primary adrenal insufficiency [3, 11, 12], and type 1 diabetes mellitus [13]. Consistent with the fact that the thyroid gland is vulnerable to autoimmune attack [14], thyroid dysfunction has been reported to be one of the most frequent irAEs induced by nivolumab [15–17]. In a phase 3 clinical trial, hypothyroidism was observed in 8.6% (27/313) of patients with MM who were treated with nivolumab [10]. Osorio et al. [18] observed thyroid dysfunction in 21% (10/48) of patients with NSCLC in a phase 1 clinical trial of pembrolizumab, another anti–PD-1 Ab. In a prospective analysis of thyroid function in patients with MM treated with pembrolizumab, thyrotoxicosis and hypothyroidism occurred in 12.1% and 15.2%, respectively [19]. However, except for phase 3 clinical trials, there have been no studies prospectively analyzing the precise incidence and clinical features of endocrine irAEs induced by nivolumab.
The aim of this study was to clarify the clinical characteristics of endocrine irAEs induced by nivolumab.
1. Subjects and Methods
A. Patients
To identify the clinically relevant features of endocrine irAEs, we conducted a prospective study analyzing irAEs in patients treated with immune-checkpoint inhibitors, including nivolumab, since 2 November 2015. All patients with MM, NSCLC, RCC, or HL who started nivolumab treatment between 2 November 2015 and 17 May 2017 at Nagoya University Hospital were included in this study and were observed for 24 weeks. Written informed consent was obtained from all patients. This study was approved by the Ethical Committee of the Nagoya University Hospital. Nivolumab was administered to patients at 3 mg/kg every 2 weeks, with the exception of patients with MM, who were treated with 2 mg/kg every 3 weeks or with 3 mg/kg every 2 weeks. Nivolumab treatment was continued until progression of the disease, death, or unacceptable severe adverse events occurred or if patients withdrew consent for treatment.
B. Assessments
To examine endocrine irAEs, adrenocorticotropic hormone (ACTH), cortisol, luteinizing hormone (LH), follicle-stimulating hormone (FSH), growth hormone, insulin-like growth factor-1, prolactin, free triiodothyronine (FT3), free thyroxine (FT4), thyroid-stimulating hormone (TSH), thyroglobulin (Tg), anti-Tg Abs (TgAbs), anti–thyroid peroxidase Abs (TPOAbs), TSH receptor Abs (TRAbs), and blood glucose levels were prospectively assessed at baseline and every 6 weeks after the first administration of nivolumab for 24 weeks. Serum levels of FT3, FT4, and TSH were measured using electrochemiluminescent immunoassays (Architect-FT3 II kit, Architect FT4 kit, and Architect TSH; Abbott Diagnostics, Santa Clara, CA). The normal ranges of FT3, FT4, and TSH were 1.71 to 3.71 pg/mL, 0.70 to 1.48 ng/dL, and 0.35 to 4.94 μIU/mL, respectively. TPOAb, TgAb, and TRAb levels were measured using electrochemiluminescent immunoassays (Elecsys Anti-TPO kit, Elecsys Anti-Tg kit, and Elecsys Anti-TSHR kit, respectively; Roche Diagnostics, Mannheim, Germany). The normal ranges of TPOAbs, TgAbs, and TRAbs were <16 IU/mL, <28 IU/mL, and <2.0 IU/mL, respectively. Serum levels of ACTH, cortisol, growth hormone, LH, FSH, and prolactin were measured using electrochemiluminescent immunoassays (Elecsys ACTH kit, Elecsys cortisol kit and Elecsys hGH kit, respectively; Roche Diagnostics; Architect LH kit, Architect FSH kit, and Architect PRL kit, respectively; Abbott Diagnostics). Serum levels of insulin-like growth factor-1 were measured using an immunoradiometric assay (Fujirebio, Tokyo, Japan).
Destructive thyroiditis, defined based on criteria provided by the Japan Thyroid Association, was indicated by (1) a suppressed TSH level with an elevated level of FT3 and/or FT4 and (2) no TRAbs. In one case, destructive thyroiditis was also confirmed by the low level of thyroid uptake of 99mTc pertechnetate in scintigraphy. Ultrasonography for the thyroid glands was performed in patients who had positive TgAbs and/or TPOAbs at baseline and who had developed thyroid dysfunction after the initiation of nivolumab. Thyroid function tests and ultrasonography for thyroid glands were performed when needed clinically. All irAEs, including thyroid dysfunction, were monitored and graded using CTCAE 4.0 criteria.
C. Statistical Analysis
Continuous variables of patient characteristics (i.e., age and follow-up period) are expressed as means ± standard deviation. The differences of continuous variables were tested for significance with the two-sample t test. Values of nominal variables (i.e., malignancy, sex, history of prior treatment with immune checkpoint inhibitors, abnormality of thyroid function at baseline, positive antithyroid Abs at baseline, and incidence of other irAEs) were compared using Fishers exact test. Cumulative incidence of destructive thyroiditis was analyzed using the Kaplan-Meier method and compared using the log-lank test. All statistical tests were two-sided, and significance was defined as a P value of <0.05. All statistical analyses were performed with IBM SPSS Statistics 24 (IBM, Armonk, NY).
