Abstract
Background
Adequate visualization is known to be essential to perform arthroscopic procedures effectively and efficiently. We hypothesized that tranexamic acid may be considered as an alternative agent to reduce intra-articular bleeding during arthroscopic procedures, after comparing its potential chondrotoxicity with that of epinephrine.
Material/Methods
Seventy-two rats were randomized into 3 groups with 24 rats each. The injections were performed in the right knees, as follows: Group 1: 0.25 mL of tranexamic acid solution, Group 2: 0.25 mL of epinephrine solution, and Group 3: 0.25 mL of 0.9% saline, serving as control. One week after the injections, the animals were euthanized. Samples were evaluated histologically based on the Osteoarthritis Research Society International (OARSI) Histopathology Grading and Staging System and the “live/dead” staining technique to determine chondrocyte viability.
Results
Comparison of epinephrine and tranexamic acid revealed significantly higher OARSI scores in the epinephrine group (epinephrine: 3.42±1.31, TA: 0.92±0.90; P<0.001). The most significant difference between the 2 groups was in the number of joints diagnosed with OARSI grade III. The percentage of viability was significantly higher in the tranexamic acid group when compared with the epinephrine group (tranexamic acid: 79.74±3.343; epinephrine: 63.81±1.914; P<0.05).
Conclusions
Based on the histologic parameters and chondrocyte viability, tranexamic acid is less cytotoxic than epinephrine in rat chondrocytes at the doses typically used in irrigation fluid, and may be a good alternative to epinephrine in arthroscopic surgery.
MeSH Keywords: Arthroscopy, Chondrocytes, Epinephrine, Tranexamic Acid
Background
The role of arthroscopic procedures in the diagnosis and treatment of a wide variety of joint disorders is evolving. Among the many factors that determine the success of surgery and patient outcomes, adequate visualization is known to be essential to perform these procedures effectively and efficiently [1,2]. Numerous devices and surgical techniques have been developed for this purpose [2,3]. Epinephrine is a potent vasoconstrictor, frequently added to the irrigation fluid to reduce intra-articular bleeding, and thereby maintaining adequate visualization during the operation [4,5]. Recently, systemic complications, including pulmonary edema and cardiopulmonary arrest, have been reported, and they are attributed to the epinephrine in the irrigation fluid [6,7]. Moreover, there is an increasing number of in vitro studies showing the cytotoxic effects of epinephrine on chondrocytes, and focusing attention on the potential chondrolysis [8–10]. Thus, the presence of epinephrine in the irrigation fluid has been questioned [4,8]. Tranexamic acid (TA) is both an inhibitor of fibrinolysis and an activator of plasminogen. It is known to be effective and safe in reducing the amount of bleeding during various orthopedic interventions when administered intravenously [11,12]. Intravenous administration of TA is considered safe; however, some studies have associated intravenous TA with postoperative seizures and increased thromboembolic events [13]. Hence, the intra-articular administration of TA has been increasing over the past several years [14,15], with the benefits of higher concentration at the operative site while reducing the risk of systemic adverse effects, reduced cost, and giving more control to the surgeon [16]. However, there are few studies available on the intra-articular administration of TA, and these focus on the clinical outcomes only [17,18]. In this experimental study, we focused on investigating the effects of TA on the articular cartilage, and comparing them with the effects of epinephrine on the articular cartilage. We hypothesized that TA may be considered as an alternative agent to reduce intra-articular bleeding during arthroscopic procedures, after comparing its potential chondrotoxicity with that of epinephrine.
Material and Methods
Animals
A total of 72 adult female Sprague-Dawley rats were used, with a mean age of 12-months and weighing 250–350 g. Animals were housed under standard management conditions (5 rats/cage). The room temperature and humidity were maintained at 20–24°C and 50–60%, respectively. The light cycle was fixed at 12 hours. They were fed a standard rat diet with water ad libitum. All animal experiment protocols were approved by the Animal Research Committee at Istanbul Bezmialem University Research and Training Hospital (date: 07/02/2012, id: 2012/309).