2. Results
A. Patient Characteristics and Observed irAEs
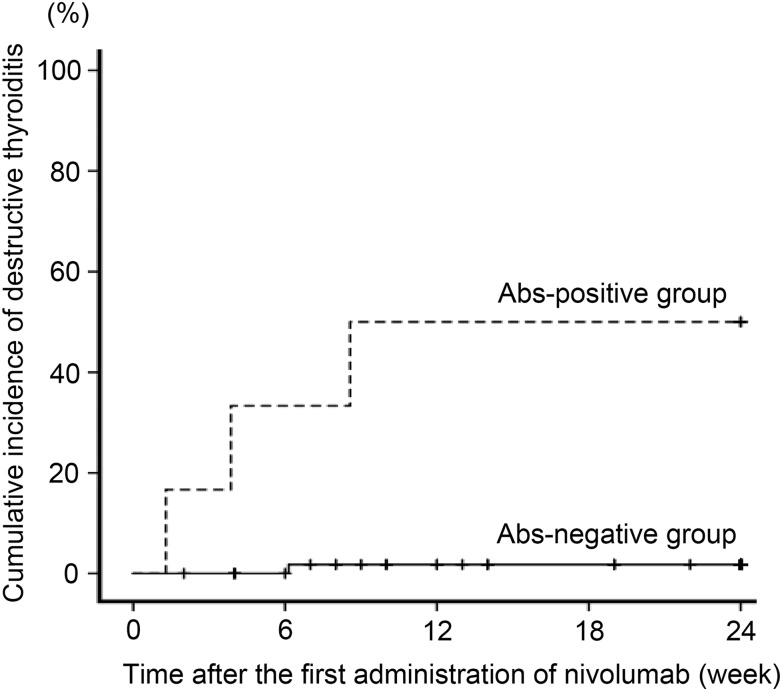
A total of 66 patients with either MM, NSCLC, RCC, or HL who started nivolumab therapy between 2 November 2015 and 17 May 2017 at Nagoya University Hospital were enrolled in this study. After the initiation of nivolumab, four patients (6.1%) developed destructive thyroiditis, although neither pituitary dysfunction, adrenal insufficiency, nor type 1 diabetes mellitus was observed during the 24 weeks. The prevalence of TgAbs and/or TPOAbs before nivolumab treatment was significantly higher in patients who developed destructive thyroiditis compared with patients who did not develop destructive thyroiditis (Table 1). There were no significant differences in the other clinical variables we examined (type of malignancy, sex, age, history of treatment with immune checkpoint inhibitors before this study, and abnormality of thyroid function at baseline). At baseline, TgAbs and/or TPOAbs were positive in six patients (Ab-positive group); neither was positive in the remaining 60 patients (Ab-negative group). During the 24 weeks after initiation of nivolumab therapy, the cumulative incidence was significantly higher in the Ab-positive group (3/6) compared with the Ab-negative group (1/60) (log lank P < 0.001) (Fig. 1). There were no significant differences in the incidence of other irAEs, including interstitial pneumonitis, rash or skin reactions, colitis or constipation, or muscle weakness, between the groups (Table 2). Although nivolumab was changed to ipilimumab due to progression of the disease in four patients with MM, there were no newly developed irAEs during the 24-week observation period. Five patients had been treated with ipilimumab before nivolumab. Two of these patients developed hypophysitis during ipilimumab treatment, and one patient who had positive TPOAbs before ipilimumab treatment developed thyrotoxicosis during nivolumab treatment (Table 1).
Table 1.
Patient Characteristics
| Total (n = 66) | Destructive Thyroiditis |
P Value | ||
|---|---|---|---|---|
| Negative (n = 62) | Positive (n = 4) | |||
| Tumor type, n | ||||
| MM | 19 | 17 | 2 | 0.759 |
| NSCLC | 39 | 37 | 2 | |
| RCC | 7 | 7 | ||
| HL | 1 | 1 | ||
| Sex, male/female | 42/24 | 39/23 | 3/1 | 1.000 |
| Age, y (range) | 65 ± 11 (42–85) | 66 ± 11 (42–85) | 58 ± 9 (51–70) | 0.202 |
| Follow-up period, wk | 20 ± 7 | 20 ± 7 | 24 ± 0.5 | 0.260 |
| History of prior immunotherapy, n | 5 | 4 | 1 | 0.276 |
| Abnormal thyroid function at baseline, n | 7 | 7 | 0 | 1.000 |
| Positive antithyroid Ab at baseline, n | 6 | 3 | 3 | 0.002 |
Figure 1.