Study groups
Seventy-two rats were randomized into 3 groups with 24 rats in each. The injections were performed in the right knees, as follows: Group 1: 0.25 mL of TA solution (1 g of TA is diluted in 50 mL of saline). Group 2: 0.25 mL of epinephrine solution (50 mL of epinephrine diluted to 1: 200 000). Group 3: 0.25 mL of 0.9% saline, serving as control. One week after the injections, the animals were euthanized with high doses of intraperitoneal thiopental (200 mg/kg). Twelve specimens from each group were separated for the histological analysis and the chondrocyte viability evaluation.
Tissue preparation and histological analysis
Tibiofemoral joints of the rats were excised immediately after the rats were euthanized. Femoral condyles and tibia samples were fixed in 10% buffered formalin and decalcified in 8% formic acid. Then, the tissues were rinsed with tap water, and routine tissue processing was performed using a Shandon™ Excelsior™ tissue processor. Slices of 0.4-μm thickness were prepared from the paraffin-embedded tissue. The slides were stained with hematoxylin and eosin and toluidine blue. Samples were evaluated histologically for the presence of inflammation and osteoarthritis grade (OARSI grades 0–6), osteoarthritis stage (OA stages 04), and osteoarthritis scoring (OA scoring: [OARSI grade] × [OA stage]), according to the recommendation of the International Cartilage Repair Society’s osteoarthritis and cartilage histopathology grading and staging system [19]. Hematoxylin and eosin stains were used to evaluate the structure, the chondrocytes, and inflammation. Loss of proteoglycan staining intensity was assessed by toluidine blue staining. The samples were examined and assessed independently by 2 blinded investigators.
Chondrocyte viability analysis
The “live/dead” or “red/green” staining technique was used, relying on cell membrane integrity and intracellular esterase activity to produce products that can be visualized on confocal microscopy [20]. Full-thickness cartilage was removed from subchondral bone and sectioned into approximately 0.5-mm-thick coronal slices. Tissues were stained with calcein-AM (Cal-AM) and ethidium homodimer (Et-HD) for 35 to 45 minutes in a nutrient medium. After staining, the tissues were rinsed 3 times with the nutrient medium, for 2 minutes each. The stained tissues were examined under a confocal microscope (Zeiss LSM 780). Three coronal slices were imaged at 3 random locations within each of the superficial, middle, and deep chondrocyte regions, for a total of 9 images per specimen. The Cal-AM and Et-HD stained cells were counted separately and the percentage of viability was calculated.
Statistical analysis
Inter-observer reliability was evaluated using a two-way random absolute agreement method. Data were evaluated using the SPSS for Windows 15.0 (SPSS Inc., Chicago, IL, USA) software. The independence between the groups was analyzed using the Pearson’s chi-square test. In the absence of the conditions provided, Monte Carlo simulation was performed. Ordinal variables between 2 independent groups were compared by the Mann-Whitney U test. The Kruskal-Wallis and Dunn tests were used for comparing the parameters. An alpha level of 0.05 was chosen to assess statistical significance. P-values <0.05 were regarded as significant.
Results
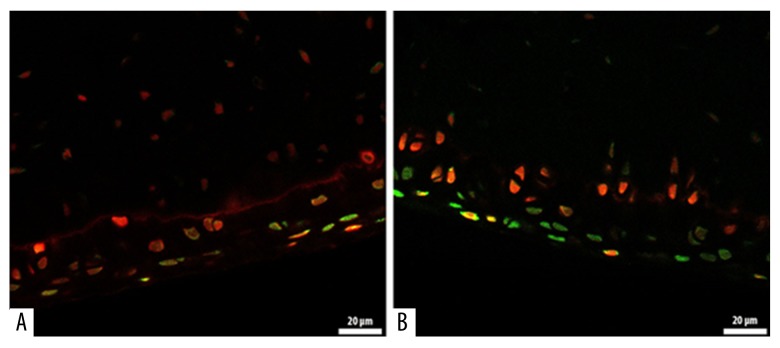
Comparison of epinephrine and TA revealed significantly higher OARSI scores in the epinephrine group (epinephrine: 3.42±1.31, TA: 0.92±0.90; P<0.001), (Table 1). The most significant difference between the 2 groups was in the number of joints diagnosed with OARSI grade III. In the epinephrine group, 9 specimens showed grade III changes, whereas only 1 specimen showed grade III changes in the TA group (Table 2). Representative histological lesions assessed as grade III were: extension of matrix cracks into the mid-zone to form vertical clefts, slightly irregular surface layer, cluster of cells focally, as well as hypocellularity, chondrocyte deaths, loss of stainable proteoglycan matrix, and moderate toluidine blue staining. No specimens were determined as grade V or VI where the subchondral bone was involved. According to the calcein-AM and ethidium homodimer-1 staining of chondrocytes, the live cells were stained green and the dead cells were stained red (Figure 1). The percentage of viability was significantly higher in the TA group compared with the epinephrine group (TA: 79.74±3.343; epinephrine: 63.81±1.914; P<0.05) (Figure 2).