Cumulative incidence of destructive thyroiditis in patients treated with nivolumab. Of the 66 patients included, six patients had positive TgAbs and/or TPOAbs at baseline (Ab-positive group), and 60 patients were negative for both (Ab-negative group). The cumulative incidence of destructive thyroiditis was significantly higher in the Ab-positive group than in the Ab-negative group (P < 0.001).
Table 2.
Characteristics of irAEs Other Than Thyroiditis
| Total (n = 66) | Abs-Negative (n = 60) |
Abs-Positive (n = 6) |
P Value | |||
|---|---|---|---|---|---|---|
| Total number of irAEs | 14 | 12 | G1:3 | 2 | G1:1 | 0.337 |
| G2:4 | G2:1 | |||||
| G3:5 | ||||||
| Interstitial pneumonitis | 4 | 4 | G3:4 | 0 | 1.000 | |
| Rash, skin reactions | 5 | 3 | G1:1 | 2 | G1:1 | 0.061 |
| G2:2 | G2:1 | |||||
| Colitis, constipation | 4 | 4 | G1:2 | 0 | 1.000 | |
| G2:2 | ||||||
| Muscle weakness | 1 | 1 | G3:1 | 0 | 1.000 | |
Abbreviations: G, grade of irAEs based on CTCAE 4.0 criteria.
B. Clinical Features of Destructive Thyroiditis Induced by Nivolumab
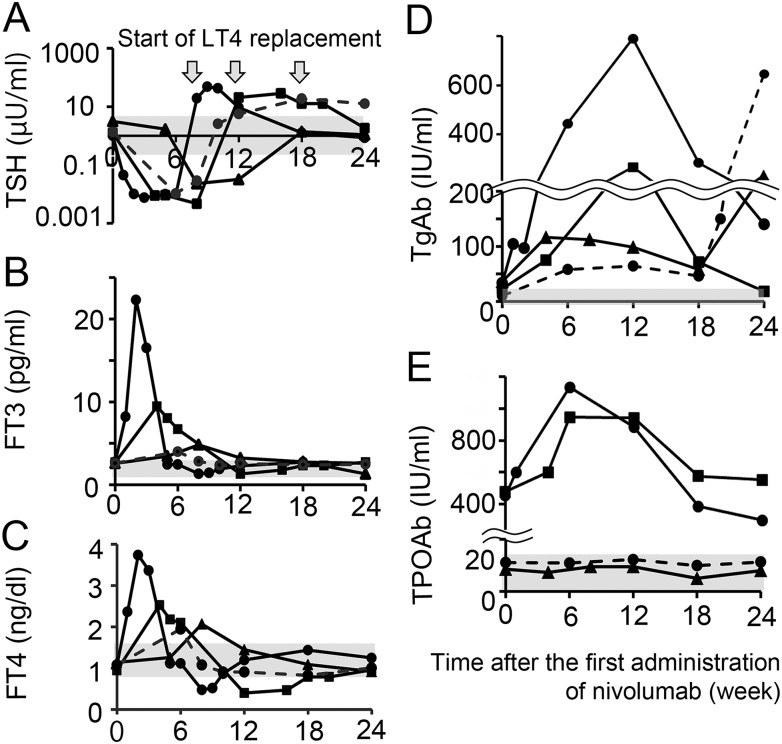
Time course changes in thyroid function and titers of TgAbs and TPOAbs in four patients who developed destructive thyroiditis are shown in Fig. 2. Thyrotoxicosis occurred from 9 to 60 days (median, 35 days) after the first nivolumab administration (Table 3). Ultrasonography in the thyroid gland before nivolumab treatment revealed mild enlargement with low echogenicity, consistent with features of Hashimoto thyroiditis in three patients (cases 021, 038, and 041) (Table 3). In two patients who developed thyrotoxicosis, changes in ultrasonography were detected, which consisted of a marked enlargement of the thyroid gland with a progression of diffuse low echogenicity (case 021) or a progression of focal low echogenicity (case 038). Three of the four patients (cases 021, 034, 038) developed hypothyroidism (Fig. 2A–2C) that required levothyroxine replacement (75 or 100 µg) during the observation period. The duration from the onset of thyrotoxicosis to the start of levothyroxine replacement was 45 to 79 days (median, 70 days) (Fig. 2A). In the remaining patient who showed no changes in the thyroid ultrasonography (case 041), thyrotoxicosis was followed by normalization of thyroid function.
Figure 2.
Time course changes of thyroid function in patients who developed thyrotoxicosis. Each line represents time course changes of TSH (A), FT3 (B), FT4 (C), TgAbs (D), or TPOAbs (E) in patients who developed thyrotoxicosis. Gray areas indicate the normal range of each value. Black circle, black triangle, and black square with solid lines indicate case 021, 041, and 038, respectively (Ab-positive group). Dashed lines indicate case 034 (Ab-negative group).
Table 3.
Clinical Characteristics of Patients Developing Destructive Thyroiditis
| Case | Tumor Type | TPOAb (IU/mL) |
TgAb (IU/mL) |
G | Onseta (d) | Ultrasonography Findings |
|||
|---|---|---|---|---|---|---|---|---|---|
| 0 w | Peak | 0 w | Peak | Before Nivolumab | At Diagnosis | ||||
| 021 | NSCLC | 454b | 1134b | 33.7b | 790.4b | 3 | 9 | Mild enlargement, low echogenicity | Marked enlargement, progression of low echogenicity |
| 034 | NSCLC | 11.1 | 12.4 | 10 | 648.4b | 1 | 43 | N/A | Mild enlargement |
| 038 | MM | 478.1b | 947b | 22.8 | 266.7b | 2 | 27 | Mild enlargement, low echogenicity | Progression of focal low echogenicity |
| 041 | MM | 8.5 | 9.5 | 35.9b | 116.3b | 1 | 60 | Mild enlargement, low echogenicity | Not changed |
Abbreviations: G, grade of irAEs based on CTCAE 4.0 criteria; N/A, not available.
Days to the onset of destructive thyroiditis from the first administration of nivolumab.
Titer above the upper normal range.
In the Ab-positive group, two patients with TPOAbs showed positive changes in TgAbs; one patient developed destructive thyroiditis (case 038) (Table 3; Fig. 2D), and the other did not. In the Ab-negative group, TPOAb or TgAb became positive during the treatment in five patients (three for TgAb and two for TPOAb, respectively), including one patient who developed destructive thyroiditis (case 034) (Table 3). The titers were still high at 24 weeks in three patients (cases 018, 034, 056), whereas the increases were transient in two patients (cases 040, 043) (Table 4). Thyroid ultrasonography revealed findings characteristic of Hashimoto thyroiditis in three of the four patients examined (cases 034, 040, 043) (Tables 3 and 4).
Table 4.
Time Course Changes of TgAb and TPOAb Titers and Ultrasonography Findings in Patients Who Developed Positive Antithyroid Abs but Not Thyroid Dysfunction
| Antibodies | Case | Week |
Findings of Ultrasonography After Developing Ab | ||||
|---|---|---|---|---|---|---|---|
| 0 | 6 | 12 | 18 | 24 | |||
| TgAb, IU/mL | 043 | 13.2 | 63.4a | N/A | 10.7 | 7.4 | Diffuse enlargement, diffuse low echogenicity |
| 056 | 10 | 22.5 | 353.8a | 387a | 433a | N/A | |
| TPOAb, IU/mL | 018 | 12.7 | 21.8a | 16.9a | 19.5a | 16.7a | Normal |
| 040 | 13.5 | 14.8 | 12.3 | 17.6a | 13.0 | Mild atrophy, diffuse low echogenicity | |
Abbreviations: N/A, not available.
Titer above the upper normal range.
Based on the CTCAE 4.0 criteria, cases 034 and 041 had grade 1 thyrotoxicosis, case 038 had grade 2 thyrotoxicosis, and case 021 had grade 3 thyrotoxicosis (Table 3). The latter two cases had relatively high titers of TPOAbs at baseline (Fig. 2E), suggesting that the severity of thyroidal irAEs might be correlated with the titers of pre-existing antithyroid Abs.
Although nivolumab was readministered in all patients who developed destructive thyroiditis after temporal suspension in case 021 with grade 3 and case 038 with grade 2, thyroid function was not exacerbated following the administration of nivolumab.