Table 1.
Comparison of the groups according to the OARSI scores.
| Group | P | ||||
|---|---|---|---|---|---|
| Epinephrine | Saline | TA | |||
| MD | 3.42 | 0.33 | 0.92 | <0.001 | |
| SD | 1.31 | 0.65 | 0.90 | ||
| Median | 3 | 0 | 1 | ||
| Percentiles | 25 | 3 | 0 | 0 | |
| 50 | 3 | 0 | 1 | ||
| 75 | 3.75 | 0.75 | 1 | ||
OARSI – Osteoarthritis Research Society International; TA – tranexamic acid.
Table 2.
Histological findings of OARSI assessment.
| OARSI scores | Group | P | ||||||
|---|---|---|---|---|---|---|---|---|
| Epinephrine | Saline | TA | ||||||
| n | % | n | % | n | % | |||
| Grade | 0 | 0 | 0.0 | 9 | 75.0 | 4 | 33.3 | <0.001 |
| 1 | 0 | 0.0 | 3 | 25.0 | 6 | 50.0 | ||
| 2 | 3 | 25.0 | 0 | 0.0 | 1 | 8.3 | ||
| 3 | 9 | 75.0 | 0 | 0.0 | 1 | 8.3 | ||
| Stage | 0 | 0 | 0.0 | 9 | 75.0 | 4 | 33.3 | <0.001 |
| 1 | 9 | 75.0 | 2 | 16.7 | 8 | 66.7 | ||
| 2 | 3 | 25.0 | 1 | 8.3 | 0 | 0.0 | ||
| Score | 0 | 0 | 0.0 | 9 | 75.0 | 4 | 33.3 | <0.001 |
| 1 | 0 | 0.0 | 2 | 16.7 | 6 | 50.0 | ||
| 2 | 2 | 16.7 | 1 | 8.3 | 1 | 8.3 | ||
| 3 | 7 | 58.3 | 0 | 0.0 | 1 | 8.3 | ||
| 4 | 1 | 8.3 | 0 | 0.0 | 0 | 0.0 | ||
| 6 | 2 | 16.7 | 0 | 0.0 | 0 | 0.0 | ||
Figure 1.
Calcein-AM and ethidium homodimer-1 staining of chondrocytes. The live cells are stained green, and the dead cells are stained red (A: Epinephrine group) (B: TA group).
Figure 2.
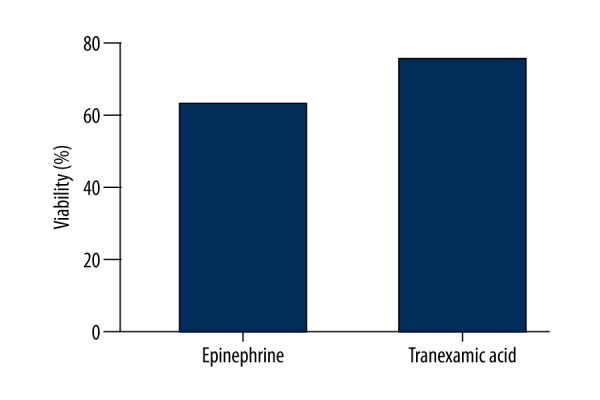
Viability stains in cartilage.
Discussion
Based on the histologic parameters and chondrocyte viability, the principal finding of this study is that TA is less cytotoxic than epinephrine in rat chondrocytes at the doses typically used in irrigation fluid, and may be a good alternative to epinephrine in arthroscopic surgery. Moreover, this study is the first to compare the cytotoxic effects of TA and epinephrine. Intra-operative bleeding during arthroscopic procedures is a challenging problem for orthopedic surgeons. Epinephrine is a potent vasoconstrictor, frequently added to the arthroscopic irrigation fluid to reduce intra-articular bleeding and thereby maintain adequate visualization [1,4,5,21]. However, recent experimental studies have shown that epinephrine is cytotoxic to the chondrocytes and may cause chondrolysis [22]. Rao et al. demonstrated that epinephrine is toxic to chondrocytes, even at concentrations as low as 1: 1000 mL, and its addition to irrigation fluid may not be advisable [23]. Alan et al. compared the effects of low-dose (1: 3 000 000) versus high-dose (1: 300 000) epinephrine on chondrocyte viability, reporting a significant decrease in chondrocyte survivability at the doses typically used in arthroscopic irrigation fluids. They also emphasized that high-dose epinephrine results in a significant increase in chondrocyte death over a very brief period (1 hour) [24]. In this in vitro study, the OARSI grades were significantly higher in all epinephrine subgroups (1-hour, 24-hour, and 1-week subgroups) when compared with the control groups: a finding similar to the previous reports.
Tranexamic acid is a well-known agent that effectively suppresses bleeding in various orthopedic surgical interventions, and may be administered intravenously or topically [18,25]. Only a few studies have evaluated the outcomes of intra-articular application, and only 1 has investigated the effect of TA on the cartilage and synovial tissues [11,26,27]. Tuttle et al. conducted a study to understand the in vitro effects of TA on bovine cartilage and murine chondrocytes. They studied TA at 3 doses (25, 50, and 100 mg/mL), reporting that 50-mg/mL and 100-mg/mL doses of TA have a cytotoxic effect on the chondrocytes and damaged the cartilage. However, 25 mg/mL may be an effective and safe dose for intra-articular use of TA in native joints [27]. Intra-articular use of TA is a simple procedure, with the inherent advantage of inducing partial microvascular hemostasis in the area by preventing fibrin clot dissolution [28]. When injected intra-articularly, TA is rapidly absorbed in the joint fluid, with an average biological half-life of 3 hours. Thus, a high concentration inside the joint for maximum effect can be achieved with limited systemic adverse effects [18,29]. In this experimental study, we determined the cytotoxicity by using histological parameters according to the OARSI grading-scoring system and chondrocyte viability percentage. OARSI system is a conventional histologic analysis showing changes in cartilage and synovial membrane. According to the OARSI system (in 1-week subgroups), the epinephrine group had significantly higher OARSI grades compared with the TA group. The most important cause of this significant difference in the 1-week subgroup is the greater number of chondrocyte deaths and cellular abnormalities in the epinephrine group vs. the TA group. Additionally, complex chondrins containing multiple and disoriented chondrocytes are seen as being more prominent in the epinephrine group, as described by Pritzker et al. [29]. The “live/dead” or “red/green” staining technique relies on the cell membrane integrity and provides a direct assessment of cell viability at the time of tissue harvest. This technique has been used to determine chondrocyte viability [20]. The percentage of viability at the time of tissue harvest was significantly higher in the TA group (only in the 1-week subgroup), and corresponded well to histologic findings, when compared with the epinephrine group. This study was designed as a safety study in regards to the cartilage viability, and the efficacy of TA in used doses was not within the scope of this study. The efficacy of intra-articular TA in these doses should be assessed in future studies.
Conclusions
In conclusion, TA is less cytotoxic to rat chondrocytes than epinephrine at the doses typically used in irrigation fluid. Hence, TA may be considered as a good alternative to epinephrine for reducing intra-articular bleeding during arthroscopic procedures, and can be safely used in partial arthroplasty cases.