C. A Case With Grade 3 Thyroidal irAE
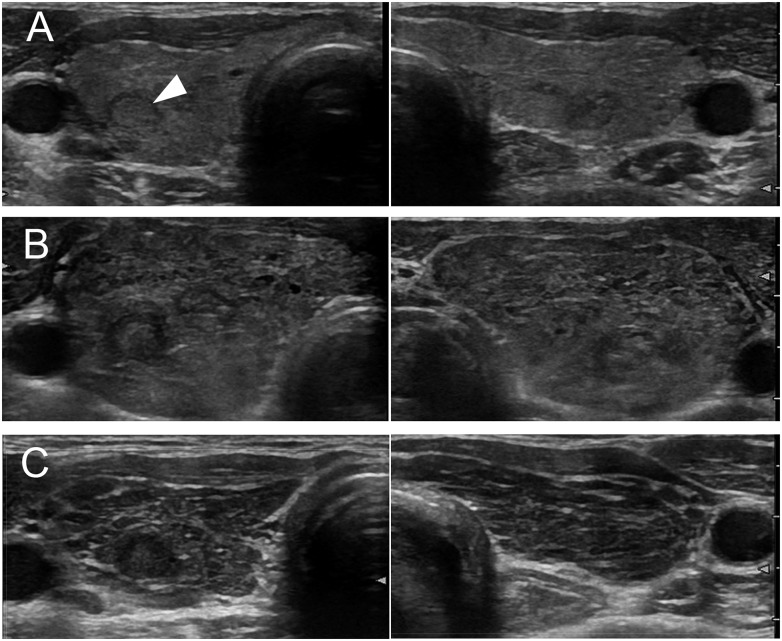
Case 021 was a 51-year-old woman diagnosed with lung adenocarcinoma with stage cT2aN0M0 at the age of 49. The lung tumor relapsed 1 year after surgical resection. The patient was then treated with a first-line chemotherapy (carboplatin, pemetrexed, and bevacizumab), which was discontinued due to progression of the disease. As a second-line therapy, nivolumab at 3 mg/kg every 2 weeks was initiated. The serum levels of TSH, FT3, and FT4 were 1.01 µIU/mL, 2.76 pg/mL, and 1.08 ng/dL, respectively, and antithyroid Abs were positive (TgAbs, 33.7 IU/mL; TPOAbs, 454 IU/mL) prior to nivolumab treatment. Ultrasonography revealed slightly diffuse swelling of the thyroid (Fig. 3A). Nine days after the first administration of nivolumab, the patient complained of anxiety and a feeling of pressure on the anterior neck. The serum levels of TSH, FT3, and FT4 were 0.05 µIU/mL, 8.21 pg/mL, and 2.37 ng/dL, respectively. Ultrasonography revealed marked enlargement of both thyroid lobes with low echogenicity (Fig. 3B). The uptake of 99mTc pertechnetate was low. Based on these results, the patient was diagnosed with destructive thyroiditis and hospitalized. Serum levels of FT3 and FT4 increased to 22.3 pg/mL and 4.02 ng/dL, respectively. The patient complained of palpitation and anxiety, and propranolol (30 mg/d) was administered for 7 days. Thereafter, thyroid hormone levels started to decrease, followed by hypothyroidism, which required levothyroxine replacement (100 µg/d). After transient interruption, nivolumab was restarted. Two months after the onset of destructive thyroiditis, ultrasonography revealed a progression of low echogenicity and decreased size of the thyroid gland (Fig. 3C).
Figure 3.
Time course changes of thyroid ultrasonography in case 021. Thyroid ultrasonography revealed slightly diffuse swelling of the thyroid gland with low echogenicity before nivolumab treatment, which was compatible with Hashimoto thyroiditis (A), the marked enlargement of the both thyroid lobes at the onset of destructive thyroiditis (B), and the progression of internal hypo-echoic changes and the decrease in size of both thyroid lobes 2 months after diagnosis (C). Arrowhead indicates a small nodule suggesting adenomatous goiter.
3. Discussion
This study prospectively examined endocrine irAEs associated with nivolumab treatment in a real-world clinical practice and demonstrated that destructive thyroiditis was the most frequent endocrine irAE induced by nivolumab. Furthermore, we clearly showed that patients with TgAbs and/or TPOAbs were prone to develop destructive thyroiditis after initiation of nivolumab treatment.
Verma et al. [20] reported a patient with NSCLC with elevated TgAbs at the time of diagnosis who developed thyrotoxicosis after nivolumab treatment. Tanaka et al. [21] reported three cases with MM who developed thyroid dysfunction induced by nivolumab, one of whom had positive TPOAbs and TgAbs before nivolumab treatment. Yamauchi et al. [22] also reported five cases with thyroid dysfunction induced by nivolumab, three of whom had positive TPOAbs and TgAbs at baseline. Among patients undergoing pembrolizumab treatment, several cases have been reported to have positive TgAbs or TPOAbs after the development of thyroid dysfunction, some of whom also had them at baseline [18, 19]. However, no studies examined TgAbs and/or TPOAbs in all subjects before the initiation of treatment. In this study, we prospectively examined endocrine irAEs induced by nivolumab and verified the higher incidence of destructive thyroiditis in the Ab-positive group compared with the Ab-negative group (50% vs 1.7%), suggesting a clinical benefit in evaluating TgAbs and TPOAbs before nivolumab treatment.
It has been reported that thyroidal irAEs induced by nivolumab occurred with high frequency and low severity [10]. Although cases 034 and 041 (grade 1) showed no subjective symptoms and case 38 (grade 2) showed only general fatigue, case 021 (grade 3) showed severe thyrotoxicosis symptoms that required monitoring of general conditions, including heart rate and mental status, under hospitalization. Furthermore, three of the four patients, including two patients who exhibited changes in ultrasonography at the onset (cases 021 and 038), progressed to hypothyroidism after thyrotoxicosis and needed replacement of levothyroxine. The other patient who showed no changes in ultrasonography (case 041) did not develop hypothyroidism after destructive thyroiditis. It is therefore important to closely follow each patient’s general conditions, perform thyroid ultrasonography, and measure thyroid function in patients treated with nivolumab who develop thyrotoxicosis.