Footnotes
Source of support: Bezmialem Vakif University Institutional Research Fund
References
- 1.Jensen KH, Werther K, Stryger V, et al. Arthroscopic shoulder surgery with epinephrine saline irrigation. Arthroscopy. 2001;17:578–81. doi: 10.1053/jars.2001.23590. [DOI] [PubMed] [Google Scholar]
- 2.Hsiao MS, Kusnezov N, Sieg RN, et al. Use of an irrigation pump system in arthroscopic procedures. Orthopedics. 39(3):e474–78. doi: 10.3928/01477447-20160427-01. [DOI] [PubMed] [Google Scholar]
- 3.Morrison DS, Schaefer RK, Friedman RL. The relationship between subacromial space pressure, blood pressure, and visual clarity during arthroscopic subacromial decompression. Arthroscopy. 1995;11:557–60. doi: 10.1016/0749-8063(95)90131-0. [DOI] [PubMed] [Google Scholar]
- 4.Avery DM, Gibson BW, Carolan GF. Surgeon-rated visualization in shoulder arthroscopy: A randomized blinded controlled trial comparing irrigation fluid with and without epinephrine. Arthroscopy. 31(1):12–18. doi: 10.1016/j.arthro.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 5.Van Montfoort DO, van Kampen PM, Huijsmans PE. Epinephrine diluted saline-irrigation fluid in arthroscopic shoulder surgery: A significant improvement of clarity of visual field and shortening of total operation time. A randomized controlled trial. Arthroscopy. 2015;32(3):436–44. doi: 10.1016/j.arthro.2015.08.027. [DOI] [PubMed] [Google Scholar]
- 6.Karns JL. Epinephrine-induced potentially lethal arrhythmia during arthroscopic shoulder surgery: A case report. AANA J. 1999;67(5):419–21. [PubMed] [Google Scholar]
- 7.Mazzocca AD, Meneghini RM, Chhablani R, et al. Epinephrine-induced pulmonary edema during arthroscopic knee surgery. A case report. J Bone Joint Surg Am. 2003;85-A(5):913–15. doi: 10.2106/00004623-200305000-00023. [DOI] [PubMed] [Google Scholar]
- 8.Buchko JZ, Gurney-Dunlop T, Shin JJ. Knee chondrolysis by infusion of bupivacaine with epinephrine through an intra-articular pain pump catheter after arthroscopic ACL reconstruction. Am J Sports Med. 2015;43(2):337–44. doi: 10.1177/0363546514555667. [DOI] [PubMed] [Google Scholar]
- 9.Dragoo JL, Korotkova T, Kim HJ, Jagadish A. Chondrotoxicity of low pH, epinephrine, and preservatives found in local anesthetics containing epinephrine. Am J Sports Med. 2010;38(6):1154–59. doi: 10.1177/0363546509359680. [DOI] [PubMed] [Google Scholar]
- 10.Dragoo JL, Braun HJ, Kim HJ, et al. The in vitro chondrotoxicity of single-dose local anesthetics. Am J Sports Med. 2012;40(4):794–99. doi: 10.1177/0363546511434571. [DOI] [PubMed] [Google Scholar]
- 11.Konig G, Hamlin BR, Waters JH. Topical tranexamic acid reduces blood loss and transfusion rates in total hip and total knee arthroplasty. J Arthroplasty. 2013;28(9):1473–76. doi: 10.1016/j.arth.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karaaslan F, Karaoğlu S, Mermerkaya MU, Baktir A. Reducing blood loss in simultaneous bilateral total knee arthroplasty: combined intravenous-intra-articular tranexamic acid administration. A prospective randomized controlled trial. Knee. 2015;22(2):131–35. doi: 10.1016/j.knee.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 13.Murkin JM, Falter F, Granton J, et al. High-dose tranexamic acid is associated with nonischemic clinical seizures in cardiac surgical patients. Anesth Analg. 2010;110(2):350–53. doi: 10.1213/ANE.0b013e3181c92b23. [DOI] [PubMed] [Google Scholar]
- 14.Morrison JJ, Dubose JJ, Rasmussen TE, Midwinter MJ. Military application of tranexamic acid in trauma emergency resuscitation (MATTERs) study. Arch Surg. 2012;147(2):113–19. doi: 10.1001/archsurg.2011.287. [DOI] [PubMed] [Google Scholar]