It has been reported that readministration of immune checkpoint inhibitors, including nivolumab, can worsen irAEs such as pneumonitis. For example, 25% of patients with nivolumab-induced pneumonitis exhibited recurrence after nivolumab readministration [23]. In contrast, all four patients with destructive thyroiditis in this study were readministered nivolumab, but further exacerbation of thyrotoxicosis was not observed. It is important to confirm our findings in future studies with a larger cohort and longer periods of observation.
It has been reported that a patient treated with nivolumab developed destructive thyroiditis followed by isolated ACTH deficiency [22] and that another patient treated with ipilimumab developed type 1 diabetes followed by pan-hypopituitarism [24]. In addition, the incidence of endocrine irAEs was suggested to be increased by the simultaneous administration of nivolumab and ipilimumab [10]. Consistent with these previous studies, one patient in our Ab-positive group who developed destructive thyroiditis had a history of isolated ACTH deficiency during prior ipilimumab treatment. This finding suggests that patients who develop autoimmunity against a self-antigen after immune checkpoint blockade are susceptible to develop immune responses against heterologous antigens and that the use of two types of immune checkpoint inhibitors increases the risk of endocrine irAEs. Further study is required to support this hypothesis.
Patients with Hashimoto thyroiditis have been reported to have both thyroid peroxidase and thyroglobulin recognized by CD8 T cells [25]. The progression of low echogenicity in ultrasonography in this study is consistent with the possible infiltration of inflammatory cells such as lymphocytes into the thyroid glands. Although the precise immunological mechanism remains unclear, drastic changes in the Ab titers and ultrasonography findings in this study suggest that the immune responses to thyroglobulin and/or thyroid peroxidase may be involved in the pathogenesis of destructive thyroiditis induced by nivolumab. This hypothesis is further supported by the findings that titers of TPOAbs and/or TgAbs increased not only in patients who developed thyrotoxicosis but also in some patients who did not. It is an objective for future studies to clarify if the magnitude of changes in the titers is associated with the development of thyrotoxicosis and hypothyroidism.
There are some limitations in this study. First, the follow-up period was only 24 weeks, and therefore we cannot exclude the possibility that the patients who had positive antithyroid Abs but not thyroid dysfunction during the observation period will develop destructive thyroiditis or hypothyroidism in the future. Indeed, a previous study suggested that long-term observations are necessary to clarify the role of autoimmune mechanisms underlying hypothyroidism development in patients treated with sunitinib [26]. However, it has been reported that most nivolumab-induced adverse events occurred within the first 16 weeks of treatment [9] and that irAEs in the thyroid mostly developed within the first 3 months [27]. This was also true in this study; the longest duration from the initiation of nivolumab to the onset of thyrotoxicosis was 60 days (9 weeks). Therefore, the degree of underestimation of destructive thyroiditis is quite low. Second, the sample size in this study was small (n = 66), and other endocrine irAEs reported to date, such as pituitary dysfunction [28], adrenal insufficiency [29], and type 1 diabetes mellitus [13], were not observed in this study. Nevertheless, our study clearly showed that destructive thyroiditis was induced in a substantial number of patients and that the existence of TgAbs and/or TPOAbs at baseline was significantly correlated with the development of destructive thyroiditis, suggesting clinical benefit in the examination of thyroid Abs when considering nivolumub treatment as well as monitoring thyroid Ab and thyroid function during the treatment.
It is unclear from this study whether irAEs are associated with better clinical responses of nivolumab. A previous study reported that cutaneous irAEs were associated with improved outcomes in patients with MM treated with nivolumab [30]. Whether this is also the case with thyroidal irAEs awaits further examination.
In summary, destructive thyroiditis was a frequently observed endocrine irAE induced by nivolumab treatment and was significantly associated with the existence of TgAbs and/or TPOAbs prior to treatment.
Acknowledgments
Clinical Trial Information: www.umin.ac.jp no.UMIN000019024 (registered 15 September 2015).
Author Contributions: S.I. designed the study. T.K. and S.I. performed the clinical study. T.K., S.I., and H.A. analyzed the data. T.K., S.I., and H.A. wrote the manuscript. All authors treated patients, discussed the data, and were involved in revising the manuscript.
Disclosure Summary: S.I. is a consultant/advisory board member on endocrinological adverse events for Ono Pharmaceutical Company and Bristol-Myers Squibb and reports speaker fees, consultant and advisory fees, and fees for writing published materials from Ono Pharmaceutical Company and Bristol-Myers Squibb. H.A. reports fees for writing published materials from Ono Pharmaceutical Company and Bristol-Myers Squibb. The remaining authors have nothing to disclose.
Glossary
Abbreviations:
- Ab
antibody
- ACTH
adrenocorticotropic hormone
- FSH
follicle-stimulating hormone
- FT3
free triiodothyronine
- FT4
free thyroxine
- HL
Hodgkin lymphoma
- irAE
immune-related adverse event
- LH
luteinizing hormone
- MM
metastatic melanoma
- NSCLC
non–small cell lung cancer
- PD-1
programmed cell death-1
- RCC
renal cell carcinoma
- TgAb
thyroglobulin antibody
- TPOAb
thyroid peroxidase antibody
- TRAb
thyroid-stimulating hormone receptor antibody
- TSH
thyroid-stimulating hormone.
References and Notes
- 1. Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci USA. 2002;99(19):12293–12297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Boussiotis VA. Molecular and biochemical aspects of the PD-1 checkpoint pathway. N Engl J Med. 2016;375(18):1767–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C, Kalinka-Warzocha E, Savage KJ, Hernberg MM, Lebbé C, Charles J, Mihalcioiu C, Chiarion-Sileni V, Mauch C, Cognetti F, Arance A, Schmidt H, Schadendorf D, Gogas H, Lundgren-Eriksson L, Horak C, Sharkey B, Waxman IM, Atkinson V, Ascierto PA. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320–330. [DOI] [PubMed] [Google Scholar]
- 4. Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, Barlesi F, Kohlhäufl M, Arrieta O, Burgio MA, Fayette J, Lena H, Poddubskaya E, Gerber DE, Gettinger SN, Rudin CM, Rizvi N, Crinò L, Blumenschein GR Jr, Antonia SJ, Dorange C, Harbison CT, Graf Finckenstein F, Brahmer JR. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G, Plimack ER, Castellano D, Choueiri TK, Gurney H, Donskov F, Bono P, Wagstaff J, Gauler TC, Ueda T, Tomita Y, Schutz FA, Kollmannsberger C, Larkin J, Ravaud A, Simon JS, Xu LA, Waxman IM, Sharma P; CheckMate 025 Investigators . Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373(19):1803–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ferris RL, Blumenschein G Jr, Fayette J, Guigay J, Colevas AD, Licitra L, Harrington K, Kasper S, Vokes EE, Even C, Worden F, Saba NF, Iglesias Docampo LC, Haddad R, Rordorf T, Kiyota N, Tahara M, Monga M, Lynch M, Geese WJ, Kopit J, Shaw JW, Gillison ML. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375(19):1856–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, Schuster SJ, Millenson MM, Cattry D, Freeman GJ, Rodig SJ, Chapuy B, Ligon AH, Zhu L, Grosso JF, Kim SY, Timmerman JM, Shipp MA, Armand P. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015;372(4):311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kang YK, Boku N, Satoh T, Ryu MH, Chao Y, Kato K, Chung HC, Chen JS, Muro K, Kang WK, Yeh KH, Yoshikawa T, Oh SC, Bai LY, Tamura T, Lee KW, Hamamoto Y, Kim JG, Chin K, Oh DY, Minashi K, Cho JY, Tsuda M, Chen LT. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390(10111):2461–2471. [DOI] [PubMed] [Google Scholar]
- 9. Scott LJ. Nivolumab: a review in advanced melanoma. Drugs. 2015;75(12):1413–1424. [DOI] [PubMed] [Google Scholar]
- 10. Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, Ferrucci PF, Hill A, Wagstaff J, Carlino MS, Haanen JB, Maio M, Marquez-Rodas I, McArthur GA, Ascierto PA, Long GV, Callahan MK, Postow MA, Grossmann K, Sznol M, Dreno B, Bastholt L, Yang A, Rollin LM, Horak C, Hodi FS, Wolchok JD. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(1):23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. González-Rodríguez E, Rodríguez-Abreu D; Spanish Group for Cancer Immuno-Biotherapy (GETICA) . Immune checkpoint inhibitors: review and management of endocrine adverse events. Oncologist. 2016;21(7):804–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH, Brahmer JR, Lawrence DP, Atkins MB, Powderly JD, Leming PD, Lipson EJ, Puzanov I, Smith DC, Taube JM, Wigginton JM, Kollia GD, Gupta A, Pardoll DM, Sosman JA, Hodi FS. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J. Clin Oncol. 2014;32:1020–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hughes J, Vudattu N, Sznol M, Gettinger S, Kluger H, Lupsa B, Herold KC Precipitation of autoimmune diabetes with anti-PD-1 immunotherapy. Diabetes Care. 2015;38:e55–e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hasham A, Tomer Y. Genetic and epigenetic mechanisms in thyroid autoimmunity. Immunol Res. 2012;54(1-3):204–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Boutros C, Tarhini A, Routier E, Lambotte O, Ladurie FL, Carbonnel F, Izzeddine H, Marabelle A, Champiat S, Berdelou A, Lanoy E, Texier M, Libenciuc C, Eggermont AM, Soria JC, Mateus C, Robert C. Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination. Nat Rev Clin Oncol. 2016;13(8):473–486. [DOI] [PubMed] [Google Scholar]