- 15.Ker K, Prieto-Merino D, Roberts I. Systematic review, meta-analysis and meta-regression of the effect of tranexamic acid on surgical blood loss. Br J Surg. 2013;100(10):1271–79. doi: 10.1002/bjs.9193. [DOI] [PubMed] [Google Scholar]
- 16.Alshryda S, Mason J, Vaghela M, et al. Topical (intra-articular) tranexamic acid reduces blood loss and transfusion rates following total knee replacement: A randomized controlled trial (TRANX-K) J Bone Joint Surg Am. 2013;95(21):1961–68. doi: 10.2106/JBJS.L.00907. [DOI] [PubMed] [Google Scholar]
- 17.Yuan ZF, Yin H, Ma WP, Xing DL. The combined effect of administration of intravenous and topical tranexamic acid on blood loss and transfusion rate in total knee arthroplasty: Combined tranexamic acid for TKA. Bone Joint Res. 2016;5(8):353–61. doi: 10.1302/2046-3758.58.BJR-2016-0001.R2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goyal N, Chen DB, Harris IA, et al. Intravenous vs. intra-articular tranexamic acid in total knee arthroplasty: A randomized, double-blind trial. J Arthroplasty. 2017;32(1):28–32. doi: 10.1016/j.arth.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 19.Pritzker KP, Gay S, Jimenez SA, et al. Osteoarthritis cartilage histopathology: Grading and staging. Osteoarthritis Cartilage. 2006;14(1):13–29. doi: 10.1016/j.joca.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 20.Kaplan LD. The analysis of articular cartilage after thermal exposure: “Is red really dead?”. Arthroscopy. 2003;19(3):310–13. doi: 10.1053/jars.2003.50087. [DOI] [PubMed] [Google Scholar]
- 21.Chierichini A, Frassanito L, Vergari A, et al. The effect of norepinephrine versus epinephrine in irrigation fluid on the incidence of hypotensive/bradycardic events during arthroscopic rotator cuff repair with interscalene block in the sitting position. Arthroscopy. 2015;31(5):800–6. doi: 10.1016/j.arthro.2015.02.030. [DOI] [PubMed] [Google Scholar]
- 22.Buchko JZ, Gurney-Dunlop T, Shin JJ. Knee chondrolysis by infusion of bupivacaine with epinephrine through an intra-articular pain pump catheter after arthroscopic ACL reconstruction. Am J Sports Med. 2015;43(2):337–44. doi: 10.1177/0363546514555667. [DOI] [PubMed] [Google Scholar]
- 23.Rao AJ, Johnston TR, Harris AH, et al. Inhibition of chondrocyte and synovial cell death after exposure to commonly used anesthetics: Chondrocyte apoptosis after anesthetics. Am J Sports Med. 2014;42(1):50–58. doi: 10.1177/0363546513507426. [DOI] [PubMed] [Google Scholar]
- 24.Dang AB, McCarthy MB, Dang AB, et al. Effects of adding epinephrine to arthroscopic irrigation fluid on cultured chondrocyte survival in vitro. Arthroscopy. 2011;27(8):1118–22. doi: 10.1016/j.arthro.2011.02.036. [DOI] [PubMed] [Google Scholar]
- 25.Tengborn L, Blombäck M, Berntorp E. Tranexamic acid – an old drug still going strong and making a revival. Thromb Res. 2015;135(2):231–42. doi: 10.1016/j.thromres.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 26.Digas G, Koutsogiannis I, Meletiadis G, et al. Intra-articular injection of tranexamic acid reduce blood loss in cemented total knee arthroplasty. Eur J Orthop Surg Traumatol. 2015;25(7):1181–88. doi: 10.1007/s00590-015-1664-8. [DOI] [PubMed] [Google Scholar]
- 27.Tuttle JR, Feltman PR, Ritterman SA, Ehrlich MG. Effects of tranexamic acid cytotoxicity on in vitro chondrocytes. Am J Orthop (Belle Mead NJ) 2015;44(12):E497–502. [PubMed] [Google Scholar]
- 28.Mannucci PM. Hemostatic drugs. N Engl J Med. 1998;339(4):245–53. doi: 10.1056/NEJM199807233390407. [DOI] [PubMed] [Google Scholar]
- 29.Seo JG, Moon YW, Park SH, et al. The comparative efficacies of intra-articular and IV tranexamic acid for reducing blood loss during total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2013;21(8):1869–74. doi: 10.1007/s00167-012-2079-2. [DOI] [PubMed] [Google Scholar]