- 16. Shang YH, Zhang Y, Li JH, Li P, Zhang X. Risk of endocrine adverse events in cancer patients treated with PD-1 inhibitors: a systematic review and meta-analysis. Immunotherapy. 2017;9(3):261–272. [DOI] [PubMed] [Google Scholar]
- 17. Morganstein DL, Lai Z, Spain L, Diem S, Levine D, Mace C, Gore M, Larkin J. Thyroid abnormalities following the use of cytotoxic T-lymphocyte antigen-4 and programmed death receptor protein-1 inhibitors in the treatment of melanoma. Clin Endocrinol (Oxf). 2017;86(4):614–620. [DOI] [PubMed] [Google Scholar]
- 18. Osorio JC, Ni A, Chaft JE, Pollina R, Kasler MK, Stephens D, Rodriguez C, Cambridge L, Rizvi H, Wolchok JD, Merghoub T, Rudin CM, Fish S, Hellmann MD. Antibody-mediated thyroid dysfunction during t-cell checkpoint blockade in patients with non-small cell lung cancer. Ann Oncol. 2017;28(3):583–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. de Filette J, Jansen Y, Schreuer M, Everaert H, Velkeniers B, Neyns B, Bravenboer B. Incidence of thyroid-related adverse events in melanoma patients treated with pembrolizumab. J Clin Endocrinol Metab. 2016;101(11):4431–4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Verma I, Modi A, Tripathi H, Agrawal A. Nivolumab causing painless thyroiditis in a patient with adenocarcinoma of the lung. BMJ Case Rep. 2016;2016:pii: bcr2015213692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tanaka R, Fujisawa Y, Maruyama H, Nakamura Y, Yoshino K, Ohtsuka M, Fujimoto M. Nivolumab-induced thyroid dysfunction. Jpn J Clin Oncol. 2016;46(6):575–579. [DOI] [PubMed] [Google Scholar]
- 22. Yamauchi I, Sakane Y, Fukuda Y, Fujii T, Taura D, Hirata M, Hirota K, Ueda Y, Kanai Y, Yamashita Y, Kondo E, Sone M, Yasoda A, Inagaki N.. Clinical features of nivolumab-induced thyroiditis: a case series study. Thyroid. 2017;27:894–901. [DOI] [PubMed] [Google Scholar]
- 23. Naidoo J, Wang X, Woo KM, Iyriboz T, Halpenny D, Cunningham J, Chaft JE, Segal NH, Callahan MK, Lesokhin AM, Rosenberg J, Voss MH, Rudin CM, Rizvi H, Hou X, Rodriguez K, Albano M, Gordon RA, Leduc C, Rekhtman N, Harris B, Menzies AM, Guminski AD, Carlino MS, Kong BY, Wolchok JD, Postow MA, Long GV, Hellmann MD. Pneumonitis in patients treated with anti-programmed death-1/programmed death ligand 1 therapy. J Clin Oncol. 2017;35(7):709–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tsiogka A, Jansky GL, Bauer JW, Koelblinger P. Fulminant type 1 diabetes after adjuvant ipilimumab therapy in cutaneous melanoma. Melanoma Res. 2017;27(5):524–525. [DOI] [PubMed] [Google Scholar]
- 25. Ehlers M, Thiel A, Bernecker C, Porwol D, Papewalis C, Willenberg HS, Schinner S, Hautzel H, Scherbaum WA, Schott M. Evidence of a combined cytotoxic thyroglobulin and thyroperoxidase epitope-specific cellular immunity in Hashimoto’s thyroiditis. J Clin Endocrinol Metab. 2012;97(4):1347–1354. [DOI] [PubMed] [Google Scholar]
- 26. Pani F, Atzori F, Baghino G, Boi F, Tanca L, Ionta MT, Mariotti S. Thyroid dysfunction in patients with metastatic carcinoma treated with sunitinib: is thyroid autoimmunity involved? Thyroid. 2015;25(11):1255–1261. [DOI] [PubMed] [Google Scholar]
- 27. Sznol M, Postow MA, Davies MJ, Pavlick AC, Plimack ER, Shaheen M, Veloski C, Robert C. Endocrine-related adverse events associated with immune checkpoint blockade and expert insights on their management. Cancer Treat Rev. 2017;58:70–76. [DOI] [PubMed] [Google Scholar]
- 28. Ishikawa M, Oashi K. Case of hypophysitis caused by nivolumab. J Dermatol. 2017;44(1):109–110. [DOI] [PubMed] [Google Scholar]
- 29. Trainer H, Hulse P, Higham CE, Trainer P, Lorigan P. Hyponatraemia secondary to nivolumab-induced primary adrenal failure. Endocrinol Diabetes Metab Case Rep. 2016;2016:16–0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Freeman-Keller M, Kim Y, Cronin H, Richards A, Gibney G, Weber JS. Nivolumab in resected and unresectable metastatic melanoma: characteristics of immune-related adverse events and association with outcomes. Clin Cancer Res. 2016;22(4):886–894. [DOI] [PMC free article] [PubMed] [Google Scholar]